A Novel Simple Anti-Ice Aluminum Coating: Synthesis and In-Lab Comparison with a Superhydrophobic Hierarchical Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Superhydrophobic Surface (SHP)
2.3. Preparation of Slippery Surface (SLS)
2.4. Characterization Methods
2.5. Durability Performance Tests
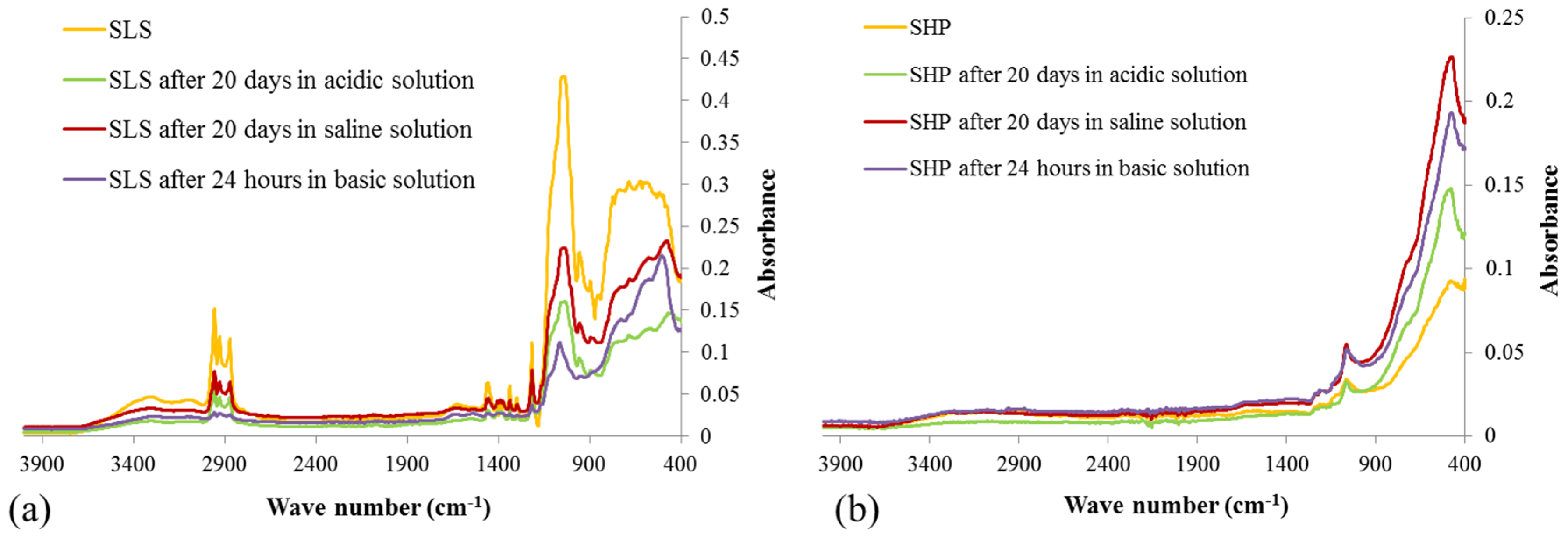
2.5.1. Resistance in Wet Chemicals
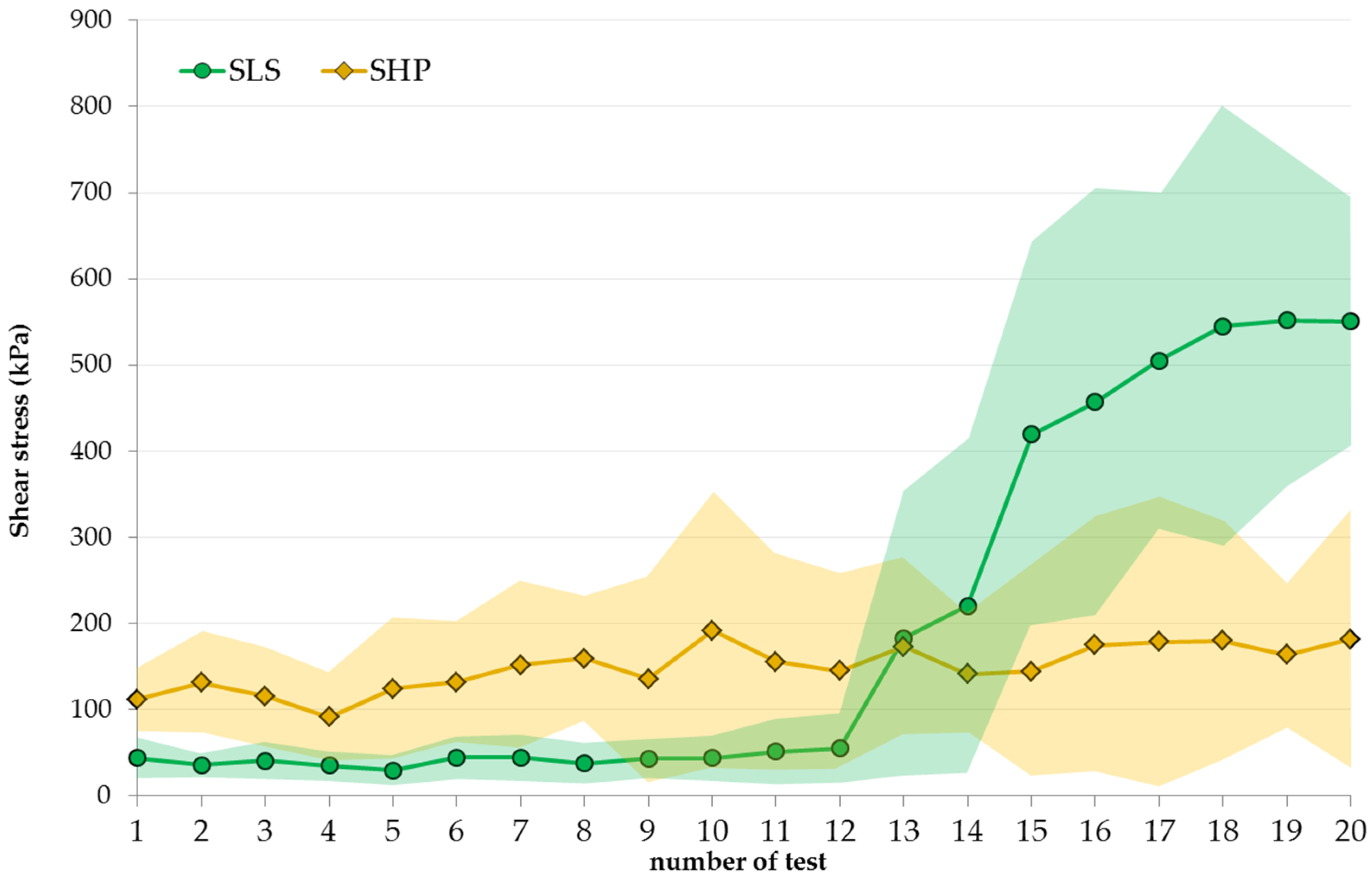
2.5.2. Resistance to Ice Shedding
3. Results and Discussion
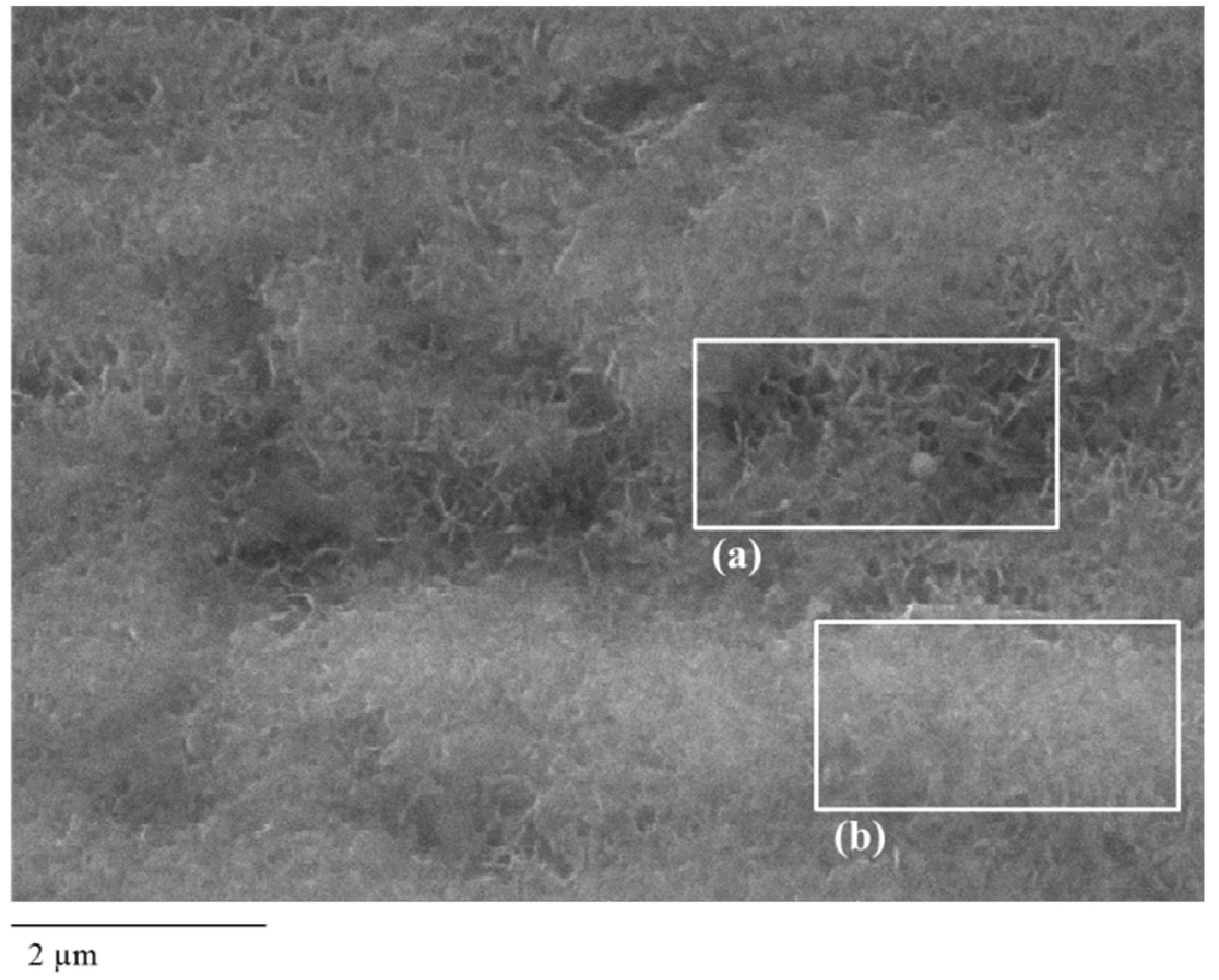
3.1. Morphological and Chemical Evaluation
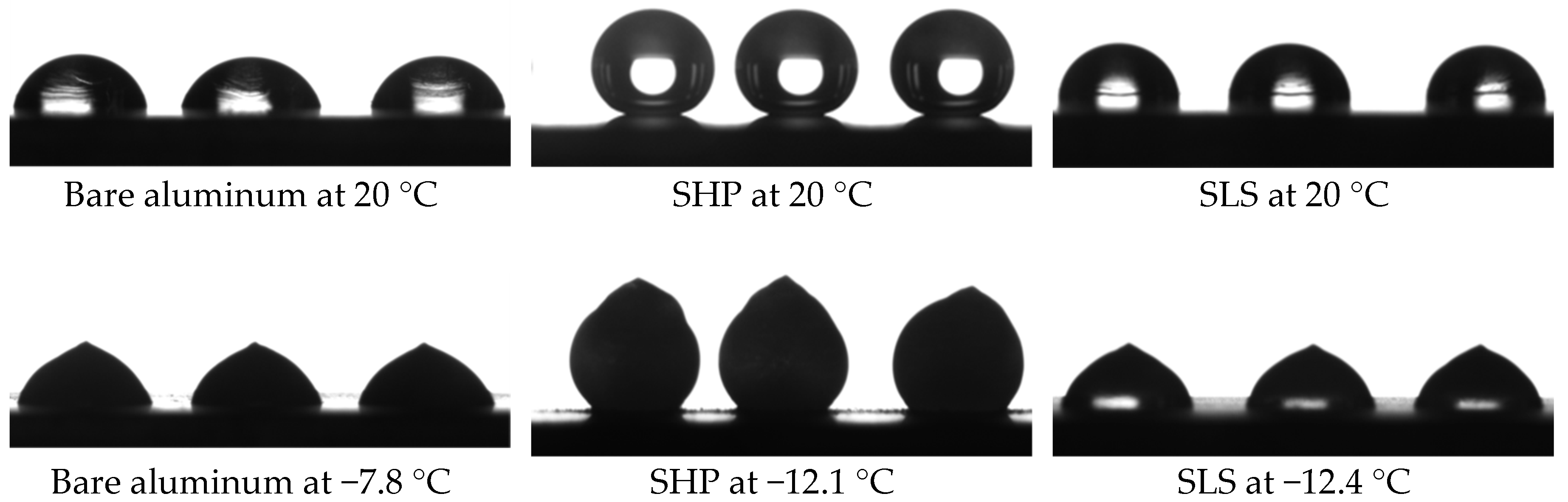
3.2. Hydrophobic Properties at Room and Low Temperatures
3.3. Ice-Proof Performances
3.3.1. Anti-Icing Properties
3.3.2. Ice-Phobic Properties
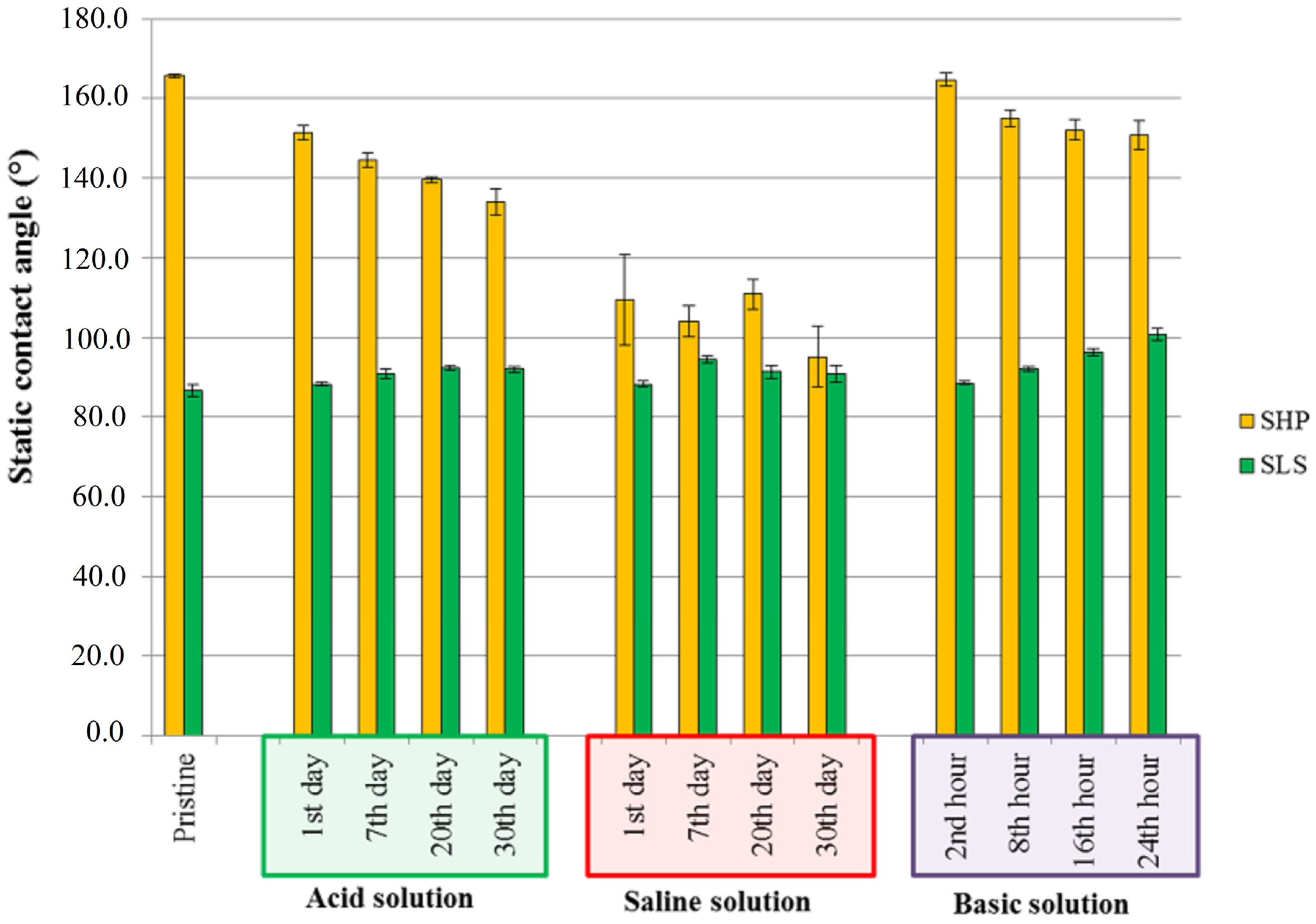
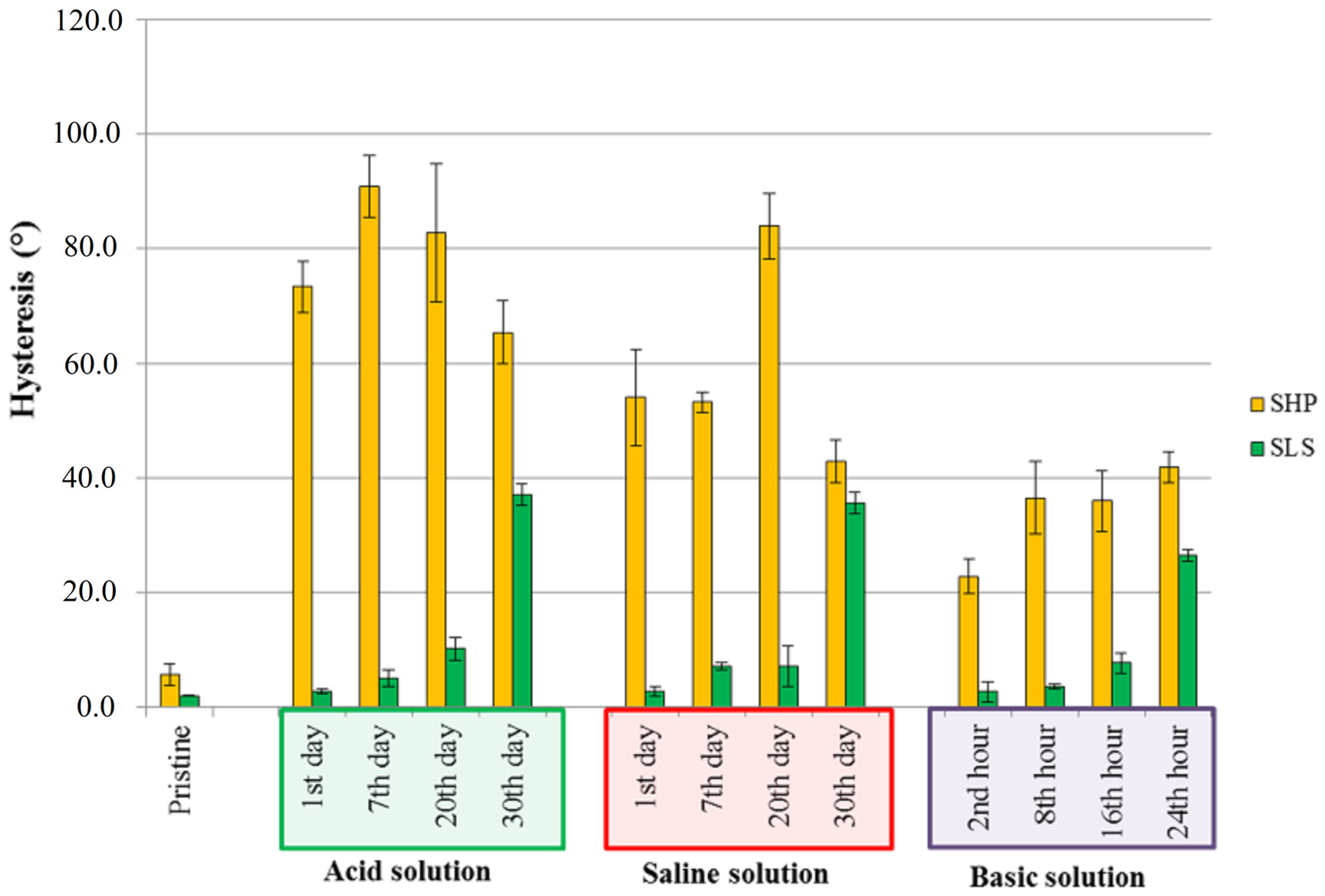
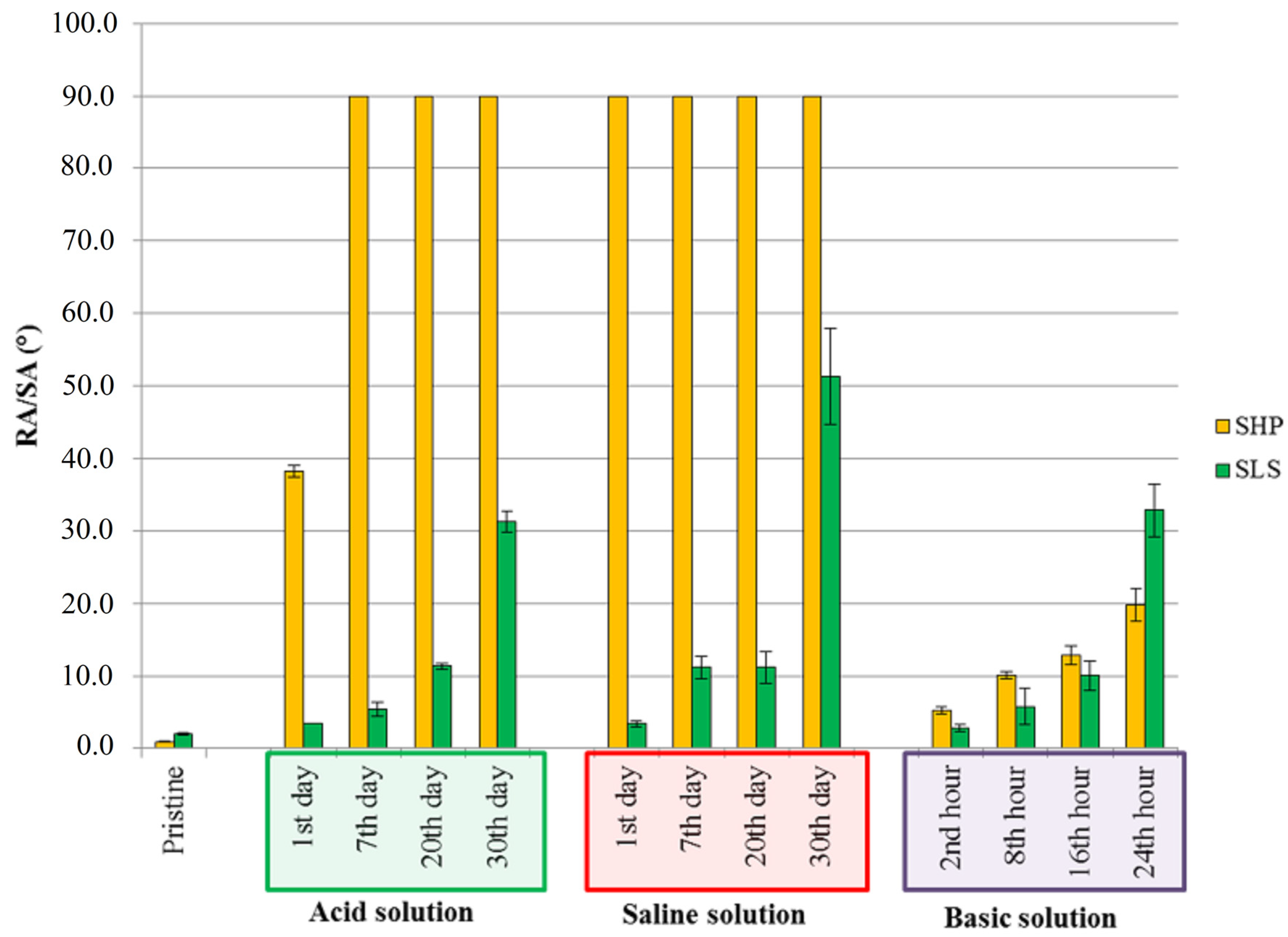
3.4. Durability of Wettability Properties
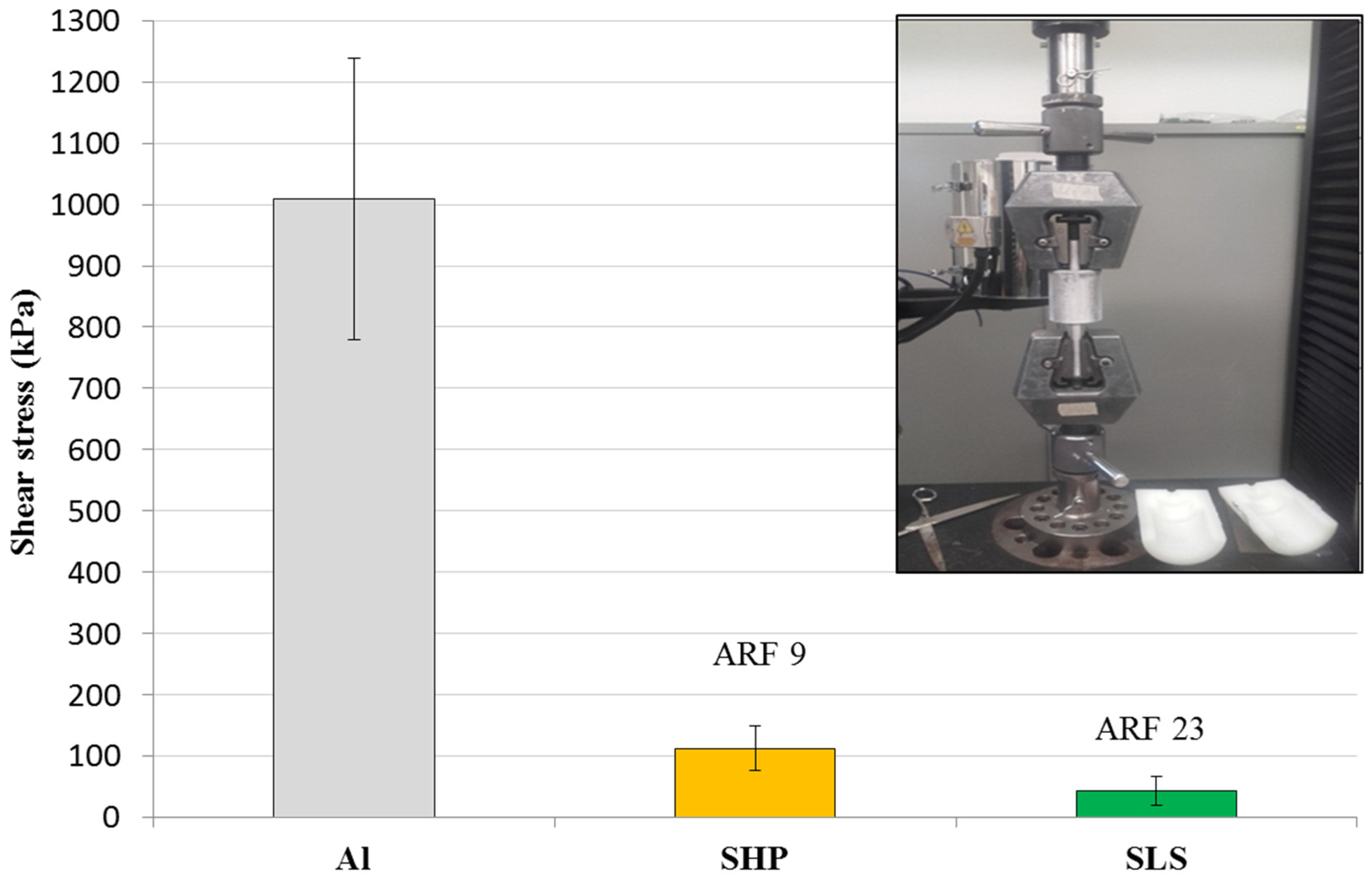
3.5. Durability of Ice-Phobic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Latthe, S.S.; Sutar, R.S.; Bhosale, A.K.; Nagappan, S.; Ha, C.-S.; Sadasivuni, K.K.; Liu, S.; Xing, R. Recent developments in air-trapped superhydrophobic and liquid-infused slippery surfaces for anti-icing application. Prog. Org. Coat. 2019, 137, 105373. [Google Scholar] [CrossRef]
- Farzaneh, M.; Gauthier, H.; Castellana, G.; Engelbrecht, C.; Elìasson, Á.J.; Fikke, S.M.; Greyling, C.; Gutman, I.; Hayashi, T.; Jakl, F. Coatings for Protecting Overhead Power Network Equipment in Winter Conditions; CIGRE: Paris, France, 2015; ISBN 978-2-85873-334-7. [Google Scholar]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Gao, D. Anti-icing superhydrophobic coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Dorrestijn, M.; Raps, D.; Das, A.; Megaridis, C.M.; Poulikakos, D. Are superhydrophobic surfaces best for icephobicity? Langmuir 2011, 27, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Narhe, R.D.; Beysens, D.A. Growth dynamics of water drops on a square-pattern rough hydrophobic surface. Langmuir 2007, 23, 6486–6489. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; He, M.; Li, K.; Cui, D.; Zhang, Q.; Zeng, X.; Zhang, Y.; Wang, J.; Song, Y. Superhydrophobic surfaces cannot reduce ice adhesion. Appl. Phys. Lett. 2012, 101, 111603. [Google Scholar] [CrossRef]
- Varanasi, K.K.; Deng, T.; Smith, J.D.; Hsu, M.; Bhate, N. Frost formation and ice adhesion on superhydrophobic surfaces. Appl. Phys. Lett. 2010, 97, 234102. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farhadi, S.; Nose, K.; Du, X.W. Superhydrophobic surfaces: are they really ice-repellent? Langmuir 2011, 27, 25–29. [Google Scholar] [CrossRef]
- Verho, T.; Bower, C.; Andrew, P.; Franssila, S.; Ikkala, O.; Ras, R.H.A. Mechanically durable superhydrophobic surfaces. Adv. Mater. 2011, 23, 673–678. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Emelyanenko, K.A.; Modin, E.B. Modus operandi of protective and anti-icing mechanisms underlying the design of longstanding outdoor icephobic coatings. ACS Nano 2019, 13, 4335–4346. [Google Scholar] [CrossRef]
- Balordi, M.; Cammi, A.; Santucci de Magistris, G.; Chemelli, C. Role of micrometric roughness on anti-ice properties and durability of hierarchical super-hydrophobic aluminum surfaces. Surf. Coat. Technol. 2019, 374, 549–556. [Google Scholar] [CrossRef]
- Occurrence and use of Highly Fluorinated Substances and Alternatives; 7/15; Swedish Chemical Agency: Stockholm, Sweden, 2015; p. 112.
- Kreder, M.J.; Alvarenga, J.; Kim, P.; Aizenberg, J. Design of anti-icing surfaces: smooth, textured or slippery? Nat. Rev. Mater. 2016, 1, 15003. [Google Scholar] [CrossRef]
- Wang, N.; Xiong, D.; Lu, Y.; Pan, S.; Wang, K.; Deng, Y.; Shi, Y. Design and fabrication of the lyophobic slippery surface and its application in anti-icing. J. Phys. Chem. C 2016, 120, 11054–11059. [Google Scholar] [CrossRef]
- Kim, P.; Wong, T.-S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.-S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Y.; Huang, M.; Zhou, Y.; Liu, Y.; Liang, X. Durability of a lubricant-infused electrospray silicon rubber surface as an anti-icing coating. Appl. Surf. Sci. 2015, 346, 68–76. [Google Scholar] [CrossRef]
- Smith, J.D.; Dhiman, R.; Anand, S.; Reza-Garduno, E.; Cohen, R.E.; McKinley, G.H.; Varanasi, K.K. Droplet mobility on lubricant-impregnated surfaces. Soft Matter 2013, 9, 1772–1780. [Google Scholar] [CrossRef]
- Lee, H.; Alcaraz, M.L.; Rubner, M.F.; Cohen, R.E. Zwitter-wettability and antifogging coatings with frost-resisting capabilities. ACS Nano 2013, 7, 2172–2185. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, C.; Tu, J. Robust slippery coating with superior corrosion resistance and anti-icing performance for az31b mg alloy protection. ACS Appl. Mater. Interfaces 2017, 9, 11247–11257. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, J.; Chen, R.; Liu, Q.; Liu, J.; Song, D.; Liu, P.; Gao, L.; Wang, J. Highly transparent and robust slippery lubricant-infused porous surfaces with anti-icing and anti-fouling performances. J. Alloys Compd. 2019, 803, 51–60. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Park, S.; Jung, Y.; Lim, H. Effects of hydrophobicity and lubricant characteristics on anti-icing performance of slippery lubricant-infused porous surfaces. J. Ind. Eng. Chem. 2019, 69, 99–105. [Google Scholar] [CrossRef]
- Veronesi, F.; Boveri, G.; Raimondo, M. Amphiphobic nanostructured coatings for industrial applications. Materials 2019, 12, 787. [Google Scholar] [CrossRef] [PubMed]
- Jafari, R.; Farzaneh, M. Fabrication of superhydrophobic nanostructured surface on aluminum alloy. Appl. Phys. A 2011, 102, 195–199. [Google Scholar] [CrossRef]
- Michael, N.; Bhushan, B. Hierarchical roughness makes superhydrophobic states stable. Microelectron. Eng. 2007, 84, 382–386. [Google Scholar] [CrossRef]
- Saifaldeen, Z.S.; Khedir, K.R.; Cansizoglu, M.F.; Demirkan, T.; Karabacak, T. Superamphiphobic aluminum alloy surfaces with micro and nanoscale hierarchical roughness produced by a simple and environmentally friendly technique. J. Mater. Sci. 2014, 49, 1839–1853. [Google Scholar] [CrossRef]
- Heydari, G.; Tyrode, E.; Visnevskij, C.; Makuska, R.; Claesson, P.M. Temperature-dependent deicing properties of electrostatically anchored branched brush layers of poly(ethylene oxide). Langmuir 2016, 32, 4194–4202. [Google Scholar] [CrossRef]
- Malavasi, I.; Bernagozzi, I.; Antonini, C.; Marengo, M. Assessing durability of superhydrophobic surfaces. Surf. Innov. 2015, 3, 49–60. [Google Scholar] [CrossRef]
- Geiculescu, A. A microstructural investigation of low-temperature crystalline alumina films grown on aluminum. Thin Solid Films 2003, 426, 160–171. [Google Scholar] [CrossRef]
- Rider, A.N. The influence of porosity and morphology of hydrated oxide films on epoxy-aluminium bond durability. J. Adhes. Sci. Technol. 2001, 15, 395–422. [Google Scholar] [CrossRef]
- Motta, A.; Cannelli, O.; Boccia, A.; Zanoni, R.; Raimondo, M.; Caldarelli, A.; Veronesi, F. A mechanistic explanation of the peculiar amphiphobic properties of hybrid organic–inorganic coatings by combining xps characterization and dft modeling. ACS Appl. Mater. Interfaces 2015, 7, 19941–19947. [Google Scholar] [CrossRef]
- Rio, E.; Boulogne, F. Withdrawing a solid from a bath: how much liquid is coated? Adv. Colloid Interface Sci. 2017, 247, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Damayanti, N.P. Preparation of superhydrophobic pet fabric from al2o3–sio2 hybrid: geometrical approach to create high contact angle surface from low contact angle materials. J. Sol-Gel Sci. Technol. 2010, 56, 47–52. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Lv, Y.; Lv, F.; Du, Y. Ice accretion on superhydrophobic aluminum surfaces under low-temperature conditions. Cold Reg. Sci. Technol. 2010, 62, 29–33. [Google Scholar] [CrossRef]
- Prysiazhnyi, V. Plasma treatment of aluminum using a surface barrier discharge operated in air and nitrogen: Parameter optimization and related effects. Plasma Sci. Technol. 2013, 15, 794–799. [Google Scholar] [CrossRef]
- Niemelä-Anttonen, H.; Koivuluoto, H.; Tuominen, M.; Teisala, H.; Juuti, P.; Haapanen, J.; Harra, J.; Stenroos, C.; Lahti, J.; Kuusipalo, J. Icephobicity of slippery liquid infused porous surfaces under multiple freeze-thaw and ice accretion-detachment cycles. Adv. Mater. Interfaces 2018, 5, 1800828. [Google Scholar] [CrossRef]
- Sojoudi, H.; Wang, M.; Boscher, N.D.; McKinley, G.H.; Gleason, K.K. Durable and scalable icephobic surfaces: Similarities and distinctions from superhydrophobic surfaces. Soft Matter 2016, 12, 1938–1963. [Google Scholar] [CrossRef]
- Meuler, A.J.; Smith, J.D.; Varanasi, K.K.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Relationships between water wettability and ice adhesion. ACS Appl. Mater. Interfaces 2010, 2, 3100–3110. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Z. Fundamentals of icing and common strategies for designing biomimetic anti-icing surfaces. J. Mater. Chem. A 2018, 6, 13549–13581. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.-Y. Control of ice nucleation: Freezing and antifreeze strategies. Chem. Soc. Rev. 2018, 47, 7116–7139. [Google Scholar] [CrossRef]
- He, M.; Wang, J.; Li, H.; Song, Y. Super-hydrophobic surfaces to condensed micro-droplets at temperatures below the freezing point retard ice/frost formation. Soft Matter 2011, 7, 3993. [Google Scholar] [CrossRef]
- Zhang, Q.; Jin, B.; Wang, B.; Fu, Y.; Zhan, X.; Chen, F. Fabrication of a highly stable superhydrophobic surface with dual-scale structure and its antifrosting properties. Ind. Eng. Chem. Res. 2017, 56, 2754–2763. [Google Scholar] [CrossRef]
- Anand, S.; Paxson, A.T.; Dhiman, R.; Smith, J.D.; Varanasi, K.K. Enhanced condensation on lubricant-impregnated nanotextured surfaces. ACS Nano 2012, 6, 10122–10129. [Google Scholar] [CrossRef]
- Zhan, X.; Yan, Y.; Zhang, Q.; Chen, F. A novel superhydrophobic hybrid nanocomposite material prepared by surface-initiated aget atrp and its anti-icing properties. J. Mater. Chem. A 2014, 2, 9390–9399. [Google Scholar] [CrossRef]
- Song, J.; Zhao, D.; Han, Z.; Xu, W.; Lu, Y.; Liu, X.; Liu, B.; Carmalt, C.J.; Deng, X.; Parkin, I.P. Super-robust superhydrophobic concrete. J. Mater. Chem. A 2017, 5, 14542–14550. [Google Scholar] [CrossRef]
- Wei, C.; Jin, B.; Zhang, Q.; Zhan, X.; Chen, F. Anti-icing performance of super-wetting surfaces from icing-resistance to ice-phobic aspects: Robust hydrophobic or slippery surfaces. J. Alloys Compd. 2018, 765, 721–730. [Google Scholar] [CrossRef]
- Bi, Y.; Cabriolu, R.; Li, T. Heterogeneous ice nucleation controlled by the coupling of surface crystallinity and surface hydrophilicity. J. Phys. Chem. C 2016, 120, 1507–1514. [Google Scholar] [CrossRef]
- Fu, Q.; Wu, X.; Kumar, D.; Ho, J.W.C.; Kanhere, P.D.; Srikanth, N.; Liu, E.; Wilson, P.; Chen, Z. Development of sol–gel icephobic coatings: Effect of surface roughness and surface energy. ACS Appl. Mater. Interfaces 2014, 6, 20685–20692. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, J.; Wang, Q.; Chen, Q.; Ding, J. Verification of icephobic/anti-icing properties of a superhydrophobic surface. ACS Appl. Mater. Interfaces 2013, 5, 3370–3381. [Google Scholar] [CrossRef]
- Bharathidasan, T.; Kumar, S.V.; Bobji, M.S.; Chakradhar, R.P.S.; Basu, B.J. Effect of wettability and surface roughness on ice-adhesion strength of hydrophilic, hydrophobic and superhydrophobic surfaces. Appl. Surf. Sci. 2014, 314, 241–250. [Google Scholar] [CrossRef]
- Susoff, M.; Siegmann, K.; Pfaffenroth, C.; Hirayama, M. Evaluation of icephobic coatings—screening of different coatings and influence of roughness. Appl. Surf. Sci. 2013, 282, 870–879. [Google Scholar] [CrossRef]
- Sojoudi, H.; Arabnejad, H.; Raiyan, A.; Shirazi, S.A.; McKinley, G.H.; Gleason, K.K. Scalable and durable polymeric icephobic and hydrate-phobic coatings. Soft Matter 2018, 14, 3443–3454. [Google Scholar] [CrossRef] [PubMed]
- Juuti, P.; Haapanen, J.; Stenroos, C.; Niemelä-Anttonen, H.; Harra, J.; Koivuluoto, H.; Teisala, H.; Lahti, J.; Tuominen, M.; Kuusipalo, J.; et al. Achieving a slippery, liquid-infused porous surface with anti-icing properties by direct deposition of flame synthesized aerosol nanoparticles on a thermally fragile substrate. Appl. Phys. Lett. 2017, 110, 161603. [Google Scholar] [CrossRef]
- Coady, M.J.; Wood, M.; Wallace, G.Q.; Nielsen, K.E.; Kietzig, A.-M.; Lagugné-Labarthet, F.; Ragogna, P.J. Icephobic behavior of uv-cured polymer networks incorporated into slippery lubricant-infused porous surfaces: Improving slips durability. ACS Appl. Mater. Interfaces 2018, 10, 2890–2896. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.; Li, W.; Wang, B.; Deng, B.; Ma, F.; Yu, Z. Preparation and anti-icing behavior of superhydrophobic surfaces on aluminum alloy substrates. Langmuir 2013, 29, 8482–8491. [Google Scholar] [CrossRef] [PubMed]
- Sarshar, M.A.; Swarctz, C.; Hunter, S.; Simpson, J.; Choi, C.-H. Effects of contact angle hysteresis on ice adhesion and growth on superhydrophobic surfaces under dynamic flow conditions. Colloid Polym. Sci. 2013, 291, 427–435. [Google Scholar] [CrossRef]
- Amirfazli, A. Superomniphobic Surfaces for Military Applications: Nano- and Micro-fabrication Methods; Defence Research and Development Canada: Ottawa, ON, Canada, 2011; p. 130. [Google Scholar]
- Boinovich, L.B.; Emelyanenko, A.M.; Ivanov, V.K.; Pashinin, A.S. Durable icephobic coating for stainless steel. ACS Appl. Mater. Interfaces 2013, 5, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Kulinich, S.A.; Honda, M.; Zhu, A.L.; Rozhin, A.G.; Du, X.W. The icephobic performance of alkyl-grafted aluminum surfaces. Soft Matter 2015, 11, 856–861. [Google Scholar] [CrossRef]
- Momen, G.; Jafari, R.; Farzaneh, M. Ice repellency behaviour of superhydrophobic surfaces: Effects of atmospheric icing conditions and surface roughness. Appl. Surf. Sci. 2015, 349, 211–218. [Google Scholar] [CrossRef]
- Lazauskas, A.; Guobienė, A.; Prosyčevas, I.; Baltrušaitis, V.; Grigaliūnas, V.; Narmontas, P.; Baltrusaitis, J. Water droplet behavior on superhydrophobic sio2 nanocomposite films during icing/deicing cycles. Mater. Charact. 2013, 82, 9–16. [Google Scholar] [CrossRef]
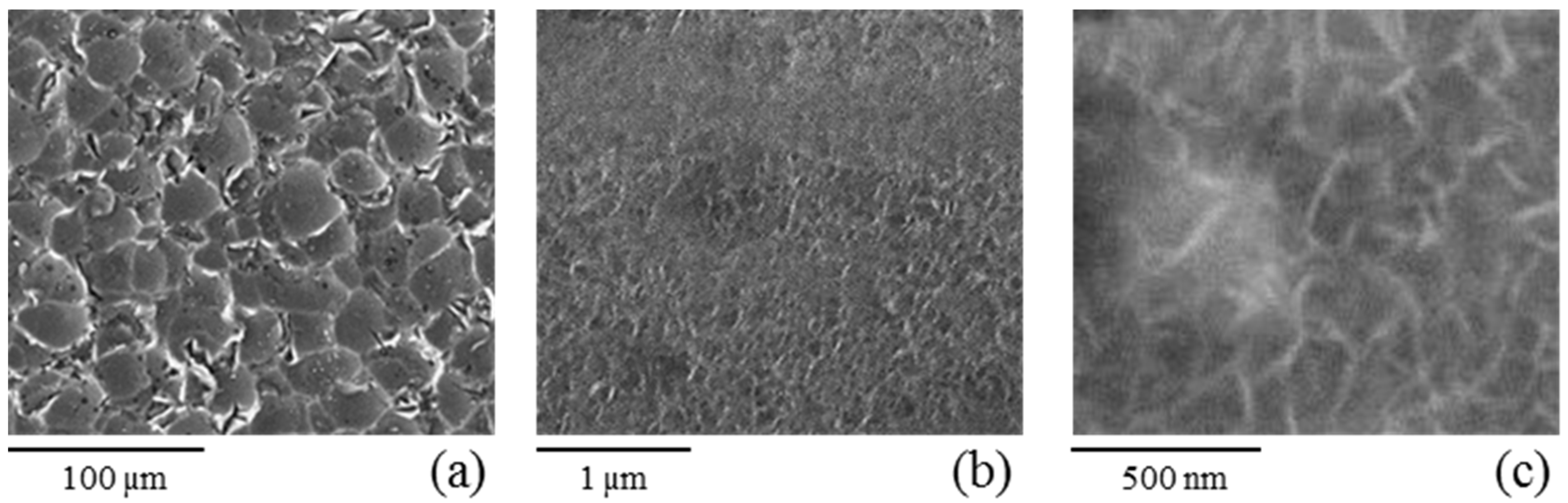
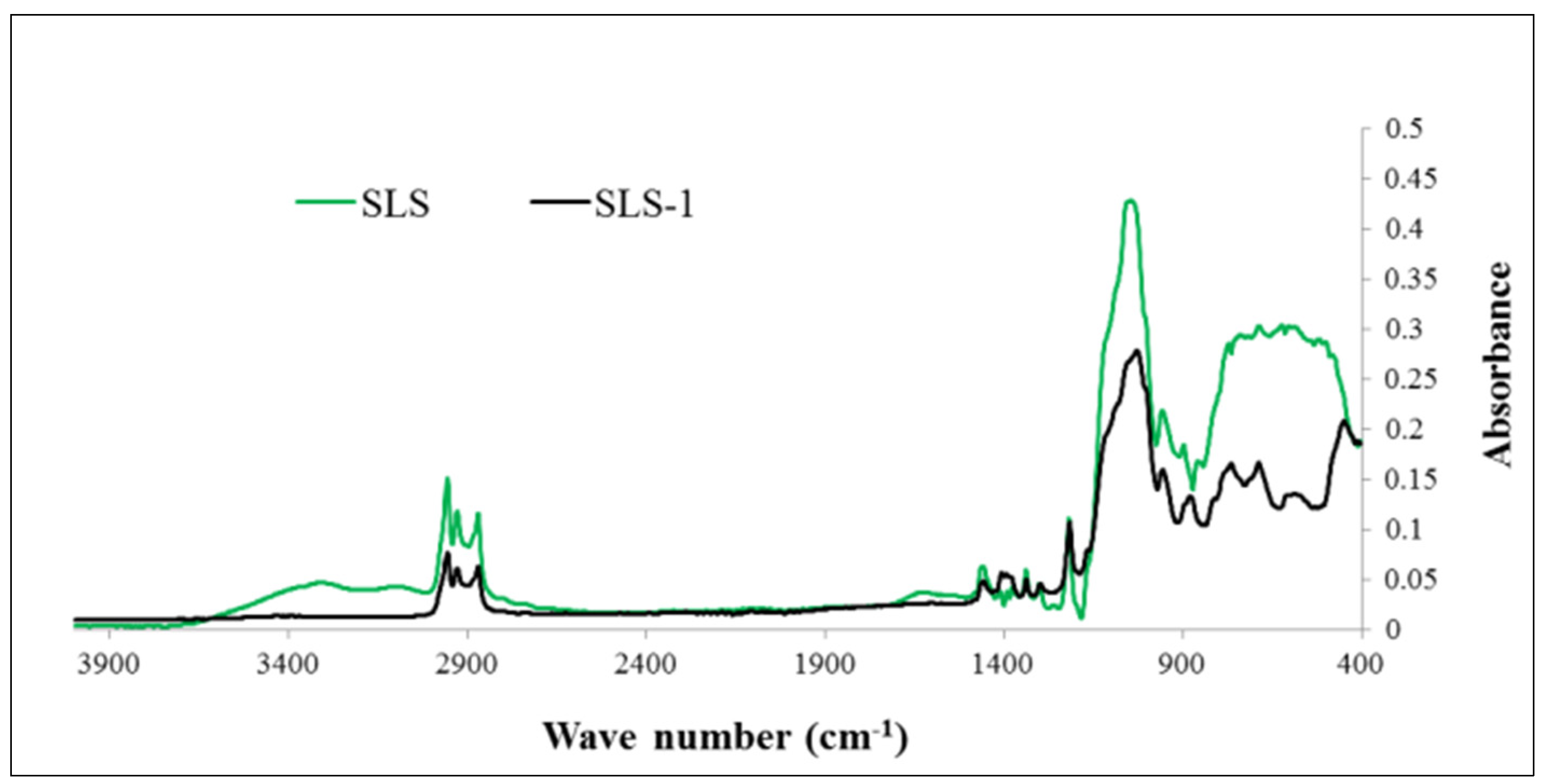
| Sample | Si (at.%) | Al (at.%) | O (at.%) | C (at.%) |
|---|---|---|---|---|
| SLS | 4 | 56 | 17 | 23 |
| SLS-1 | 1 | 67 | 8 | 24 |
| Sample | Room Temperature | Low Temperature | |||||
|---|---|---|---|---|---|---|---|
| WCA (°) | CAH (°) | RA/SA (°) | SFE (mJ/m2) | WCA (°) | CAH (°) | RA/SA (°) | |
| SHP | 170.2 ± 2.7 | 4.2 ± 0.7 | 2 ± 1 | 0.11 ± 0.03 | 143.2 ± 2.7 | 32.3 ± 2.7 | >90 |
| SLS | 86.7 ± 1.5 | 2.0 ± 0.1 | 2.0 ± 0.2 | 38.50 ± 0.02 | 81.5 ± 0.9 | 8.6 ± 0.8 | 16.0 ± 2.0 |
| Sample | Acid Solution | Saline Solution | Basic Solution | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 7 Days | 15 Days | 24 h | |||||||
| CA (°) | CAH (°) | RA/SA (°) | CA (°) | CAH (°) | RA/SA (°) | CA (°) | CAH (°) | RA/SA (°) | |
| SLS | 89.1 | 6.7 | 6.1 | 92.8 | 6.6 | 8.2 | 100.6 | 26.4 | 32.8 |
| SLS-1 | 88.6 | 26.3 | 44.5 | 99.5 | 38.9 | 56.9 | 99.4 | 33.4 | 39.8 |
| SHP | 144.6 | 90.9 | no roll | 87.1 | 46.2 | no roll | 150.9 | 41.7 | 19.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balordi, M.; Santucci de Magistris, G.; Chemelli, C. A Novel Simple Anti-Ice Aluminum Coating: Synthesis and In-Lab Comparison with a Superhydrophobic Hierarchical Surface. Coatings 2020, 10, 111. https://doi.org/10.3390/coatings10020111
Balordi M, Santucci de Magistris G, Chemelli C. A Novel Simple Anti-Ice Aluminum Coating: Synthesis and In-Lab Comparison with a Superhydrophobic Hierarchical Surface. Coatings. 2020; 10(2):111. https://doi.org/10.3390/coatings10020111
Chicago/Turabian StyleBalordi, Marcella, Giorgio Santucci de Magistris, and Cristina Chemelli. 2020. "A Novel Simple Anti-Ice Aluminum Coating: Synthesis and In-Lab Comparison with a Superhydrophobic Hierarchical Surface" Coatings 10, no. 2: 111. https://doi.org/10.3390/coatings10020111
APA StyleBalordi, M., Santucci de Magistris, G., & Chemelli, C. (2020). A Novel Simple Anti-Ice Aluminum Coating: Synthesis and In-Lab Comparison with a Superhydrophobic Hierarchical Surface. Coatings, 10(2), 111. https://doi.org/10.3390/coatings10020111







