1. Introduction
Coatings have been used for many purposes such as protection, decoration, and functional. For protection, the coating has been applied in many construction applications as passive fire protection such as a suspended ceiling, cladding, or intumescent coating [
1]. Due to the fact that substrates used in construction work such as steelwork or aluminium are prone to buckling, bending, or deflecting under a certain load, it is necessary for the coating material to be sufficiently flexible so as not to exhibit any tears, cracks, or debonding. Coating material that failed under load stress probably has weak adhesion bonding and is subjected to undercutting at the bend region. Two properties which are flexural strength and flexural modulus have been considered in evaluating the properties of coating material. Flexural strength is the maximum stress that the coating material can withstand before reaching a breaking point. Flexural modulus represents the coating material stiffness which measures the ability to resist bending or displacement when force is applied.
In previous literature, three-point bending tests were used to determine the flexural performance of various coating materials. Yang et al. carried out a three-point bending test to study the mechanical and bonding properties of the Al
2O
3/Ni surface that infiltrated the composite layer. The result showed that the peak load was inversely proportional to the content of Al
2O
3 [
2]. Li et al. also used a three-point bending test to investigate the mechanical properties of different NiCrAlY coating overlayers. It was found that the damage behavior was mostly influenced by the interfacial adhesion and the overlayer (brittle or ductile) property [
3]. To date, no research on the flexural properties of the geopolymer composite coating (GCC) has been conducted and therefore this research may provide useful information for the improvement of coating material in the building construction industry.
Geopolymer is a material that has attracted wide interests among many scientists around the world due to its excellent properties which include high compressive strength, low shrinkage, acid, and fire resistance [
4,
5,
6]. Studies have been conducted on different forms of geopolymer including geopolymer concrete [
7,
8], precast paving cement-free brick [
9], and lightweight geopolymer composite [
10]. As an inorganic polymer which is based on synthetic aluminosilicate materials [
11], geopolymer has a potential in coating application as a surface protector and insulator for various surfaces including metal [
12]. Fly ash, metakaolin, and blast furnace slag are the example of pozzolanic waste and were used widely to form GCC and coated either on ceramic or metal substrate [
12,
13]. However, to the best of our knowledge, there were no publications on the application of optimization method to obtain the best composition of GCC to produce sufficiently flexible coating and the application of rice husk ash as geopolymer coating on a metal substrate. In this research, the rice husk ash will be used as the main component in producing GCC and response surface methodology (RSM) used as the optimization method to elucidate the best coating composition that can be achieved. This method will also be used to validate the predicted optimal value.
Rice husk contains approximately 50, 25%–30% and 15%–20% of cellulose, lignin and silica contents, respectively. When it is burnt under controlled temperature and environment, more than 85% of silica is left behind together with RHA. For every 100 kg of paddy milled, nearly 20 kg which is 20% of husk is produced and about 5 kg (25%) of RHA is generated after burning [
14]. RHA contains around 93% and 0.4% of silicon dioxide (SiO
2) and aluminum oxide (Al
2O
3), respectively, compared to fly ash, which has about 41 and 21.6% of SiO
2 and Al
2O
3, respectively [
15]. This suggests that RHA contains higher amount of silica as compared to fly ash. However, their relative quantity differs according to their origin. Higher silica contains higher compressive strength. According to Songpiriyakij et al., a higher Si/Al ratio will lead to higher compressive strength, but lower compressive strength is observed when the Si/Al ratio exceeds 15 [
16]. De Silva et al. obtained similar results for the Si/Al ratio that is between 2.5 and 5. They found that a higher content of alumina provides lower mechanical strength [
17].
In order to maximize the properties of geopolymer based on RHA, many parameters have to be considered including the ratio of activated alkaline (AA) solution, concentration of sodium hydroxide (NaOH) solution, curing temperature and time, RHA/AA ratio, sintering temperature and time, water content of mixture, and size of materials aggregate. It is laborious and time-consuming to develop a process that involves a large number of factors in order to obtain the optimum result using classical method (which is usually involves one factor at a time) of the data analysis. Response Surface Methodology (RSM) consists of a combination of mathematical and statistical techniques which is useful in modeling, analyzing, and optimizing responses that are influenced by several factors. It was originally coined by Box and Wilson [
18] particularly to study the relationship between factors and responses. Besides minimizing the number of experiments needed (for a high number of levels) to produce relevant information for a set of inputs, the choice of using RSM for optimization is considered a quick and easy way to screen wide experimental field and elucidate the roles of every component involved [
19,
20].
Table 1 shows a comparison between full factorial design and RSM based on 5-level factors. Compared to a classical method such as factorial design which requires 25 runs, RSM that involves two factors only requires 13 runs to complete an experiment. RSM is very beneficial to be used especially when it involves more than two factors. This is not only resulting in lower overall costs (material and testing), but it also shortens the experimental time.
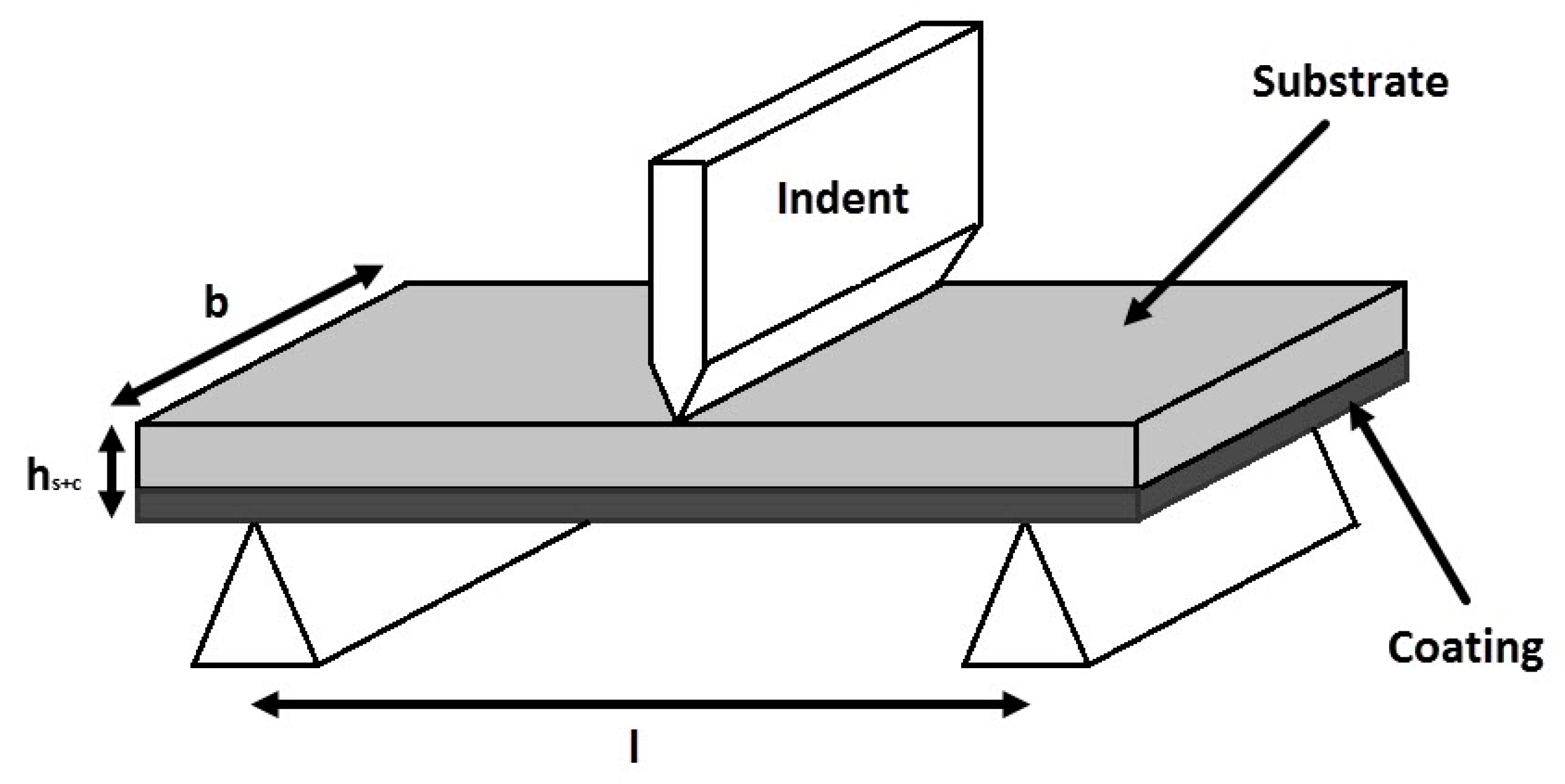
The main objective of this paper is to elucidate whether the material coating, such as polymer, may be adversely affected by load-bending moments on coated structures. The effect of factors including the ratio of AA solution, RHA/AA ratio, curing temperature, water to solid (W/S) ratio, and silica to alumina (Si/Al) ratio on flexural properties were studied. In addition, microstructure, flexural properties, and damage mechanism that followed a three-point bending test were evaluated and discussed.
3. Results and Discussion
Complete design matrix and responses values of flexural strength and flexural modulus are given in
Table 6 and data were analyzed using MINITAB (version 16.2).
3.1. Statistical Analysis of Flexural Strength
The regression analysis, after a backward elimination, is shown in
Table 7. The results showed that there was no quadratic effect (interaction between different factors including
V1*
V2,
V2*
V3, and
V1*
V3) that can be fitted into the model, thus indicating that there was no significant relationship between the factors.
The value of R2 (coefficient of determination) was 0.8380, which indicates that 83.80% of the sample variation in the flexural strength was attributed to the factors (V1, V2, and V3). For example, if R2 was 1.0000, the regression model produced would predict the optimum value with high accuracy. Square models are best fitted to the response. However, only the RHA/AA ratio (V2) and curing temperature (V3) showed significant linear and square effects on the response since the P was smaller than 0.050. The V2 showed the lowest p, which meant that it had the strongest effect on flexural strength for both linear (p < 0.013) and square effects (p < 0.005). The significant square effects indicated that the relationship between V2 and response followed a curved line instead of a straight one.
The ratio of AA solution (
V1) had no significant effect on flexural strength. Equation (1) represents the final reduced regression model equation for flexural strength:
YFS represents the response of flexural strength, while V1, V2, and V3 are the decoded values of the ratio of AA solution, RHA/AA ratio, and curing temperature, respectively. This regression model can be used to calculate and analyze the effect of factors on the flexural strength of geopolymer composite coating (GCC).
3.2. Effect of Factors on Flexural Strength
ANOVA and regression models were used to analyze the effect of various factors on flexural strength. Contour plots were used for better illustration.
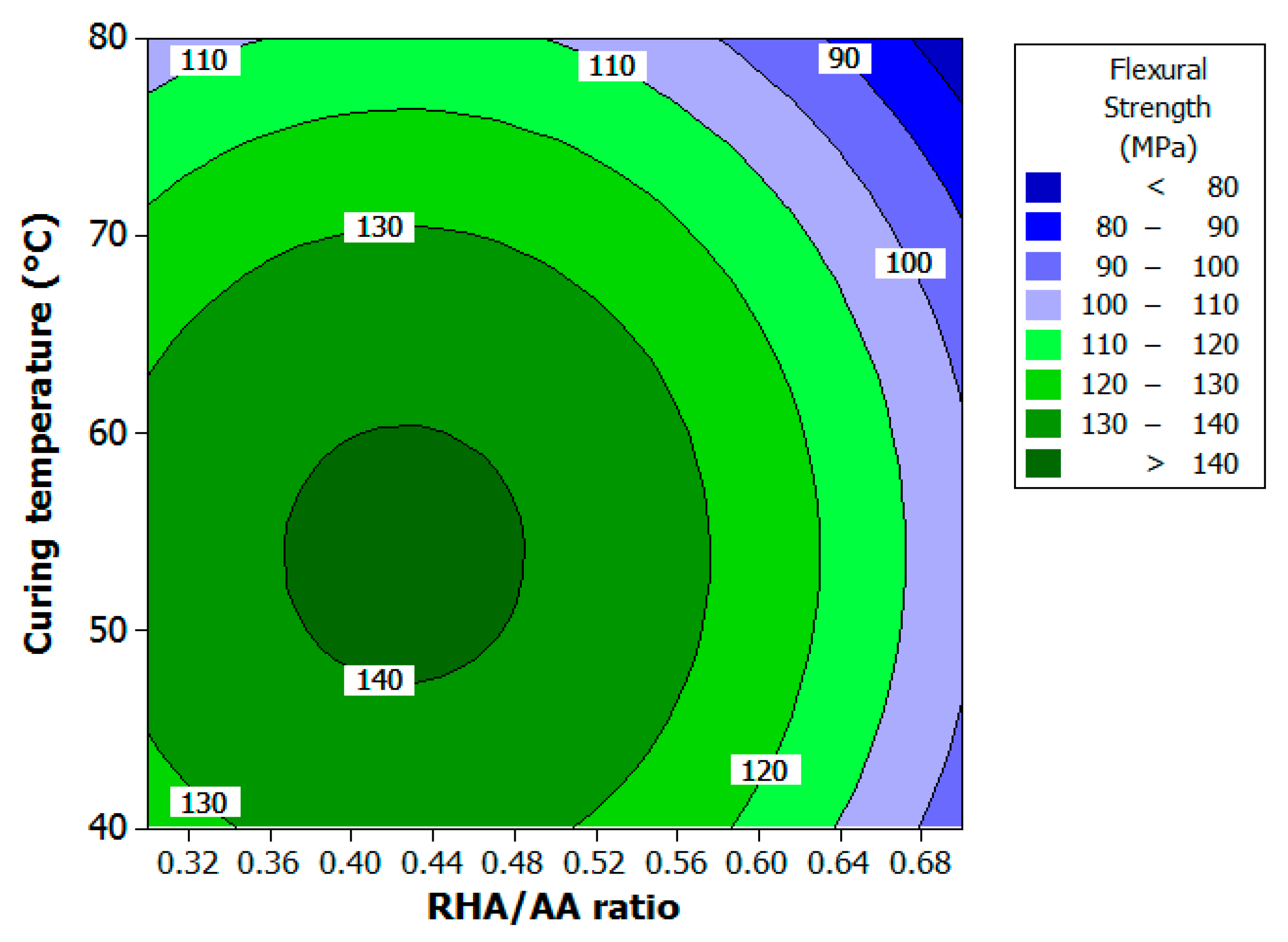
Figure 3 illustrates the effect of RHA/AA ratio (
V2) and curing temperature (
V3) on flexural strength and is shown as numbered contours.
V1 was held constant at 2.5 since this factor did not have any effects on the response. It is clear that flexural strength increases with lower
V3 and low
V2. High flexural strength was observed for geopolymer cured in the range of 47 to 60 °C and within 0.36 to 0.48 for
V2. As the value for both factors increased, flexural strength decreased. An increment of the RHA/AA ratio beyond 0.48 meant that the mixture had less workability as shown in
Figure 3. Different aluminosilicate sources had different optimum strength and workability. For example, Kong et al. [
36] found that metakaolin-based geopolymer paste had optimum workability at the water to solid (W/S) ratio of 1.25, whereas fly ash (FA)-based geopolymer had its optimum at 0.33.
Hardjito et al. [
37] found that the increment of the curing temperature to above 60 °C will not necessarily increase the sample strength. At a higher temperature, the loss of moisture will occur and consequently affect the sample strength. According to Van Jaarsveld et al. [
38], curing at higher temperature breaks down the structure of the geopolymer mixture resulting in dehydration and excessive shrinkage as the gel contracts without transforming to a more semi-crystalline form. Without retaining small amounts of water in the geopolymer matrix, cracking cannot be reduced resulted in low mechanical strength. Chindaprasirt et al. [
39] stated that the presence of moisture is important for geopolymerization in order to develop good strength.
Based on
Figure 4, RHA-based geopolymer showed optimum workability at a W/S ratio of slightly higher than 1.25. The higher W/S ratio compared to that of metakaolin- and FA-based geopolymer indicated that RHA required higher liquid demand due probably to its finer particle size that has been identified in previous research [
36]. Higher flexural strength was achieved when the W/S ratio is between 1.22 and 1.29, and Si/Al ratio between 98 and 104.
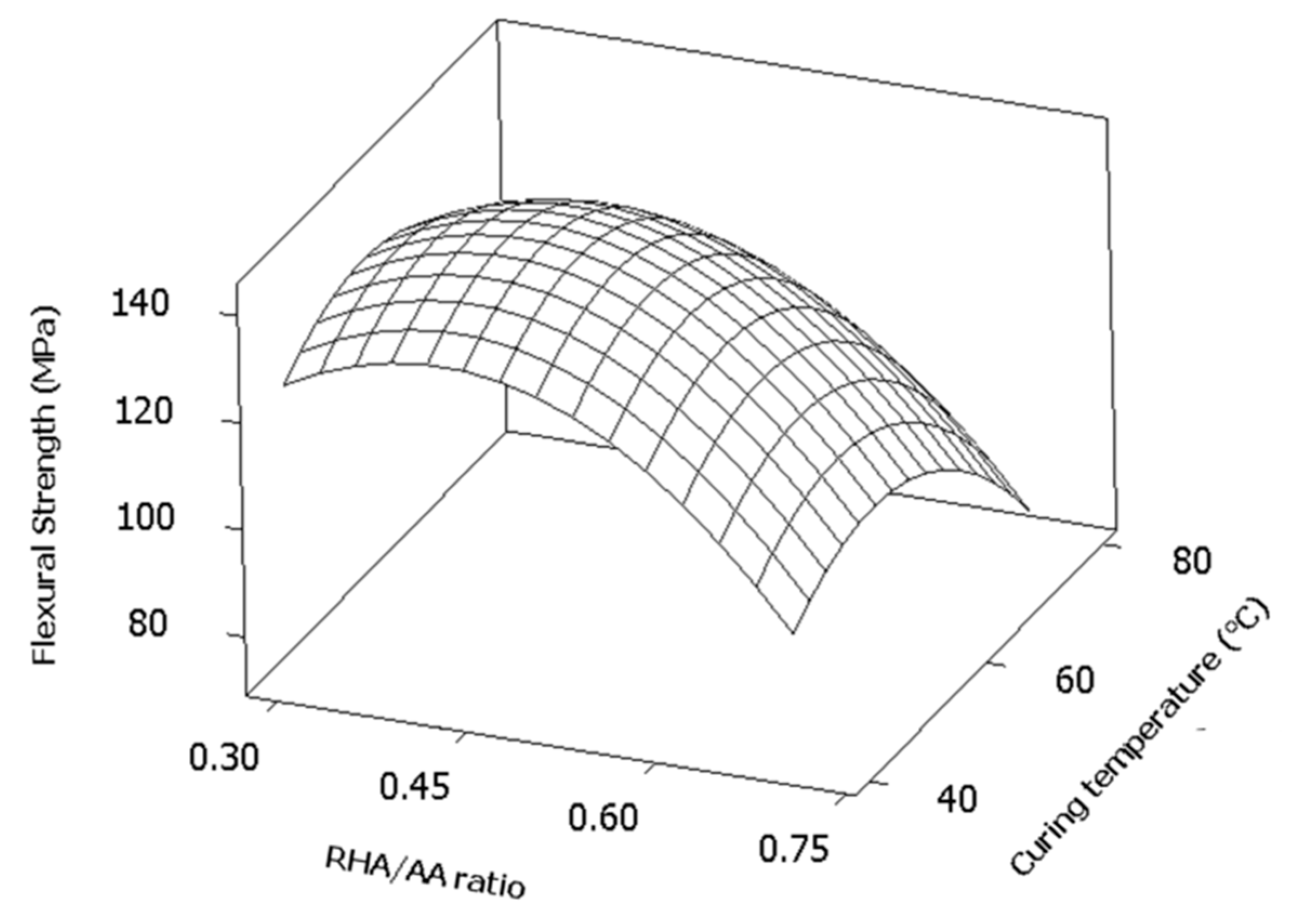
Figure 5 shows the surface response plot of curing temperature (
V3) versus RHA/AA ratio (
V2). The plot represents a surface where the maximum point is located inside the experimental region. It also proves that both factors have a square effect on the response.
3.3. Statistical Analysis of Flexural Modulus
The ANOVA results in
Table 8 showed that there was no relationship between the factors analyzed. Single-factor mainly influenced the response compared to square and interaction effects. Therefore, the linear model best fitted the response.
The value of the coefficient of determination showed that R2 was 0.6475 which indicated that 64.75% of the sample variation in the flexural modulus was attributed to the factors. The R2 value would be sufficient to indicate the right direction to take for further improvements in obtaining higher response values. While the ratio of AA solution (V1) had p well above 0.050, only the RHA/AA ratio (V2) and curing temperature (V3) showed a significant effect on the response. V3 had stronger effect (p < 0.003) compared to that of V2 (p < 0.018) on the flexural modulus.
Equation (2) represents regression model for flexural modulus with Y
FM represents the response of flexural modulus while
V1,
V2, and
V3 are the decoded values of the ratio of AA solution, RHA/AA ratio, and curing temperature, respectively:
3.4. Effect of Factors on Flexural Modulus
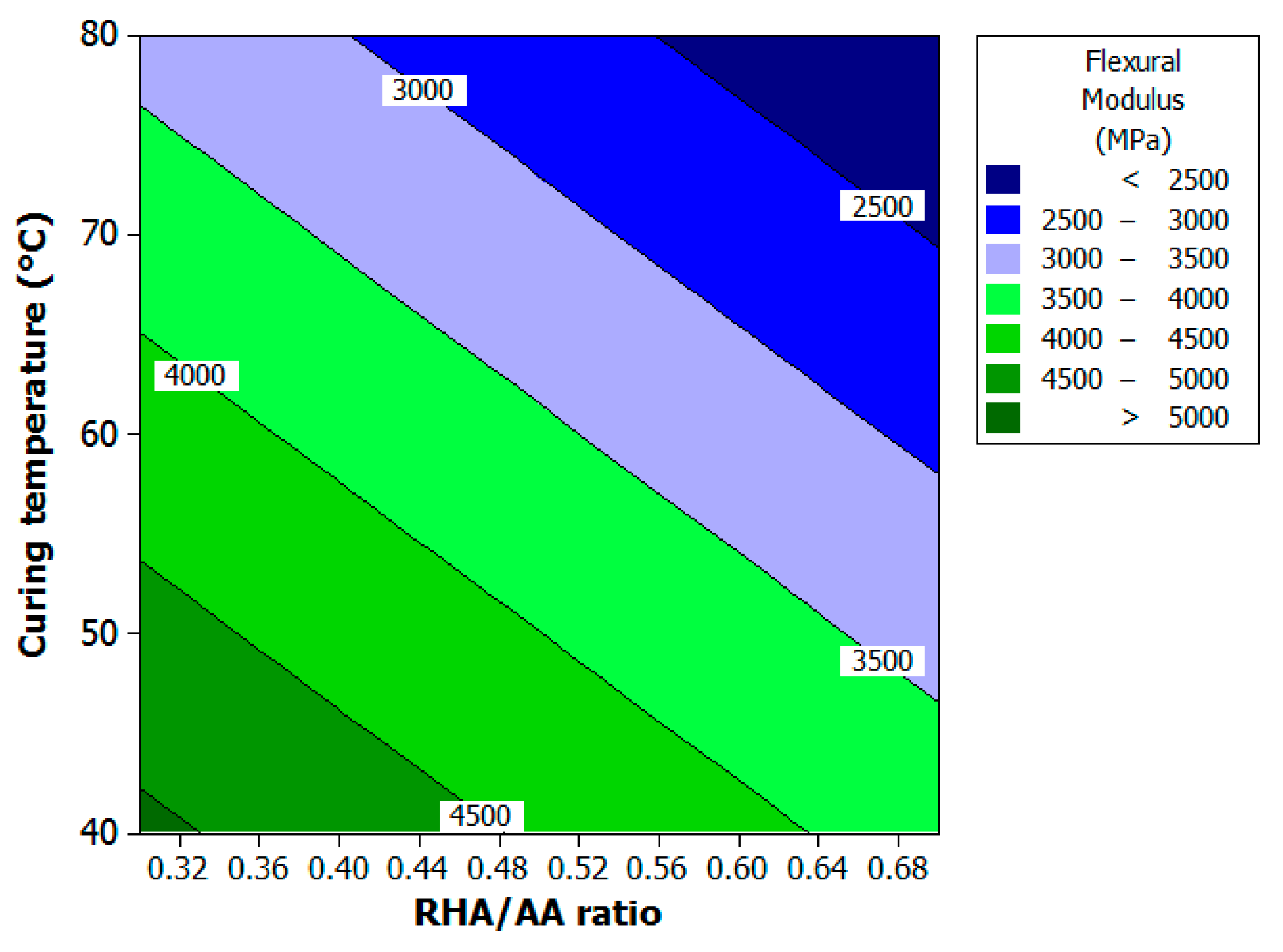
Figure 6 and
Figure 7 show the contour and surface plots, respectively. The ratio of AA solution (
V1) in
Figure 6 is held at 2.5 since this factor does not influence the response. The increment in RHA/AA ratio (
V2) and curing temperature (
V3) will decrease the flexural modulus. High flexural modulus of greater than 5000 MPa can be achieved when V
2 is approximately 0.32 and there is curing temperature of 41 °C.
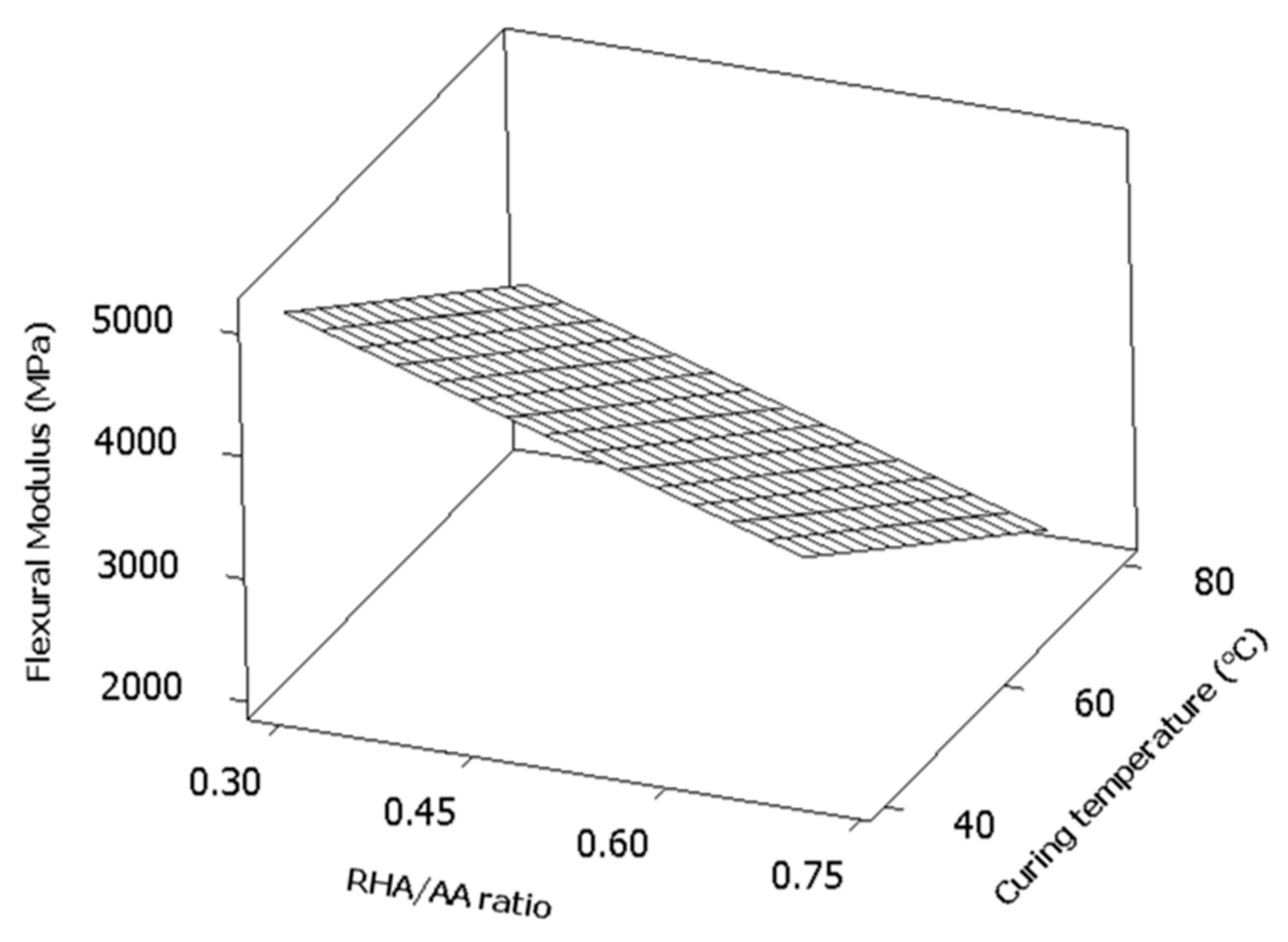
In
Figure 7, the surface plotted was a flat plane which indicated that the factors linearly affected the response. The highest flexural modulus could be achieved when the values for both factors were the lowest and the converse was also true.
As the curing temperature increased, the sample exhibited loss of moisture due to continuous evaporation of capillary water in the specimens. Since the content of moisture in GCC was reduced, continuous moisture loss on the surface of the specimen might lead to low strength and less flexibility as confirmed by Nagral et al. [
40].
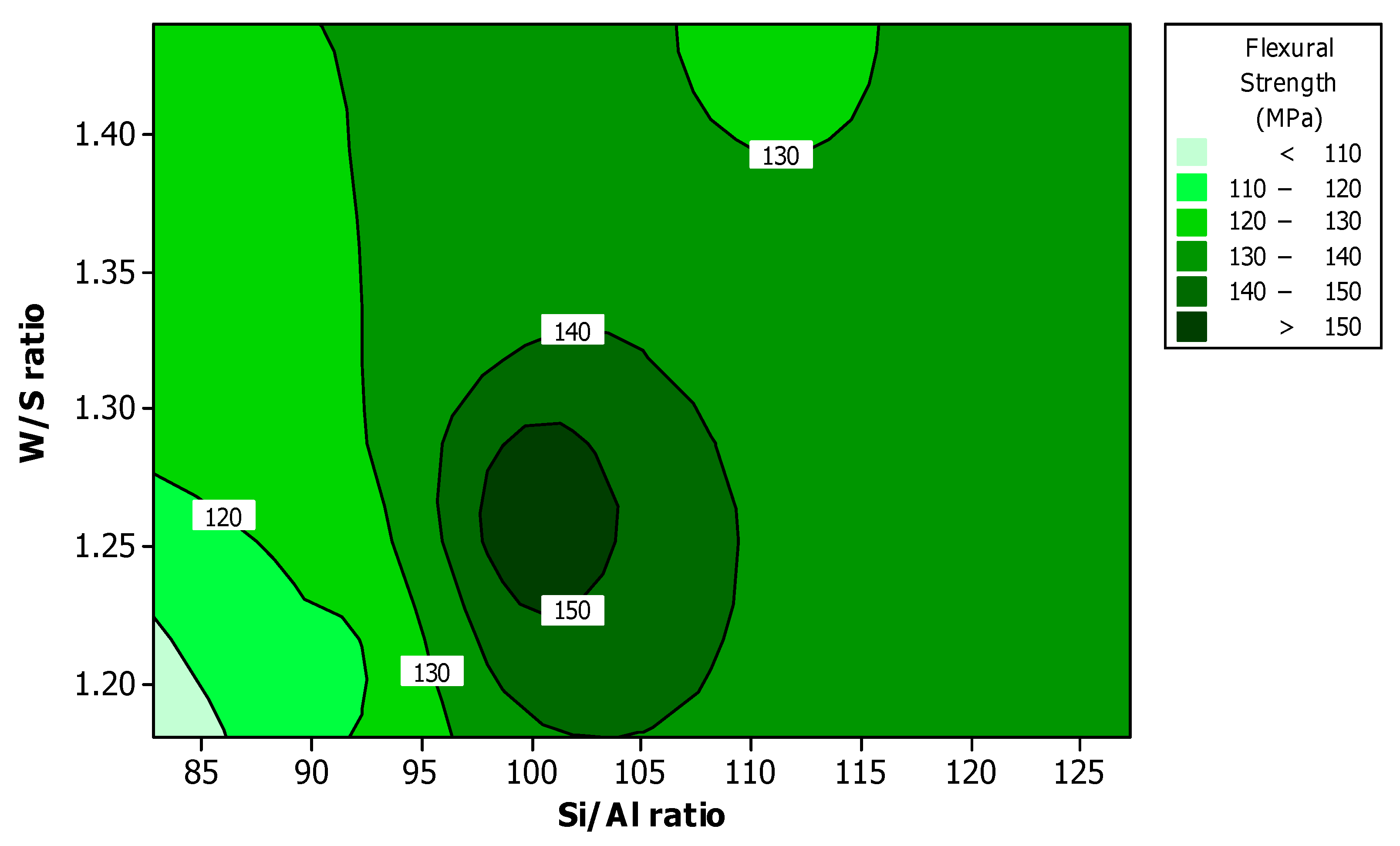
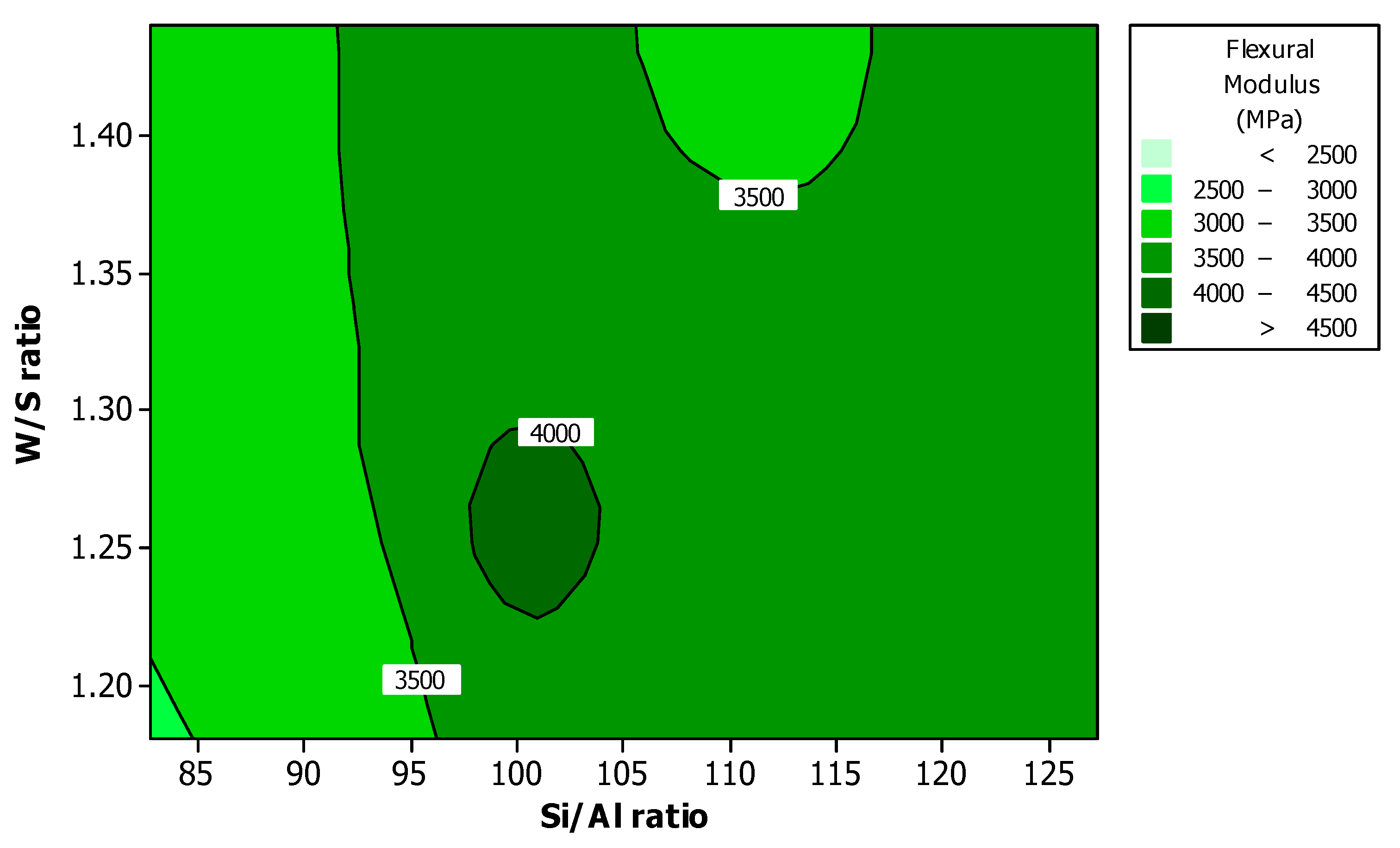
Figure 8 shows the effect of Si/Al ratio and W/S ratio on the flexural modulus. The high value of flexural modulus appeared at the Si/Al ratio between 97 and 104, and W/S ratio between 1.23 and 1.30. The result is similar to that of flexural strength shown in
Figure 4.
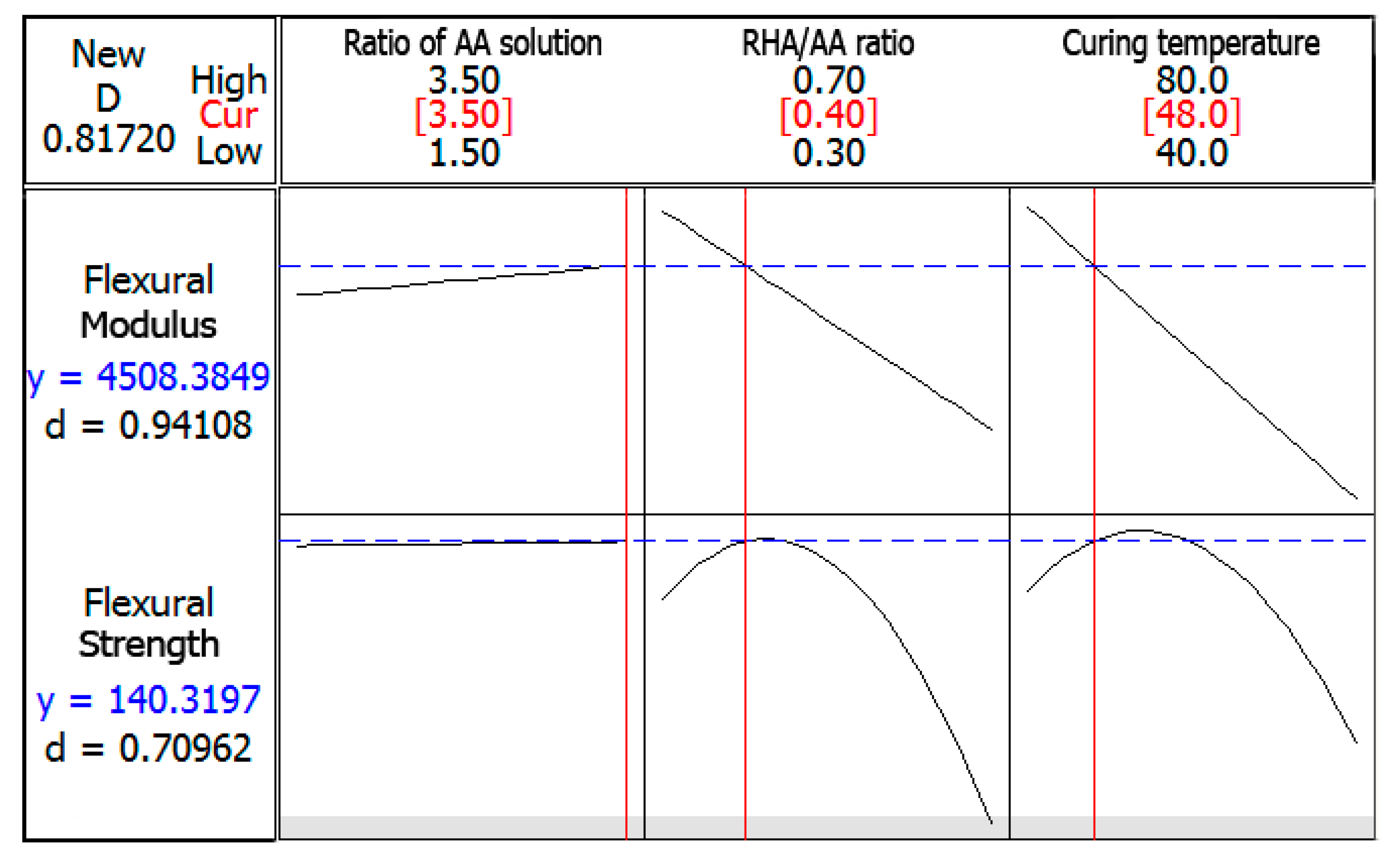
3.5. Optimization of the Responses
Optimization plot for both responses is shown in
Figure 9. The objective was to maximize both responses. The composite desirability value was calculated to be close to 1; therefore, the parameters were within their working range. The optimized values of factors were at
V1 = 3.5,
V2 = 0.40, and
V3 = 48 °C.
3.6. Experimental Validation
In the modelling process, experimental validation is the final step in order to verify the accuracy of the model. In order to verify the reproducibility of the developed regression model and the RSM model, three validation experiments were carried out under the optimal conditions obtained from the optimization plot (see
Figure 9). According to Sivaraos et al. [
41], for a nonlinear process, the optimization and validity of the model are only confirmed when the average difference between experimental and predicted values is less than 15%. As shown in
Table 9, the average error for flexural strength was slightly below 15% while the flexural modulus was well below this value at only 8.42%. It was concluded that the developed regression model established using this method was able to optimize the value for flexural modulus which was more accurate compared to the value for flexural strength.
Figure 10 shows sample SV
1 after a validation test. With a relatively high flexural strength and modulus as shown in
Table 9, the coating for sample SV
1, SV
2, and SV
3 showed no crack or debonding. Compared with previous studies [
2,
3,
42], it can be concluded that the coating was very flexible and has good adhesion bonding with the substrate since no surface cracking and debonding occurred. Both properties are very important, and the optimized coating has potential in many applications, which require flexible coating.
3.7. Flexural Properties and Microstructure Analysis
The mechanical properties of the coating of the selected samples were compared using the load–displacement curves and observation on the onset of cracking. To study the influence of the microstructure on the flexural properties, the coated mild steel with highest flexural strength (S14), lowest flexural strength (S17), and a sample suspected to have ductile behavior (S13) were selected. The uncoated mild steel served as control. The control sample showed very high bending stress at 691.10 MPa compared to the coated samples. This is due to its low strength in resisting the bending load as reported by Umesh et al. [
43].
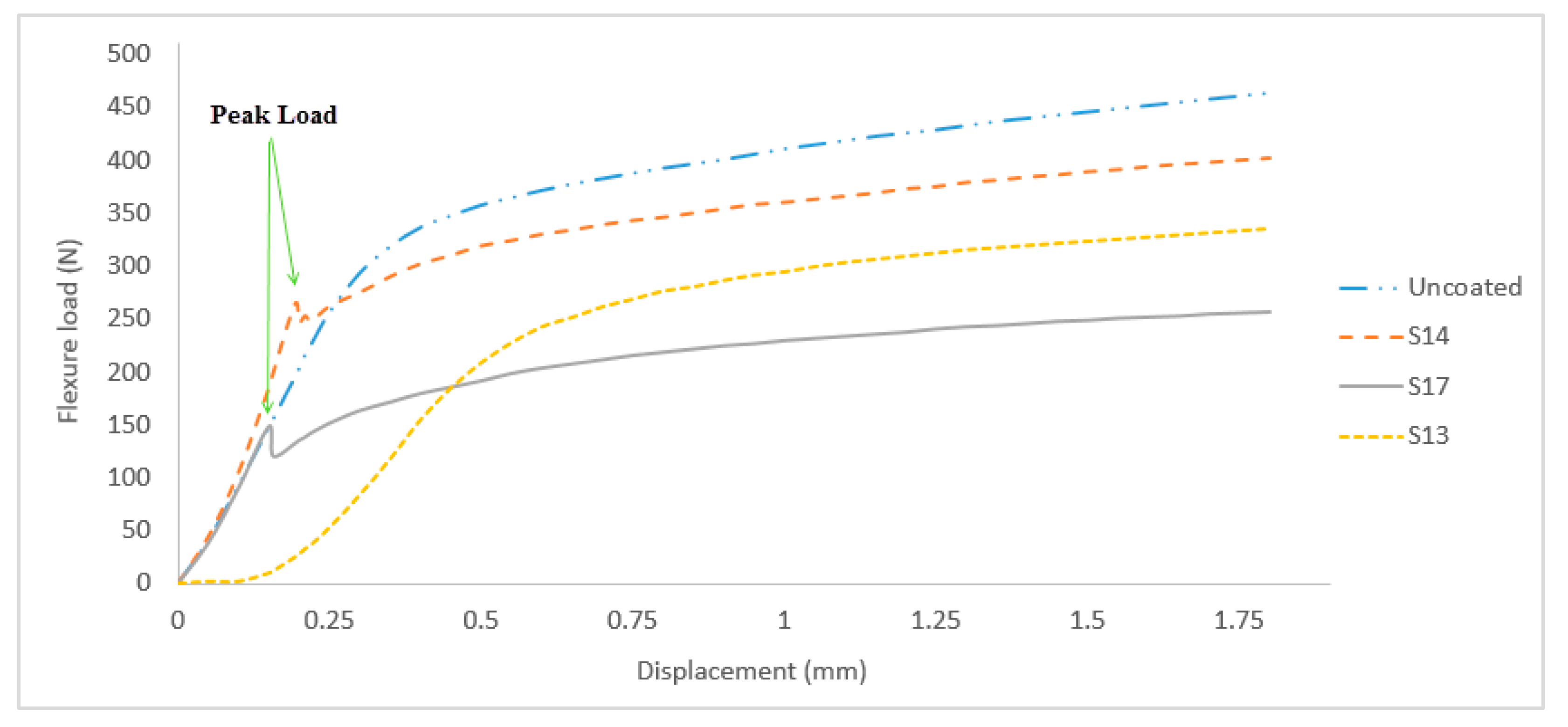
Figure 11 shows the load–extension characteristics of sample S17, S14, and the substrate during a three-point bending test.
S17 and S14 produced the lowest at 102.71 MPa and the highest flexural strength at 156.92 MPa, respectively, while S13 showed ductile behavior with relative high flexural strength at 143.15 MPa. Although the flexural strength of sample S13 was higher than that of sample S17, it exhibited ductile behavior due to a very high flexural modulus, which is 4636.30 MPa compared to 2912.19 MPa. During the bending process, the outer coating surface was subjected to tensile stress while compression stress occurred between indenter and the surface of the substrate. The crack on both samples extended further at the peak load.
S14 exhibited higher peak load at approximately 0.20 mm of displacement which indicated stronger interfacial strength and adhesion strength between coating and substrate as compared to S17 which exhibited lower peak load at approximately 0.15 mm of displacement. Similar results were previously recorded by Yang et al. [
2]. From all 20 samples tested, only three samples which were S2, S4, and S13 showed no peak load, which indicated high ductility coating. Substrate without coating and samples S2, S4, and S13 showed no holding load while other samples were subjected to holding load between 0.1 and 0.5 mm of displacement.
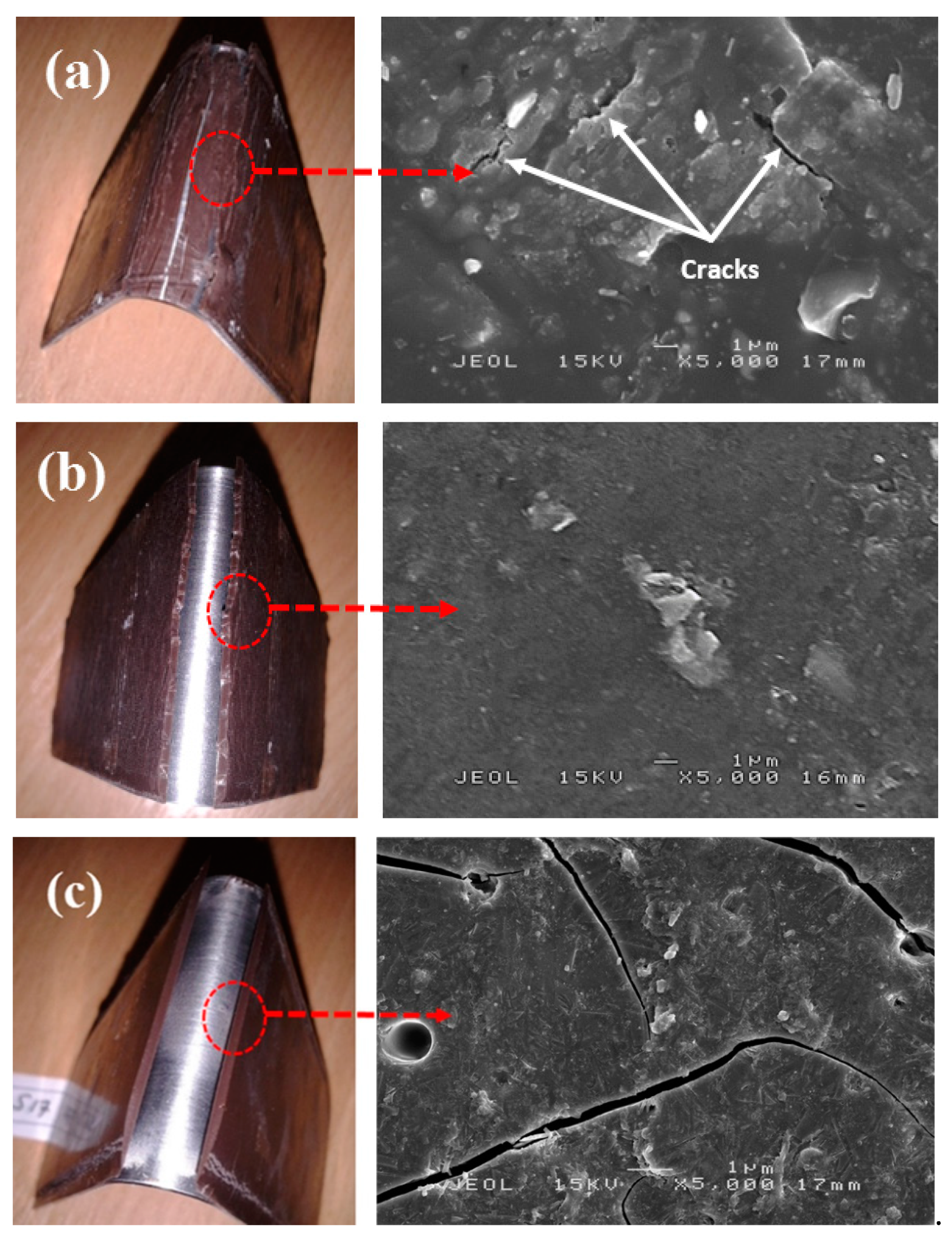
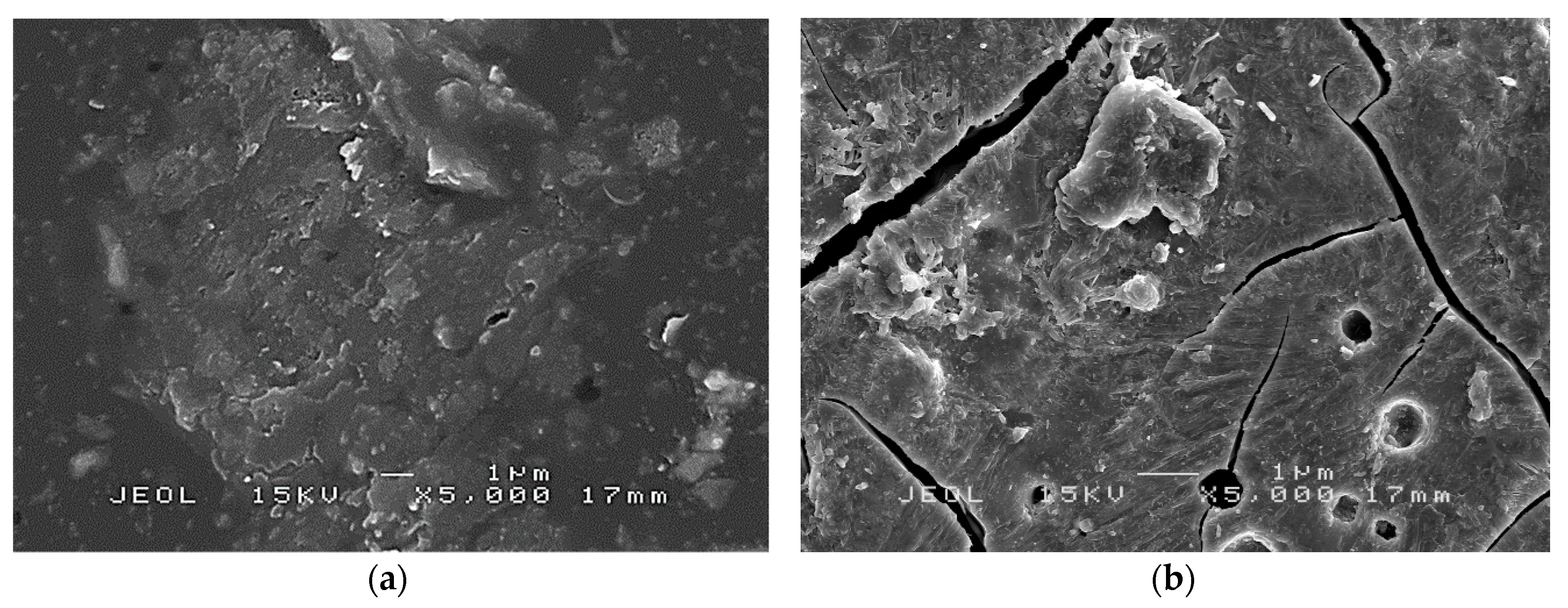
Figure 12 shows SEM micrographs of sample S13 which exhibited no peak load, S14 which produced the highest flexural strength and exhibited peak load, and S17 which produced the lowest flexural strength and displayed peak load. The absence of the peak load indicated that the coating was ductile. During the bending process, the coating was subjected to a high plastic deformation rate causing it to expand and develop cracks. However, due to adequate bonding between coating and substrate in S13, the expansion was delayed. Only with the additional load applied to the sample, cracks appeared along the interface causing damage to the system. The propagation of cracks was observed in S13 and S17 are respectively shown in
Figure 12a,c.
Ductile coating in sample S13 showed minor and rough cracks while the brittle coating in S17 displayed a crack with clean linear cut. Results showed that the expansion in S13 was delayed while S17 failed to withstand high flexural load causing it to break without any resistant. S14 as shown in
Figure 12b, which had a smoother surface compared to S17, showed no formation of crystal particles on its surface or within its geopolymer matrix. It is possible that S14 had undergone a higher rate of geopolymerization, which resulted in a highly homogeneous mixture [
44]. Bonding between RHA particles and AA solution is, therefore, stronger and was able to produce a higher flexural strength compared to that in S17.
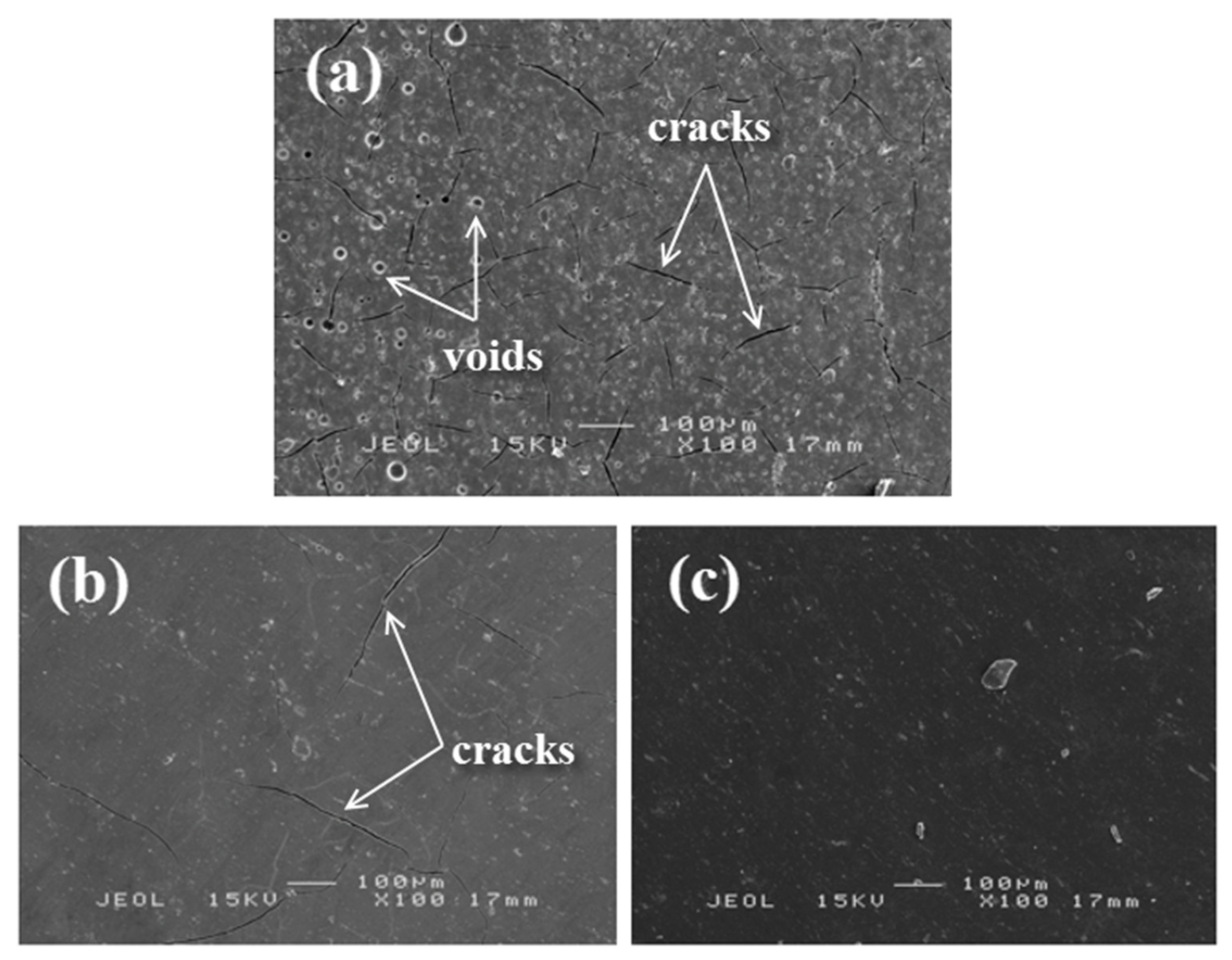
Figure 13 shows microscopic images of cracks formed on the surface of three samples that exhibited different damage behaviors during bending tests. Compared to S14, adhesion bonding between substrate and coating for S17 was weak and the coating showed brittle behavior.
S17 was also subjected to higher residual stress at the interface, which resulted in lower interfacial adhesion. When the load was applied, the coated sample stored the strain energy which would potentially cause crack formation between the coating layer and the substrate [
45]. Upon debonding at the coating–substrate interface, the energy was released and caused cracks to nucleate from voids and propagate along with the interface. Since S17 had a lower energy release rate compared to S14, multiple cracking formed with a larger crack width in the coating layer and which subsequently lowered the overall flexural strength and modulus. When further load was applied, the sample failed to endure high plastic deformation causing the brittle coating layer to rupture and delaminate from the substrate as shown in
Figure 13a,b. Sample S13 in
Figure 13c was less porous and a homogeneous phase was formed in the geopolymer matrix. The number of cracks which occurred in the ductile coating was very minimal.
In addition to crack formation, particle agglomeration of RHA-based geopolymer had also contributed to low flexural strength. As shown in
Figure 14, the particles of RHA-based geopolymer in S17 (
Figure 14b) were highly agglomerated as compared to that of sample S13 (
Figure 14a). The agglomeration was probably influenced by RHA/AA ratio and water to solid (W/S) ratio with the silica component not homogeneously dispersed into the geopolymer matrix. S17 had a lower W/S ratio of 1.18 compared to S13 with 1.68. A lower W/S ratio resulted in a low degree of geopolymerization resulted in a partial reaction process. During the bending process, stress would be concentrated at the area where the RHA agglomerated leading to initiation of cracks.
4. Conclusions
The response surface methodology was proved to be an adequate tool to study the relationship between factors that significantly influenced the flexural properties. From the analysis and investigation, the RHA/AA ratio (V2) showed the most significant effect on the response followed by curing temperature (V3). The ratio of AA solution provided no effect to the flexural strength and modulus. From surface and contour plots, high flexural strength was observed for a geopolymer cured in the range of 47 to 60 °C and within 0.36 to 0.48 for the RHA/AA ratio. In addition, high flexural strength was achieved when the W/S ratio is between 1.22 and 1.29, and Si/Al ratio between 98 and 104. High flexural modulus of greater than 5000 MPa can be achieved when the RHA/AA ratio is approximately 0.32 and curing temperature of 41 °C. The high value of a flexural modulus appeared at the Si/Al ratio between 97 and 104, and the W/S ratio between 1.23 and 1.30. The maximum value of both flexural strength and flexural modulus could be achieved by using V1 = 3.5, V2 = 0.39, and V3 = 45.7 °C. The validated coating samples experienced no cracks or debonding, which means that the coating is flexible and has good adhesion bonding with the substrate. A geopolymer composite coating (GCC) sample with different properties showed varying behavior. Sample S17 had multiple cracking formed with a larger crack width in the coating layer, which leads to the overall flexural strength and modulus. The latter also differed according to their flexural strength. GCC with high strength at 156.92 MPa was more brittle and had more cracks than GCC with lower strength at 102.71 MPa. Sample S17, which was highly agglomerated compared to sample S13, resulted in low flexural strength due to the higher content of silica. Several potential studies have been identified including an XRD test to investigate the homogeneity of the geopolymer binder and involving different types of substrate such as glass, wood, and fiber.