Influence of SiO2 Content and Exposure Periods on the Anticorrosion Behavior of Epoxy Nanocomposite Coatings
Abstract
:1. Introduction
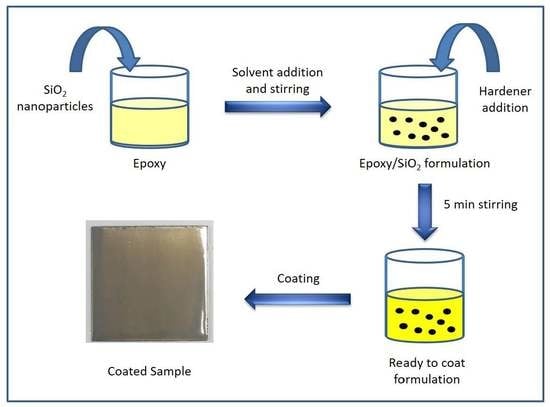
2. Materials and Methods
3. Results and Discussion
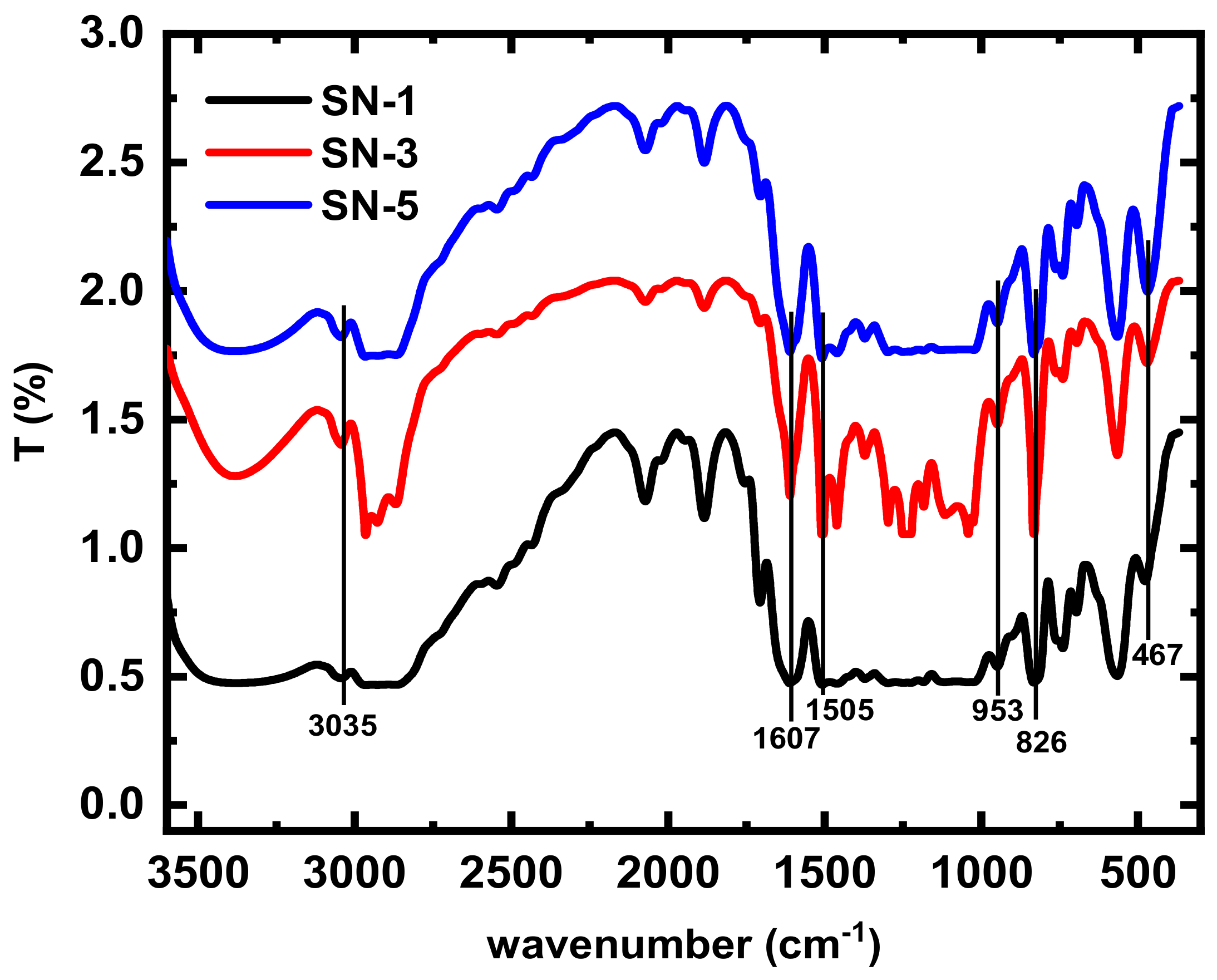
3.1. Fourier Transforms Infrared Spectroscopy
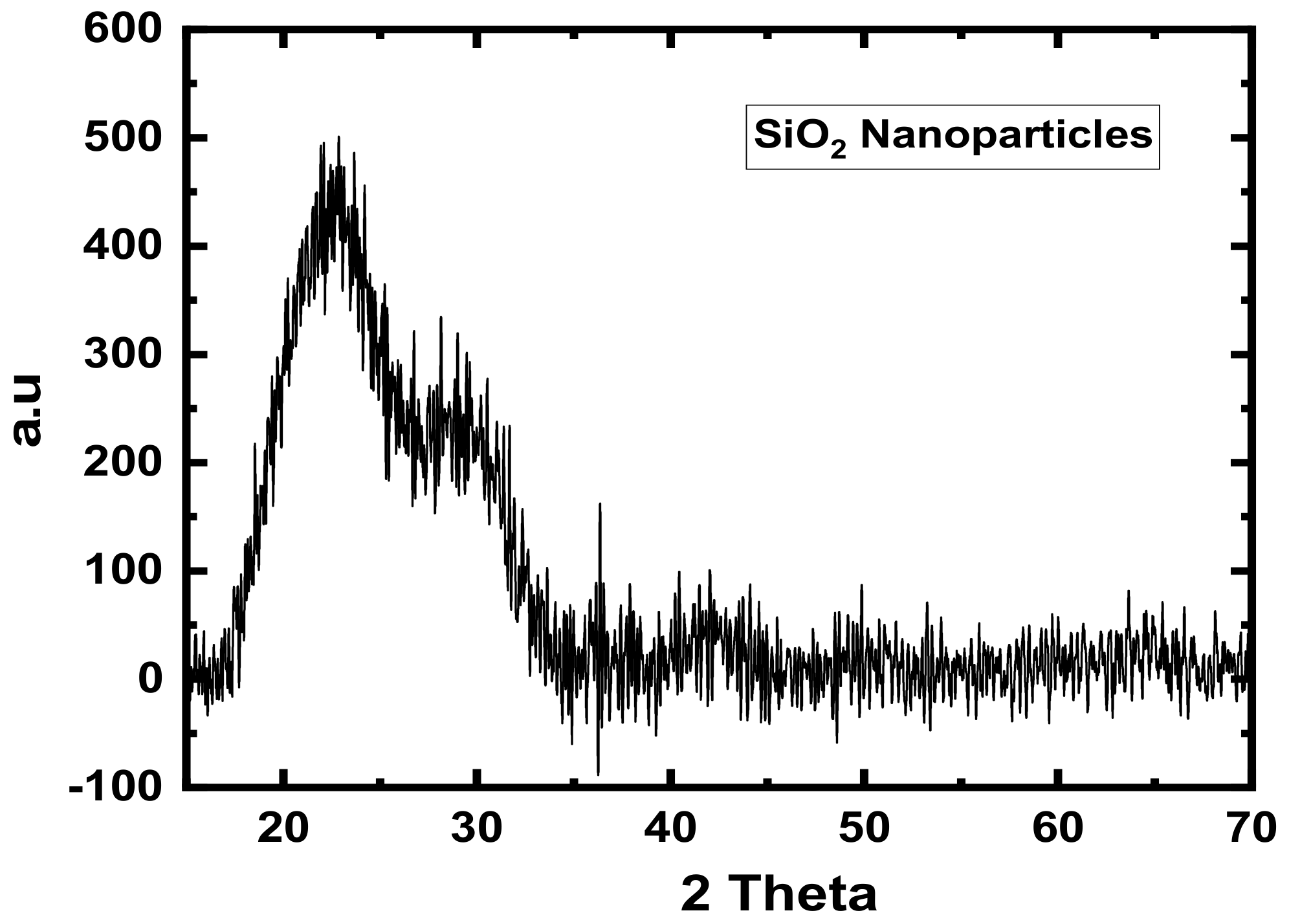
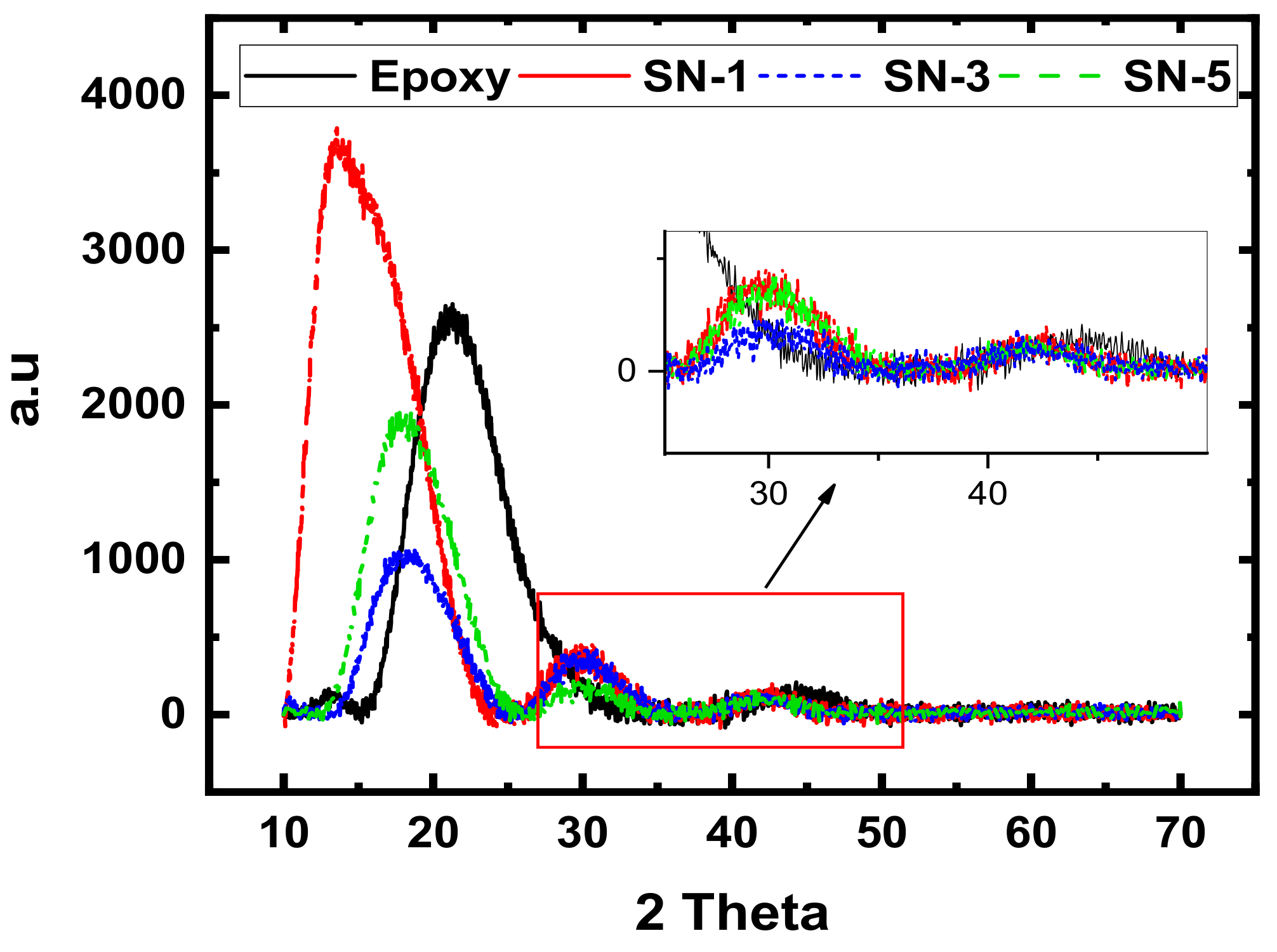
3.2. XRD Analyses
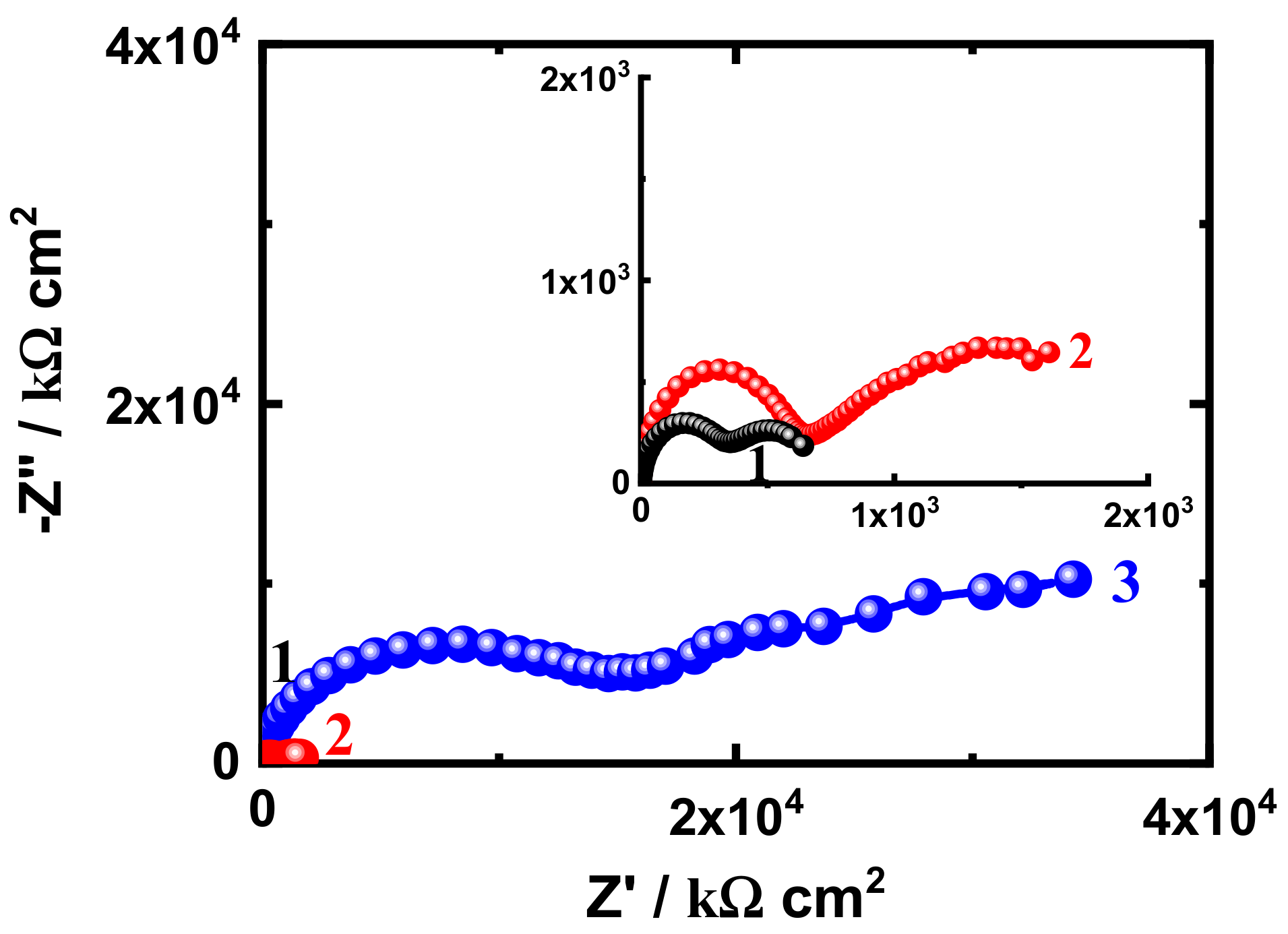
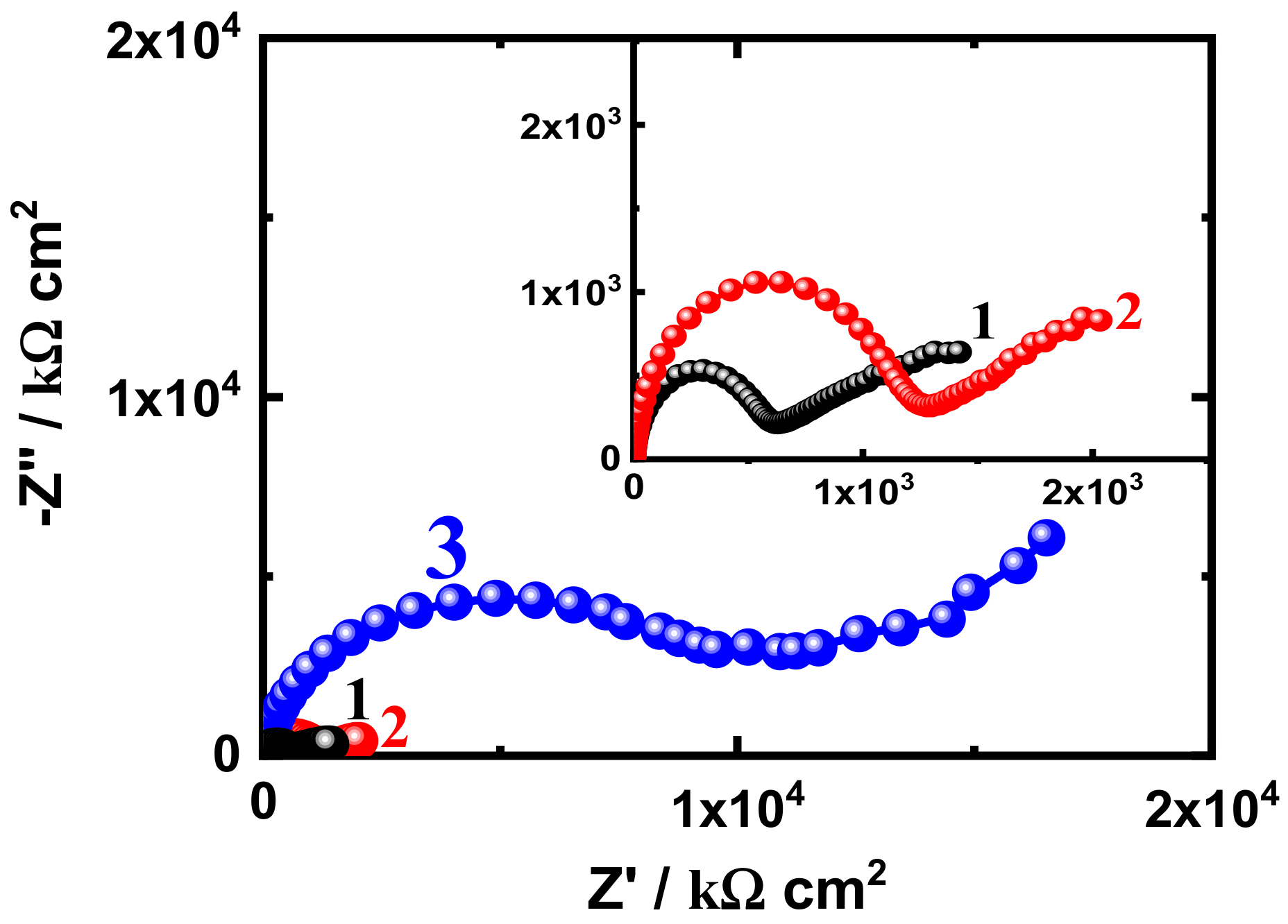
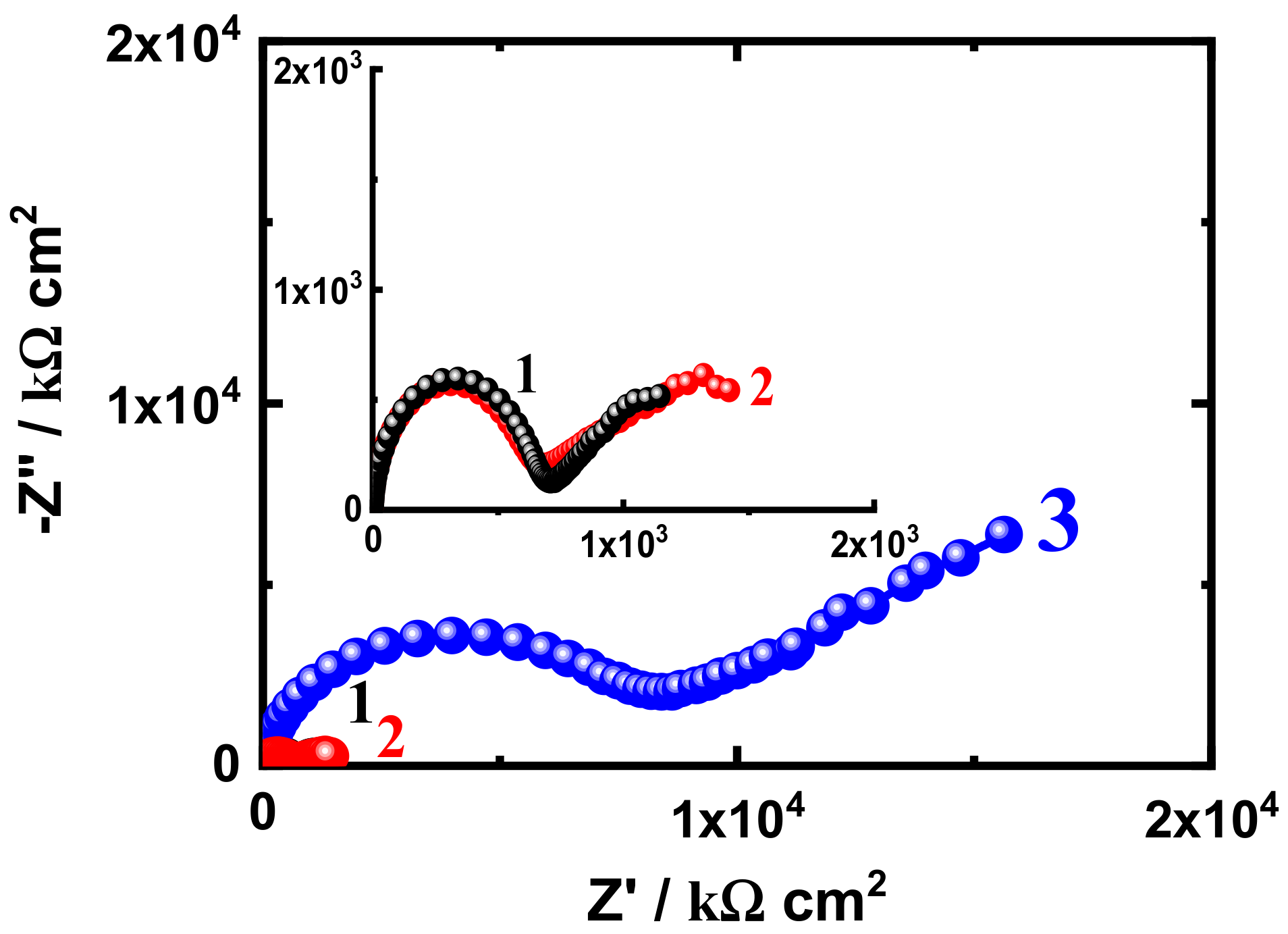
3.3. EIS Measurement
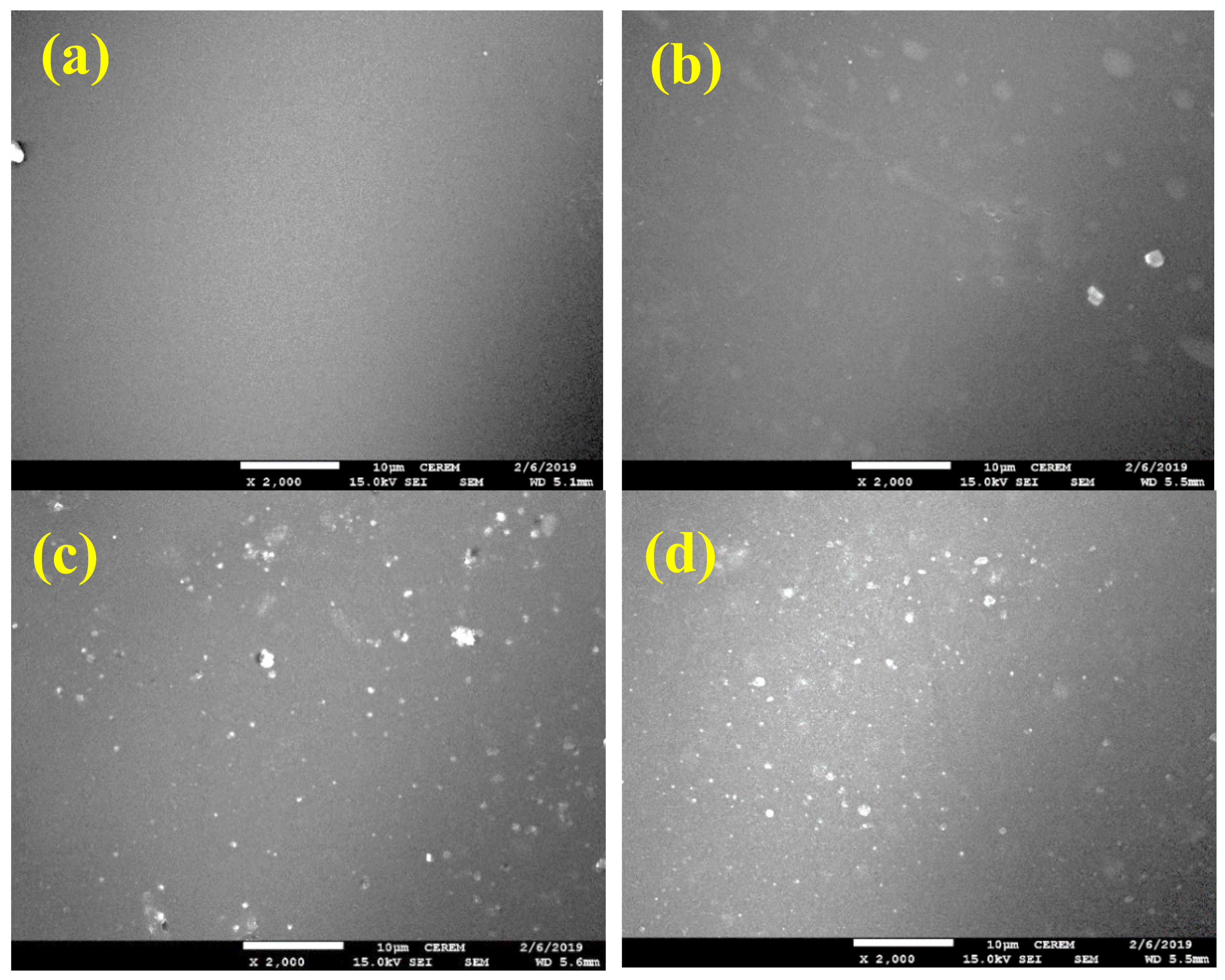
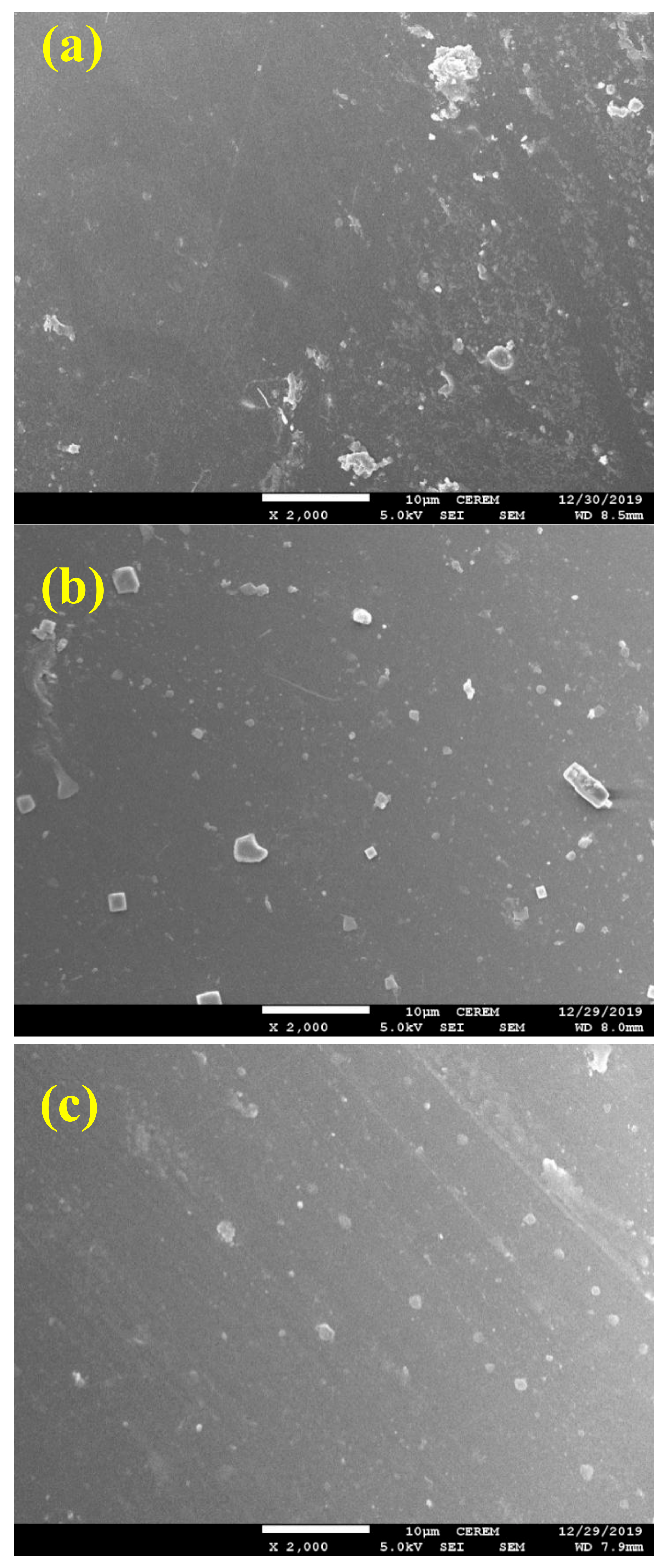
3.4. SEM Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferreira, E.S.; Giacomelli, C.; Giacomelli, F.C.; Spinelli, A. Evaluation of the inhibitor effect of L-ascorbic acid on the corrosion of mild steel. Mater. Chem. Phys. 2004, 83, 129–134. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Siju, C.; Mahanta, D.; Patil, S.; Madras, G. Conducting polyaniline–nano-TiO2 composites for smart corrosion resistant coatings. Electrochim. Acta 2009, 54, 1249–1254. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M.; Farzam, M. A study on the anticorrosion performance of the epoxy–polyamide nanocomposites containing ZnO nanoparticles. Prog. Org. Coat. 2011, 72, 410–422. [Google Scholar] [CrossRef]
- Behzadnasab, M.; Mirabedini, S.M.; Kabiri, K.; Jamali, S. Corrosion performance of epoxy coatings containing silane treated ZrO2 nanoparticles on mild steel in 3.5% NaCl solution. Corros. Sci. 2011, 53, 89–98. [Google Scholar] [CrossRef]
- Dolatzadeh, F.; Moradian, S.; Jalili, M.M. Influence of various surface treated silica nanoparticles on the electrochemical properties of SiO2/polyurethane nanocoatings. Corros. Sci. 2011, 53, 4248–4257. [Google Scholar] [CrossRef]
- Barna, E.; Bommer, B.; Kursteiner, J.; Vital, A.; Von Trzebiatowski, O.; Koch, W.; Schmid, B.; Graule, T. Innovative, scratch proof nanocomposites for clear coatings. Compos. Part A Appl. S. 2005, 36, 473–480. [Google Scholar] [CrossRef]
- Ghanbari, A.; Attar, M.M. A study on the anticorrosion performance of epoxy nanocomposite coatings containing epoxy-silane treated nano-silica on mild steel substrate. J. Ind. Eng. Chem. 2015, 23, 145–153. [Google Scholar] [CrossRef]
- Conradi, M.; Kocijan, A.; Zorko, M.; Verpoest, I. Damage resistance and anticorrosion properties of nanosilica-filled epoxy-resin composite coatings. Prog. Org. Coat. 2015, 80, 20–26. [Google Scholar] [CrossRef]
- Conradi, M.; Kocijan, A.; Kek-Merl, D.; Zorko, M.; Verpoest, I. Mechanical and anticorrosion properties of nanosilica-filled epoxy-resin composite coatings. Appl. Surf. Sci. 2014, 292, 432–437. [Google Scholar] [CrossRef]
- Blum, F.D. Silane Coupling Agents. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons: New York, NY, USA, 2016; Volume 8, pp. 38–50. [Google Scholar]
- Zhou, H.J.; Rong, M.Z.; Zhang, M.Q.; Friedrich, K. Effects of reactive compatibilization on the performance of nano-silica filled polypropylene composites. J. Mater. Sci. 2006, 41, 5767–5770. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Rong, M.Z.; Zeng, H.M.; Schmitt, S.; Wetzel, B.; Friedrich, K. Atomic force microscopy study on structure and properties of irradiation grafted silica particles in polypropylene-based nanocomposites. J. Appl. Polym. Sci. 2001, 80, 2218–2227. [Google Scholar] [CrossRef]
- Ajayan, P.; Schadler, L.; Braun, P. Polymer-based and polymer-filled nanocomposites. In Nanocomposite Science and Technology; John Wiley & Sons: New York, NY, USA, 2003; p. 112. [Google Scholar]
- Alam, M.A.; Sherif, E.S.M.; Al-Zahrani, S.M. Fabrication of Various Epoxy Coatings for Offshore Applications and Evaluating Their Mechanical Properties and Corrosion Behavior. Int. J. Electrochem. Sci. 2013, 8, 3121–3131. [Google Scholar]
- Matin, E.; Attar, M.M.; Ramezanzadeh, B. Investigation of corrosion protection properties of an epoxy nanocomposite loaded with polysiloxane surface modified nanosilica particles on the steel substrate. Prog. Org. Coat. 2015, 78, 395–403. [Google Scholar] [CrossRef]
- Khan, R.; Azhar, M.R.; Anis, A.; Alam, M.A.; Boumaza, M.; Al-Zahrani, S.M. Facile synthesis of epoxy nanocomposite coatings using inorganic nanoparticles for enhanced thermo-mechanical properties: a comparative study. J. Coat. Technol. Res. 2016, 13, 159–169. [Google Scholar] [CrossRef]
- Tran, T.N.; Pham, T.V.A.; Le, M.L.P.; Nguyen, T.P.T.; Tran, V.M. Synthesis of amorphous silica and sulfonic acid functionalized silica used as reinforced phase for polymer electrolyte membrane. Adv. Nat. Sci Nanosci. 2013, 4, 045007. [Google Scholar] [CrossRef] [Green Version]
- Samad, U.A.; Alam, M.A.; Chafidz, A.; Al-Zahrani, S.M.; Alharthi, N.H. Enhancing mechanical properties of epoxy/polyaniline coating with addition of ZnO nanoparticles: Nanoindentation characterization. Prog. Org. Coat. 2018, 119, 109–115. [Google Scholar] [CrossRef]
- Rong, Z.D.; Sun, W.; Xiao, H.J.; Jiang, G. Effects of nano-SiO2 particles on the mechanical and microstructural properties of ultra-high performance cementitious composites. Cement Concrete Comp. 2015, 56, 25–31. [Google Scholar] [CrossRef]
- Chen, Q.G.; Yang, H.D.; Wang, X.Y.; Liu, H.Q.; Zhou, K.; Ning, X. Dielectric Properties of Epoxy Resin Impregnated Nano-SiO2 Modified Insulating Paper. Polymers 2019, 11, 393. [Google Scholar] [CrossRef] [Green Version]
- Shinde, V.; Patil, P.P. Evaluation of corrosion protection performance of poly(o-ethyl aniline) coated copper by electrochemical impedance spectroscopy. Mater. Sci. Eng. B Adv. 2010, 168, 142–150. [Google Scholar] [CrossRef]
- Floyd, F.L.; Avudaiappan, S.; Gibson, J.; Mehta, B.; Smith, P.; Provder, T.; Escarsega, J. Using electrochemical impedance spectroscopy to predict the corrosion resistance of unexposed coated metal panels. Prog. Org. Coat. 2009, 66, 8–34. [Google Scholar] [CrossRef]
- Gao, W.J.; Cao, S.; Yang, Y.Z.; Wang, H.; Li, J.; Jiang, Y.M. Electrochemical impedance spectroscopy investigation on indium tin oxide films under cathodic polarization in NaOH solution. Thin Solid Films 2012, 520, 6916–6921. [Google Scholar] [CrossRef]
- Darowicki, K.; Krakowiak, S.; Slepski, P. Evaluation of pitting corrosion by means of dynamic electrochemical impedance spectroscopy. Electrochim. Acta 2004, 49, 2909–2918. [Google Scholar] [CrossRef]
- Rehim, S.S.A.; Hassan, H.H.; Amin, M.A. Corrosion and corrosion inhibition of Al and some alloys in sulphate solutions containing halide ions investigated by an impedance technique. Appl. Surf. Sci. 2002, 187, 279–290. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Almajid, A.A. Anodic Dissolution of API X70 Pipeline Steel in Arabian Gulf Seawater after Different Exposure Intervals. J. Chem. N. Y. 2014, 2014. [Google Scholar] [CrossRef]
- Khalil, K.A.; Sherif, E.S.M.; Almajid, A.A. Corrosion Passivation in Simulated Body Fluid of Magnesium/Hydroxyapatite Nanocomposites Sintered by High Frequency Induction Heating. Int. J. Electrochem. Sci. 2011, 6, 6184–6199. [Google Scholar]
- Sherif, E.M.; Abdo, H.S.; Almajid, A.A. Corrosion Behavior of Cast Iron in Freely Aerated Stagnant Arabian Gulf Seawater. Materials 2015, 8, 2127–2138. [Google Scholar] [CrossRef] [Green Version]
- Alharthi, N.; Sherif, E.M.; Abdo, H.S.; Alharbi, H.F.; Misiolek, W.Z. Effect of extrusion welding locations on the corrosion of AM30 alloy extrudate. J. Mater. Res. Technol. 2019, 8, 2280–2289. [Google Scholar] [CrossRef]
- Sherif, E.-S.; Abdo, H.; Khalil, K.; Nabawy, A. Corrosion properties in sodium chloride solutions of Al–TiC composites in situ synthesized by HFIHF. Metals 2015, 5, 1799–1811. [Google Scholar] [CrossRef] [Green Version]
- Sherif, E.M.; Park, S.M. Inhibition of copper corrosion in 3.0% NaCl solution by N-phenyl-1,4-phenylenediamine. J. Electrochem. Soc. 2005, 152, B428–B433. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.A.; Sherif, E.S.M.; Al-Zahrani, S.M. Mechanical Properties and Corrosion Behavior of Different Coatings Fabricated by Diglycidyl Ether of Bisphenol-A Epoxy Resin and Aradur®-3282 Curing Agent. Int. J. Electrochem. Sci. 2013, 8, 3388–83400. [Google Scholar]
- Sherif, E.-S.M.; Seikh, A.H. Effect of Exposure Period and Temperature on the Corrosion of Incoloy® Alloy 800™ in Hydrochloric Acid Pickling Solutions. Int. J. Electrochem. Sci. 2015, 10, 1843–1854. [Google Scholar]
- Zhang, Z.; Chen, S.; Li, Y.; Li, S.; Wang, L. A study of the inhibition of iron corrosion by imidazole and its derivatives self-assembled films. Corros. Sci. 2009, 51, 291–300. [Google Scholar] [CrossRef]

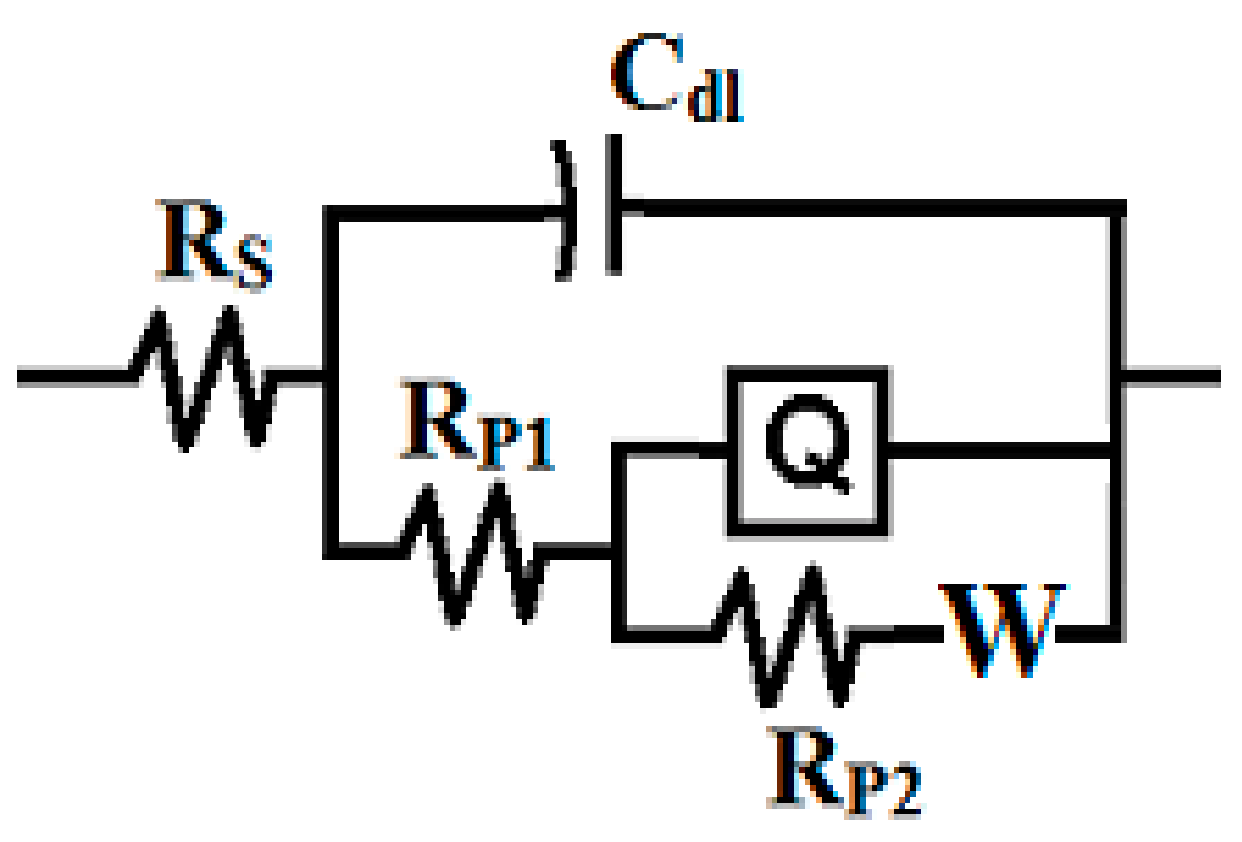
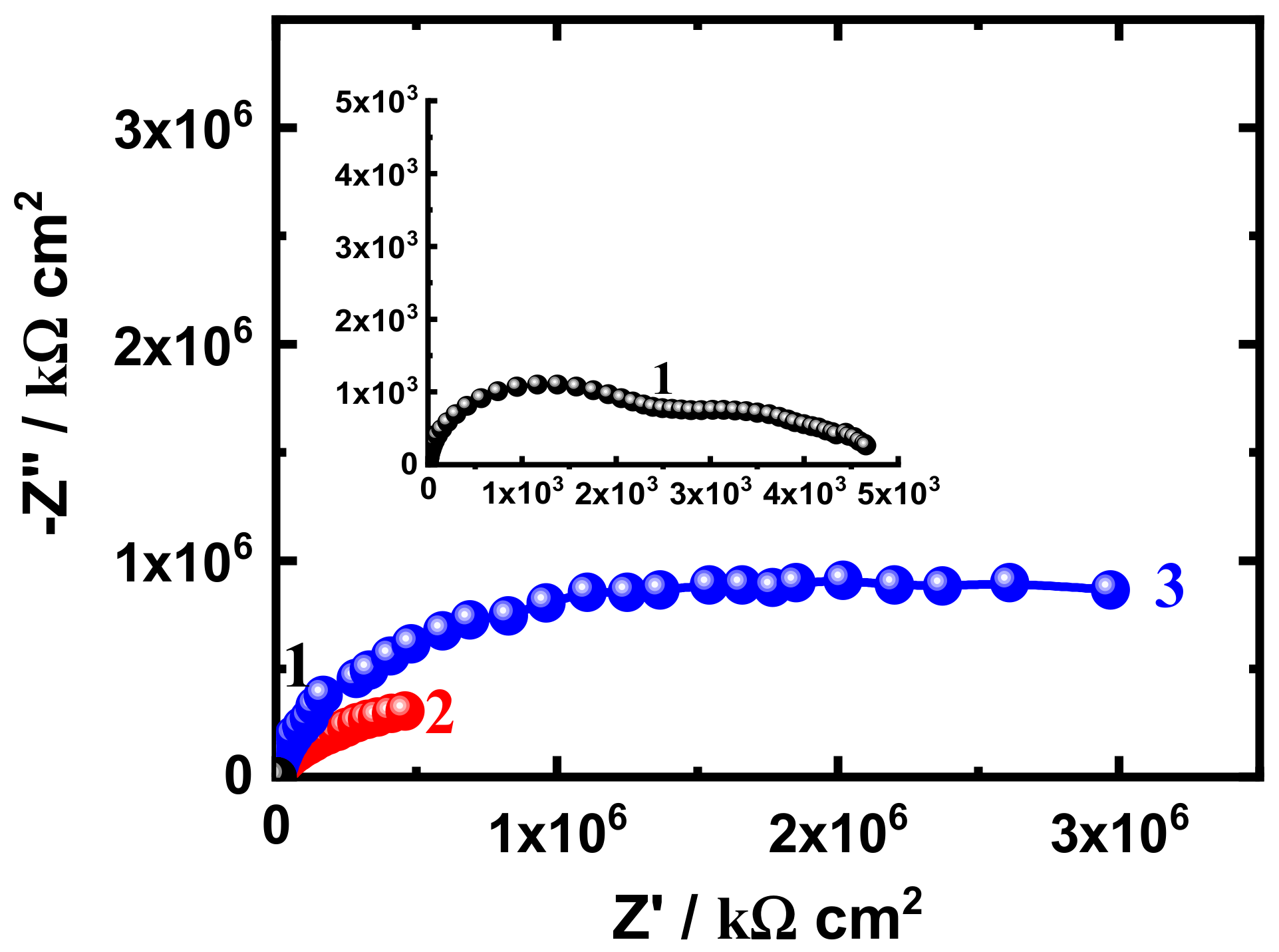
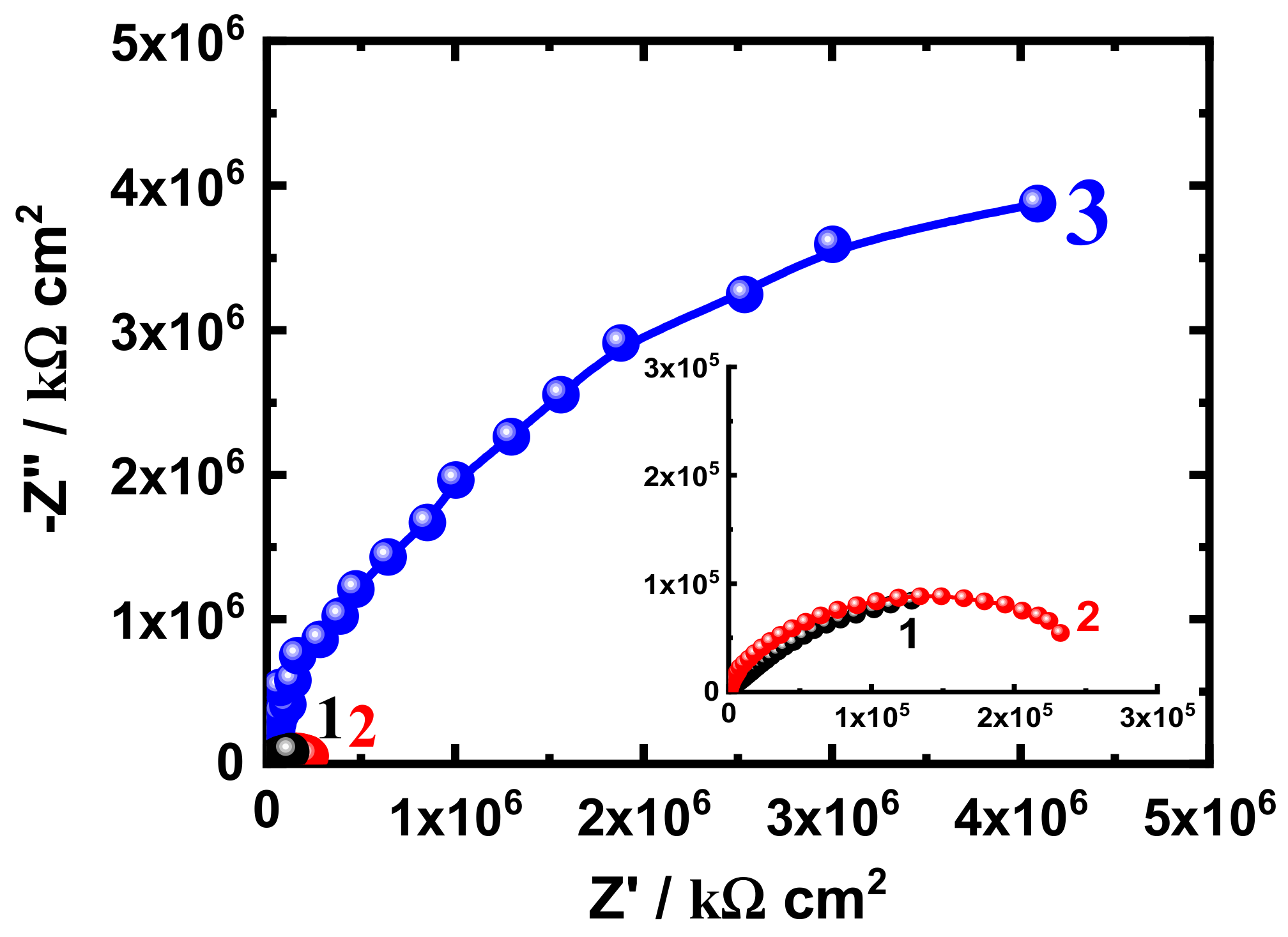
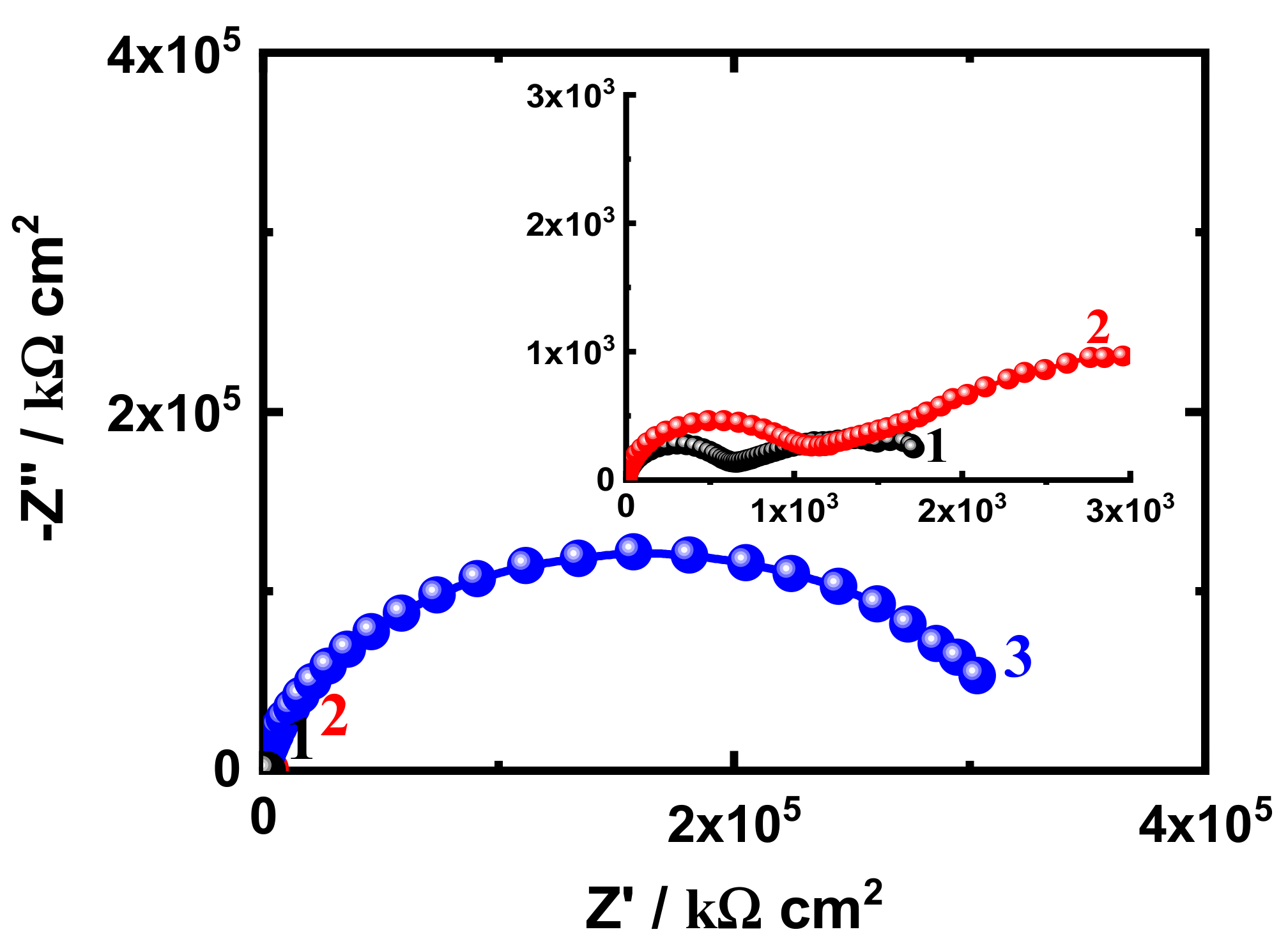
| Formulation Code | * Epoxy Resin (g) | MIBK (ml) | Xylene (ml) | Silane (g) | SiO2 Nanoparticles (g) | * D-450 Hardener (g) |
|---|---|---|---|---|---|---|
| Neat | 83.34 | 8 | 8 | 2 | 0 | 16.66 |
| SN-1 | 83.34 | 8 | 8 | 2 | 1 | 16.66 |
| SN-3 | 83.34 | 8 | 8 | 2 | 3 | 16.66 |
| SN-5 | 83.34 | 8 | 8 | 2 | 5 | 16.66 |
| EIS Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Coating/Time | RS/ Ω cm2 | Cdl/ F cm−2 | RP1/ k Ω cm2 | Q | RP2/ k Ω cm2 | W/ Ω −1/2 | |
| YQ/F cm−2 | n | ||||||
| SN1-1 h | 36.04 | 2.204 × 10−9 | 1593 | 2.268 × 10−8 | 0.54 | 2.246 × 105 | 1.690 × 10−5 |
| SN3-1 h | 42.04 | 2.077 × 10−9 | 1727 | 4.857 × 10−9 | 0.57 | 2.441 × 105 | 9.950 × 10−8 |
| SN5-1 h | 55.48 | 2.648 × 10−9 | 5574 | 8.999 × 10−6 | 0.64 | 2.961 × 105 | 4.136 × 10−8 |
| SN1-5 d | 37.54 | 2.179 × 10−9 | 1180 | 1.470 × 10−7 | 0.43 | 0.328 × 103 | 1.787 × 10−5 |
| SN3-5 d | 44.11 | 2.508 × 10−9 | 5273 | 6.10 × 10−10 | 0.53 | 1.002 × 103 | 4.890 × 10−9 |
| SN5-5 d | 57.96 | 2.123 × 10−9 | 6571 | 2.999 × 10−6 | 0.59 | 3.493 × 103 | 5.345 × 10−8 |
| SN1-10 d | 38.27 | 2.190 × 10−9 | 3806 | 3.313 × 10−7 | 0.37 | 4.977 × 103 | 1.097 × 10−8 |
| SN3-10 d | 48.32 | 2.424 × 10−9 | 4209 | 4.416 × 10−7 | 0.56 | 1.475 × 104 | 3.590 × 10−9 |
| SN5-10 d | 58.12 | 5.815 × 10−9 | 6987 | 8.125 × 10−8 | 0.75 | 4.516 × 103 | 2.222 × 10−8 |
| SN1-15 d | 41.39 | 2.239 × 10−9 | 3904 | 6.408 × 10−7 | 0.32 | 1.261 × 103 | 3.186 × 10−6 |
| SN3-15 d | 49.14 | 2.452 × 10−9 | 8121 | 3.286 × 10−7 | 0.46 | 2.895 × 103 | 5.060 × 10−7 |
| SN5-15 d | 58.41 | 2.333 × 10−8 | 9298 | 2.087 × 10−8 | 0.80 | 5.361 × 103 | 4.358×10−8 |
| SN1-20 d | 43.68 | 2.200 × 10−9 | 4185 | 6.537 × 10−7 | 0.30 | 1.415 × 103 | 4.989 × 10−6 |
| SN3-20 d | 50.18 | 2.358 × 10−9 | 6655 | 1.295 × 10−9 | 0.43 | 2.181 × 103 | 6.460 × 10−8 |
| SN5-20 d | 58.96 | 8.087 × 10−9 | 8112 | 3.793 × 10−9 | 0.83 | 1.476 × 104 | 1.787 × 10−7 |
| SN1-25 d | 45.58 | 2.218 × 10−9 | 4266 | 7.443 × 10−7 | 0.29 | 1.681 × 103 | 6.071 × 10−6 |
| SN3-25 d | 53.41 | 2.486 × 10−9 | 6987 | 1.166 × 10−6 | 0.36 | 2.136 × 103 | 2.350 × 10−7 |
| SN5-25 d | 59.32 | 2.421 × 10−9 | 9501 | 3.673 × 10−9 | 0.85 | 9.711 × 103 | 2.80 × 10−7 |
| SN1-30 d | 47.94 | 2.21 × 10−9 | 4523 | 7.265 × 10−7 | 0.28 | 1.816 × 103 | 6.903 × 10−6 |
| SN3-30 d | 54.28 | 2.479 × 10−9 | 5415 | 3.253 × 10−6 | 0.35 | 2.631 × 103 | 2.151 × 10−8 |
| SN5-30 d | 59.87 | 7.64 × 10−10 | 9685 | 3.757 × 10−9 | 0.88 | 7.957 × 103 | 3.810 × 10−7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.A.; Samad, U.A.; Sherif, E.-S.M.; Poulose, A.M.; Mohammed, J.A.; Alharthi, N.; Al-Zahrani, S.M. Influence of SiO2 Content and Exposure Periods on the Anticorrosion Behavior of Epoxy Nanocomposite Coatings. Coatings 2020, 10, 118. https://doi.org/10.3390/coatings10020118
Alam MA, Samad UA, Sherif E-SM, Poulose AM, Mohammed JA, Alharthi N, Al-Zahrani SM. Influence of SiO2 Content and Exposure Periods on the Anticorrosion Behavior of Epoxy Nanocomposite Coatings. Coatings. 2020; 10(2):118. https://doi.org/10.3390/coatings10020118
Chicago/Turabian StyleAlam, Mohammad Asif, Ubair Abdus Samad, El-Sayed M. Sherif, Anesh Manjaly Poulose, Jabair Ali Mohammed, Nabeel Alharthi, and Saeed M. Al-Zahrani. 2020. "Influence of SiO2 Content and Exposure Periods on the Anticorrosion Behavior of Epoxy Nanocomposite Coatings" Coatings 10, no. 2: 118. https://doi.org/10.3390/coatings10020118
APA StyleAlam, M. A., Samad, U. A., Sherif, E.-S. M., Poulose, A. M., Mohammed, J. A., Alharthi, N., & Al-Zahrani, S. M. (2020). Influence of SiO2 Content and Exposure Periods on the Anticorrosion Behavior of Epoxy Nanocomposite Coatings. Coatings, 10(2), 118. https://doi.org/10.3390/coatings10020118