Bio-Inspired Facile Synthesis of Graphene-Based Nanocomposites: Elucidation of Antimicrobial and Biofilm Inhibitory Potential against Foodborne Pathogenic Bacteria
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Properties: XRD
2.2. Optical Properties: UV-VIS
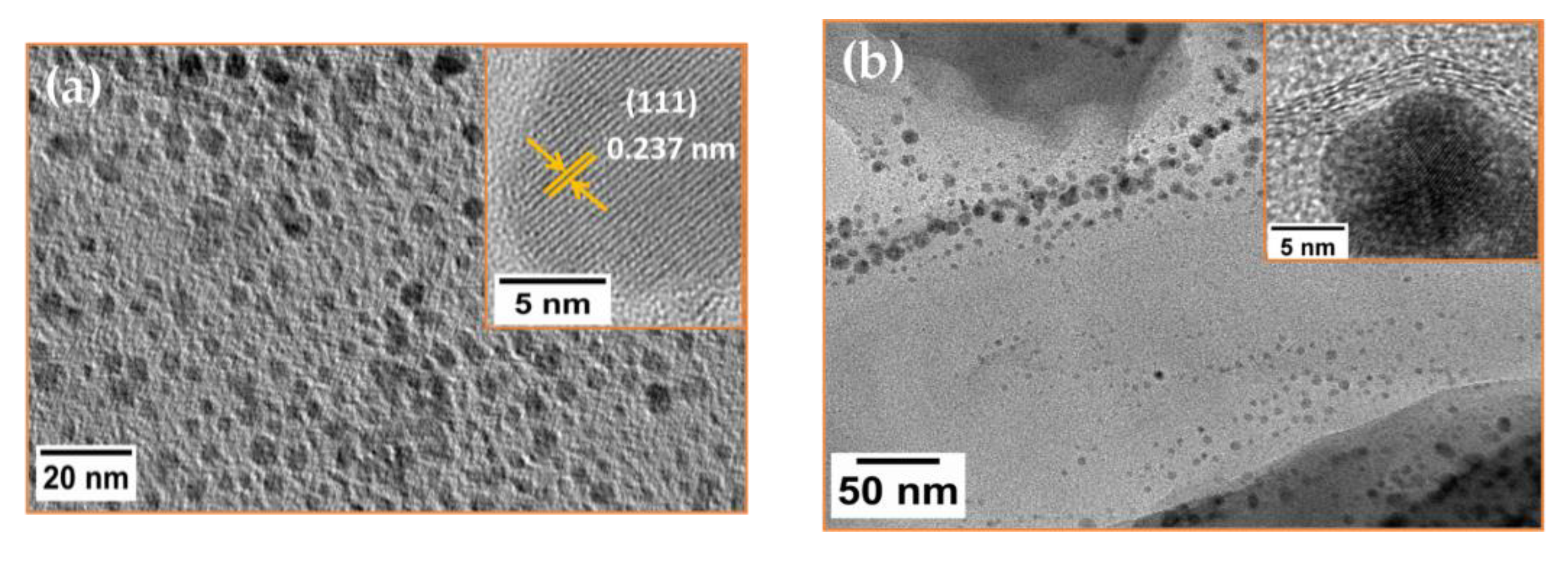
2.3. Morphological Studies: TEM
2.4. Raman Spectroscopy
2.5. Antibacterial Activity: Determination of Minimum Inhibitory Concentration (MICs)
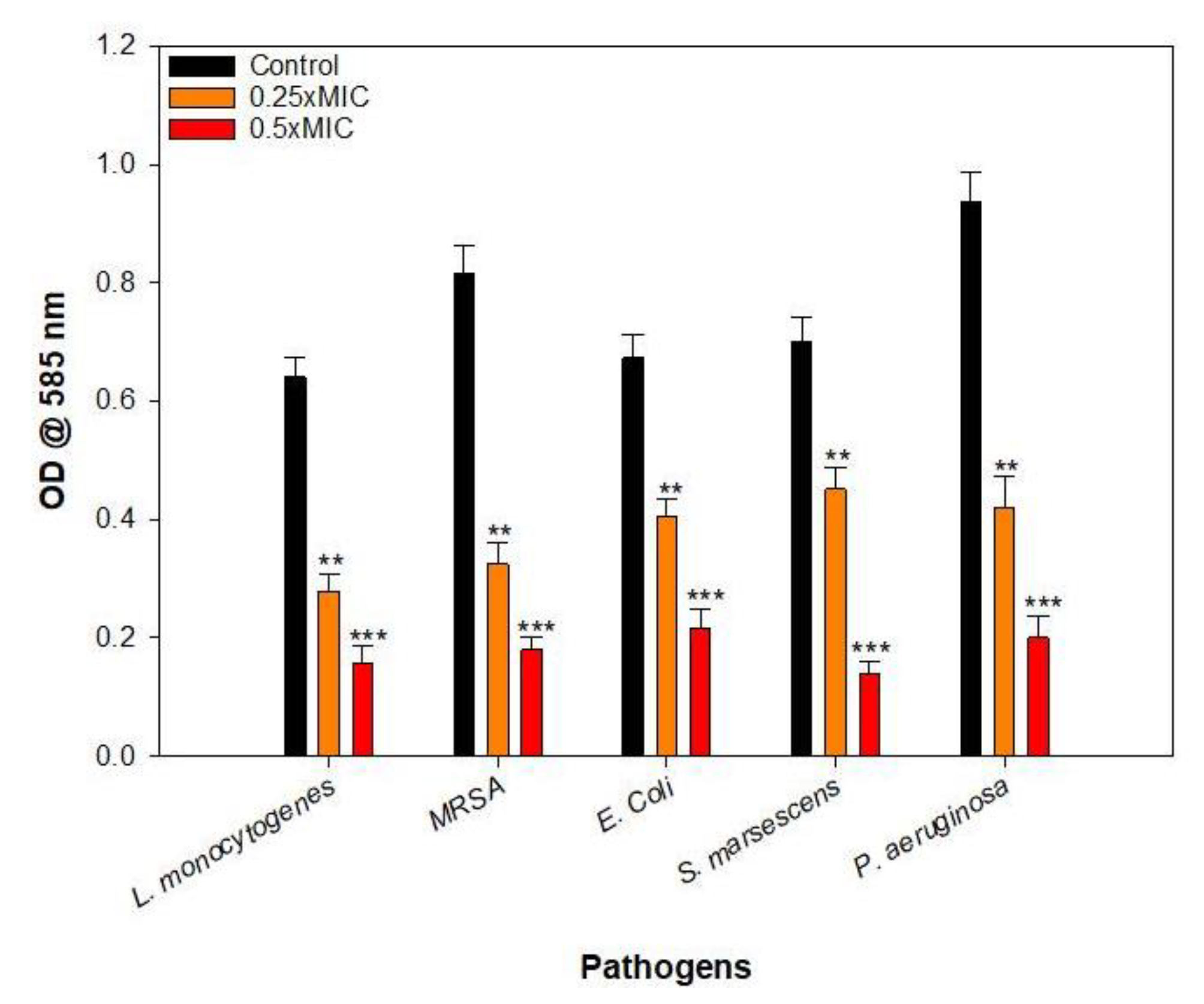
2.6. Effect of Sub-MICs on the Growth of Test Bacteria
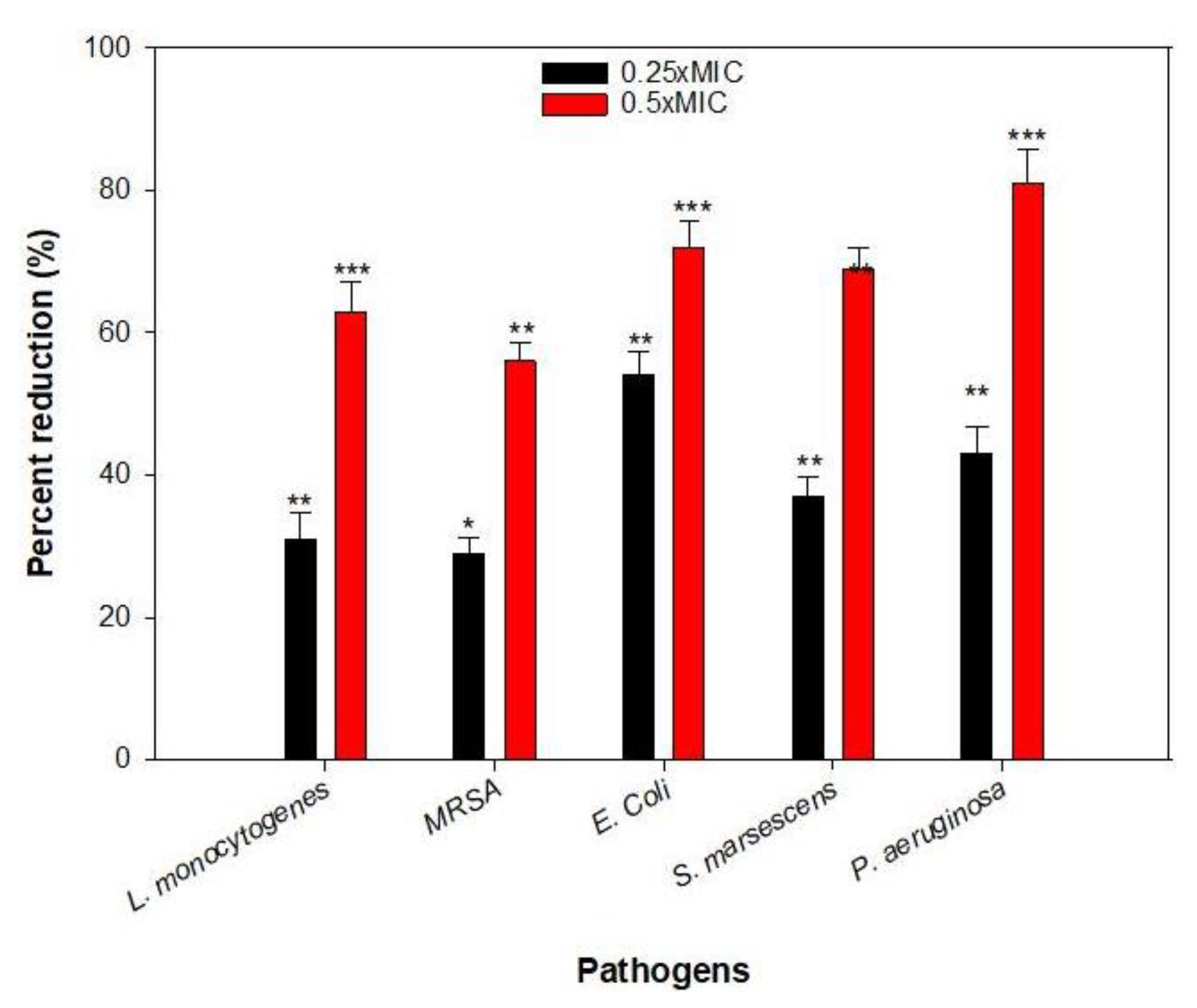
2.7. Inhibition of Biofilm Formation
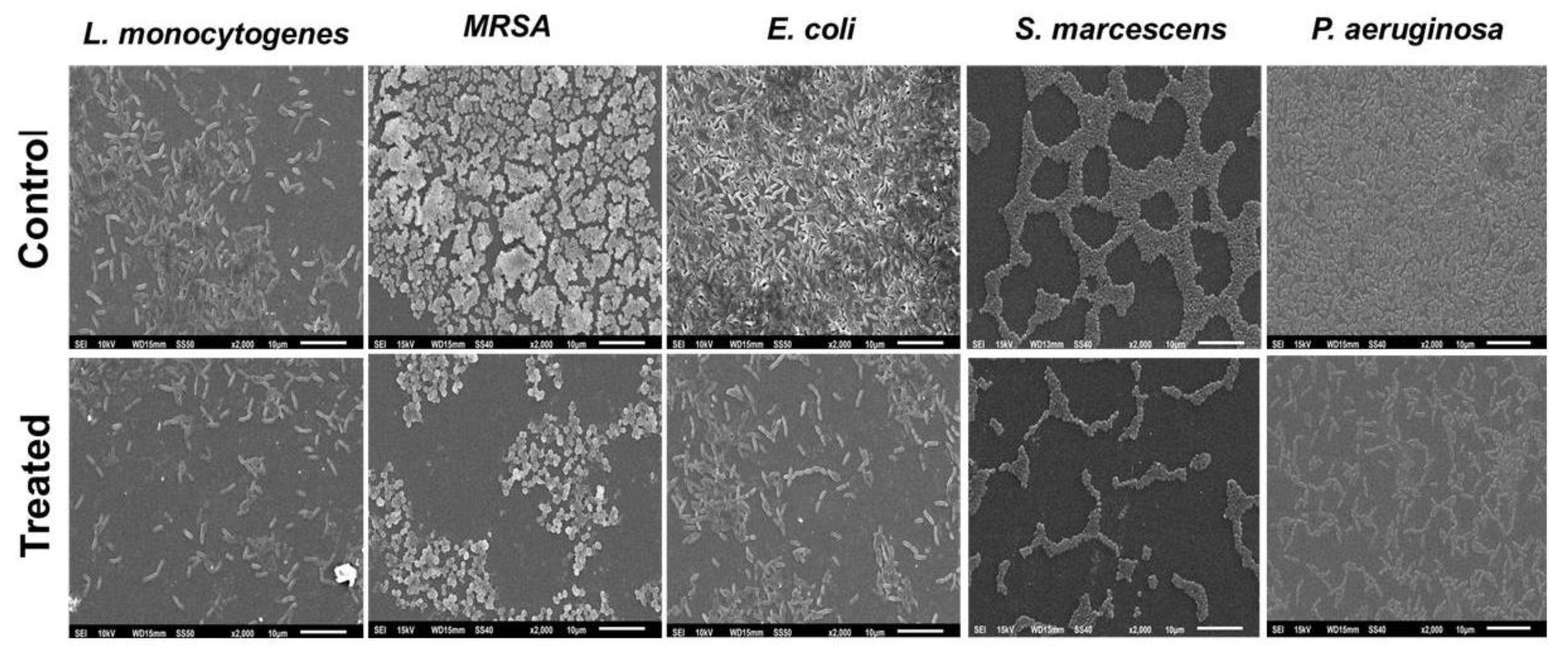
2.8. Microscopic Analysis
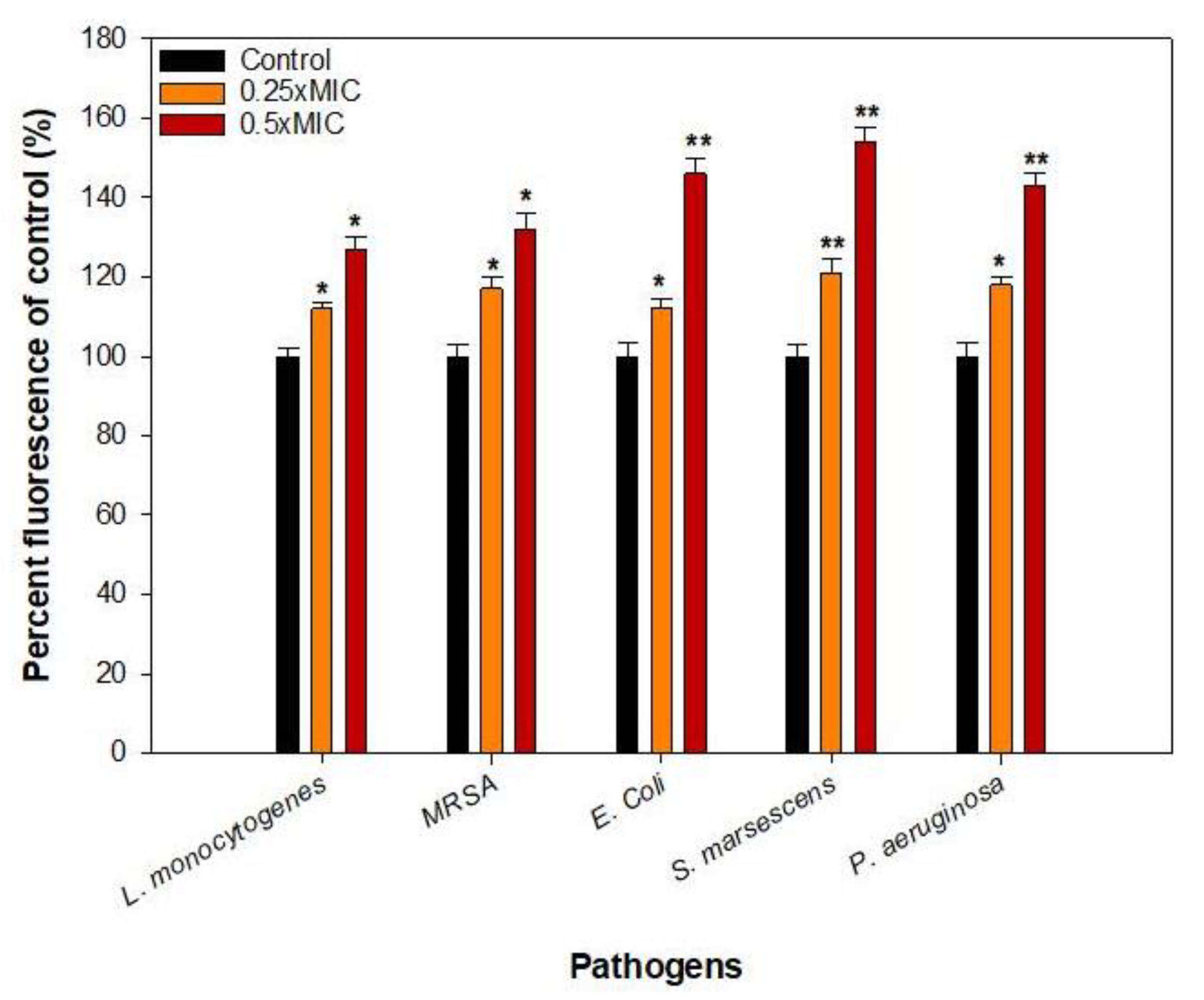
2.9. Effect on the EPS Production
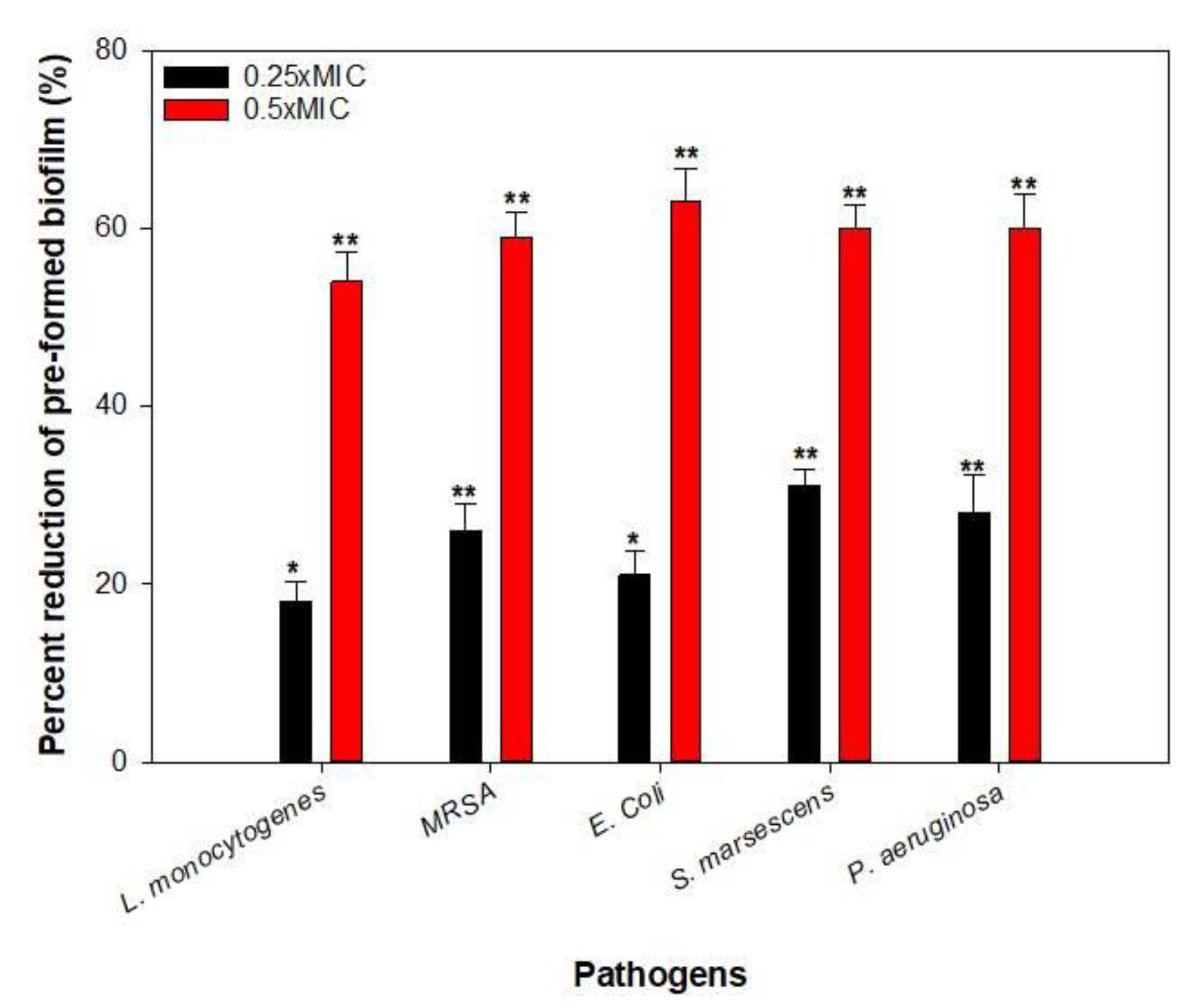
2.10. Disruption of Pre-Formed Biofilms
2.11. Mode of Biofilm Inhibition by Au-RGO
2.12. Cytotoxicity on HEK-293 Cell Line
3. Material and Methods
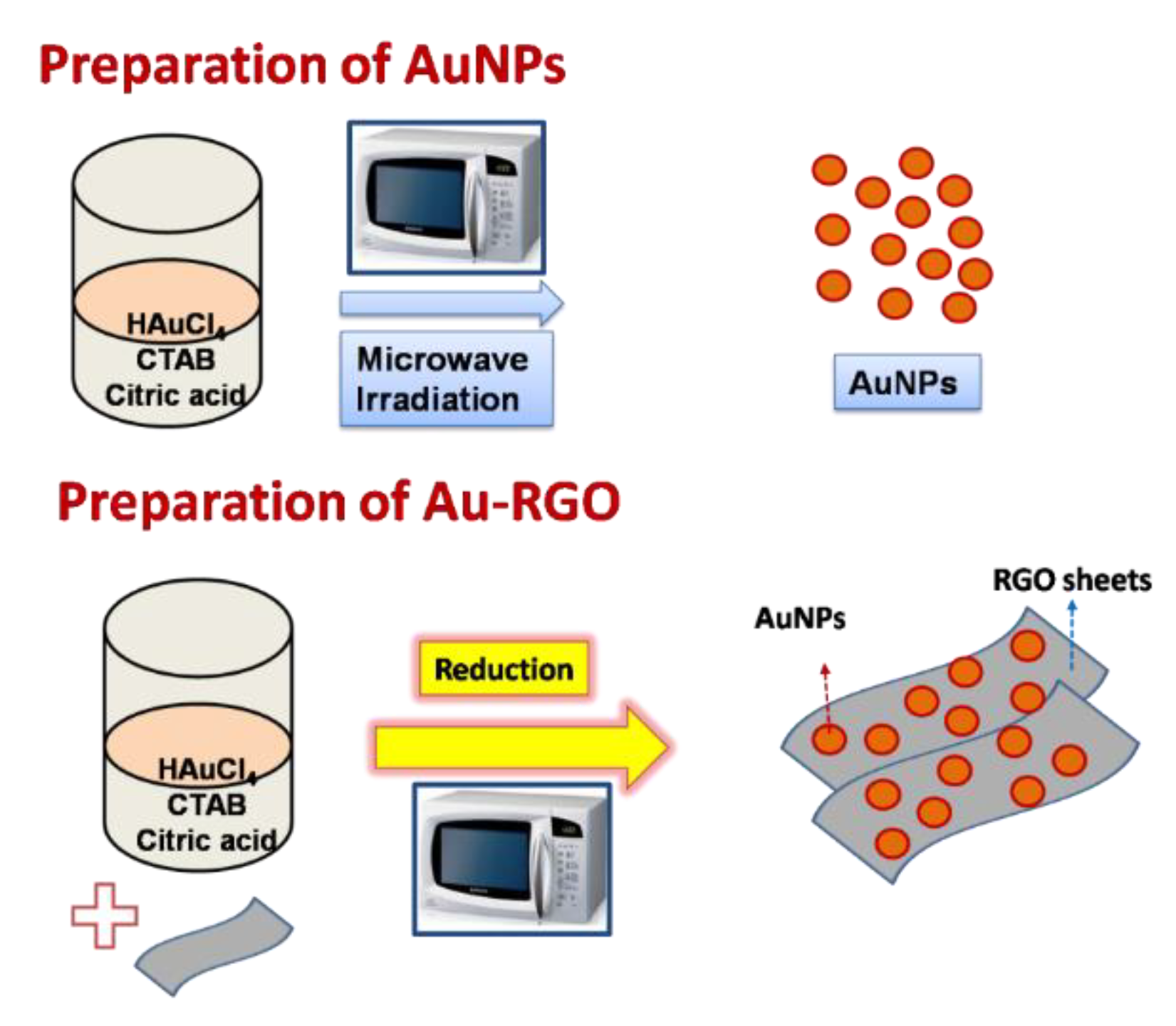
3.1. Preparation of Gold Nanoparticles (AuNPs)
3.2. Preparation of Graphene Oxide (GO)
3.3. Preparation of Reduced Graphene Oxide (RGO)
3.4. Preparation of Au/RGO Nanocomposites
3.5. Characterizations
3.6. Strains and Culture Condition
3.7. Antibacterial Activity
3.8. Growth Kinetics Studies
3.9. Biofilm Inhibition
3.10. Microscopic Evaluation of Biofilm Inhibition
3.11. Effect on Exopolysaccharide Production
3.12. Disruption of Pre-Formed Biofilms
3.13. ROS Generation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar]
- De Souza, E.L.; Meira, Q.G.S.; Barbosa, I.D.M.; Athayde, A.J.A.A.; Conceição, M.L.D.; Siqueira Júnior, J.P.D. Biofilm formation by Staphylococcus aureus from food contact surfaces in a meat-based broth and sensitivity to sanitizers. Braz. J. Microbiol. 2014, 45, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, H.-C.F.J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S.A.R.S. Biofilms: An emergent form of bacterial life. Nat. Rev. Genet. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, M.; Morris, D.; De Lappe, N.; O’Connor, J.; Lalor, P.; Dockery, P.; Cormican, M. Commonly Used Disinfectants Fail to Eradicate Salmonella enterica Biofilms from Food Contact Surface Materials. Appl. Environ. Microbiol. 2014, 80, 1507–1514. [Google Scholar] [CrossRef]
- Husain, F.M.; Khan, M.S.; Siddiqui, S.; Khan, A.; Arshad, M.; Alyousef, A.A.; Rahman, M.; Al-Shabib, N.A.; Ahmad, I. Nanoparticles as New Emerging Antibacterials: Potentials and Limitations. Antibact. Drug Discov. Combat MDR 2019, 561–579. [Google Scholar] [CrossRef]
- Yu, Q.; Li, J.; Zhang, Y.; Wang, Y.; Liu, L.; Li, M. Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci. Rep. 2016, 6, 26667. [Google Scholar] [CrossRef]
- Benassai, E.; Del Bubba, M.; Ancillotti, C.; Colzi, I.; Gonnelli, C.; Calisi, N.; Salvatici, M.C.; Casalone, E.; Ristori, S. Green and cost-effective synthesis of copper nanoparticles by extracts of non-edible and waste plant materials from Vaccinium species: Characterization and antimicrobial activity. Mater. Sci. Eng. C 2020, C, 111453. [Google Scholar] [CrossRef]
- Duffy, L.; Osmond-McLeod, M.J.; Judy, J.; King, T. Investigation into the antibacterial activity of silver, zinc oxide and copper oxide nanoparticles against poultry-relevant isolates of Salmonella and Campylobacter. Food Control 2018, 92, 293–300. [Google Scholar] [CrossRef]
- Vithiya, K.; Rajendran, K.; Shampa, S. Antimicrobial Activity of Biosynthesized Silver Oxide Nanoparticles. J. Pure Appl. Microbiol. 2014, 4, 3263–3268. [Google Scholar]
- Alsharaeh, E.; Alazzam, S.; Ahmed, F.; Arshi, N.; Al-Hindawi, M.; Sing, G.K. Green Synthesis of Silver Nanoparticles and Their Reduced Graphene Oxide Nanocomposites as Antibacterial Agents: A Bio-inspired Approach. Acta Met. Sin. Engl. Lett. 2016, 30, 45–52. [Google Scholar] [CrossRef][Green Version]
- Liu, L.; Bai, H.; Liu, J.; Sun, D.D. Multifunctional graphene oxide-TiO2-Ag nanocomposites for high performance water disinfection and decontamination under solar irradiation. J. Hazard. Mater. 2013, 261, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, D.; Kim, J.; Kim, T.; Kim, W.J. Photothermally Triggered Cytosolic Drug Delivery via Endosome Disruption Using a Functionalized Reduced Graphene Oxide. ACS Nano 2013, 7, 6735–6746. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhu, B.; Zhao, Y.; Shi, M.; He, X.; Xu, H.; Zhou, Q. One-Step Eco-Friendly Synthesis of Ag-Reduced Graphene Oxide Nanocomposites for Antibiofilm Application. J. Mater. Eng. Perform. 2020, 29, 2551–2559. [Google Scholar] [CrossRef]
- Jaworski, S.; Wierzbicki, M.; Sawosz, E.; Jung, A.; Gielerak, G.; Biernat, J.; Jaremek, H.; Łojkowski, W.; Woźniak, B.; Wojnarowicz, J.; et al. Graphene Oxide-Based Nanocomposites Decorated with Silver Nanoparticles as an Antibacterial Agent. Nanoscale Res. Lett. 2018, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Radzig, M.; Nadtochenko, V.A.; Koksharova, O.; Kiwi, J.; Lipasova, V.; Khmel, I. Antibacterial effects of silver nanoparticles on gram-negative bacteria: Influence on the growth and biofilms formation, mechanisms of action. Colloids Surf. B Biointerfaces 2013, 102, 300–306. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Antibacterial Action of Nanoparticle Loaded Nanocomposites Based on Graphene and Its Derivatives: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 3563. [Google Scholar] [CrossRef]
- Hussain, N.; Gogoi, A.; Sarma, R.K.; Sharma, P.; Barras, A.; Boukherroub, R.; Saikia, R.; Sengupta, P.; Das, M.R. Reduced Graphene Oxide Nanosheets Decorated with Au Nanoparticles as an Effective Bactericide: Investigation of Biocompatibility and Leakage of Sugars and Proteins. ChemPlusChem 2014, 79, 1774–1784. [Google Scholar] [CrossRef]
- Chuang, M.-K.; Chen, F.-C.; Hsu, C.-S. Gold Nanoparticle-Graphene Oxide Nanocomposites That Enhance the Device Performance of Polymer Solar Cells. J. Nanomater. 2014, 2014, 736879. [Google Scholar] [CrossRef]
- Hong, W.; Bai, H.; Xu, Y.; Yao, Z.; Gu, Z.; Shi, G. Preparation of Gold Nanoparticle/Graphene Composites with Controlled Weight Contents and Their Application in Biosensors. J. Phys. Chem. C 2010, 114, 1822–1826. [Google Scholar] [CrossRef]
- Da Silva, M.K.; Cesarino, I. Evaluation of a Nanocomposite Based on Reduced Graphene Oxide and Gold Nanoparticles as an Electrochemical Platform for Detection of Sulfamethazine. J. Compos. Sci. 2019, 3, 59. [Google Scholar] [CrossRef]
- Szustakiewicz, P.; Kołsut, N.; Leniart, A.; Lewandowski, W. Universal Method for Producing Reduced Graphene Oxide/Gold Nanoparticles Composites with Controlled Density of Grafting and Long-Term Stability. Nanomaterials 2019, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Aoki, K.; Chen, J.; Yang, N.; Nagasava, H. Charge-Transfer Reactions of Silver Stearate-Coated Nanoparticles in Suspensions. Langmuir 2003, 19, 9904–9909. [Google Scholar] [CrossRef]
- Nath, S.S.; Chakdar, D.; Gope, G. Synthesis of CdS and ZnS quantum dots and their applications in electronics. Nanotrends 2007, 2, 3. [Google Scholar]
- Nath, S.S.; Chakdar, D.; Gope, G.; Avasthi, D.K. Effect of 100 MeV nickel ions on silica coated ZnS quantum dots. J. Nanoelectron. Optoelectron. 2008, 3, 180–183. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, M.S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 168–171. [Google Scholar] [CrossRef]
- Fred, F.; Lidorikis, E.; Geim, A.K.; Grigorenko, A.N.; Novoselov, K.S.; Ferrari, A.C. Surface-enhanced Raman spectroscopy of graphene. ACS Nano 2010, 4, 5617–5626. [Google Scholar]
- Park, S.; Mohanty, N.; Suk, J.W.; Nagaraja, A.; An, J.; Piner, R.D.; Cai, W.; Dreyer, D.R.; Berry, V.; Ruoff, R.S. Biocompatible, Robust Free-Standing Paper Composed of a TWEEN/Graphene Composite. Adv. Mater. 2010, 22, 1736–1740. [Google Scholar] [CrossRef]
- Rai, A.; Prabhune, A.; Perry, C.C. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J. Mater. Chem. 2010, 20, 6789–6798. [Google Scholar] [CrossRef]
- Bindhu, M.; Umadevi, M. Antibacterial activities of green synthesized gold nanoparticles. Mater. Lett. 2014, 120, 122–125. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Q.; Xu, L.; Zhang, S.; Feng, L.; Cheng, L.; Xu, H.; Liu, Z.; Peng, R. Graphene Oxide–Silver Nanocomposite As a Highly Effective Antibacterial Agent with Species-Specific Mechanisms. ACS Appl. Mater. Interfaces 2013, 5, 3867–3874. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, S.; Qayyum, S.; Khan, A.U. Antibiofilm efficacy of green synthesized graphene oxide-silver nanocomposite using Lagerstroemia speciosa floral extract: A comparative study on inhibition of gram-positive and gram-negative biofilms. Microb. Pathog. 2017, 103, 167–177. [Google Scholar] [CrossRef]
- Cossu, A.; Si, Y.; Sun, G.; Nitin, N. Antibiofilm effect of poly(vinyl alcohol-co-ethylene) halamine film against Listeria innocua and Escherichia coli O157:H7. Appl. Environ. Microbiol. 2017, 83, e00975-17. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-Q.; Cheng, H.; Duan, Q.; Huang, W. Sodium houttuyfonate inhibits biofilm formation and alginate biosynthesis-associated gene expression in a clinical strain of Pseudomonas aeruginosa in vitro. Exp. Ther. Med. 2015, 10, 753–758. [Google Scholar] [CrossRef]
- Kannappan, A.; Santhakumari, S.; Srinivasan, R.; Pandian, S.K.; Ravi, A.V. Hemidesmus indicus, a traditional medicinal plant, targets the adherence of multidrug-resistant pathogens to form biofilms. Biocatal. Agric. Biotechnol. 2019, 21, 101338. [Google Scholar] [CrossRef]
- Herzberg, M.; Yang, Y.; Ziemann, E.; Be’Er, A.; Bashouti, M.Y.; Elimelech, M.; Bernstein, R. One-step sonochemical synthesis of a reduced graphene oxide–ZnO nanocomposite with antibacterial and antibiofouling properties. Environ. Sci. Nano 2019, 6, 3080–3090. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Nadeem, M.; Khan, M.S.; Al-Qurainy, F.; Alyousef, A.A.; Arshad, M.; Khan, A.; Khan, J.M.; Alam, P.; et al. Bio-inspired facile fabrication of silver nanoparticles from in vitro grown shoots of Tamarixnilotica: Explication of its potential in impeding growth and biofilms of Listeria monocytogenes and assessment of wound healing ability. RSC Adv. 2020, 10, 30139–30149. [Google Scholar] [CrossRef]
- Mussa, Y.; Ahmed, F.; Arsalan, M.; Alsharaeh, E.H. Two dimensional (2D) reduced graphene oxide (RGO)/hexagonal boron nitride (h-BN) based nanocomposites as anodes for high temperature rechargeable lithium-ion batteries. Sci. Rep. 2020, 10, 1882. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Qais, F.A.; Ahmad, N.; Khan, A.; Alyousef, A.A.; Arshad, M.; Noor, S.; Khan, J.M.; Alam, P.; et al. Phyto-Mediated Synthesis of Porous Titanium Dioxide Nanoparticles From Withaniasomnifera Root Extract: Broad-Spectrum Attenuation of Biofilm and Cytotoxic Properties Against HepG2 Cell Lines. Front. Microbiol. 2020, 11, 1680. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.M.; Ansari, A.A.; Khan, A.; Ahmad, N.; Albadri, A.; AlBalawi, T.H. Mitigation of acyl-homoserine lactone (AHL) based bacterial quorum sensing, virulence functions, and biofilm formation by yttrium oxide core/shell nanospheres: Novel approach to combat drug resistance. Sci. Rep. 2019, 9, 18476. [Google Scholar] [CrossRef] [PubMed]
- Huston, A.L.; Methe, B.; Deming, J.W. Purification, Characterization, and Sequencing of an Extracellular Cold-Active Aminopeptidase Produced by Marine Psychrophile Colwelliapsychrerythraea Strain 34H. Appl. Environ. Microbiol. 2004, 70, 3321–3328. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Ahmad, N.; Qais, F.A.; Khana, A.; Khan, A.; Khan, M.S.; Khan, J.M.; Shahzad, S.A.; Ahmad, I. Facile Synthesis of Tin Oxide Hollow Nanoflowers Interfering with Quorum Sensing-Regulated Functions and Bacterial Biofilms. J. Nanomater. 2018, 2018, 6845026. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Ahmed, F.; Khan, R.A.; Khan, M.S.; Ansari, F.A.; Alam, M.Z.; Ahmed, M.A.; Khan, M.S.; Baig, M.H.; et al. Low Temperature Synthesis of Superparamagnetic Iron Oxide (Fe3O4) Nanoparticles and Their ROS Mediated Inhibition of Biofilm Formed by Food-Associated Bacteria. Front. Microbiol. 2018, 9, 2567. [Google Scholar] [CrossRef]


| Sample | Test Pathogens (MIC Values in µg/mL) | ||||
|---|---|---|---|---|---|
| L. monocytogenes | MRSA | E. coli | S. marcescens | P. aeruginosa | |
| Au-RGO | 32 | 64 | 16 | 8 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljaafari, A.; Ahmed, F.; Husain, F.M. Bio-Inspired Facile Synthesis of Graphene-Based Nanocomposites: Elucidation of Antimicrobial and Biofilm Inhibitory Potential against Foodborne Pathogenic Bacteria. Coatings 2020, 10, 1171. https://doi.org/10.3390/coatings10121171
Aljaafari A, Ahmed F, Husain FM. Bio-Inspired Facile Synthesis of Graphene-Based Nanocomposites: Elucidation of Antimicrobial and Biofilm Inhibitory Potential against Foodborne Pathogenic Bacteria. Coatings. 2020; 10(12):1171. https://doi.org/10.3390/coatings10121171
Chicago/Turabian StyleAljaafari, Abdullah, Faheem Ahmed, and Fohad Mabood Husain. 2020. "Bio-Inspired Facile Synthesis of Graphene-Based Nanocomposites: Elucidation of Antimicrobial and Biofilm Inhibitory Potential against Foodborne Pathogenic Bacteria" Coatings 10, no. 12: 1171. https://doi.org/10.3390/coatings10121171
APA StyleAljaafari, A., Ahmed, F., & Husain, F. M. (2020). Bio-Inspired Facile Synthesis of Graphene-Based Nanocomposites: Elucidation of Antimicrobial and Biofilm Inhibitory Potential against Foodborne Pathogenic Bacteria. Coatings, 10(12), 1171. https://doi.org/10.3390/coatings10121171







