Abstract
Two fluoroalkylated vinyltrimethoxysilane oligomer (RF-(CH2CHSi(OMe)3)n-RF; n = 2, 3; RF = CF(CF3)OC3F7:RF-VMSi) in methanol reacted with aqueous sodium carbonate solution containing 2-hydroxy-4,6-dichloro-1,3,5-triazine sodium salt (TAZ) to provide two fluoroalkylated oligomeric silica/TAZ nanocomposites (RF-VMSiO2/TAZ). The original cotton fabric gives an oleophilic/hydrophilic property on its surface; however, modified cotton fabric surface with RF-VMSiO2/TAZ composites was demonstrated to provide highly oleophobic/superhydrophobic property on the surface. We can observe a remarkable time-dependent decrease of the contact angle of dodecane (oil) on the modified surfaces, and the contact angles of dodecane were found to decrease effectively from 55°–83° to 0° over 5–30 s to supply superoleophilicity with keeping the superhydrophobic property on the surfaces. The modified cotton fabric having superoleophilic/superhydrophobic property was applicable to the separation membrane to separate oil and water. Interestingly, modified cotton fabric was found to adsorb efficiently only droplets of oil spread on the water interface due to its unique surface wettability.
1. Introduction
In the textile industry, cotton fabric as a typical textile is of particular importance as it provides a variety of preferable characteristics, such as low cost, wearing comfortability, breathability, absorptivity toward water, flexibility, and environmental friendliness [1]. Most textile fabrics including cotton are in general likely to absorb significant amounts of water and numerous oils [1,2,3,4,5,6,7]. From this point of view, the modified textile fabrics with surface treated water- and oil-repellent properties have been among the most intensively studied subjects in order to create additional functionalities, such as fire retardancy, antibacterial activity, and UV protection [8,9,10,11]. There has heretofore been a considerable interest in the fabrication of superhydrophobic textile fabrics from the developmental point of view of novel practical applications such as self-cleaning, anti-corrosion, friction reduction, and anti-icing characteristics [2,3,4,5,6,7]. We can find a variety of reports on the exploration of superhydrophobic textiles based on the creation of the roughness surface by the grafting polymerization [12,13], layer-by-layer assembly [14,15], dip-coating [16,17,18,19,20], chemical vapor depositing [21,22,23], and spray coating [24,25,26,27,28]. During our comprehensive studies on the exploration of superhydrophilic/superoleophobic, superamphiphobic, and superhydrophobic/superoleophilic surfaces by using two fluoroalkylated oligomer [26,27,28,29,30], two fluoroalkylated vinyltrimethoxysilane oligomer (RF-(CH2CHSi(OMe)3)n-RF:RF-VMSi) was shown to be a useful tool for the surface modification of glass, giving not only good oleophobicity but also a perfectly superhydrophobic characteristic with non-wetting properties against water droplets [31]. RF-VMSi oligomer was also developed to create two fluoroalkylated oligomeric talc/Pst composites through the reaction of the fluorinated oligomer in the presence of micrometer sized polystyrene particles (Pst) and the talc under alkaline conditions, applying to the packing material possessing superoleophilic/superhydrophobic properties to separate oil/water [32]. Now, we would like to report herein that two fluoroalkylated vinyltrimethoxysilane oligomeric silica/triazine derivative (TAZ) nanocomposites, which were prepared by reaction of the fluoroalkylated oligomer with TAZ in the presence of sodium carbonate, were applicable to surface modification of cotton fabric, suppling superoleophilic/superhydrophobic properties toward the surface. The modified cotton fabric was also applicable to the separation membrane for the separation of oil/water. Additionally, it was clarified that the obtained modified cotton fabric could adsorb only oil droplets spread on the water interface.
2. Experimental
2.1. Measurements
Particle size of the composites was determined by the use of dynamic light scattering (DLS) analyses (Otsuka Electronics ELSZ-2000ZS: Tokyo, Japan). Contact angles of water and dodecane were recorded using Kyowa Interface Science Drop Master 300: Saitama, Japan. Field emission scanning electron micrography (FE-SEM) was determined by the use of a JEOL JSM-7000F (Tokyo, Japan). Energy dispersive X-ray (EDX) spectra were detected by a JEOL JSM-7000F (Tokyo, Japan). Stress–strain curve testing was examined using an A & D STB-1225S (Tokyo, Japan).
2.2. Materials
Vinyltrimethoxysilane and 10% aqueous 2-hydroxy-4,6-dichloro-1,3,5-triazine sodium salt were purchased from Dow Corning Toray Co., Ltd. (Tokyo, Japan) and Fujifilm Holdings Corp. (Tokyo, Japan), respectively. Two fluoroalkylated vinyltrimethoxysilane oligomer (RF-(CH2-CHSi(OMe)3)n-RF:the mixture of dimer and trimer; RF = CF(CF3)OC3F7 (RF-VMSi)) was prepared based on our previously reported method [33]. Cotton fabric (JIS L0803) was obtained from Japanese Standard Association (Tokyo, Japan). Acid Yellow 3 and Red G were received from Chugaikasei Co., Ltd. (Fukushima, Japan).
2.2.1. Preparation of Two Fluoroalkylated Vinyltrimethoxysilane Oligomeric Silica/Triazine Derivative Nanocomposites (RF-VMSiO2/TAZ)
A 0.05 mol/dm3 aqueous sodium carbonate solution (2 mL) was added into a methanol solution (5 mL) containing fluorinated oligomer (RF-VMSi) (30 mg), 10% TAZ aqueous solution (100 mg) and water (3 mL). The mixture was stirred for 5 h at room temperature. The solvent was evaporated off under reduced pressure, and then methanol was added to the obtained crude products. The obtained methanol solution was stirred at room temperature for one day, and successively was centrifuged for 30 min. The expected fluorinated oligomeric silica/TAZ nanocomposites thus obtained were easily separated from the methanol solution and successively washed with methanol several times. The obtained product was dried in vacuum at 50 °C for one day to give the purified fluorinated nanocomposites (17 mg). Other nanocomposites that were prepared under similar conditions are shown in Table 1.

Table 1.
Synthesis of RF-VMSiO2/TAZ nanocomposites.
2.2.2. Fabrication of Modified Cotton Fabric with RF-VMSiO2/TAZ Nanocomposites by Casting Method
The aqueous methanol solution (8 mL: MeOH/water (5/3 (vol/vol)) containing the RF-VMSi oligomer (30 mg), 10% TAZ aqueous solution (100 mg), and cotton fabric swatch (a square of 20 mm × 20 mm) was stirred for 1 h at room temperature. A 0.05 mol/dm3 aqueous sodium carbonate solution (2 mL) was added to this solution. The obtained mixture was stirred at room temperature for 5 h. Modified cotton fabric was lifted from the solutions at a constant rate (0.5 mm/min) and subjected to the treatment for 5 min at 150 °C. This fabric swatch was washed with water and was dried under vacuum for one day at room temperature. We have measured the dodecane and water contact angle values by the deposit of each droplet (2 μL) on the modified cotton fabric surface. To verify adhesion ability of the modified cotton swatch, this fabric was soaked into the petri dish containing water (30 mL) and was stirred for 10 min at room temperature. We measured the dodecane and water contact angle values on this surface under similar conditions after this swatch was dried under vacuum. Similarly, repeated measurements of the contact angle values of dodecane and water on this modified swatch surface were conducted after washing this swatch with water and then acetone a few times, and finally after drying under vacuum in each cycle.
3. Results and Discussion
It is well-known that cyanuric chloride can react with hydroxy groups in cotton fiber under alkaline conditions to produce the cyanuric chloride-immobilized cotton fibers [34,35,36]. Thus, we tried to react RF-VMSi oligomer with 2-hydroxy-4,6-dichloro-1,3,5-triazine sodium salt (TAZ) under alkaline conditions. The trimethoxysilyl groups –Si(OMe)3 in RF-VMSi oligomer are suggested to cause the sol–gel reactions in the presence of sodium carbonate to give the hydroxy groups, reacting with TAZ to afford fluoroalkylated silica/TAZ composites. As shown in Scheme 1 and Table 1, the reaction of RF-VMSi oligomer with TAZ in the presence of sodium carbonate proceeded to supply the RF-VMSiO2/TAZ composites in 43%–87% yields. The composites thus obtained were shown to give good dispersibility for water and methanol. Thus, the particle size of the composites was determined at 25 °C by using the dynamic light scattering measurements. The results are illustrated in Table 1.
Scheme 1.
Synthesis of RF-VMSiO2/TAZ nanocomposites.
As illustrated in Table 1, the size of the obtained composites was nanometer sized fine particles from 59 to 65 nm levels. In this way, RF-VMSi oligomer was found to react effectively with TAZ at room temperature in the presence of sodium carbonate to give the fluoroalkylated oligomeric silica/TAZ nanocomposites (RF-VMSiO2/TAZ). Thus, the surface modification of the cotton fabric swatch was conducted by dipping technique with the aqueous methanol sol solutions containing RF-VMSi oligomer and TAZ in the presence of sodium carbonate. The surface appearance of the modified cotton swatch is illustrated in Figure 1.

Figure 1.
Photograph of original cotton fabric (A) and modified cotton swatch with RF-VMSiO2/TAZ nanocomposites (B); used oligomer: 30 mg; 10% aq. TAZ solution: 100 mg (Run 1 in Table 1).
We have the similar surface appearance of the modified cotton fabric to that of the original one as depicted in Figure 1. Thus, the contact angle value of dodecane was measured on the modified cotton swatch surface with that of the pristine cotton fabric surface, for comparison. The results are illustrated in Table 2.

Table 2.
Dodecane contact angles on the modified cotton fabric with the RF-VMSiO2/TAZ nanocomposites.
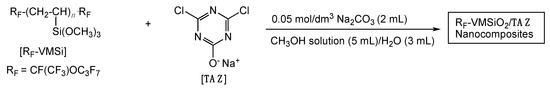
The contact angle values of dodecane after dropping dodecane (oil) droplets were 55°–83° as depicted in Table 2, affording a higher oleophobic property toward the modified cotton fabric surface. In contrast, the pristine cotton fiber gave a superoleophilic property (dodecane contact angle value: 0°). However, we could observe a remarkable time-dependent decrease of dodecane contact angle values on the cotton fabric. It was demonstrated that contact angles for dodecane decreased effectively from 55°–83° to 0° within 5–30 s, giving superoleophilicity on the modified surface as illustrated in Figure 2A (Figure 2A exhibits the decrease of the contact angle value of dodecane on the surface (Run 1 in Table 2)). This result indicates that we can observe the smooth replacement of hydrophobic fluoroalkyl segments in the composites at the interface with dodecane by the oleophilic TAZ within 5–50 s to provide the superoleophilic characteristic.
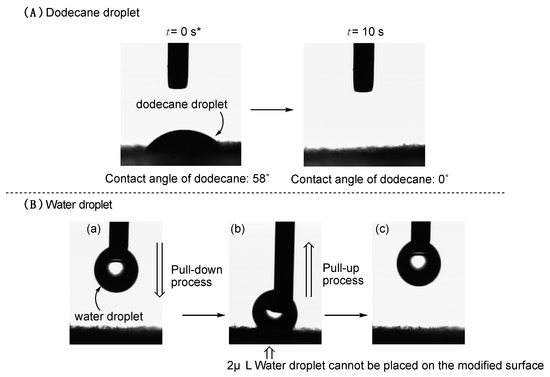
Figure 2.
Photograph of the droplet of dodecane (A) and the droplet of water (B) on the modified cotton fabric surface with RF-VMSiO2/TAZ composites (Run 1 in Table 2): (*) “0 s” approximately corresponds to “a few seconds.” (a) Water drop on the needlepoint, (b) water drop on the fabric surface, (c) pull-up of needlepoint from the modified cotton fabric surface.
On the other hand, the original cotton fabric surface afforded a superhydrophilic property; however, we observed the superhydrophobic property on each modified cotton swatch surface illustrated in Runs 1–4 in Table 2. The 2 μL water droplet was not placed on the modified surface as shown in Figure 2(B–b) and (B–c). In fact, it is impossible to deposit water droplet on the surface owing to the superhydrophobic characteristic even after the pull-up of the needlepoint from the surface depicted in Figure 2(B–a) and (B–c) (we call such wettability the superhydrophobic characteristic for convenience in this case). The creation of the superhydrophobic surface at the interface with water is related to the smooth replacement from the oleophilic TAZ moieties in the nanocomposites to the hydrophobic fluoroalkyl groups. Such flip-flop motion between the oleophilic TAZ and the fluoroalkyl groups would be effectively observed in the composite matrices under the environmental change from oil to water on the surface. We can observe the similar flip-flop motion between the longer fluoroalkyl groups and hydrophilic segments to provide the hydrophilic property with an oleophobicity on the surfaces [37]. In contrast, the RF-VMSi oligomer had already been applied to the surface modification of PET (poly(ethylene terephthalate)) to supply the oleophobic/hydrophobic surface [38]. Thus, the present unique wettability was related to the presence of the TAZ moieties in the composites. Especially owing to the lower surface tension of oil droplets such as dodecane compared to water, the oil droplet could smoothly penetrate the small orifice between the cotton fibers to show a superoleophilic property on the modified surface as shown in Figure 3C. In contrast, the fluoroalkyl groups in the composites should be arranged on the modified cotton surfaces, providing the superhydrophobic property due to the architecture of the roughness surface (see SEM pictures illustrated in Figure 3B). Heretofore, it is well-known that the superhydrophobic surface is realized by enhancing the surface roughness [39]. Therefore, the modified cotton surface coated with RF-VMSiO2/TAZ nanocomposites illustrated in Figure 3B should provide a superhydrophobic property by the fluoroalkyl groups in the composites on the roughness surface.
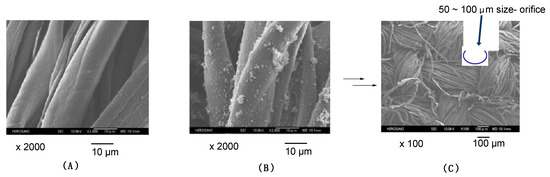
Figure 3.
SEM pictures of pristine cotton swatch (A), the modified cotton watch with RF-VMSiO2/TAZ composites (Run 1 in Table 2) (B,C).
To verify the adhesion ability of the modified cotton swatch, we studied the relationship between the contact angle values of dodecane on the modified cotton swatch and cycle to wash the modified fabric with water. The results are depicted in Figure 4.
Figure 4.
Relationship between the dodecane contact angle values on the modified cotton swatch and cycle of washing the modified cotton swatch with water.
Figure 4 shows that the contact angle values of dodecane could keep quite similar values to the superhydrophobic property illustrated in Figure 2B even after three cycles, indicating that the RF-VMSiO2/TAZ nanocomposites possess a strong adhesion ability toward the cotton fabric to give a superoleophilic/superhydrophobic property on the surface (contact angle value of dodecane in each cycle decreased to 0° after 6–15 s). Moreover, the similar contact angle values of dodecane (32°–53°; these values in each cycle changed to 0° within 6–12 s) were observed with keeping the superhydrophobic characteristic from 4 to 10 cycles. In fact, EDX analyses indicate that the similar atomic values of not only fluorine and silicon related to the fluorinated oligomer but also nitrogen and chlorine related to TAZ in composites on the cotton swatch were observed in each cycle (see Table 3).

Table 3.
The atomic contents of fluorine, silicon, nitrogen, and chlorine on the modified cotton fabric in each washing cycle.
Furthermore, we could observe the uniformly dispersed fluorine atoms in the nanocomposites corresponding to the blue-colored area on the modified cotton fabric even after three cycles (Figure 5C), compared to that of the parent cotton swatch (Figure 5A) or the modified cotton swatch treated with the fluoroalkylated composites (Figure 5B).
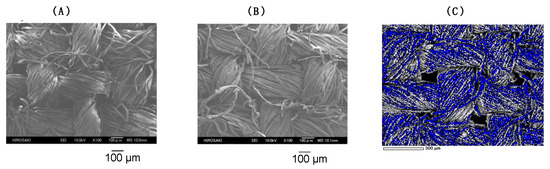
Figure 5.
FE-SEM pictures of the parent cotton swatch (A) and modified cotton swatch with RF-VMSiO2/TAZ nanocomposites (B), and EDX mapping micrograph of the fluorine atoms on the cotton surface with RF-VMSiO2/TAZ nanocomposites after three cycles of washing with water (C).
The adhesion ability of the present modified cotton fabric with RF-VMSiO2/TAZ nanocomposites (Run 1 in Table 2) was also studied with the strong acid solution (0.1 mol/dm3 HCl (pH: 1)) and alkaline solution (1.0 mol/dm3 NaOH (pH: 14)) through the measurements of contact angle values of dodecane on the cotton swatch surface. The results are depicted in Table 4.

Table 4.
Relationship between dodecane contact angle values on the modified cotton fabric and immersion time (min) into the strong acid and alkaline solutions.
As indicated in Table 4, the adhesion ability of present modified cotton swatch is strong enough even after immersion into the strong acidic solution (pH: 1.0) for 120 min, because we can keep the same contact angle values of dodecane with superhydrophobic property shown in Figure 2B in each case. In contrast, the contact angle values of dodecane decreased from 58° to 29° after immersion in the strong alkaline solution for 120 m. Similarly, the surface wettability change from superhydrophobic to hydrophobic (water contact angle value: 141°) was observed under such conditions, indicating that the siloxane bond units in the nanocomposites are likely to suffer the nucleophilic attach of hydroxy anion under the strong alkaline conditions.
We have studied the mechanical property of the modified cotton swatch (square of 10 mm × 20 mm × 244 μm (fabric thickness): Run 1 in Table 2). The original cotton fabric (square of 10 mm × 20 mm × 225 μm (fabric thickness)) was examined for comparison. Typical stress–strain curves for the modified cotton fabric with the original one at the crosshead speed of 200 mm/min at room temperature are shown in Figure 6.
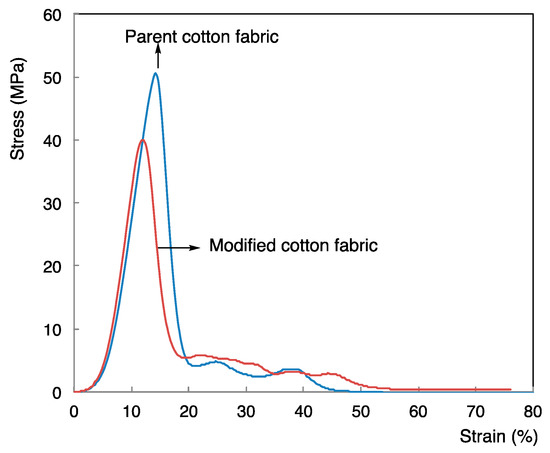
Figure 6.
Stress–strain plots of the parent cotton swatch and the modified cotton swatch treated with RF-VMSiO2/TAZ nanocomposites (Run 1 in Table 2).
Figure 6 shows that the modified cotton swatch gave a similar Young’s modulus (613 MPa) to that (617 MPa) of the parent cotton fabric. In addition, we could observe a similar maximum stress value (40 MPa) and elongation at break (56%) to those (51 MPa and 52%) of the original one, indicating that the RF-VMSiO2/TAZ composites are useful for uniform surface modification of cotton fabric.
It is generally well-known that superoleophilic surfaces can give strong affinity for organic oils. Thus, surfaces bearing superoleophilic/superhydrophobic characteristic simultaneously repel water and efficiently absorb oils. Such a unique finding was applied to oil/water separation membranes and self-cleaning [40,41,42]. Thus, the present RF-VMSiO2/TAZ nanocomposites were applied to the separation membrane to separate the blue-colored water (CuSO4·5H2O was used for the preparation of blue-colored water) and 1,2-dichlororethane. The results are illustrated in Figure 7 and Supplementary Materials Movie S1.
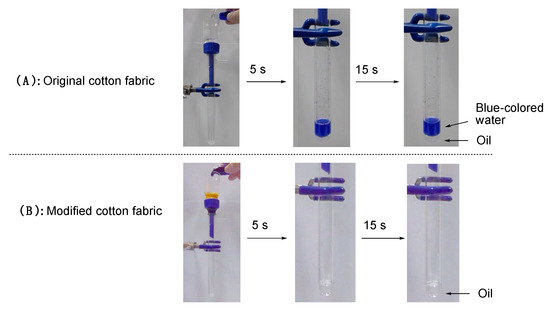
Figure 7.
Separation of blue-colored water that was colored with CuSO4·5H2O and 1,2-dichloroethane using pristine cotton fabric (A) and the modified cotton fabric with RF-VMSiO2/TAZ composites (Run 1 in Table 2) (B).
As illustrated in Figure 7A and Supplementary Materials Movie S (1A), we could not separate the oil/water mixture using the original cotton fabric. However, we could have the smooth separation of the mixture of oil and water using the modified cotton fabric with the RF-VMSiO2/TAZ composites (Run 1 in Table 2) to isolate the transparent colorless oil (1,2-dichloroethane) due to its superoleophilic/superhydrophobic characteristic (see Figure 7B and Supplementary Materials Movie S (1B)).
As indicated above, most textile fabrics including cotton can absorb both water and numerous oils effectively. Thus, our present cotton fabric bearing a superoleophilic/superhydrophobic property is quite interesting from the developmental point of textile fabrics having a unique surface wettability. In fact, the adsorption ability of the oil drop spread on water interface was studied by the use of the present modified cotton swatch. The results are depicted in Figure 8 and Supplementary Materials Movie S2.
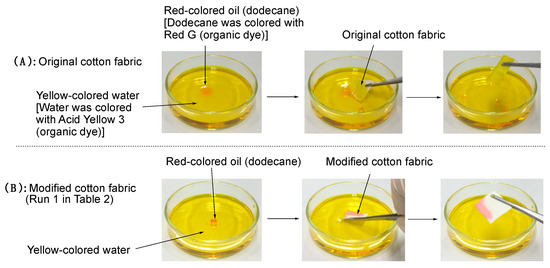
Figure 8.
Adsorption of red-colored oil (dodecane) droplets spread on yellow-colored water interface using pristine cotton fabric (A) and the modified cotton swatch with RF-VMSiO2/TAZ nanocomposites (Run 1 in Table 2) (B).
Figure 8A and Supplementary Materials Movie S (2A) show that the original cotton swatch adsorbed not only red-colored oil (oil:dodecane; dodecane was colored with organic dye (Red G)) but also yellow-colored water (water was colored with organic dye (Acid Yellow 3)); however, we could find the residual red-colored oil droplets spread on yellow-colored water interface. On the other hand, interestingly, the present modified cotton swatch could adsorb only red-colored oil droplets spread on the yellow-colored water interface under similar conditions. Thus, we could not detect the adsorbed yellow-colored water into the modified cotton fabric at all, due to its superoleophilic/superhydrophobic property (see Figure 8B and Supplementary Materials Movie S (2B)).
4. Conclusions
We found that the RF-VMSiO2/TAZ nanocomposites were obtained by the reaction of the fluoroalkylated oligomer in the presence of TAZ and sodium carbonate. The RF-VMSiO2/TAZ composites thus obtained were applied to the surface modification of cotton swatch, affording superoleophilic/superhydrophobic properties on the modified surface. Adhesion ability toward modified cotton swatch was strong enough even after washing the corresponding fabric with water ten cycles, giving the similar superoleophilic/superhydrophobic characteristic on the surface. Modified cotton fabric was applied to the separation membrane for the separation of oil/water, isolating to transparent colorless oil. Modified cotton fabric was also applied to the perfect adsorption of oil droplets spread on water interface due to its superoleophilic/superhydrophobic characteristic, although the original cotton fabric can adsorb not only oil droplets but also water. Therefore, we believe that the present modified cotton fabric swatch has high potential for application into numerous fields including the cosmetic materials such as oil-absorbing facial paper.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6412/10/2/174/s1, Movie S1: Separation of blue-colored water and 1,2-dichloroethane using the pristine cotton swatch (A) and the modified cotton swatch with RF-VMSiO2/TAZ composites (Run 1 in Table 2) (B); Movie S2: Adsorption of red-colored oil (dodecane) droplets spread on yellow-colored water interface using pristine cotton swatch (A) and modified cotton swatch with RF-VMSiO2/TAZ composites (Run 1 in Table 2) (B).
Author Contributions
Conceptualization, H.S. and A.Y.; methodology, H.S.; software, H.S.; validation, H.S., K.Y. and A.Y.; formal analysis, K.Y.; investigation, K.Y.; resources, H.S.; data curation, K.Y. and H.S.; writing—original draft preparation, K.Y.; writing—review and editing, H.S.; visualization, H.S.; supervision, H.S.; project administration, H.S.; funding acquisition, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by a Grant-in-Aid for Scientific Research 19K05027 from the Ministry of Education, Science, Sports, and Culture, Japan.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Hu, J. Active Coatings for Smart Textiles; Woodhead Publishing Series in Textile: No. 176; Woodhead Publishing: Duxford, UK, 2016. [Google Scholar]
- Lewin, M.; Sello, S.B. Handbook of Fiber Science and Technology: Volume II Chemical Processing of Fibers and Fabrics; Functional Finishes, Part B; Marcel Dekker, Inc.: New York, NY, USA, 1984. [Google Scholar]
- Xu, Q.; Shen, L.; Duan, P.; Zhang, L.; Fu, F.; Liu, X. Superhydrophobic cotton fabric with excellent healability fabricated by the “grafting to” method using a diblock copolymer mist. Chem. Eng. J. 2020. [Google Scholar] [CrossRef]
- Xu, Q.; Xie, L.; Diao, H.; Li, F.; Zhang, Y.; Fu, F.; Liu, X. Antibacterial cotton fabric with enhanced durability prepared using silver nanoparticles and carboxymethyl chitosan. Carbohydr. Polym. 2017, 177, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.-Y.; Zhao, X.-L.; Li, Y.-D.; Weng, Y.-X.; Zeng, J.-B. Robust and nanoparticle-free superhydrophobic cotton fabric fabricated from all biological resources for oil/water separation. Int. J. Biol. Macromol. 2019, 140, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Mazzon, G.; Athanassiou, A.; Bayer, I.S. Environmentally benign non-wettable textile treatments: A review of recent state-of-the-art. Adv. Colloid Interface Sci. 2019, 270, 216–250. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.J.; Williams, D.E. Surface modification by fluoroalkyl-functional silanes. J. Adhes. Sci. Technol. 1991, 5, 307–320. [Google Scholar] [CrossRef]
- Zope, I.S.; Foo, S.; Seah, D.G.; Akunuri, A.T.; Dasari, A. Development and evaluation of a water-based flame retardant spray coating for cotton fabrics. ACS Appl. Mater. Interfaces 2017, 9, 40782–40791. [Google Scholar] [CrossRef] [PubMed]
- Emam, H.E.; Abdelhameed, R.M. Anti-UV radiation textiles designed by embracing with nano-MIL (Ti, In)-metal organic framework. ACS Appl. Mater. Interfaces 2017, 9, 28034–28045. [Google Scholar] [CrossRef]
- Chen, S.; Chen, S.; Jiang, S.; Xiong, M.; Luoi, J.; Tang, J.; Ge, Z. Environmentally friendly antibacterial cotton textiles finished with siloxane sulfopropylbetaine. ACS Appl. Mater. Interfaces 2011, 3, 1154–1162. [Google Scholar] [CrossRef]
- Kawase, T.; Tanba, K.; Peng, X.; Fujii, T.; Sawada, H.; Ikematsu, Y.; Wada, K. Water repellent and antibacterial modification of cellulose using fluoroalkyl end-capped oligomers. Sen’i Gakkaishi 2000, 56, 155–162. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, J.; Li, R.; Pang, L.; Xing, Z.; Xu, L.; Wu, G. Preparation and characterization of superhydrophobic organic-inorganic hybrid cotton fabrics via γ-radiation-induced graft polymerization. Carbohydr. Polym. 2016, 149, 308–316. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Shao, J.; Zou, C. Fabrication of superhydrophobic fluorine-free films on cotton fabrics through plasma-induced grafting polymerization of 1,3,5,7-tetravinyl-1,3,5,7-tetramethylcyclotetrasiloxane. Surf. Coat. Technol. 2015, 276, 16–22. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Wang, C.; Li, J. A facile method to fabricate superhydrophobic cotton fabrics. Appl. Surf. Sci. 2012, 261, 561–566. [Google Scholar] [CrossRef]
- Amigoni, S.; de Givenchy, E.T.; Dufay, M.; Guittard, F. Covalent layer-by-layer assembled superhydrophobic organic-inorganic hybrid films. Langmuir 2009, 25, 11073–11077. [Google Scholar] [CrossRef] [PubMed]
- Mahdik, S.A.; Kavale, M.S.; Mukherjee, S.; Rao, A.V. Transparent superhydrophobic silica coatings on glass by sol-gel method. Appl. Surf. Sci. 2010, 257, 333–339. [Google Scholar] [CrossRef]
- Zhu, T.; Li, S.; Huang, J.; Mihailiasa, M.; Lai, Y. Rational design of multi-layered superhydrophobic coating on cotton fabrics for UV shielding, self-cleaning and oil-water separation. Mater. Des. 2017, 134, 342–351. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, W.; Yang, M.; Liu, C.; He, S.; Xie, Y.; Wang, Z. Facile fabrication of robust fluorine-free self-cleaning cotton textiles with superhydrophobicity, photocatalytic activity, and UV durability. Colloid Surf. A 2018, 559, 235–242. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef]
- Feng, J.; Sun, M.; Ye, Y. Ultradurable underwater superoleophobic surfaces obtained by vapor-synthesized layered polymer nanocoatings for highly efficient oil-water separation. J. Mater. Chem. A 2017, 5, 14990–14995. [Google Scholar] [CrossRef]
- Pour, F.Z.; Karimi, H.; Avargani, V.M. Preparation of a superhydrophobic and superoleophilic polyester textile by chemical vapor deposition of dichlorodimethylsilane for water-oil separation. Polyhedron 2019, 159, 54–63. [Google Scholar] [CrossRef]
- Xue, C.-H.; Fan, Q.-Q.; Guo, X.-J.; An, Q.-F.; Jia, S.-T. Fabrication of superhydrophobic cotton fabrics by grafting of POSS-based polymers on fibers. Apply. Surf. Sci. 2019, 465, 241–248. [Google Scholar] [CrossRef]
- Latthe, S.S.; Rao, A.V. Superhydrophobic SiO2 micro-particle coatings by spray method. Surf. Coat. Technol. 2012, 207, 489–492. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Zerafat, M.M. Fabrication of stable fluorine-free superhydrophobic fabrics for anti-adhesion and self-cleaning properties. Appl. Surface Sci. 2019, 464, 458–471. [Google Scholar] [CrossRef]
- Yao, H.; Lu, X.; Xin, Z.; Zhang, H.; Li, X. A durable bio-based polybenzoxazine/SiO2 modified fabric with superhydrophobicity and superoleophilicity for oil/water separation. Sep. Purif. Technol. 2019, 229, 115792. [Google Scholar] [CrossRef]
- Sawada, H. Fluorinated peroxides. Chem. Rev. 1996, 96, 1779–1808. [Google Scholar] [CrossRef]
- Sawada, H. Synthesis of self-assembled fluoroalkyl end-capped oligomeric aggregates—Applications of these aggregates to fluorinated oligomeric nanocomposites. Prog. Polym. Sci. 2007, 32, 509–533. [Google Scholar] [CrossRef]
- Sawada, H. Preparation and applications of novel fluoroalkyl end-capped oligomeric nanocomposites. Polym. Chem. 2012, 3, 46–65. [Google Scholar] [CrossRef]
- Ameduri, B.; Sawada, H. Fluorinated Polymers: Volume 1, Synthesis, Properties Processing and Simulation; RSC: Cambridge, UK, 2016. [Google Scholar]
- Ameduri, B.; Sawada, H. Fluorinated Polymers: Volume 2, Applications; RSC: Cambridge, UK, 2016. [Google Scholar]
- Sawada, H.; Suzuki, T.; Takashima, H.; Takishita, K. Preparation and properties of fluoroalkyl end-capped vinyltrimethoxysilane oligomeric nanoparticles—A new approach to facile creation of a completely superhydrophobic coating surface with these nanoparticles. Colloid Polym. Sci. 2008, 286, 1569–1574. [Google Scholar] [CrossRef]
- Oikawa, Y.; Saito, T.; Yamada, S.; Sugiya, M.; Sawada, H. Preparation and surface property of fluoroalkyl end-capped vinyltrimethoxysilane oligomer/talc composite-encapsulated organic compounds: Application for the separation of oil and water. ACS Appl. Mater. Interfaces 2015, 7, 13782–13793. [Google Scholar] [CrossRef]
- Sawada, H.; Nakayama, M. Synthesis of fluorine-containing organosilicon oligomers. J. Chem. Soc. Chem. Commun. 1991, 677–678. [Google Scholar] [CrossRef]
- Shibata, S.; Joko, K. Reactivity of the active chlorine of cyanuric chloride immobilized on cotton fibers. Sen’i Gakkaishi 2013, 69, 240–244. [Google Scholar] [CrossRef][Green Version]
- Zadorecki, P.; Flodin, P. Surface modification of cellulose fibers. I. Spectroscopic characterization of surface-modified cellulose fibers and their copolymerization with styrene. J. Appl. Polym. Sci. 1985, 30, 2419–2429. [Google Scholar] [CrossRef]
- Zadorecki, P.; Flodin, P. Surface modification of cellulose fibers. II. The effect of cellulose fiber treatment on the performance of cellulose-polyester composites. J. Appl. Polym. Sci. 1985, 30, 3971–3983. [Google Scholar] [CrossRef]
- Sawada, H.; Ikematsu, Y.; Kawase, T.; Hayakawa, Y. Synthesis and surface properties of novel fluoroalkylated flip-flop-type silane coupling agents. Langmuir 1996, 12, 3529–3530. [Google Scholar] [CrossRef]
- Kawase, T.; Yamane, M.; Fujii, T.; Minagawa, M.; Sawada, H.; Moriya, Y. Fluoroalkylation of polyester by end-capped fluoroalkyl-functional silanes. J. Adhes. Sci. Technol. 1997, 11, 1381–1397. [Google Scholar] [CrossRef]
- Liu, J.; Wan, H.; Ye, Y.; Zhou, H.; Chen, J. One-step process to fabrication of transparent superhydrophobic SiO2 paper. Appl. Surface Sci. 2012, 261, 470–472. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Wang, S.; Shi, Y.; Li, J. Fabrication of coral-like superhydrophobic coating on filter paper for water-oil separation. Appl. Surface Sci. 2012, 261, 764–769. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Wang, S.; Shi, Y.; Li, J. Fabrication of superhydrophobic cotton textiles for water-oil separation based on drop-coating route. Carbohydr. Polym. 2013, 97, 59–64. [Google Scholar] [CrossRef]
- Arbatan, T.; Zhang, L.; Fang, X.-Y.; Shen, W. Cellulose nanofibers as binder for fabrication of superhydrophobic paper. Chem. Eng. J. 2012, 210, 74–79. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).