Preparation and Characterization of Quinoxaline-Pyrene-Based Conjugated Copolymers for Organic Photovoltaic Devices
Abstract
1. Introduction
2. Materials and Methods
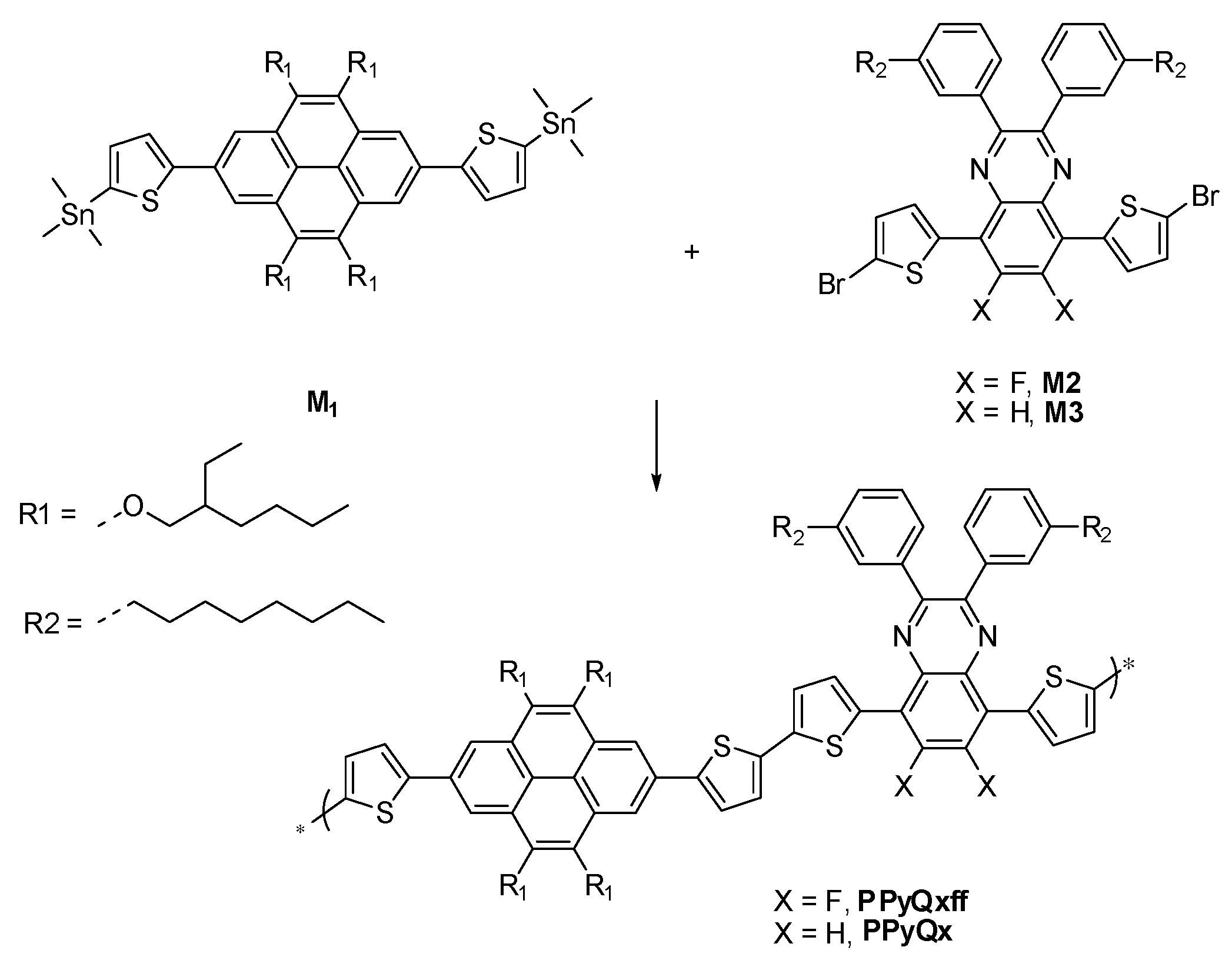
2.1. Preparation of PPyQxff
2.2. Preparation of PPyQx
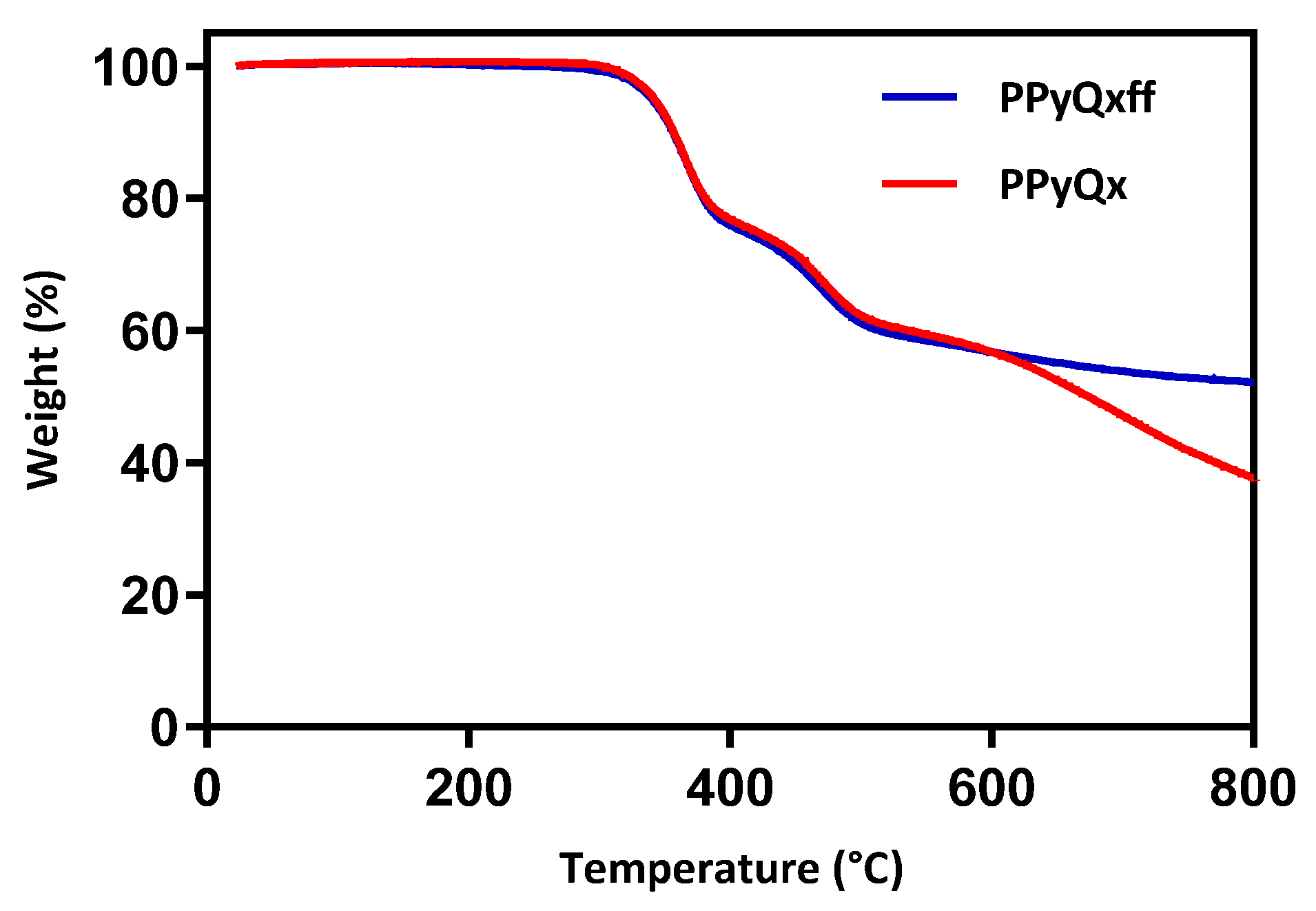
3. Results
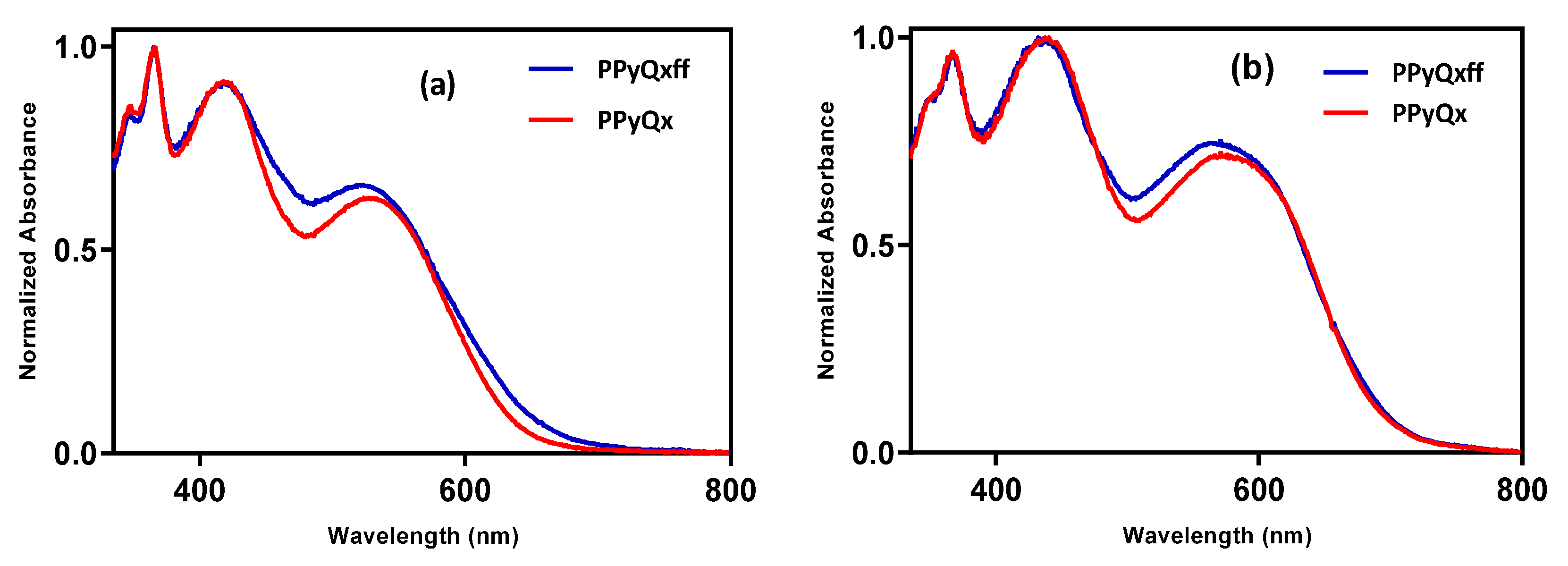
3.1. Optical Properties
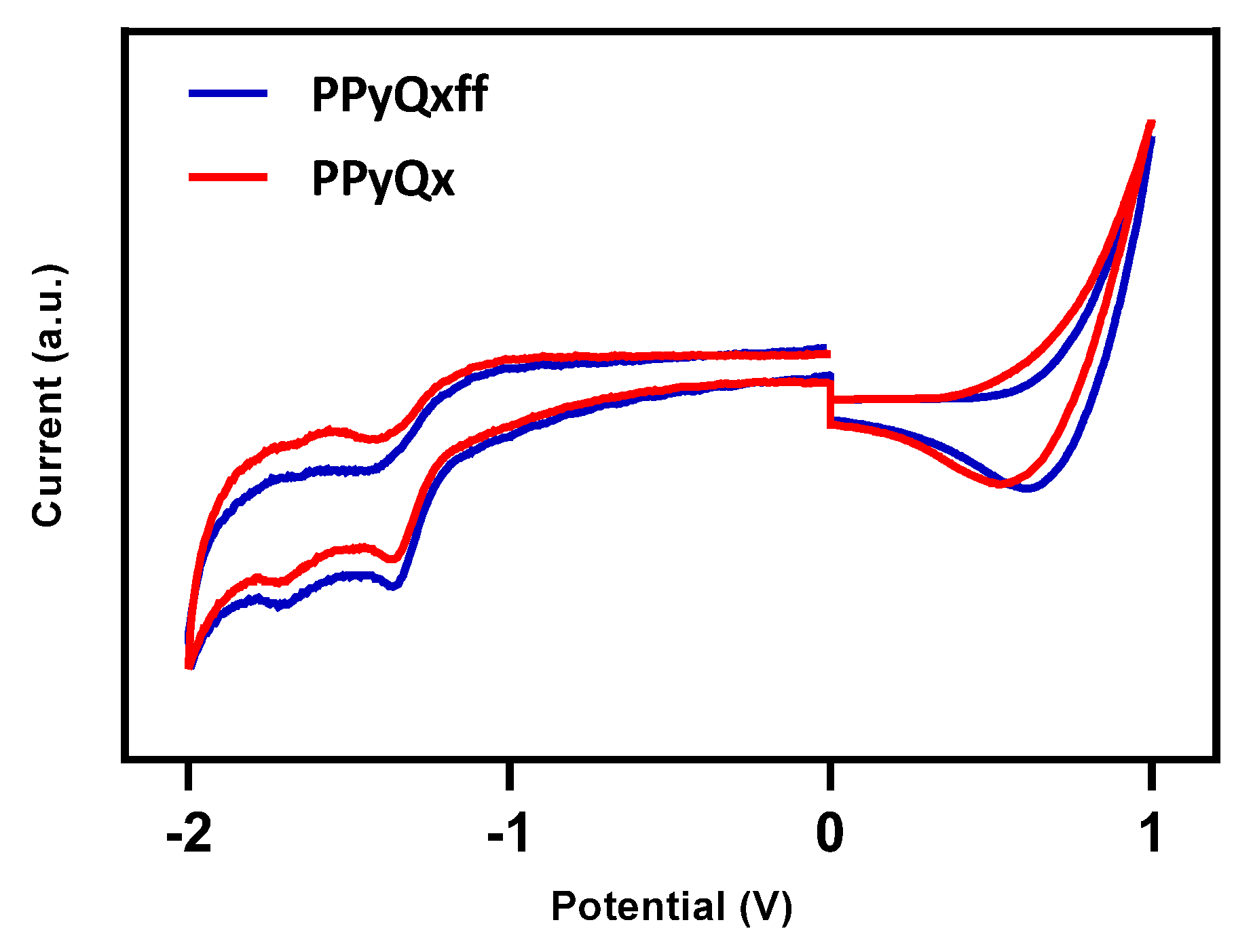
3.2. Electrochemical Properties
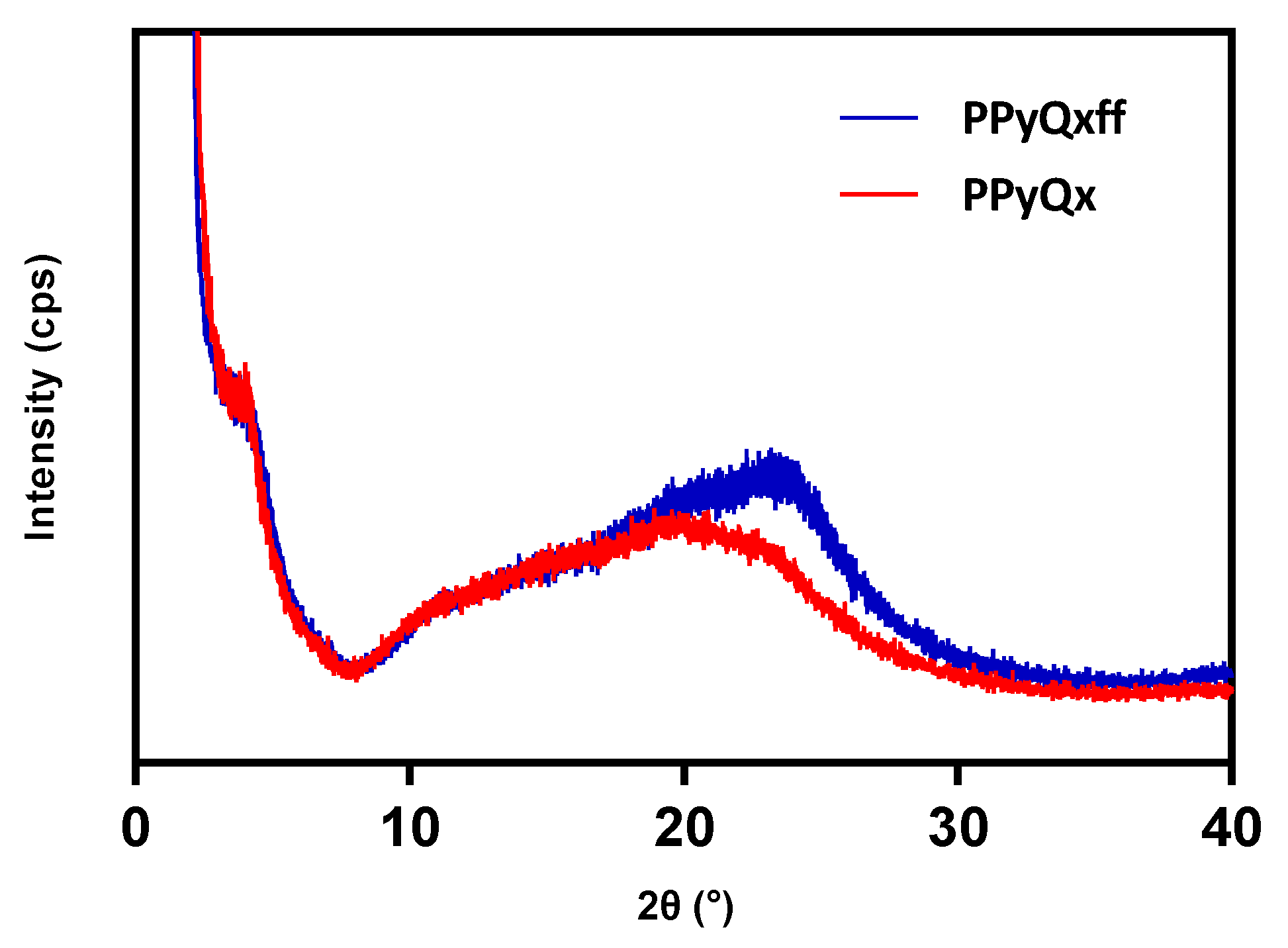
3.3. X-ray Powder Diffraction (XRD)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent advances in bulk heterojunction polymer solar cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Wang, L.; Lan, L.; Luo, C.; Zhuang, W.; Peng, J.; Cao, Y. High-performance polymer heterojunction solar cells of a polysilafluorene derivative. Appl. Phys. Lett. 2008, 92, 2–5. [Google Scholar] [CrossRef]
- Henson, Z.B.; Müllen, K.; Bazan, G.C. Design strategies for organic semiconductors beyond the molecular formula. Nat. Chem. 2012, 4, 699–704. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-fullerene bulk-heterojunction solar cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Ameri, T.; Khoram, P.; Min, J.; Brabec, C.J. Organic ternary solar cells: A review. Adv. Mater. 2013, 25, 4245–4266. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef]
- Kitamura, C.; Tanaka, S.; Yamashita, Y. Design of narrow-bandgap polymers. Syntheses and properties of monomers and polymers containing aromatic-donor and o-quinoid-acceptor units. Chem. Mater. 1996, 8, 570–578. [Google Scholar] [CrossRef]
- Price, S.C.; Stuart, A.C.; Yang, L.; Zhou, H.; You, W. Fluorine substituted conjugated polymer of medium band gap yields 7% efficiency in polymer-fullerene solar cells. J. Am. Chem. Soc. 2011, 133, 4625–4631. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, Y.H.; Liu, C.C.; Chien, Y.C.; Chou, S.W.; Chou, P.T. Prominent short-circuit currents of fluorinated quinoxaline-based copolymer solar cells with a power conversion efficiency of 8.0%. Chem. Mater. 2012, 24, 4766–4772. [Google Scholar] [CrossRef]
- Bronstein, H.; Frost, J.M.; Hadipour, A.; Kim, Y.; Nielsen, C.B.; Ashraf, R.S.; Rand, B.P.; Watkins, S.; McCulloch, I. Effect of fluorination on the properties of a donor-acceptor copolymer for use in photovoltaic cells and transistors. Chem. Mater. 2013, 25, 277–285. [Google Scholar] [CrossRef]
- Duan, R.; Ye, L.; Guo, X.; Huang, Y.; Wang, P.; Zhang, S.; Zhang, J.; Huo, L.; Hou, J. Application of two-dimensional conjugated benzo[1,2-b:4,5-b’]dithiophene in quinoxaline-based photovoltaic polymers. Macromolecules 2012, 45, 3032–3038. [Google Scholar] [CrossRef]
- Chochos, C.L.; Choulis, S.A. How the structural deviations on the backbone of conjugated polymers influence their optoelectronic properties and photovoltaic performance. Prog. Polym. Sci. 2011, 36, 1326–1414. [Google Scholar] [CrossRef]
- Wang, N.; Chen, Z.; Wei, W.; Jiang, Z. Fluorinated benzothiadiazole-based conjugated polymers for high-performance polymer solar cells without any processing additives or post-treatments. J. Am. Chem. Soc. 2013, 135, 17060–17068. [Google Scholar] [CrossRef] [PubMed]
- Alqurashy, B.A.; Li, Y.; Ko, S.J.; Park, S.Y.; Choi, H.; Nguyen, T.L.; Uddin, M.A.; Kim, T.; Hwang, S.; Kim, J.Y.; et al. Quinoxaline–thiophene based thick photovoltaic devices with an efficiency of ~8%. J. Mater. Chem. A 2016, 4, 9967–9976. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.R.; Han, S.H.; Lee, J.Y.; Seo, S.Y. Synthesis and characterization of quinoxaline derivative for high performance phosphorescent organic light-emitting diodes. Dye. Pigment. 2018, 153, 132–136. [Google Scholar] [CrossRef]
- Chang, D.W.; Lee, H.J.; Kim, J.H.; Park, S.Y. Novel quinoxaline-based organic sensitizers for dye-sensitized solar cells. Org. Lett. 2011, 13, 3880–3883. [Google Scholar] [CrossRef]
- Wang, E.; Hou, L.; Wang, Z.; Hellström, S.; Zhang, F.; Inganäs, O.; Andersson, M.R. An easily synthesized blue polymer for high-performance polymer solar cells. Adv. Mater. 2010, 22, 5240–5244. [Google Scholar] [CrossRef]
- Liu, M.; Gao, Y.; Zhang, Y.; Liu, Z.; Zhao, L. Quinoxaline-based conjugated polymers for polymer solar cells. Polym. Chem. 2017, 8, 4613–4636. [Google Scholar] [CrossRef]
- Dang, D.; Chen, W.; Yang, R.; Zhu, W.; Mammo, W.; Wang, E. Fluorine substitution enhanced photovoltaic performance of a D-A 1-D-A2 copolymer. Chem. Commun. 2013, 49, 9335–9337. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, W.; Zhang, S.; Ye, L.; Zheng, Z.; Cui, Y.; Chen, Y.; Hou, J. Highly efficient photovoltaic polymers based on benzodithiophene and quinoxaline with deeper HOMO levels. Macromolecules 2015, 48, 5172–5178. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, H.; Liu, S.; Luo, G.; Wang, W.; Guo, Z.; Wei, W.; Gao, C.; An, Z. Efficient alternating polymer based on benzodithiophene and di-fluorinated quinoxaline derivatives for bulk heterojunction photovoltaic cells. Polymer (Guildf). 2017, 116, 35–42. [Google Scholar] [CrossRef]
- Wang, N.; Bao, X.; Yan, Y.; Ouyang, D.; Sun, M.; Roy, V.A.L.; Lee, C.S.; Yang, R. Synthesis and photovoltaic properties of conjugated D-A copolymers based on thienyl substituted pyrene and diketopyrrolopyrrole for polymer solar cells. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 3198–3204. [Google Scholar] [CrossRef]
- Alqurashy, B.A. Preparation and physical characterization of pyrene and pyrrolo[3,4-c]pyrrole-1,4-dione-based copolymers. ChemistryOpen 2019, 8, 429–433. [Google Scholar] [CrossRef]
- Merz, J.; Dietz, M.; Vonhausen, Y.; Wöber, F.; Friedrich, A.; Sieh, D.; Krummenacher, I.; Braunschweig, H.; Moos, M.; Holzapfel, M.; et al. Synthesis, photophysical and electronic properties of new red-to-NIR emitting donor–Acceptor pyrene derivatives. Chem. Eur. J. 2020, 26, 438–453. [Google Scholar] [CrossRef]
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-based materials for organic electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef]
- Zöphel, L.; Beckmann, D.; Enkelmann, V.; Chercka, D.; Rieger, R.; Müllen, K. Asymmetric pyrene derivatives for organic field-effect transistors. Chem. Commun. 2011, 47, 6960–6962. [Google Scholar] [CrossRef]
- Cai, G.; Xue, P.; Chen, Z.; Li, T.; Liu, K.; Ma, W.; Lian, J.; Zeng, P.; Wang, Y.; Han, R.P.S.; et al. High-performance mid-bandgap fused-pyrene electron acceptor. Chem. Mater. 2019, 31, 6484–6490. [Google Scholar] [CrossRef]
- Liu, S.Y.; Liu, W.Q.; Xu, J.Q.; Fan, C.C.; Fu, W.F.; Ling, J.; Wu, J.Y.; Shi, M.M.; Jen, A.K.Y.; Chen, H.Z. Pyrene and diketopyrrolopyrrole-based oligomers synthesized via direct arylation for OSC applications. ACS Appl. Mater. Interfaces 2014, 6, 6765–6775. [Google Scholar] [CrossRef]
- Xu, J.; Liu, W.; Liu, S.; Ling, J.; Mai, J.; Lu, X.; Li, C.; Jen, A.K.; Chen, H. A-D-A small molecule donors based on pyrene and diketopyrrolopyrrole for organic solar cells. Sci. China Chem. 2017, 60, 561–569. [Google Scholar] [CrossRef]
- Alqurashy, B.A.; Cartwright, L.; Iraqi, A.; Zhang, Y.; Lidzey, D.G. Pyrene–benzothiadiazole-based copolymers for application in photovoltaic devices. Polym. Adv. Technol. 2017, 28, 193–200. [Google Scholar] [CrossRef]
- Alqurashy, B.A.; Iraqi, A.; Zhang, Y.; Lidzey, D.G. Preparation and photovoltaic properties of pyrene-thieno[3,4-c]pyrrole-4,6-dione-based donor-acceptor polymers. Eur. Polym. J. 2016, 85, 225–235. [Google Scholar] [CrossRef]
- Park, S.M.; Yoon, Y.; Jeon, C.W.; Kim, H.; Ko, M.J.; Lee, D.K.; Kim, J.Y.; Son, H.J.; Kwon, S.K.; Kim, Y.H.; et al. Synthesis of phenanthro[1,10,9,8-cdefg]carbazole-based conjugated polymers for organic solar cell applications. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 796–803. [Google Scholar] [CrossRef]
- Dang, D.; Xiao, M.; Zhou, P.; Shi, J.; Tao, Q.; Tan, H.; Wang, Y.; Bao, X.; Liu, Y.; Wang, E.; et al. Manipulating backbone structure with various conjugated spacers to enhance photovoltaic performance of D-A-type two-dimensional copolymers. Org. Electron. 2014, 15, 2876–2884. [Google Scholar] [CrossRef]
- You, J.; Dou, L.; Yoshimura, K.; Kato, T.; Ohya, K.; Moriarty, T.; Emery, K.; Chen, C.C.; Gao, J.; Li, G.; et al. A polymer tandem solar cell with 10.6% power conversion efficiency. Nat. Commun. 2013, 4, 1446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chien, S.C.; Chen, K.S.; Yip, H.L.; Sun, Y.; Davies, J.A.; Chen, F.C.; Jen, A.K.Y. Increased open circuit voltage in fluorinated benzothiadiazole-based alternating conjugated polymers. Chem. Commun. 2011, 47, 11026–11028. [Google Scholar] [CrossRef]
- Kroon, R.; Gehlhaar, R.; Steckler, T.T.; Henriksson, P.; Müller, C.; Bergqvist, J.; Hadipour, A.; Heremans, P.; Andersson, M.R. New quinoxaline and pyridopyrazine-based polymers for solution-processable photovoltaics. Sol. Energy Mater. Sol. Cells 2012, 105, 280–286. [Google Scholar] [CrossRef]
- Kim, J.; Yun, M.H.; Kim, G.H.; Lee, J.; Lee, S.M.; Ko, S.J.; Kim, Y.; Dutta, G.K.; Moon, M.; Park, S.Y.; et al. Synthesis of PCDTBT-based fluorinated polymers for high open-circuit voltage in organic photovoltaics: Towards an understanding of relationships between polymer energy levels engineering and ideal morphology control. ACS Appl. Mater. Interfaces 2014, 6, 7523–7534. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Z.; Wang, S.; Guo, Y.; Liu, Y. Insight into high-performance conjugated polymers for organic field-effect transistors. Chem 2018, 4, 2748–2785. [Google Scholar] [CrossRef]
| Polymers | Mn (Da) a | Mw (Da) a | PDI | λmax (nm) | Eg opt (eV) b | HOMO (eV) c | LUMO (eV) c | Eg ele (eV) d | |
|---|---|---|---|---|---|---|---|---|---|
| Solution | Film | ||||||||
| PPyQxff | 9800 | 18,400 | 1.88 | 366, 418, 530 | 367, 433, 565 | 1.77 | −5.47 | −3.62 | 1.85 |
| PPyQx | 10,800 | 19,500 | 1.80 | 366, 418, 530 | 367, 437, 570 | 1.77 | −5.41 | −3.62 | 1.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqurashy, B.A.; Altayeb, B.M.; Alfaifi, S.Y.; Alawad, M.; Iraqi, A.; Ali, I. Preparation and Characterization of Quinoxaline-Pyrene-Based Conjugated Copolymers for Organic Photovoltaic Devices. Coatings 2020, 10, 1098. https://doi.org/10.3390/coatings10111098
Alqurashy BA, Altayeb BM, Alfaifi SY, Alawad M, Iraqi A, Ali I. Preparation and Characterization of Quinoxaline-Pyrene-Based Conjugated Copolymers for Organic Photovoltaic Devices. Coatings. 2020; 10(11):1098. https://doi.org/10.3390/coatings10111098
Chicago/Turabian StyleAlqurashy, Bakhet A., Bader M. Altayeb, Sulaiman Y. Alfaifi, Majed Alawad, Ahmed Iraqi, and Imran Ali. 2020. "Preparation and Characterization of Quinoxaline-Pyrene-Based Conjugated Copolymers for Organic Photovoltaic Devices" Coatings 10, no. 11: 1098. https://doi.org/10.3390/coatings10111098
APA StyleAlqurashy, B. A., Altayeb, B. M., Alfaifi, S. Y., Alawad, M., Iraqi, A., & Ali, I. (2020). Preparation and Characterization of Quinoxaline-Pyrene-Based Conjugated Copolymers for Organic Photovoltaic Devices. Coatings, 10(11), 1098. https://doi.org/10.3390/coatings10111098






