Sorption of Methylene Blue for Studying the Specific Surface Properties of Biomass Carbohydrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Matrix Structure and Morphology Analysis
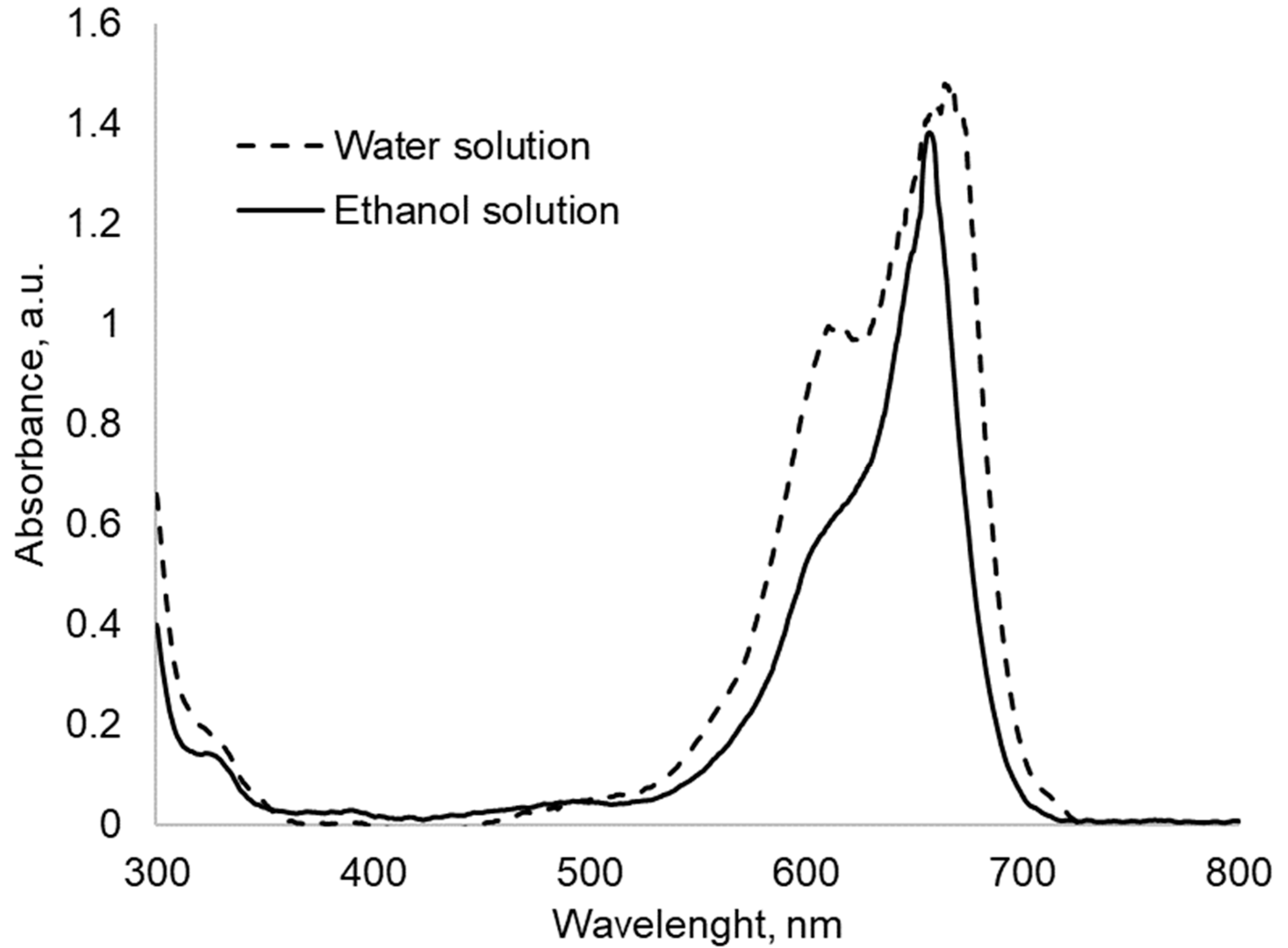
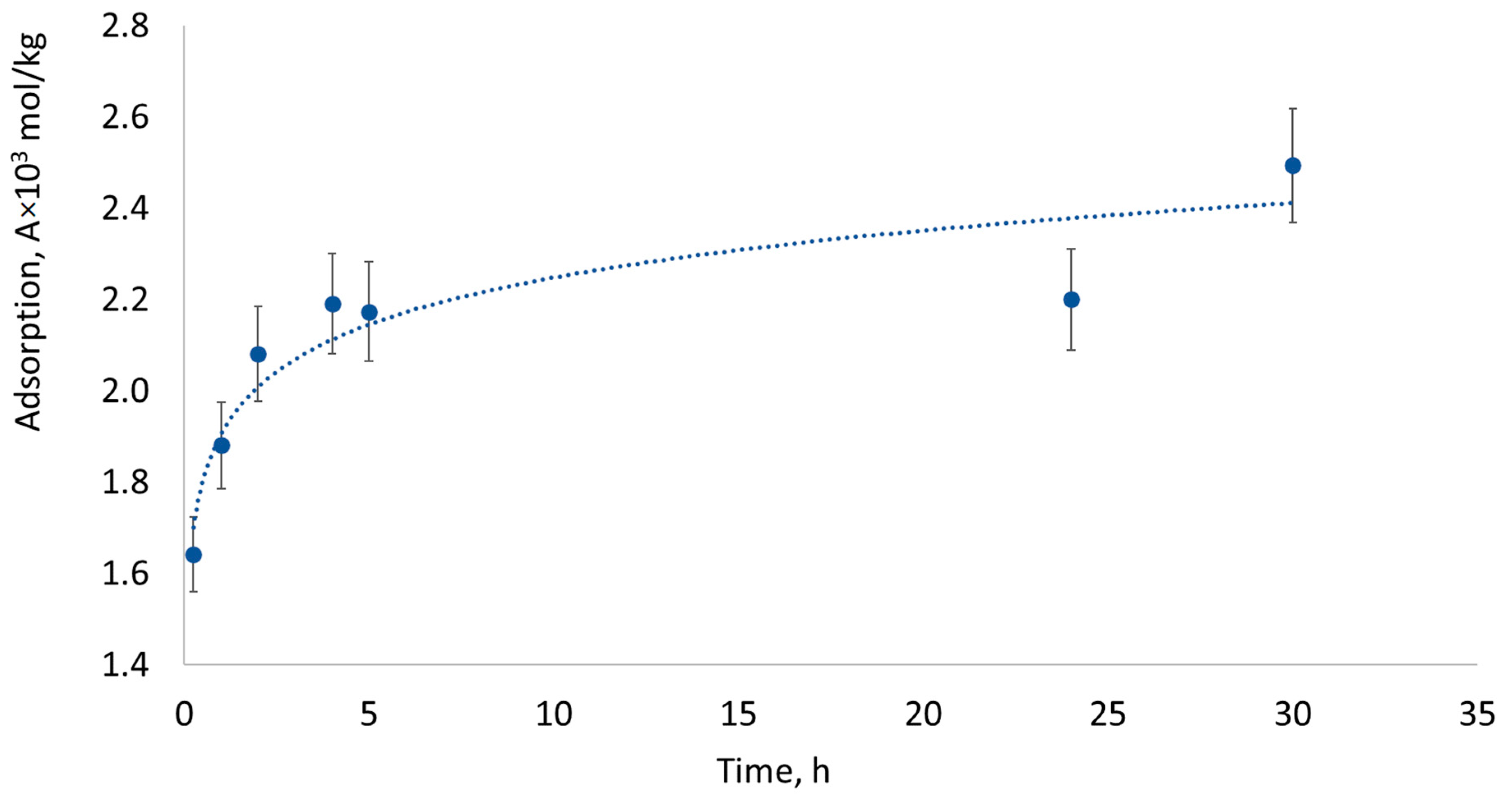
2.3. Methylene Blue Sorption and Spectrophotometric Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Q.; Liu, Z.; Pei, Y.; Li, J.; Li, B. Gelation behaviors of the konjac gum from different origins: A. guripingensis and A. rivirei. Food Hydrocoll. 2021, 111, 106152. [Google Scholar] [CrossRef]
- Semeijn, C.; Buwalda, P.L. Potato Starch. In Starch in Food; Elsevier: Amsterdam, The Netherlands, 2018; pp. 353–372. [Google Scholar]
- Kashcheyeva, E.I.; Gladysheva, E.K.; Skiba, E.A.; Budaeva, V.V. A study of properties and enzymatic hydrolysis of bacterial cellulose. Cellulose 2019, 26, 2255–2265. [Google Scholar] [CrossRef]
- Lu, M.; Li, J.; Han, L.; Xiao, W. An aggregated understanding of cellulase adsorption and hydrolysis for ball-milled cellulose. Bioresour. Technol. 2019, 273, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sinitsyn, A.P.; Gusakov, A.V.; Vlasenko, E.Y. Effect of structural and physico-chemical features of cellulosic substrates on the efficiency of enzymatic hydrolysis. Appl. Biochem. Biotechnol. 1991, 30, 43–59. [Google Scholar] [CrossRef]
- Sapozhnikov, A.N.; Kopylova, A.V.; Krainova, Y.O.; Krainov, S.A. The prospects of using spinach in flour and bakery products. Proc. Vor. State Univ. Eng. Technol. 2019, 80, 234–239. [Google Scholar] [CrossRef]
- Cova, C.M.; Luque, R. Advances in mechanochemical processes for biomass valorization. BMC Chem. Eng. 2019, 1, 16. [Google Scholar] [CrossRef]
- Nill, J.; Karuna, N.; Jeoh, T. The impact of kinetic parameters on cellulose hydrolysis rates. Process Biochem. 2018, 74, 108–117. [Google Scholar] [CrossRef]
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef]
- Fang, Z. Pretreatment Techniques for Biofuels and Biorefineries; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Staudt, P.B.; Kechinski, C.P.; Tessaro, I.C.; Marczak, L.D.F.; de Soares, R.P.; Cardozo, N.S.M. A new method for predicting sorption isotherms at different temperatures using the BET model. J. Food Eng. 2013, 114, 139–145. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, R. Modifying the BET model for accurately determining specific surface area and surface energy components of aggregates. Constr. Build. Mater. 2018, 175, 653–663. [Google Scholar] [CrossRef]
- Thompson, D.N.; Chen, H.-C.; Grethlein, H.E. Comparison of pretreatment methods on the basis of available surface area. Bioresour. Technol. 1992, 39, 155–163. [Google Scholar] [CrossRef]
- Podgorbunskikh, E.M.; Bychkov, A.L.; Lomovsky, O.I. Determination of surface accessibility of the cellulose substrate according to enzyme sorption. Polymers 2019, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefining 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Toumi, K.; Bergaoui, M.; Khalfaoui, M.; Benguerba, Y.; Erto, A.; Dotto, G.L.; Amrane, A.; Nacef, S.; Ernst, B. Computational study of acid blue 80 dye adsorption on low cost agricultural algerian olive cake waste: Statistical mechanics and molecular dynamic simulations. J. Mol. Liq. 2018, 271, 40–50. [Google Scholar] [CrossRef]
- Hong, J.; Ye, X.; Zhang, Y.-H.P. Quantitative determination of cellulose accessibility to cellulase based on adsorption of a nonhydrolytic fusion protein containing CBM and GFP with its applications. Langmuir 2007, 23, 12535–12540. [Google Scholar] [CrossRef]
- Wang, Q.Q.; He, Z.; Zhu, Z.; Zhang, Y.-H.P.; Ni, Y.; Luo, X.L.; Zhu, J.Y. Evaluations of cellulose accessibilities of lignocelluloses by solute exclusion and protein adsorption techniques. Biotechnol. Bioeng. 2012, 109, 381–389. [Google Scholar] [CrossRef]
- Bulut, Y.; Aydın, H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Li, H.; Zheng, H.; Du, Q. Equilibrium, kinetic and thermodynamic studies on methylene blue adsorption by konjac glucomannan/activated carbon aerogel. J. Polym. Environ. 2019, 27, 1342–1351. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.Ü.; Brouers, F. Methylene blue adsorption on magnetic alginate/rice husk bio-composite. Int. J. Biol. Macromol. 2020, 154, 104–113. [Google Scholar] [CrossRef]
- Dev, S.; Singh, M. Metallic sulfide nanoparticles anchored graphene oxide: Synthesis, characterization and reduction of methylene blue to leuco methylene blue in aqueous mixtures. J. Phys. Chem. Solids 2020, 139, 109335. [Google Scholar] [CrossRef]
- Hussin, M.H.; Pohan, N.A.; Garba, Z.N.; Kassim, M.J.; Rahim, A.A.; Brosse, N.; Yemloul, M.; Fazita, M.R.N.; Haafiz, M.K.M. Physicochemical of microcrystalline cellulose from oil palm fronds as potential methylene blue adsorbents. Int. J. Biol. Macromol. 2016, 92, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Batchelor-McAuley, C.; Rasche, B.; Johnston, C.; Hindle, N.; Compton, R.G. Surface area measurements of graphene and graphene oxide samples: Dopamine adsorption as a complement or alternative to methylene blue? Appl. Mater. Today 2020, 18, 100506. [Google Scholar] [CrossRef]
- International Organization for Standardization. Test Methods for Fibrous Activated Carbon; ISO 21340:2017; International organization for standardization: Geneve, Switzerland, 2017; p. 34. [Google Scholar]
- Gregg, S.J.; Sing, K.S.W. Adsorption, surface area and porosity, 2nd ed.; Academic Press: London, UK, 1982. [Google Scholar]
- Karnaukhov, A. Improvement of methods for surface area determinations. J. Colloid Interface Sci. 1985, 103, 311–320. [Google Scholar] [CrossRef]
- Van de Steeg, H.G.M.; de Keizer, A.; Stuart, M.A.C.; Bijsterbosch, B.H. Adsorption of cationic amylopectin on microcrystalline cellulose. Colloids Surfaces A Physicochem. Eng. Asp. 1993, 70, 77–89. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Özer, D.; Dursun, G.; Özer, A. Methylene blue adsorption from aqueous solution by dehydrated peanut hull. J. Hazard. Mater. 2007, 144, 171–179. [Google Scholar] [CrossRef]
- Husseien, M.; Amer, A.A.; El-Maghraby, A.; Hamedallah, N. A comprehensive characterization of corn stalk and study of carbonized corn stalk in dye and gas oil sorption. J. Anal. Appl. Pyrolysis 2009, 86, 360–363. [Google Scholar] [CrossRef]
- Xiao, L.-P.; Shi, Z.-J.; Xu, F.; Sun, R.-C. Hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef]
- Uddin, M.T.; Islam, M.A.; Mahmud, S.; Rukanuzzaman, M. Adsorptive removal of methylene blue by tea waste. J. Hazard. Mater. 2009, 164, 53–60. [Google Scholar] [CrossRef]

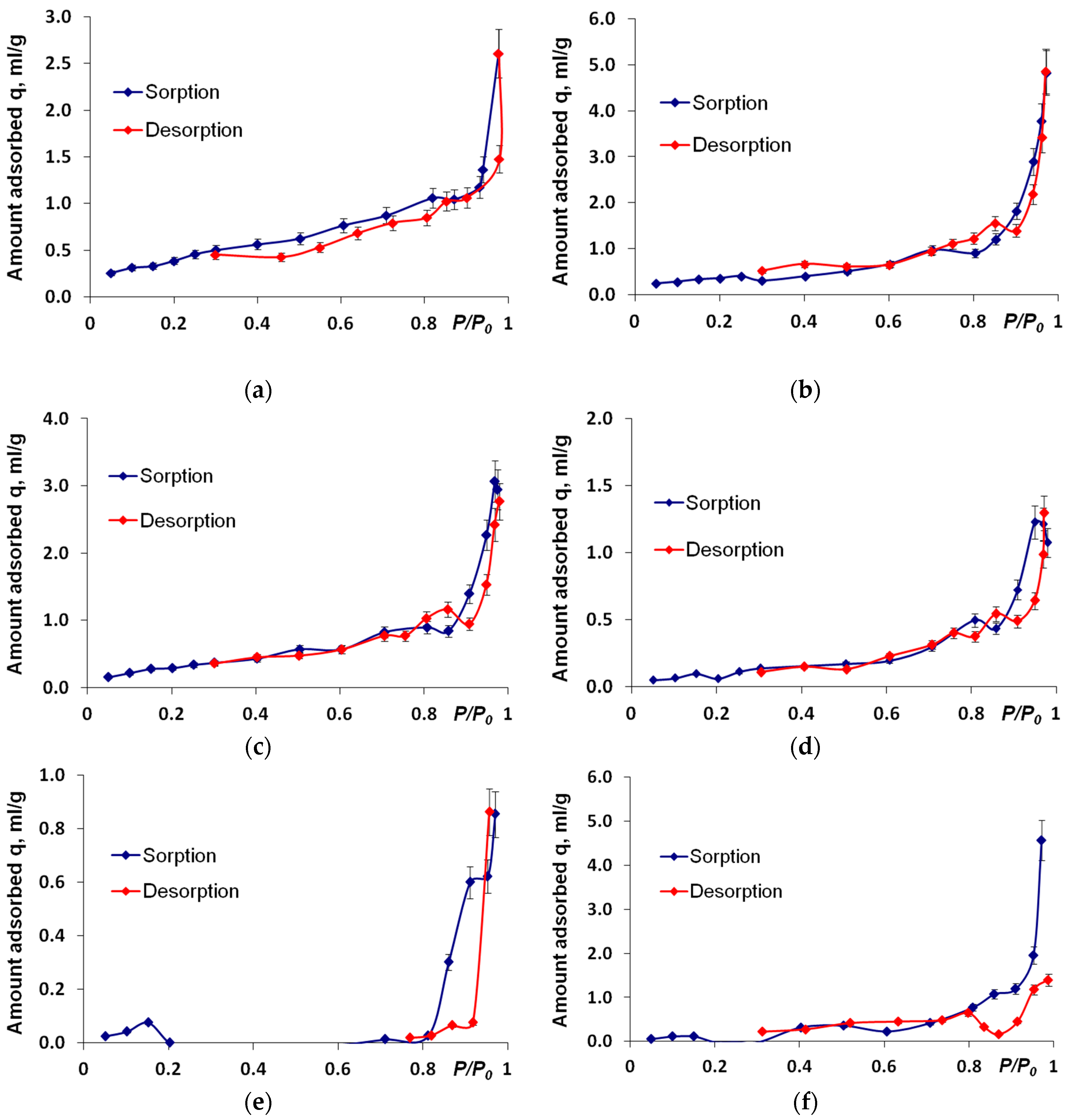
| Sample | BET Method | SSA (The Method Proposed by Gregg and Sing), m2/g | SSA (The Method Proposed by Harkins-Jura), m2/g | ||
|---|---|---|---|---|---|
| Energy Constant in the BET Equation CBET | SSA (Single-Point BET Method), m2/g | SSA (Multipoint BET Method), m2/g | |||
| α-cellulose | 89 | 1.4 ± 0.1 | 1.6 ± 0.1 | 2.3 ± 0.3 | 2.2 ± 0.3 |
| Sigmacell cellulose | 92 | 1.4 ± 0.1 | 1.40 ± 0.02 | 2.3 ± 0.9 | 2.1 ± 0.8 |
| β-glucan | 19 | 1.4 ± 0.1 | 1.4 ± 0.1 | 2.3 ± 0.2 | 2.2 ± 0.2 |
| Konjac gum | 27 | 0.2 | 0.3 | 0.5 | 0.5 |
| Potato starch | 3 | - | 0.8 | 0.9 | 0.9 |
| Corn starch | 11 | - | 0.7 | 1.3 | 1.2 |
| Sample | Sorption, A × 103 mmol/g | SSA (Multipoint BET Method), m2/g | Average Particle Size, µm | SSA Determined from Particle Distribution, m2/g |
|---|---|---|---|---|
| α-cellulose | 0.10 ± 0.04 | 1.6 ± 0.1 | 75.6 ± 0.3 | 0.10 ± 0.01 |
| Sigmacell cellulose | 9.7 ± 0.4 | 1.40 ± 0.02 | 35.6 ± 0.9 | 0.20 ± 0.01 |
| Konjac gum | 1.10 ± 0.09 | 0.3 | 104.1 ± 0.9 | 0.07 ± 0.01 |
| β-glucan | 4.0 ± 0.1 | 1.4 ± 0.1 | 106.6 ± 1.7 | 0.07 ± 0.01 |
| Potato starch | 0.30 ± 0.07 | 0.8 | 42.3 ± 0.6 | 0.17 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skripkina, T.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Sorption of Methylene Blue for Studying the Specific Surface Properties of Biomass Carbohydrates. Coatings 2020, 10, 1115. https://doi.org/10.3390/coatings10111115
Skripkina T, Podgorbunskikh E, Bychkov A, Lomovsky O. Sorption of Methylene Blue for Studying the Specific Surface Properties of Biomass Carbohydrates. Coatings. 2020; 10(11):1115. https://doi.org/10.3390/coatings10111115
Chicago/Turabian StyleSkripkina, Tatiana, Ekaterina Podgorbunskikh, Aleksey Bychkov, and Oleg Lomovsky. 2020. "Sorption of Methylene Blue for Studying the Specific Surface Properties of Biomass Carbohydrates" Coatings 10, no. 11: 1115. https://doi.org/10.3390/coatings10111115
APA StyleSkripkina, T., Podgorbunskikh, E., Bychkov, A., & Lomovsky, O. (2020). Sorption of Methylene Blue for Studying the Specific Surface Properties of Biomass Carbohydrates. Coatings, 10(11), 1115. https://doi.org/10.3390/coatings10111115






