Plant Extract Induced Biogenic Preparation of Silver Nanoparticles and Their Potential as Catalyst for Degradation of Toxic Dyes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
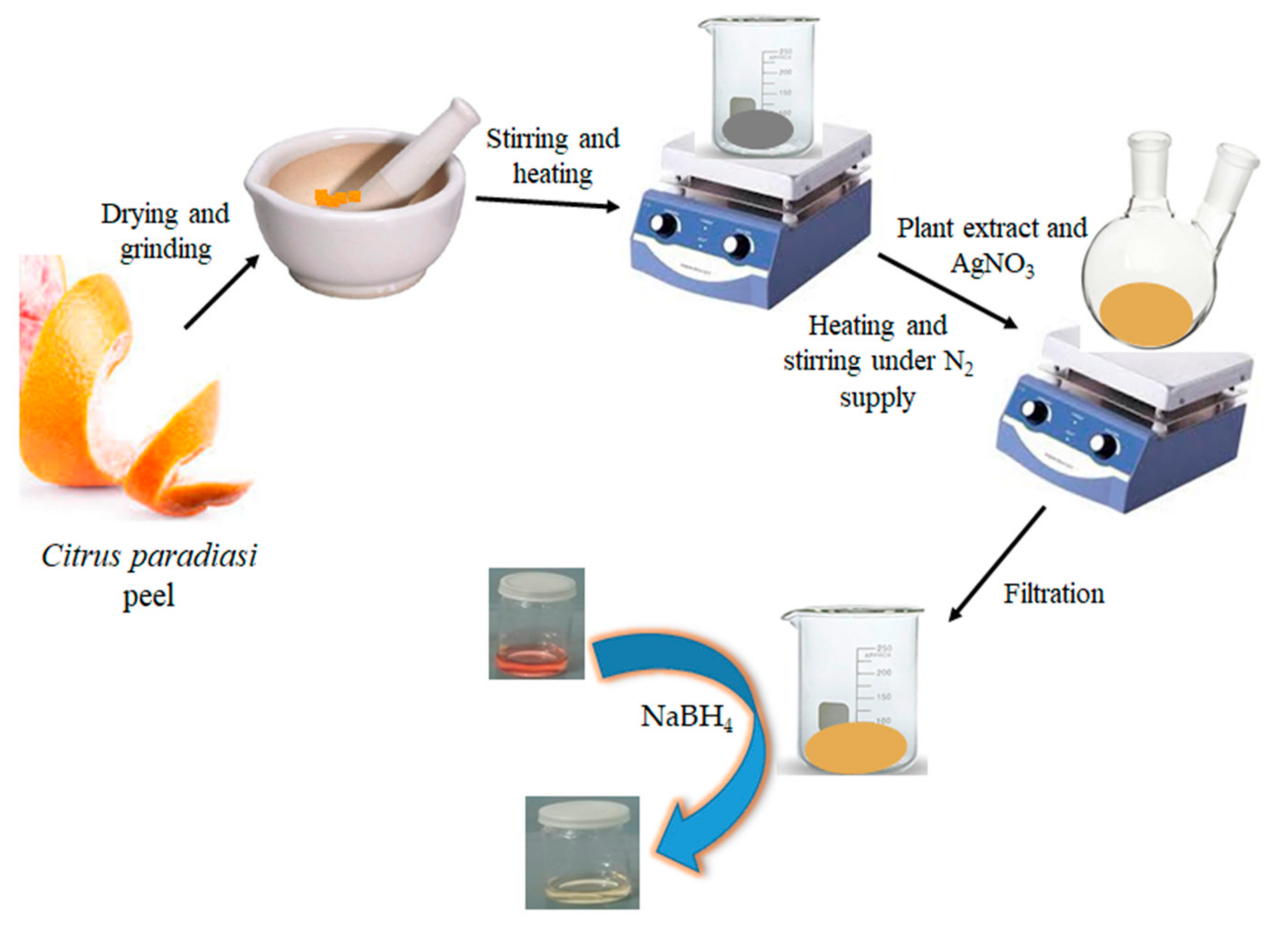
2.2. Synthesis of CAg-nPs
2.3. Characterization of CAg-nPs
2.4. Catalytic Reduction of Toxic Dyes
3. Results and Discussion
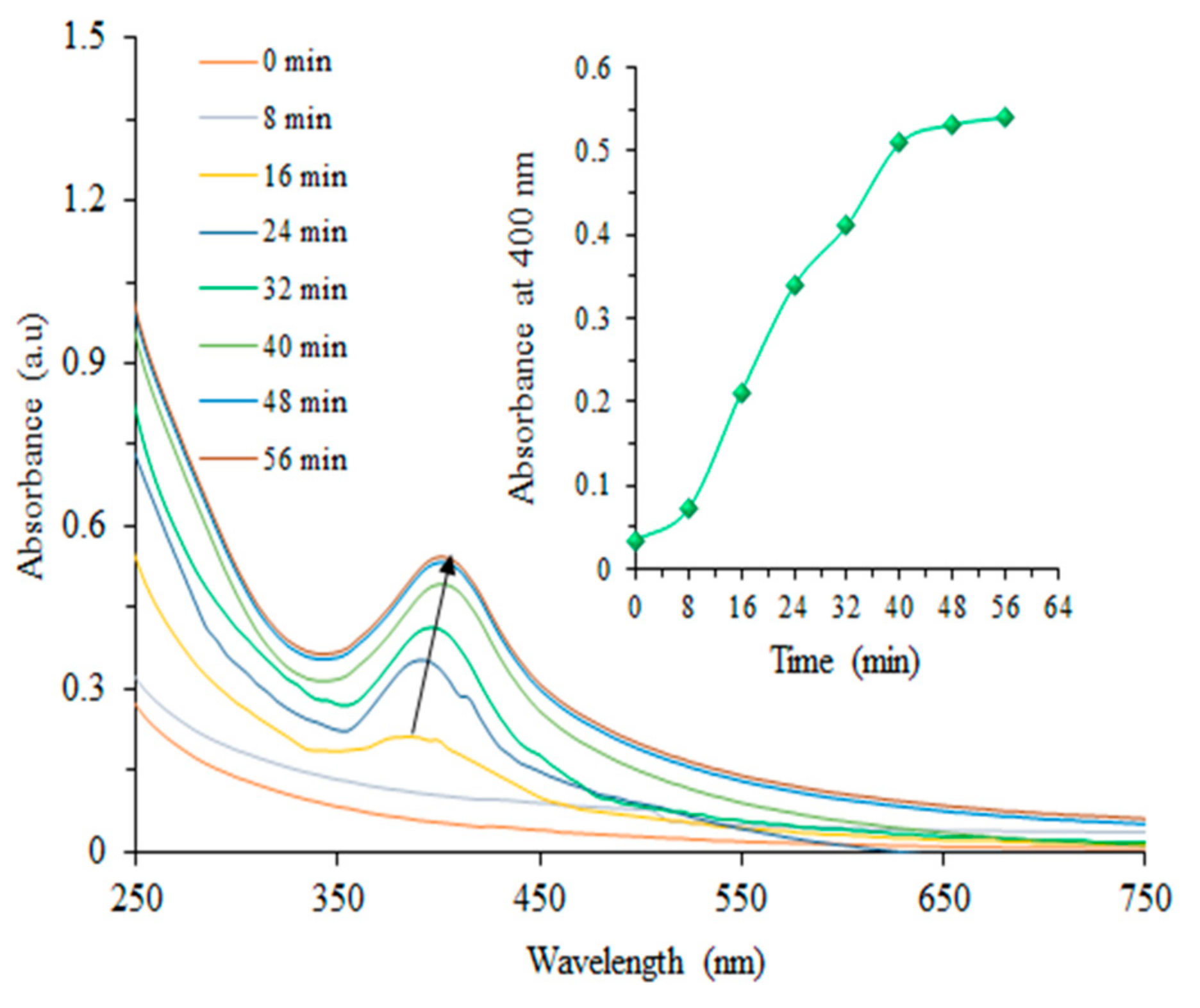
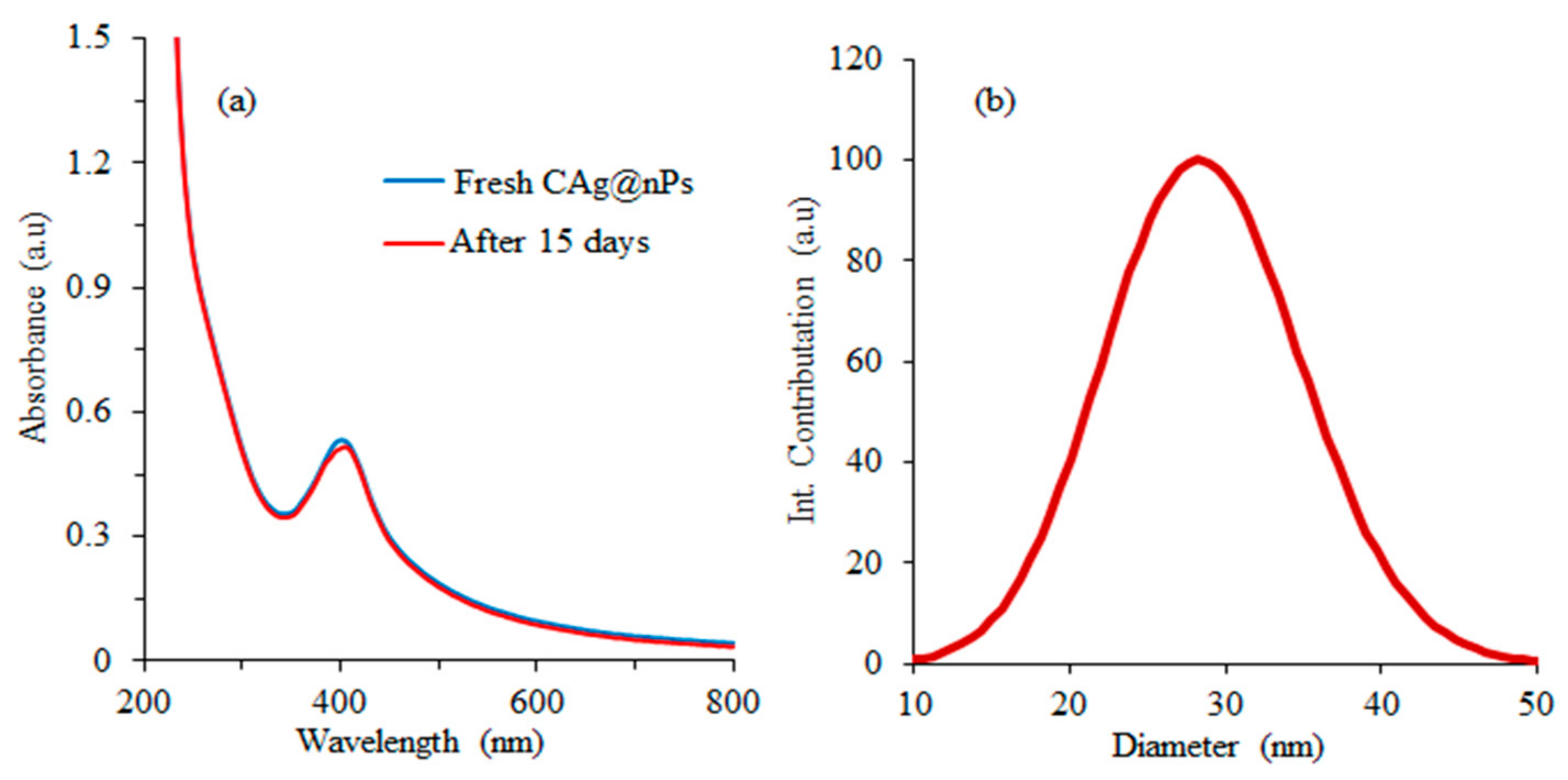
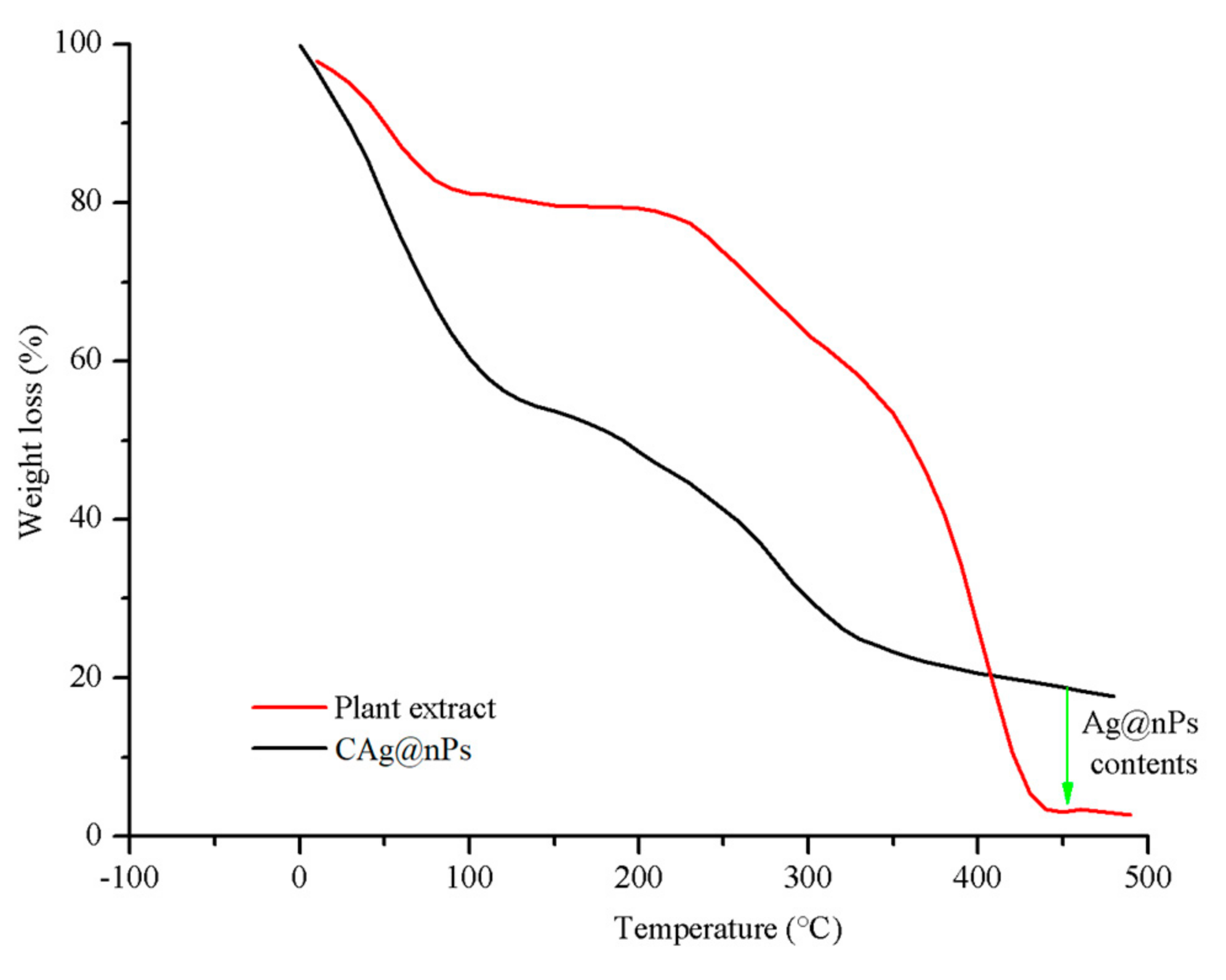
3.1. Analysis of Biosynthesized CAg-nPs
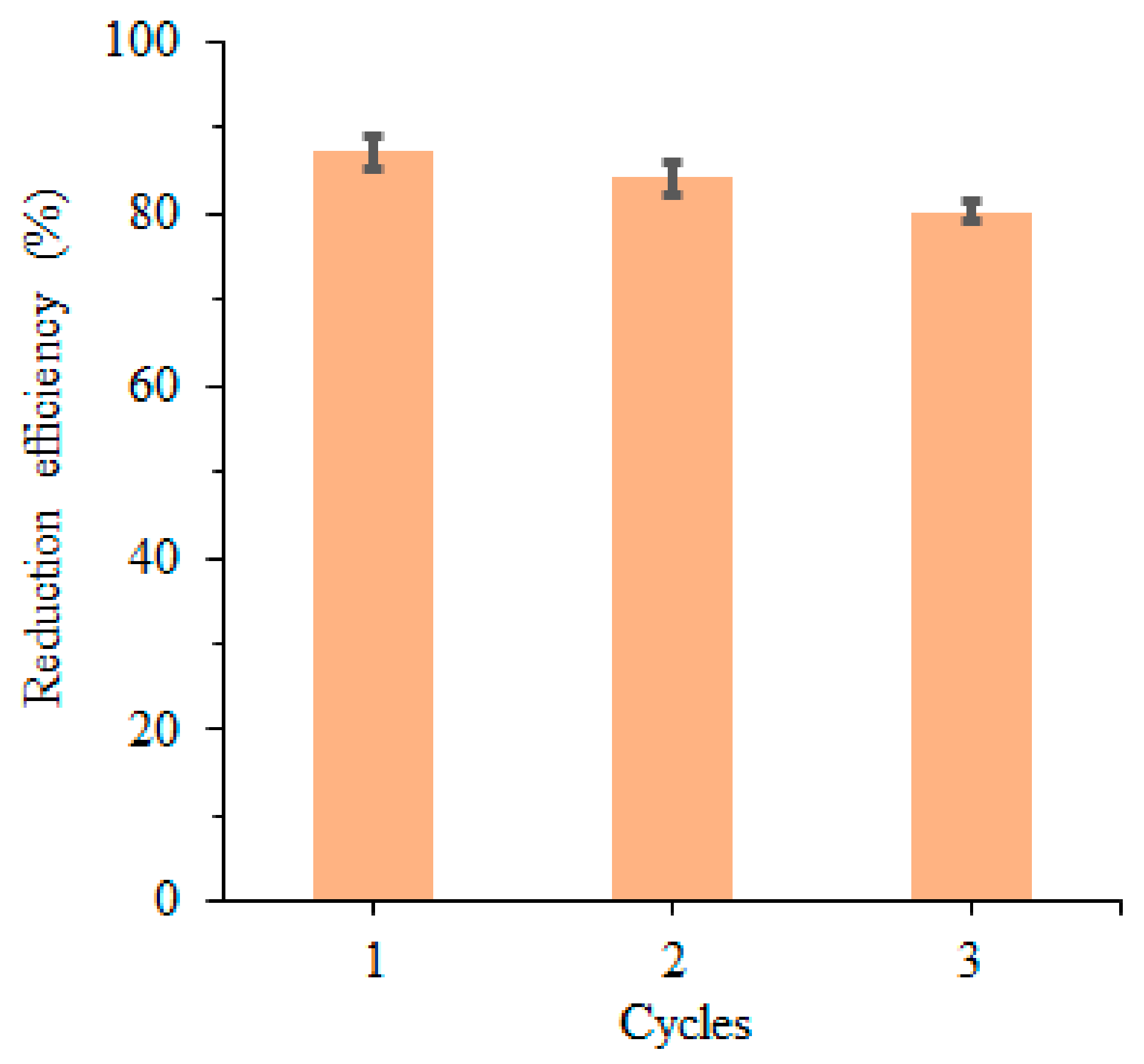
3.2. Catalytic Reduction of Toxic Dyes
3.3. Kinetic Study
3.4. Effect of Reaction Conditions
3.5. Reduction of Other Dyes and Nitroarenes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Irfan, A. Removal of Congo red dye from aqueous medium by its catalytic reduction using sodium borohydride in the presence of various inorganic nano-catalysts: A review. J. Clean. Prod. 2018, 187, 296–307. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, P.; Liu, G.; Ahmad, A.; Chen, X.; Zhu, Y.; Alothman, A.; Hussain, S.; Qiao, G. Synthesis, characterization and wettability of Cu–Sn alloy on the Si-implanted 6H–SiC. Coatings 2020, 9, 906. [Google Scholar] [CrossRef]
- Albukhari, S.M.; Ismail, M.; Akhtar, K.; Danish, E.Y. Catalytic reduction of nitrophenols and dyes using silver nanoparticles@ cellulose polymer paper for the resolution of waste water treatment challenges. Colloids Surf. Physicochem. Eng. Asp. 2019, 577, 548–561. [Google Scholar] [CrossRef]
- Aravind, M.; Ahmad, A.; Ahmad, I.; Amalanathan, M.; Naseem, K.; Mary, S.M.M.; Parvathiraja, C.; Hussain, S.; Algarni, T.S.; Pervaiz, M.; et al. Critical green routing synthesis of silver NPs using jasmine flower extract for biological activities and photocatalytical degradation of methylene blue. J. Environ. Chem. Eng. 2020, 104877. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, A.J.; Arshad, M.; Javed, M.S.; Ahmad, A.; Shah, S.S.A.; Khan, M.R.; Akram, S.; Zulfiqar; Ali, S.; et al. Charge storage in binder-free 2D-hexagonal CoMoO4 nanosheets as a redox active material for pseudocapacitors. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Ghufran, M.; Rehman, M.Z.U.; Najeeb, J.; Irfan, A.; Al-Sehemi, A.G. Poly (N-isopropylmethacrylamide-acrylic acid) microgels as adsorbent for removal of toxic dyes from aqueous medium. J. Mol. Liq. 2018, 268, 229–238. [Google Scholar] [CrossRef]
- Ahmad, A.; Jini, D.; Aravind, M.; Parvathiraja, C.; Ali, R.; Kiyani, M.Z.; Alothman, A. A novel study on synthesis of egg shell based activated carbon for degradation of methylene blue via photocatalysis. Arab. J. Chem. 2020. [Google Scholar] [CrossRef]
- Ahmad, A.; Mubharak, N.M.; Naseem, K.; Tabassum, H.; Rizwan, M.; Najda, A.; Kashif, M.; Bin-Jumah, M.; Hussain, A.; Shaheen, A.; et al. Recent advancement and development of chitin and chitosan-based nanocomposite for drug delivery: Critical approach to clinical research. Arab. J. Chem. 2020, 13, 8935–8964. [Google Scholar] [CrossRef]
- Suresh, M.; Sivasamy, A. Fabrication of graphene nanosheets decorated by nitrogen-doped ZnO nanoparticles with enhanced visible photocatalytic activity for the degradation of Methylene Blue dye. J. Mol. Liq. 2020, 317, 114112. [Google Scholar] [CrossRef]
- Ganapuram, B.R.; Alle, M.; Dadigala, R.; Dasari, A.; Maragoni, V.; Guttena, V. Catalytic reduction of methylene blue and Congo red dyes using green synthesized gold nanoparticles capped by salmalia malabarica gum. Int. Nano Lett. 2015, 5, 215–222. [Google Scholar] [CrossRef]
- Naseem, K.; Begum, R.; Wu, W.; Irfan, A.; Al-Sehemi, A.G.; Farooqi, Z.H. Catalytic reduction of toxic dyes in the presence of silver nanoparticles impregnated core-shell composite microgels. J. Clean. Prod. 2019, 211, 855–864. [Google Scholar] [CrossRef]
- Lu, Y.; Proch, S.; Schrinner, M.; Drechsler, M.; Kempe, R.; Ballauff, M. Thermosensitive core-shell microgel as a “nanoreactor” for catalytic active metal nanoparticles. J. Mater. Chem. 2009, 19, 3955–3961. [Google Scholar] [CrossRef]
- Graça, C.A.; Mendes, M.A.; Teixeira, A.C.S.; de Velosa, A.C. Anoxic degradation of chlorpyrifos by zerovalent monometallic and bimetallic particles in solution. Chemosphere 2020, 244, 125461. [Google Scholar] [CrossRef]
- Kashif, M.; Ngaini, Z.; Harry, A.V.; Vekariya, R.L.; Ahmad, A.; Zuo, Z.; Sahari, S.K.; Hussain, S.; Khan, Z.A.; Alarifi, A. An experimental and DFT study on novel dyes incorporated with natural dyes on titanium dioxide (TiO2) towards solar cell application. Appl. Phys. A 2020, 126, 1–13. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, N.; Tang, F.; Cui, Q.; He, F.; Li, L. pH-and glucose-responsive core–shell hybrid nanoparticles with controllable metal-enhanced fluorescence effects. ACS Appl. Mater. Interfaces 2012, 4, 1747–1751. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Zamanian, A.; Sangpour, P.; Shabanzadeh, P.; Abdollahi, Y.; Zargar, M. Green biosynthesis of silver nanoparticles using Curcuma longa tuber powder. Int. J. Nanomed. 2012, 7, 5603. [Google Scholar] [CrossRef]
- Rostami-Vartooni, A.; Nasrollahzadeh, M.; Alizadeh, M. Green synthesis of seashell supported silver nanoparticles using Bunium persicum seeds extract: Application of the particles for catalytic reduction of organic dyes. J. Colloid Interface Sci. 2016, 470, 268–275. [Google Scholar] [CrossRef]
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Wu, W.; Irfan, A.; Ajmal, M. Systematic study for catalytic degradation of nitrobenzene derivatives using core@ shell composite micro particles as catalyst. Colloids Surf. Physicochem. Eng. Asp. 2020, 594, 124646. [Google Scholar] [CrossRef]
- Tang, F.; Ma, N.; Tong, L.; He, F.; Li, L. Control of metal-enhanced fluorescence with pH-and thermoresponsive hybrid microgels. Langmuir 2012, 28, 883–888. [Google Scholar] [CrossRef]
- Abbasi, E.; Milani, M.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A.; Tayefi Nasrabadi, H.; Nikasa, P.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K. Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit. Rev. Microbiol. 2016, 42, 173–180. [Google Scholar] [CrossRef]
- Nava, O.; Soto-Robles, C.; Gómez-Gutiérrez, C.; Vilchis-Nestor, A.; Castro-Beltrán, A.; Olivas, A.; Luque, P. Fruit peel extract mediated green synthesis of zinc oxide nanoparticles. J. Mol. Struct. 2017, 1147, 1–6. [Google Scholar] [CrossRef]
- Kasthuri, J.; Kathiravan, K.; Rajendiran, N. Phyllanthin-assisted biosynthesis of silver and gold nanoparticles: A novel biological approach. J. Nanopart. Res. 2009, 11, 1075–1085. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.; Parsons, J.; Gomez, E.; Peralta-Videa, J.; Troiani, H.; Santiago, P.; Yacaman, M.J. Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett. 2002, 2, 397–401. [Google Scholar] [CrossRef]
- Ayinde, W.; Gitari, W.; Samie, A. Optimization of microwave-assisted synthesis of silver nanoparticle by Citrus paradisi peel and its application against pathogenic water strain. Green Chem. Lett. Rev. 2019, 12, 225–234. [Google Scholar] [CrossRef]
- Najafinejad, M.S.; Mohammadi, P.; Afsahi, M.M.; Sheibani, H. Green synthesis of the Fe3O4@ polythiophen-Ag magnetic nanocatalyst using grapefruit peel extract: Application of the catalyst for reduction of organic dyes in water. J. Mol. Liq. 2018, 262, 248–254. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Galeas, S.; Sharma, V.; Guerrero, V.H.; Debut, A.; Cumbal, L. Characterization and application of biosynthesized iron oxide nanoparticles using Citrus paradisi peel: A sustainable approach. Inorg. Chem. Commun. 2020, 119, 108116. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green approach for fabrication and applications of zinc oxide nanoparticles. Bioinorg. Chem. Appl. 2014, 2014, 523869. [Google Scholar] [CrossRef]
- Soto, K.M.; Quezada-Cervantes, C.T.; Hernández-Iturriaga, M.; Luna-Bárcenas, G.; Vazquez-Duhalt, R.; Mendoza, S. Fruit peels waste for the green synthesis of silver nanoparticles with antimicrobial activity against foodborne pathogens. LWT 2019, 103, 293–300. [Google Scholar] [CrossRef]
- Jackson, T.C.; Uwah, T.O.; Ifekpolugo, N.L.; Emmanuel, N.A. Comparison of antimicrobial activities of silver nanoparticles biosynthesized from some Citrus species. Am. J. Nano Res. Appl. 2018, 6, 54–59. [Google Scholar]
- Vankar, P.S.; Shukla, D. Biosynthesis of silver nanoparticles using lemon leaves extract and its application for antimicrobial finish on fabric. Appl. Nanosci. 2012, 2, 163–168. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Badea, N.; Ungureanu, C.; Iordache, S.M.; Constantin, M.; Purcar, V.; Rau, I.; Pirvu, C. Ecobiophysical aspects on nanosilver biogenerated from citrus reticulata peels, as potential biopesticide for controlling pathogens and wetland plants in aquatic media. J. Nanomater. 2017, 2017, 4214017. [Google Scholar] [CrossRef]
- Indana, M.K.; Gangapuram, B.R.; Dadigala, R.; Bandi, R.; Guttena, V. A novel green synthesis and characterization of silver nanoparticles using gum tragacanth and evaluation of their potential catalytic reduction activities with methylene blue and Congo red dyes. J. Anal. Sci. Technol. 2016, 7, 19. [Google Scholar] [CrossRef]
- Varadavenkatesan, T.; Selvaraj, R.; Vinayagam, R. Green synthesis of silver nanoparticles using Thunbergia grandiflora flower extract and its catalytic action in reduction of Congo red dye. Mater. Today Proc. 2020, 23, 39–42. [Google Scholar] [CrossRef]
- Saha, J.; Begum, A.; Mukherjee, A.; Kumar, S. A novel green synthesis of silver nanoparticles and their catalytic action in reduction of Methylene Blue dye. Sustain. Environ. Res. 2017, 27, 245–250. [Google Scholar] [CrossRef]
- Naraginti, S.; Sivakumar, A. Eco-friendly synthesis of silver and gold nanoparticles with enhanced bactericidal activity and study of silver catalyzed reduction of 4-nitrophenol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 357–362. [Google Scholar] [CrossRef]
- Dauthal, P.; Mukhopadhyay, M. Prunus domestica fruit extract-mediated synthesis of gold nanoparticles and its catalytic activity for 4-nitrophenol reduction. Ind. Eng. Chem. Res. 2012, 51, 13014–13020. [Google Scholar] [CrossRef]
- Kahsay, M.H.; RamaDevi, D.; Kumar, Y.P.; Mohan, B.S.; Tadesse, A.; Battu, G.; Basavaiah, K. Synthesis of silver nanoparticles using aqueous extract of Dolichos lablab for reduction of 4-Nitrophenol, antimicrobial and anticancer activities. OpenNano 2018, 3, 28–37. [Google Scholar] [CrossRef]
- Kumar, P.; Govindaraju, M.; Senthamilselvi, S.; Premkumar, K. Photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Ulva lactuca. Colloids Surf. B Biointerfaces 2013, 103, 658–661. [Google Scholar] [CrossRef]
- Begum, R.; Naseem, K.; Ahmed, E.; Sharif, A.; Farooqi, Z.H. Simultaneous catalytic reduction of nitroarenes using silver nanoparticles fabricated in poly (N-isopropylacrylamide-acrylic acid-acrylamide) microgels. Colloids Surf. Physicochem. Eng. Asp. 2016, 511, 17–26. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Naseem, K.; Begum, R.; Ijaz, A. Catalytic reduction of 2-nitroaniline in aqueous medium using silver nanoparticles functionalized polymer microgels. J. Inorg. Organomet. Polym. Mater. 2015, 25, 1554–1568. [Google Scholar] [CrossRef]
- Mata, R.; Bhaskaran, A.; Sadras, S.R. Green-synthesized gold nanoparticles from Plumeria alba flower extract to augment catalytic degradation of organic dyes and inhibit bacterial growth. Particuology 2016, 24, 78–86. [Google Scholar] [CrossRef]
- Kalia, A.; Manchanda, P.; Bhardwaj, S.; Singh, G. Biosynthesized silver nanoparticles from aqueous extracts of sweet lime fruit and callus tissues possess variable antioxidant and antimicrobial potentials. Inorg. Nano Met. Chem. 2020, 50, 1–10. [Google Scholar] [CrossRef]
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Wu, W.; Irfan, A.; Al-Sehemi, A.G. Silver Nanoparticles Engineered Polystyrene-Poly (N-isopropylmethacrylamide-acrylic acid) Core Shell Hybrid Polymer Microgels for Catalytic Reduction of Congo Red. Macromol. Chem. Phys. 2018, 219, 1800211. [Google Scholar] [CrossRef]
- Alzahrani, H.A.; Buckingham, M.A.; Wardley, W.P.; Tilley, R.D.; Ariotti, N.; Aldous, L. Gold nanoparticles immobilised in a superabsorbent hydrogel matrix: Facile synthesis and application for the catalytic reduction of toxic compounds. Chem. Commun. 2020, 56, 1263–1266. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, M.; Khan, M.A.; Akhtar, K.; Asiri, A.M.; Khan, S.B. Plant-supported silver nanoparticles: Efficient, economically viable and easily recoverable catalyst for the reduction of organic pollutants. Appl. Organomet. Chem. 2019, 33, e4971. [Google Scholar] [CrossRef]


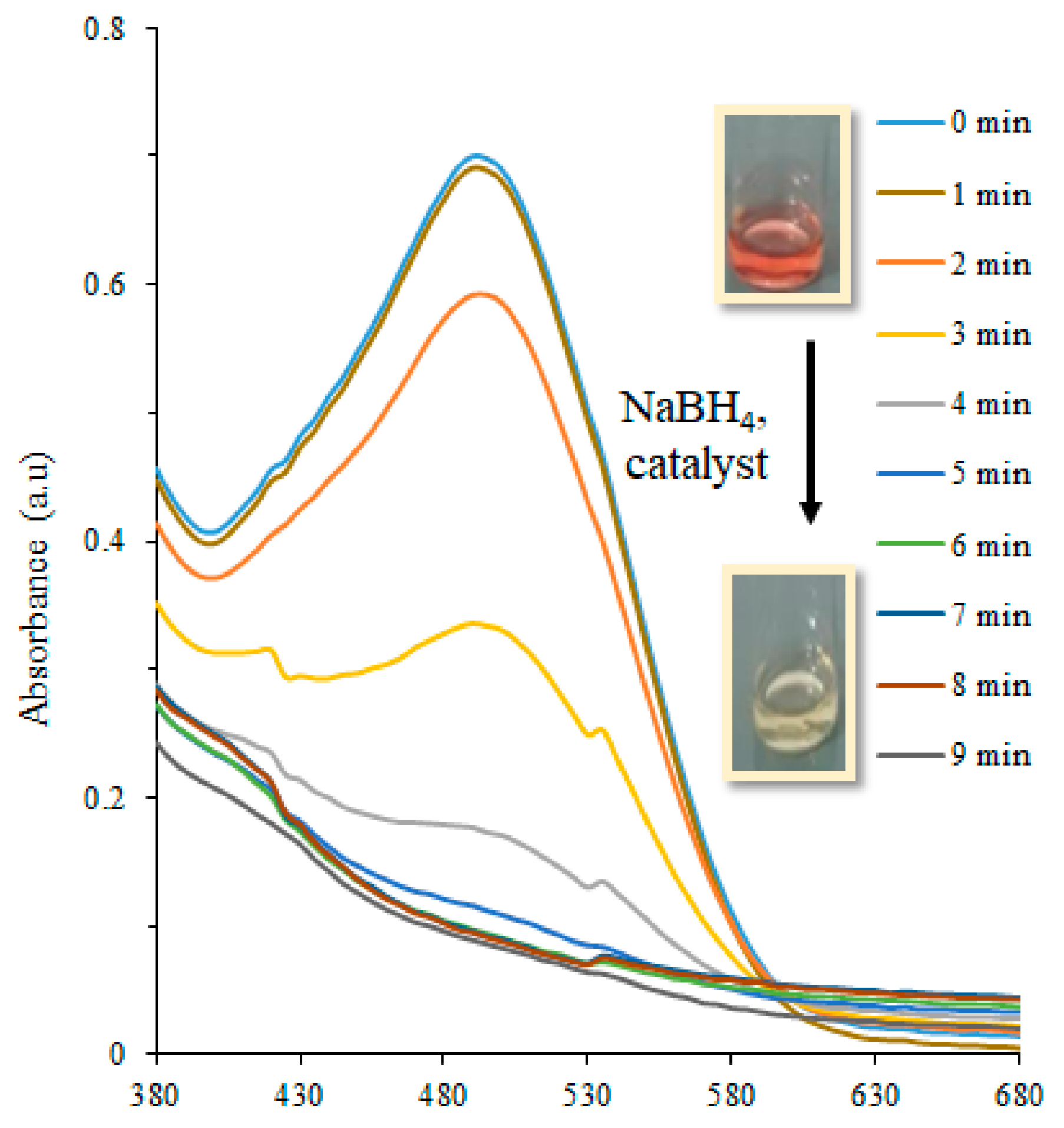
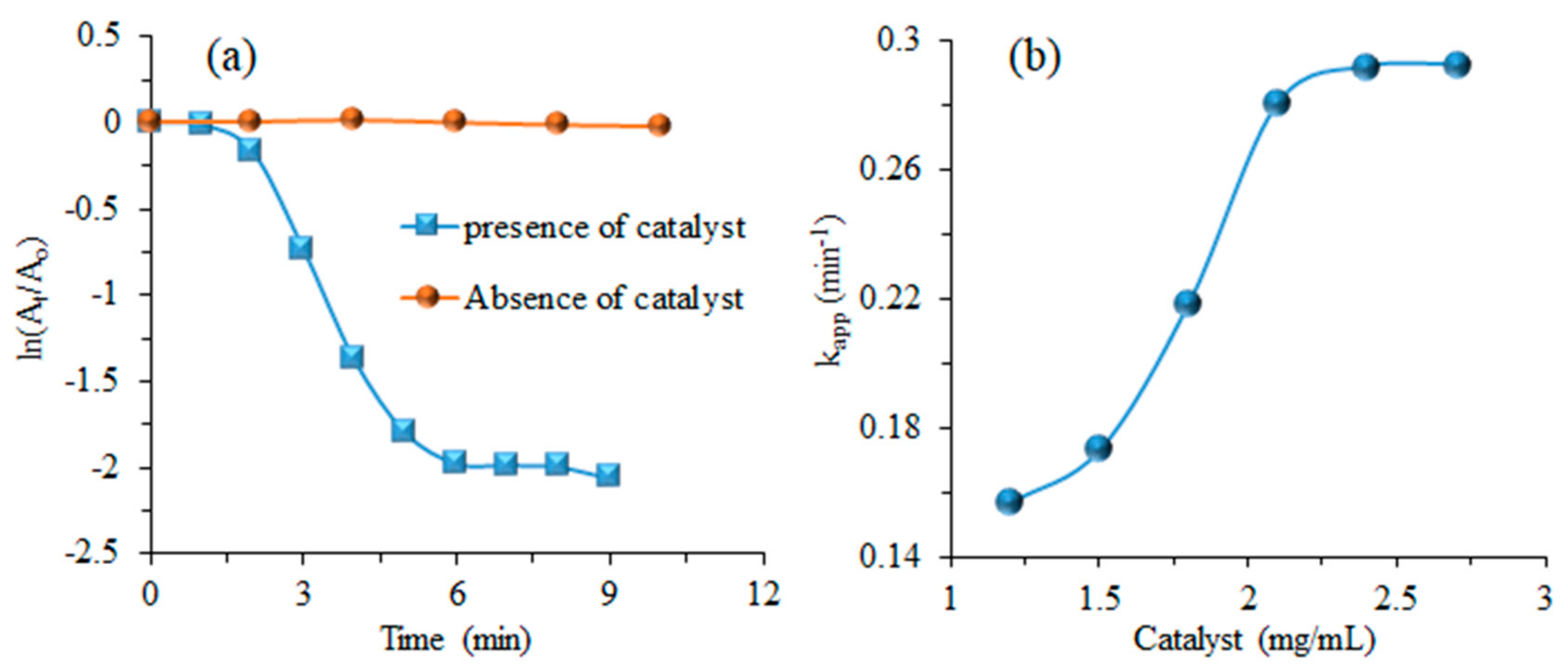
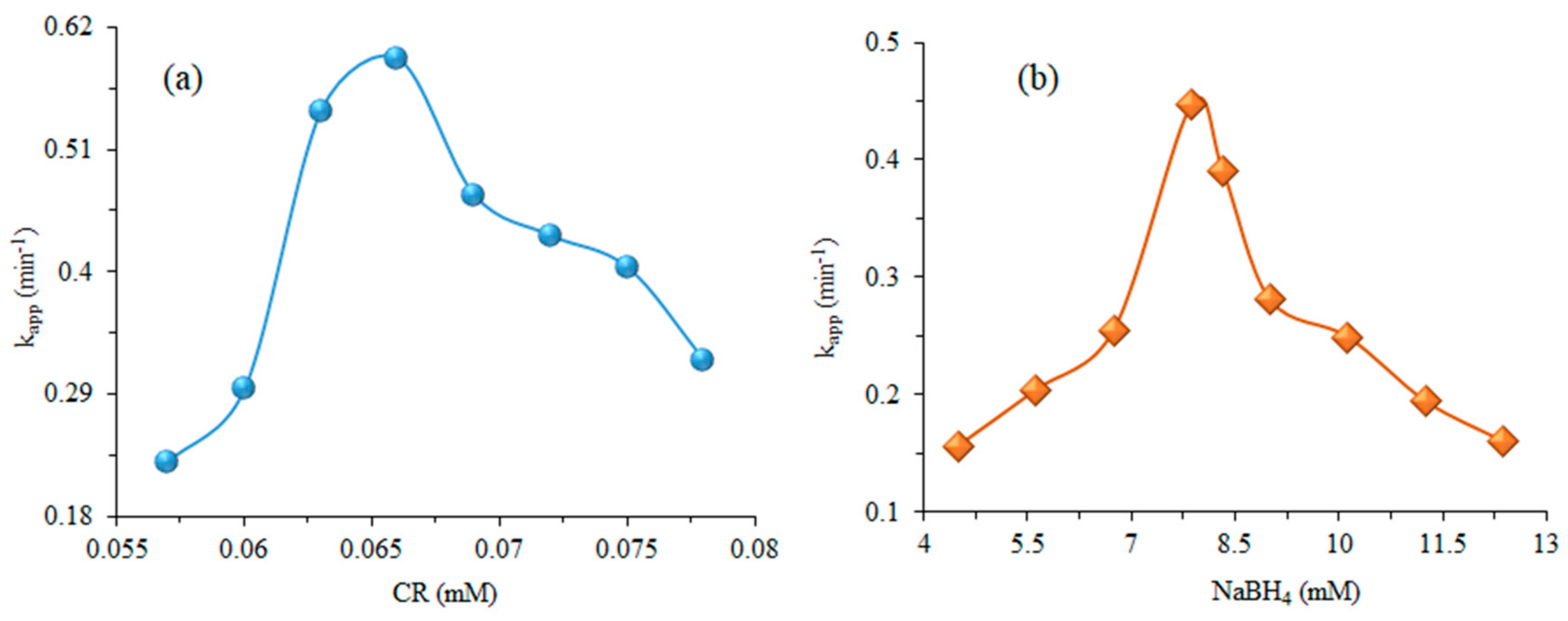
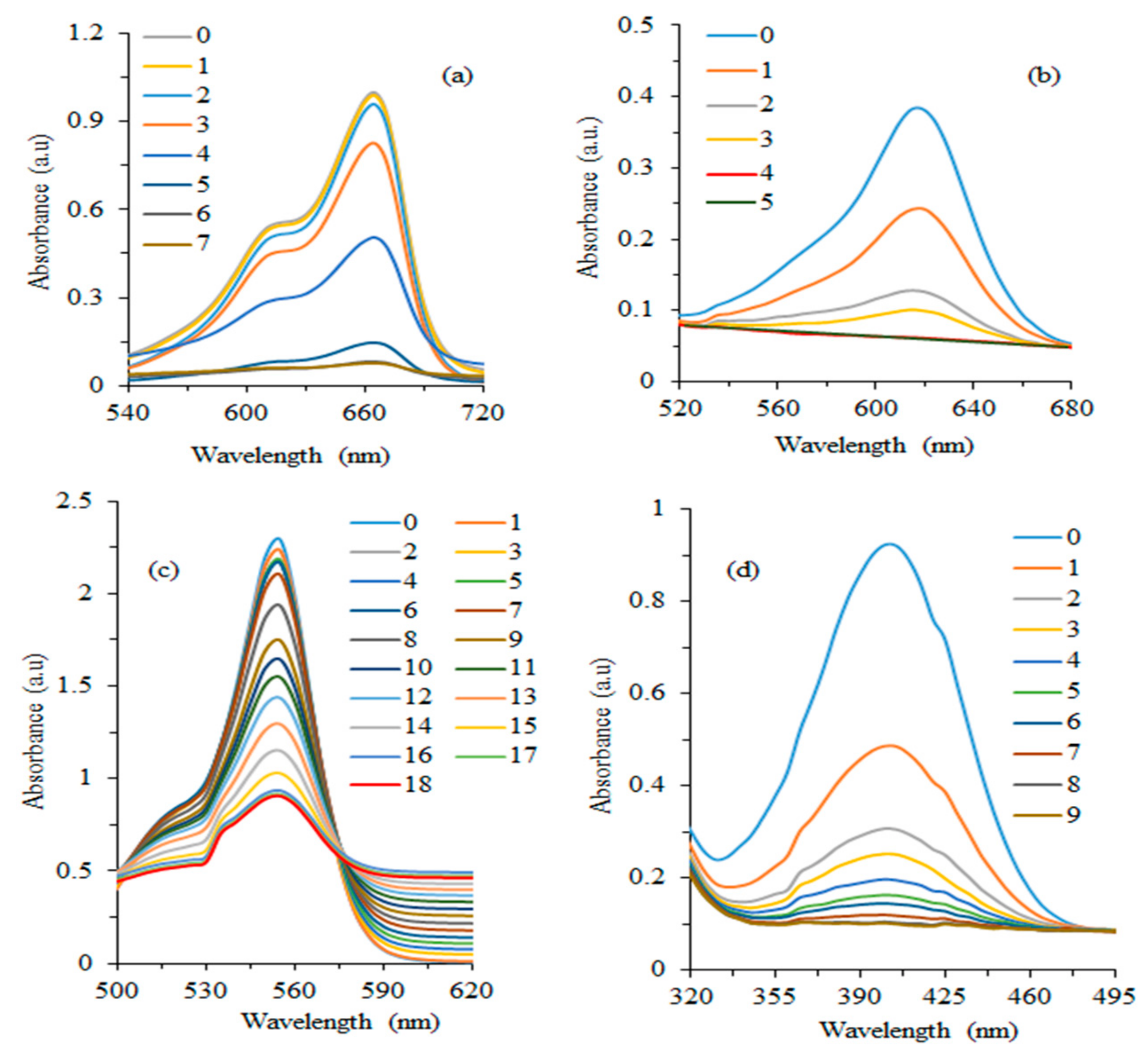
| Dye | Catalyst | Plant Extract | kapp (min−1) | Reaction Completion Time (min) | References |
|---|---|---|---|---|---|
| CR | Au-nPs | Salmalia malabarica gum | 0.236 | 10 | [10] |
| Ag-nPs | Gum tragacanth | 0.148 | 15 | [33] | |
| Ag-nPs | Thunbergia grandiflora | 0.099 | 18 | [34] | |
| Ag-nPs | Citrus paradise | 0.591 | 5 | This work | |
| MB | Au-nPs | Salmalia malabarica gum | 0.241 | 9 | [10] |
| Ag-nPs | Gum tragacanth | 0.182 | 12 | [33] | |
| Ag-nPs | Gmelina arborea | - | 10 | [35] | |
| Ag-nPs | Citrus paradise | 0.613 | 4 | This work | |
| 4-NP | Ag-nPs | Coleus forskohlii root extract | 0.101 | 24 | [36] |
| Au-nPs | Prunus domestica (plum) fruit extract | 0.114 | 9 | [37] | |
| Ag-nPs | Dolichos lablab | - | 40 | [38] | |
| Ag-nPs | Citrus paradise | 0.247 | 9 | This work |
| Contents | CR (mM) | NaBH4 (mM) | Catalyst (mg/mL) | kapp (min−1) | Half Life (min) | R2 | Induction Time (min) | Reaction Completion Time (min) |
|---|---|---|---|---|---|---|---|---|
| CR | 0.057 | 7.88 | 2.1 | 0.229 | 3.026 | 0.988 | 2 | 13 |
| 0.060 | 7.88 | 2.1 | 0.294 | 2.357 | 0.992 | 1 | 9 | |
| 0.063 | 7.88 | 2.1 | 0.544 | 1.274 | 0.951 | 0 | 4 | |
| 0.066 | 7.88 | 2.1 | 0.591 | 1.173 | 0.963 | 0 | 5 | |
| 0.069 | 7.88 | 2.1 | 0.469 | 1.478 | 0.982 | 1 | 7 | |
| 0.072 | 7.88 | 2.1 | 0.432 | 1.604 | 0.967 | 2 | 8 | |
| 0.075 | 7.88 | 2.1 | 0.404 | 1.715 | 0.995 | 2 | 9 | |
| 0.078 | 7.88 | 2.1 | 0.321 | 2.159 | 0.935 | 2 | 9 | |
| NaBH4 | 0.072 | 4.50 | 2.1 | 0.156 | 4.442 | 0.972 | 3 | 27 |
| 0.072 | 5.63 | 2.1 | 0.204 | 3.397 | 0.955 | 3 | 21 | |
| 0.072 | 6.75 | 2.1 | 0.255 | 2.718 | 0.933 | 1 | 12 | |
| 0.072 | 7.88 | 2.1 | 0.448 | 1.547 | 0.945 | 1 | 09 | |
| 0.072 | 8.33 | 2.1 | 0.390 | 1.777 | 0.9664 | 0 | 09 | |
| 0.072 | 9.00 | 2.1 | 0.281 | 2.466 | 0.9655 | 2 | 14 | |
| 0.072 | 10.13 | 2.1 | 0.249 | 2.783 | 0.9372 | 2 | 16 | |
| 0.072 | 11.25 | 2.1 | 0.194 | 3.572 | 0.978 | 2 | 20 | |
| 0.072 | 12.38 | 2.1 | 0.160 | 4.331 | 0.965 | 3 | 22 | |
| Catalyst | 0.072 | 7.88 | 1.2 | 0.157 | 4.414 | 0.997 | 3 | 26 |
| 0.072 | 7.88 | 1.5 | 0.173 | 4.006 | 0.994 | 2 | 23 | |
| 0.072 | 7.88 | 1.8 | 0.218 | 3.179 | 0.982 | 1 | 19 | |
| 0.072 | 7.88 | 2.1 | 0.281 | 2.466 | 0.978 | 1 | 15 | |
| 0.072 | 7.88 | 2.4 | 0.292 | 2.373 | 0.995 | 0 | 14 | |
| 0.072 | 7.88 | 2.7 | 0.293 | 2.365 | 0.999 | 0 | 12 |
| Dyes | kapp (min−1) | Intrinsic Rate Constant (mL·mg−1·min−1) | Reaction Completion Time (min) | Reduction Efficiency (%) |
|---|---|---|---|---|
| MB | 0.613 | 0.292 | 4 | 93.29 |
| MG | 0.451 | 0.215 | 7 | 83.73 |
| 4-NP | 0.247 | 0.118 | 9 | 88.90 |
| RhB | 0.085 | 0.041 | 18 | 60.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naseem, K.; Zia Ur Rehman, M.; Ahmad, A.; Dubal, D.; AlGarni, T.S. Plant Extract Induced Biogenic Preparation of Silver Nanoparticles and Their Potential as Catalyst for Degradation of Toxic Dyes. Coatings 2020, 10, 1235. https://doi.org/10.3390/coatings10121235
Naseem K, Zia Ur Rehman M, Ahmad A, Dubal D, AlGarni TS. Plant Extract Induced Biogenic Preparation of Silver Nanoparticles and Their Potential as Catalyst for Degradation of Toxic Dyes. Coatings. 2020; 10(12):1235. https://doi.org/10.3390/coatings10121235
Chicago/Turabian StyleNaseem, Khalida, Muhammad Zia Ur Rehman, Awais Ahmad, Deepak Dubal, and Tahani Saad AlGarni. 2020. "Plant Extract Induced Biogenic Preparation of Silver Nanoparticles and Their Potential as Catalyst for Degradation of Toxic Dyes" Coatings 10, no. 12: 1235. https://doi.org/10.3390/coatings10121235
APA StyleNaseem, K., Zia Ur Rehman, M., Ahmad, A., Dubal, D., & AlGarni, T. S. (2020). Plant Extract Induced Biogenic Preparation of Silver Nanoparticles and Their Potential as Catalyst for Degradation of Toxic Dyes. Coatings, 10(12), 1235. https://doi.org/10.3390/coatings10121235







