Room-Temperature and High-Temperature Wear Behaviors of As-Sprayed and Annealed Cr3C2-25NiCr Coatings Prepared by High Velocity Air-Fuel Spraying
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock Powder and Coating Deposition
2.2. High Temperature Annealing
2.3. Friction and Wear Tests
2.4. General Characterization
3. Results
3.1. Characterization of Coatings after Annealing
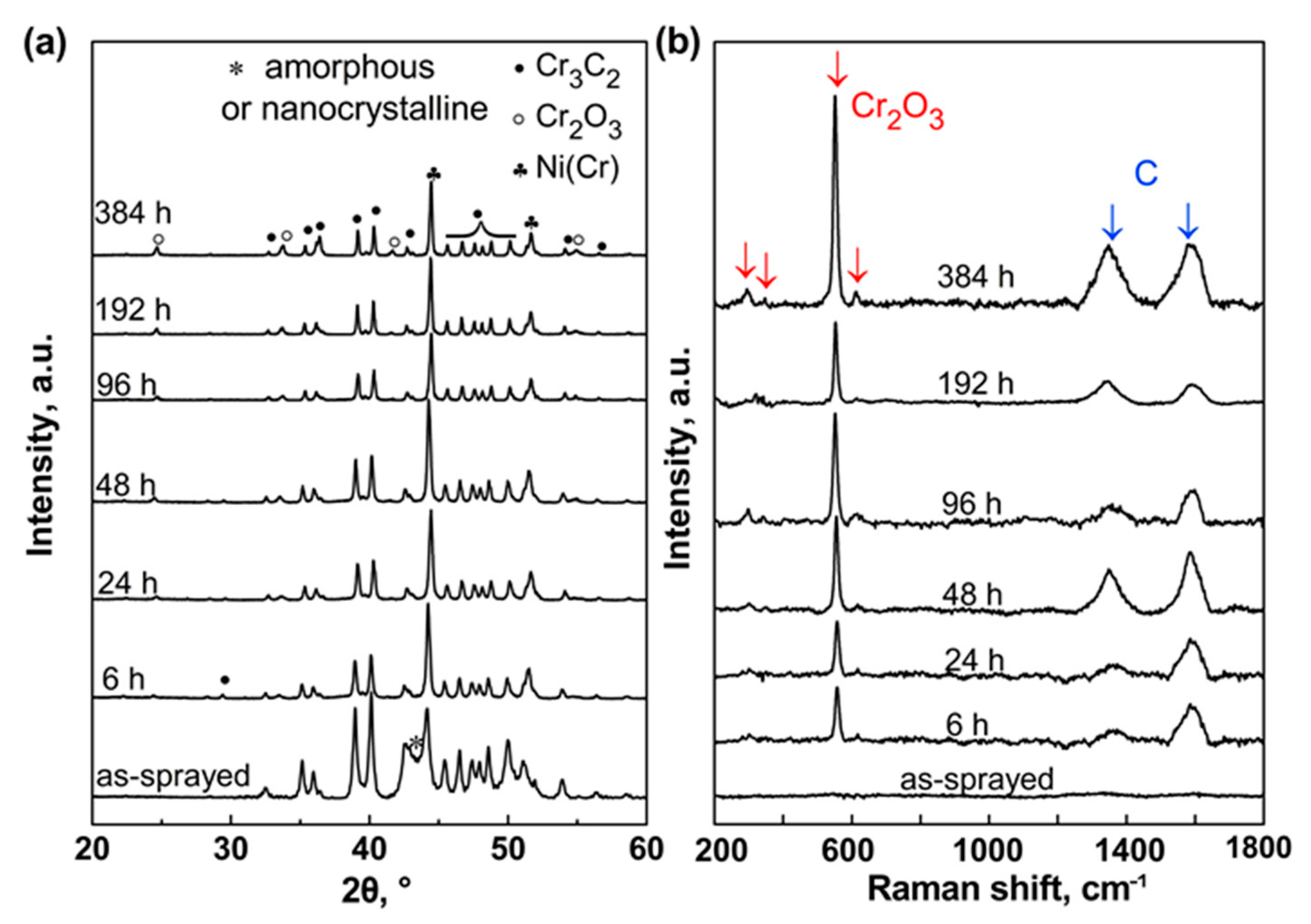
3.1.1. XRD and Raman Spectra Analysis
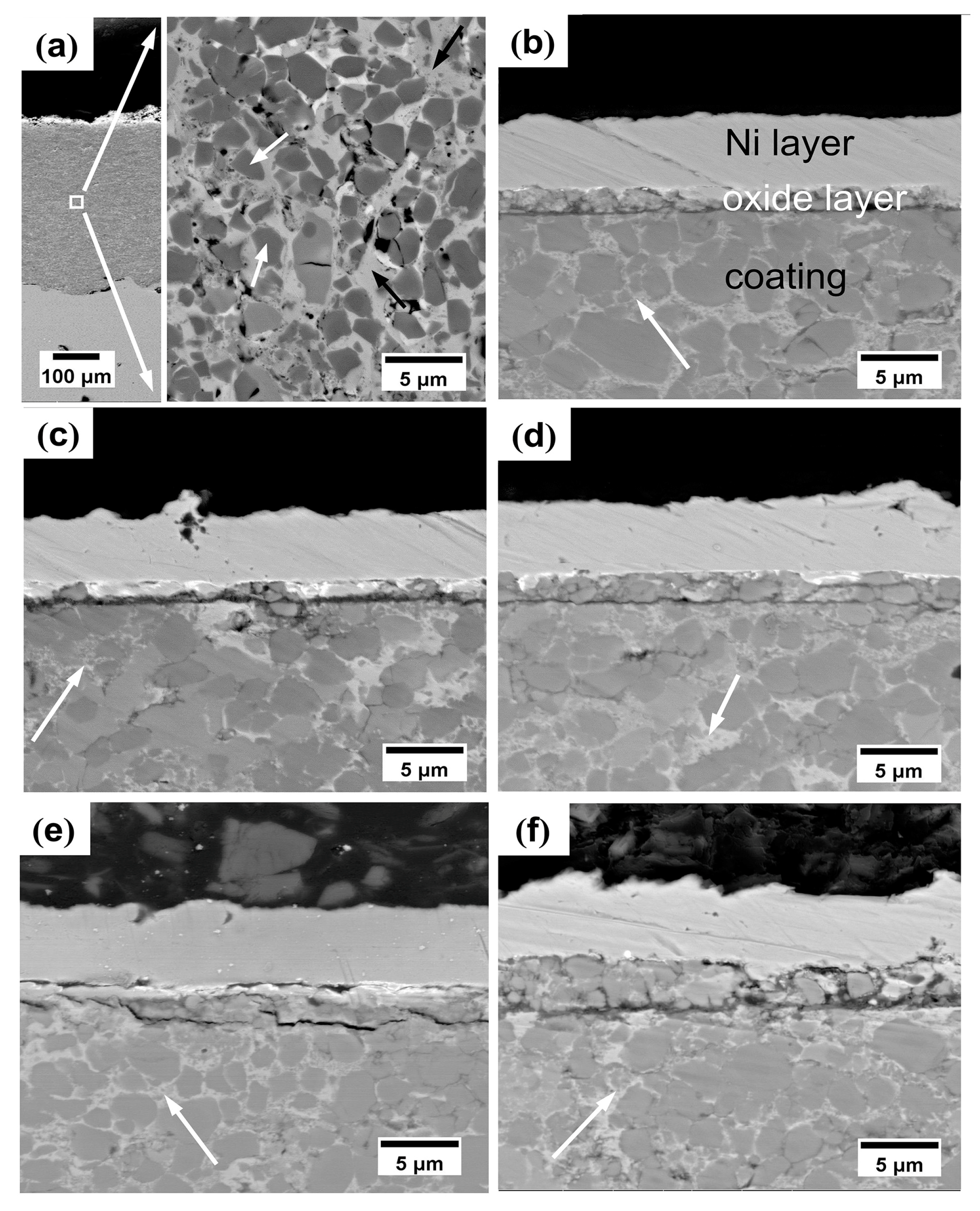
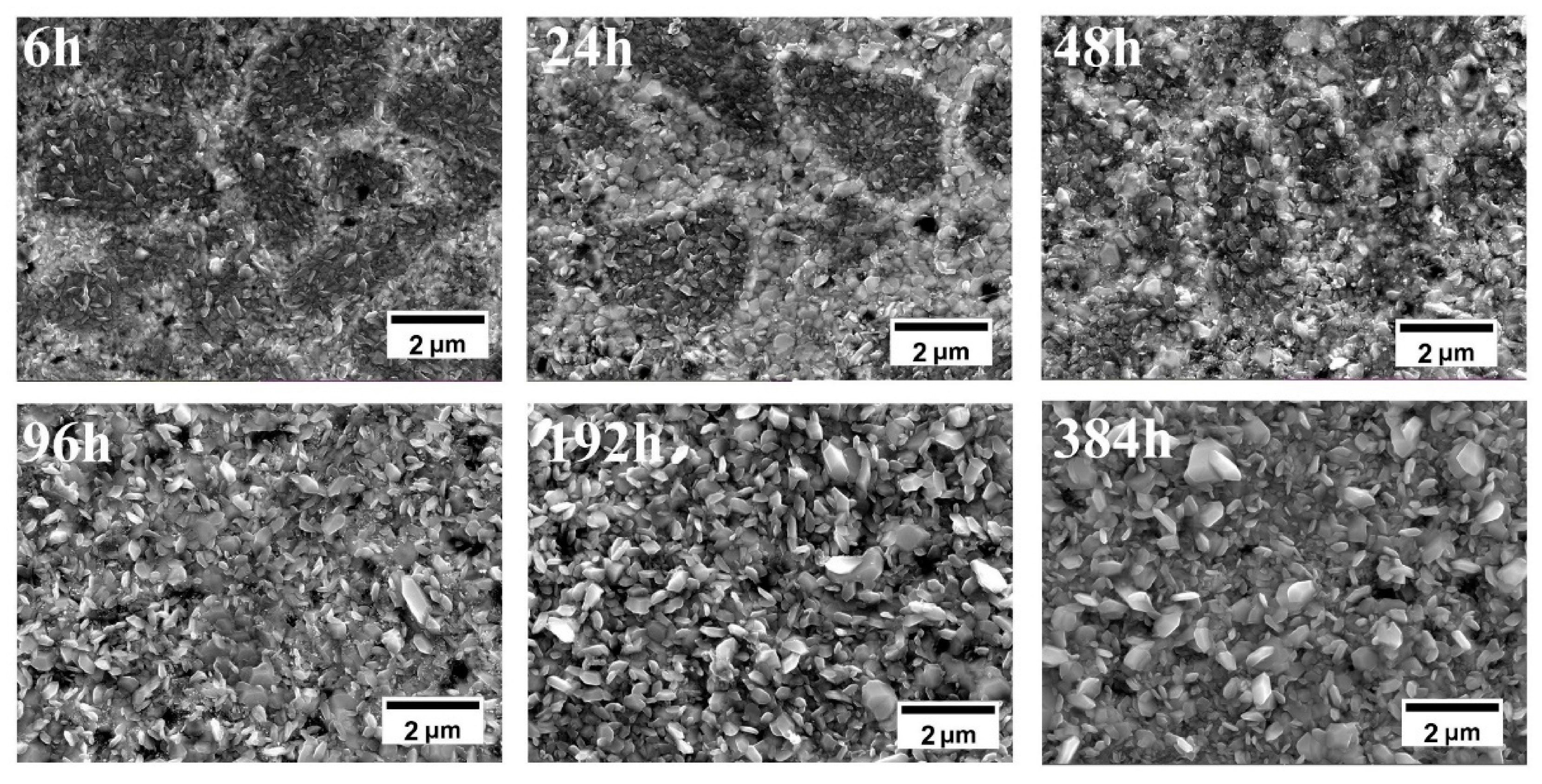
3.1.2. Microstructure Analysis
3.2. Hardness and Tribological Properties
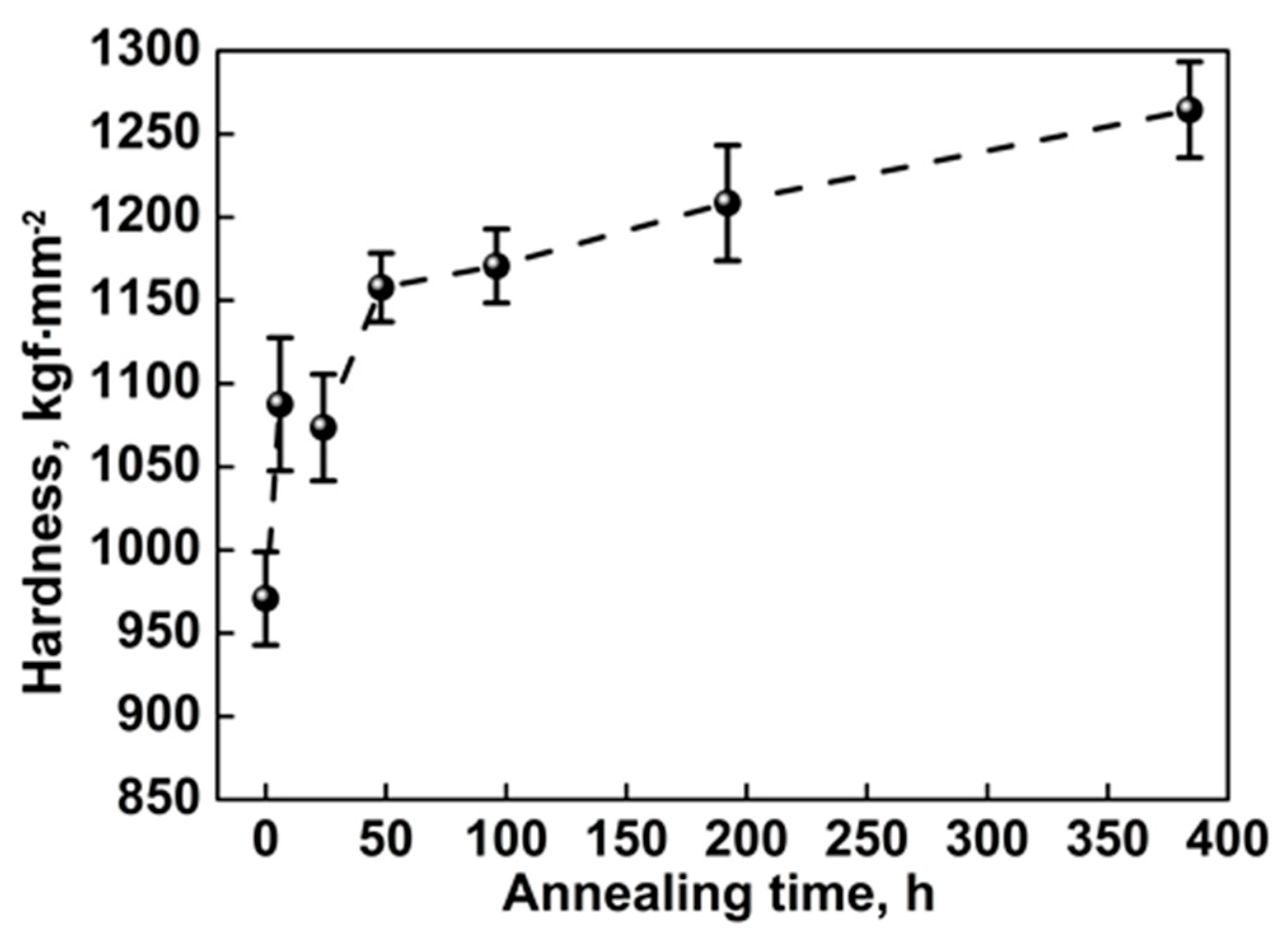
3.2.1. Hardness of the Annealed Coatings
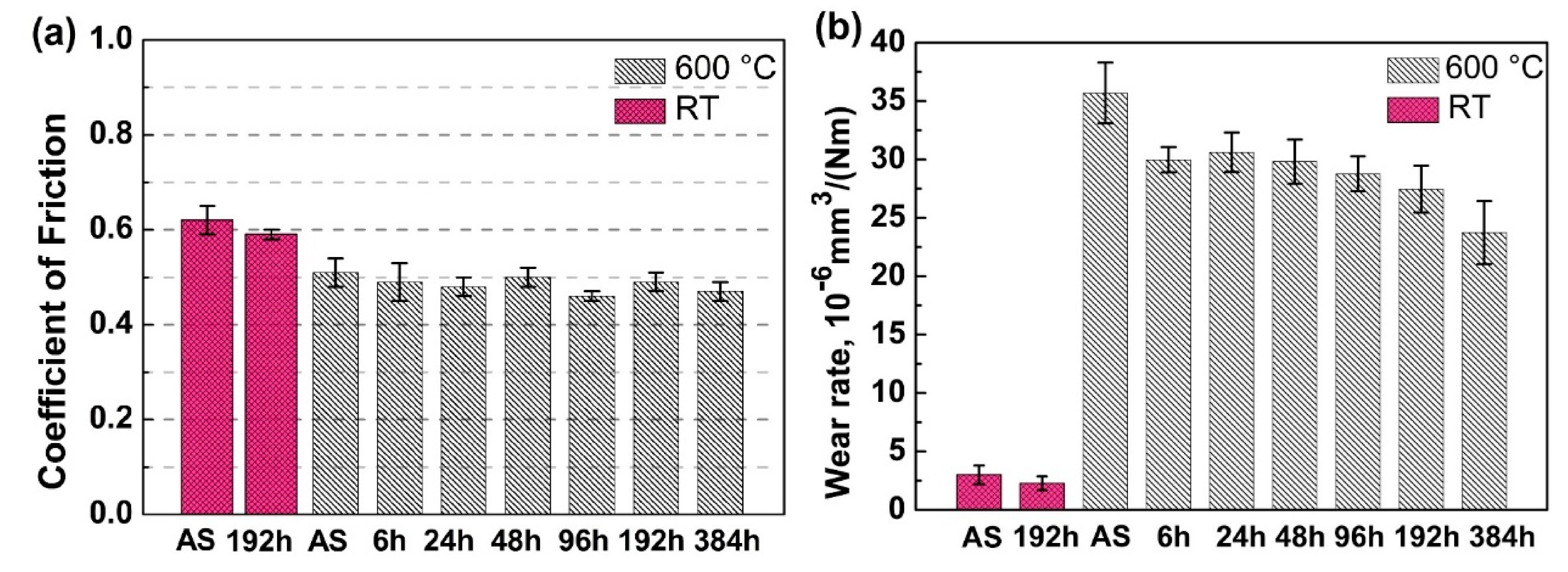
3.2.2. Friction Coefficient and Wear Rate
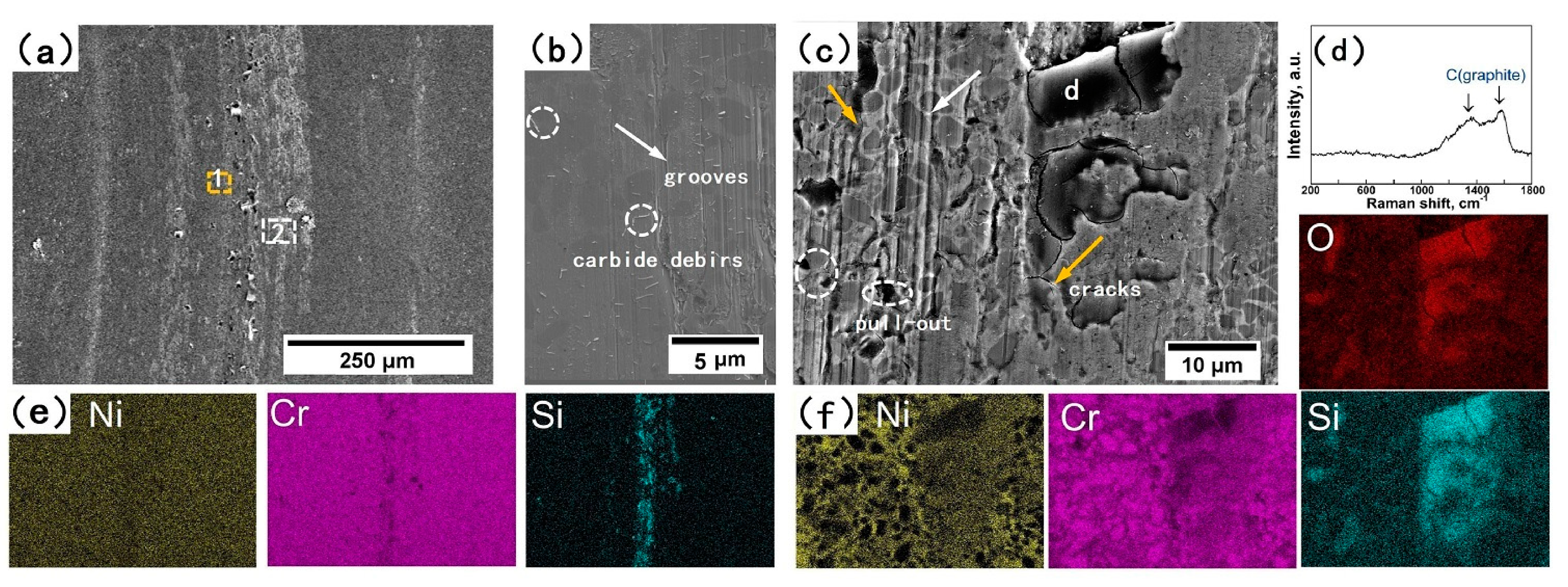
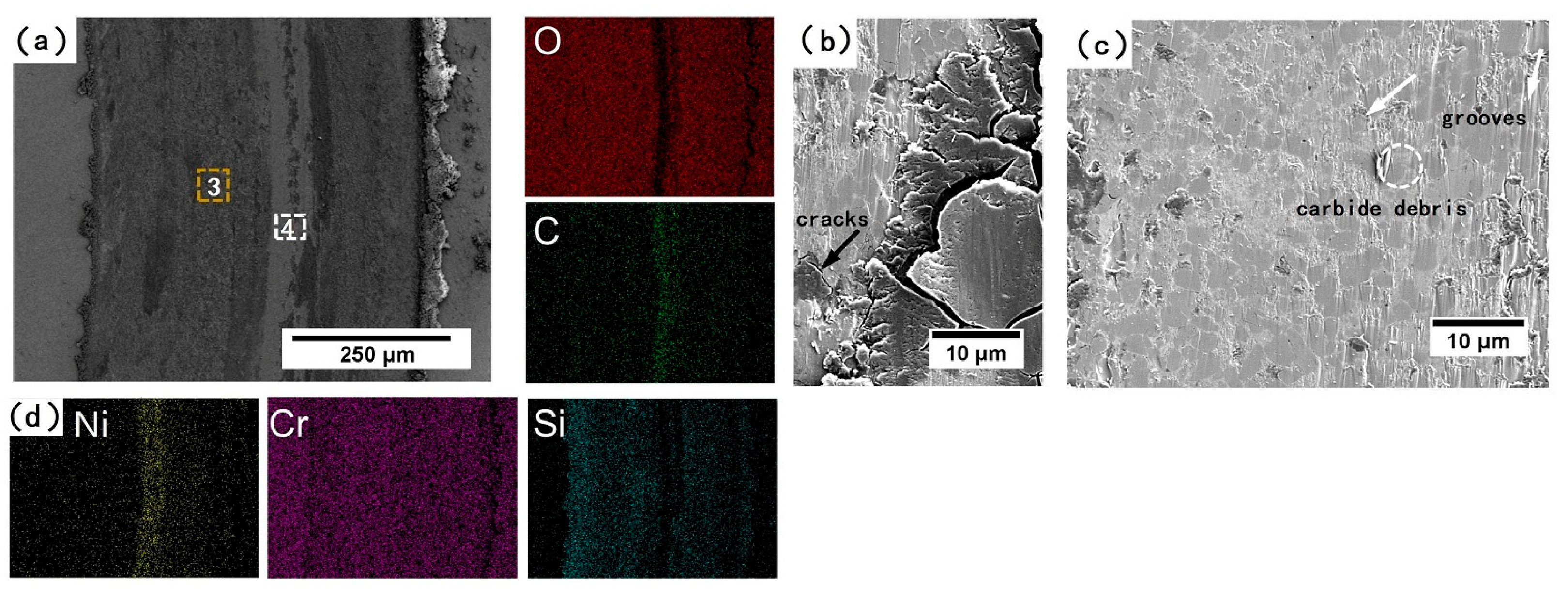
3.3. Characterization of the Wear Scar
3.3.1. Raman Spectroscopy Analysis
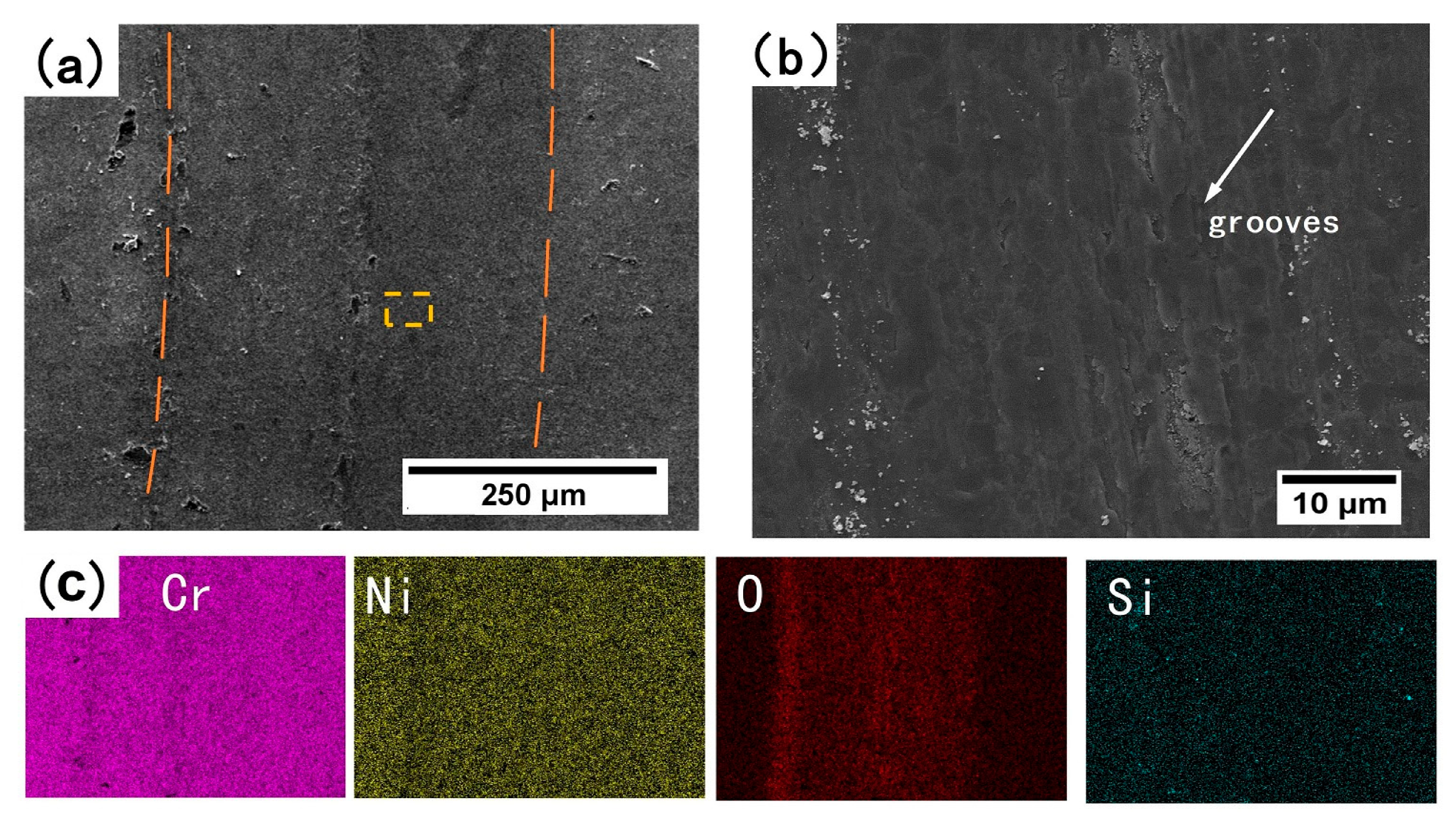
3.3.2. Surface Morphology of the Wear Scar
3.3.3. Cross-Sectional Analysis of Wear Scar
4. Discussion
4.1. Microstructure and Composition Evolution of Coatings
4.2. Wear Mechanism(s) of Coatings at RT and HT
5. Conclusions
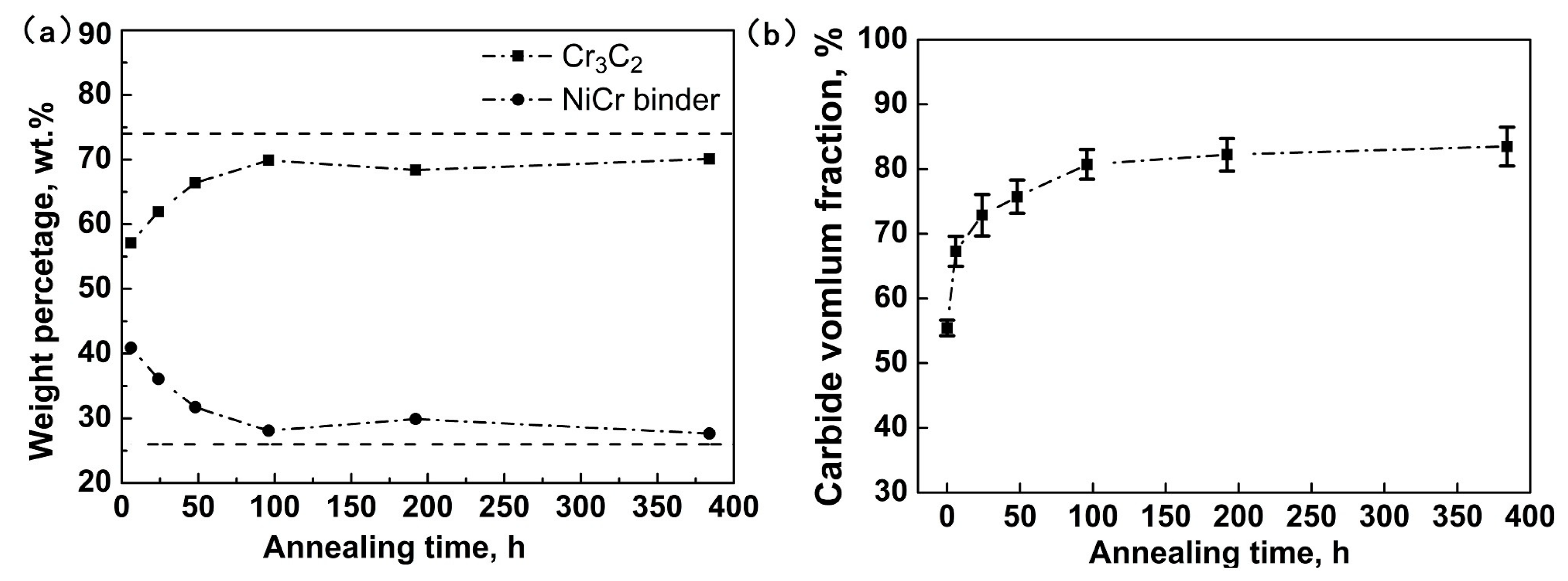
- The as-sprayed Cr3C2-NiCr coating is subjected to oxidation at 600 °C in air to form a continuous and dense Cr2O3 oxide scale on the coating surface. The oxide-scale growth follows the parabolic law that the thickness increases linearly with increasing square root of annealing time. The underlying coating experiences a compositional variation during annealing. The volume fraction of the carbide phase shows a rapid increase up to 96 h annealing and then remains stable.
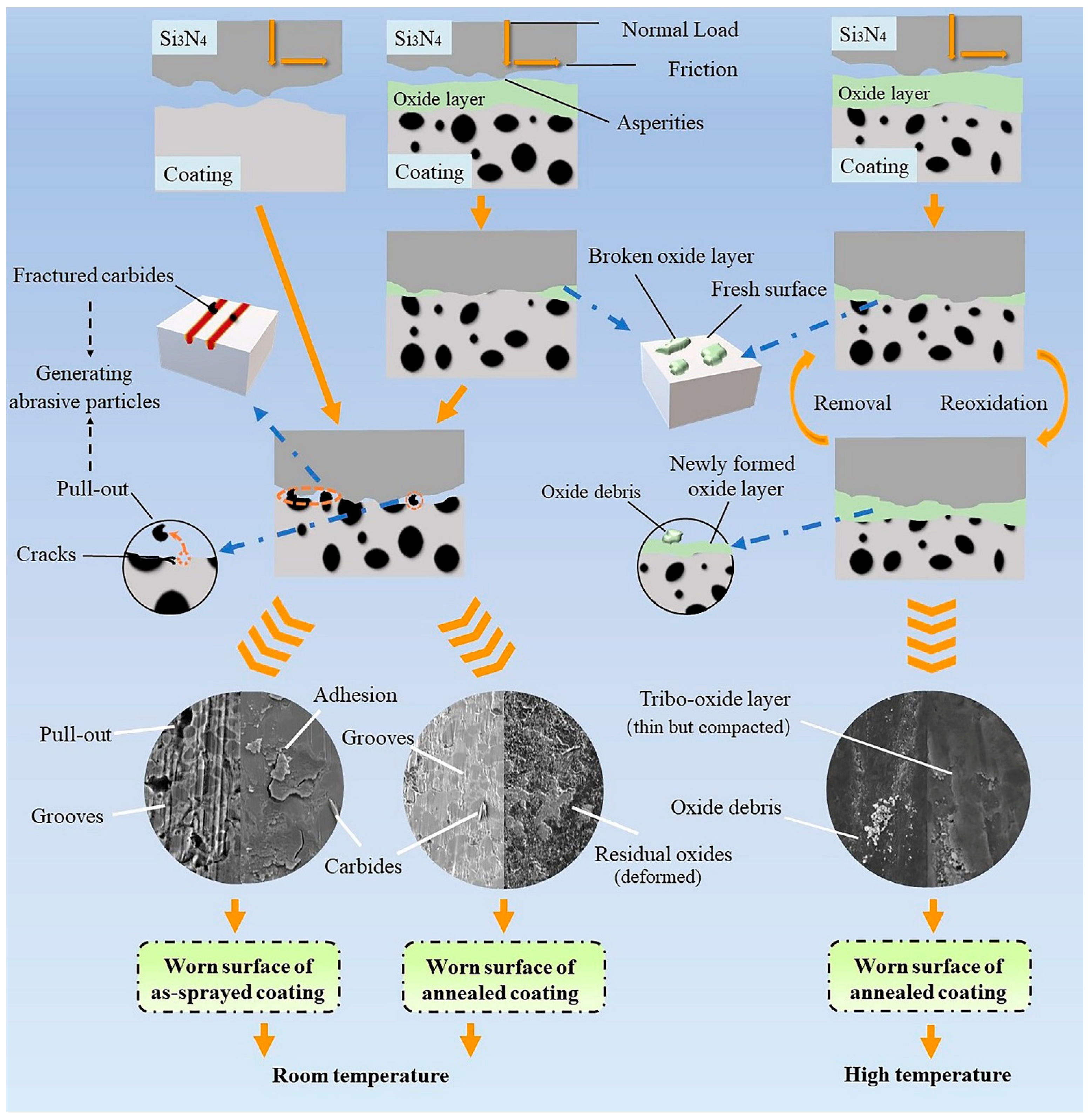
- The annealed coatings show better wear-resistance than the as-sprayed coating both at RT and at HT due to a combined effect from the formation of oxide-scale and the increased carbide fraction in the coatings after annealing.
- The wear behaviors of the annealed coatings obey different mechanisms at RT and HT. The RT wear behavior follows an abrasive wear mechanism, whereas the HT wear behavior is best described by an oxidative wear, in which formation of a tribo-oxide layer plays a critical role to reduce the friction coefficient and to protect the underlying coatings from damage.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Matthews, S.; James, B.; Hyland, M. The role of microstructure in the high temperature oxidation mechanism of Cr3C2-NiCr composite coating. Corros. Sci. 2009, 51, 1172–1180. [Google Scholar] [CrossRef]
- Matthews, S.; James, B.; Hyland, M. High temperature erosion−oxidation of Cr3C2-NiCr thermal spray coatings under simulated turbine conditions. Corros. Sci. 2013, 70, 203–211. [Google Scholar] [CrossRef]
- Ahuja, L.; Mudgal, D.; Singh, S.; Prakash, S. A comparative study to evaluate the corrosion performance of Zr incorporated Cr3C2-NiCr coating at 900 °C. Ceram. Int. 2018, 44, 6479–6492. [Google Scholar] [CrossRef]
- Matthews, S.; James, B.; Hyland, M. The effect of heat treatment of the oxidation mechanism of blended powder Cr3C2-NiCr coatings. J. Therm. Spray Technol. 2010, 19, 119–127. [Google Scholar] [CrossRef]
- Berger, L.-M. Application of hard metals as thermal spray coatings. Int. J. Refract. Met. Hard Mater. 2015, 49, 350–364. [Google Scholar] [CrossRef]
- Matthews, S.; Berger, L.-M. Long-term compositional/microstructural development of Cr3C2-NiCr coatings at 500 °C, 700 °C and 900 °C. Int. J. Refract. Met. Hard Mater. 2016, 59, 1–18. [Google Scholar] [CrossRef]
- Matthews, S.; Asadov, A.; Ruddell, S.; Berger, L.-M. Thermally induced metallurgical processes in Cr3C2-NiCr thermal spray coatings as a function of carbide dissolution. J. Alloys. Compd. 2017, 728, 445–463. [Google Scholar] [CrossRef]
- Matthews, S.; James, B.; Hyland, M. The role of microstructure in the high velocity erosion of Cr3C2-NiCr thermal spray coatings: Part 1-as-sprayed coatings. Surf. Coat. Technol. 2009, 203, 1086–1093. [Google Scholar] [CrossRef]
- Berger, L.-M.; Woydt, M.; Saaro, S. Comparison of self-mated hard metal coatings under dry sliding conditions up to 600 °C. Wear 2009, 266, 406–416. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, S.; Wang, S.; Wang, H.; Ramachandran, C.S. Wear, erosion and corrosion resistance of HVOF-sprayed WC and Cr3C2 based coatings for electrolytic hard chrome replacement. Int. J. Refract. Met. Hard Mater. 2019, 81, 242–252. [Google Scholar] [CrossRef]
- Bjordal, M.; Bardal, E.; Rogne, T.; Eggen, T.G. Combined erosion and corrosion of thermal sprayed WC and CrC coatings. Surf. Coat. Technol. 1995, 70, 215–220. [Google Scholar] [CrossRef]
- Wirojanupatump, S.; Shipway, P.H.; McCartney, D.G. The influence of HVOF powder feedstock characteristics on the abrasive wear behaviour of CrxCy-NiCr Coatings. Wear 2001, 249, 829–837. [Google Scholar] [CrossRef]
- Vashishtha, N.; Sapate, S.G.; Bagde, P.; Rathod, A.B. Effect of heat treatment on friction and abrasive wear behaviour of WC-12Co and Cr3C2-25NiCr coatings. Tribo. Int. 2018, 118, 381–399. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Miguel, J.M.; Vizcaíno, S.; Lorenzana, C.; Delgado, J.; Sánchez, J. Role of heat treatments in the improvement of the sliding wear properties of Cr3C2-NiCr coatings. Surf. Coat. Technol. 2002, 157, 207–213. [Google Scholar] [CrossRef]
- Janka, L.; Norpoth, J.; Trache, R.; Berger, L.-M. Influence of heat treatment on the abrasive wear resistance of a Cr3C2-NiCr coating deposited by an ethane-fuelled HVOF spray process. Surf. Coat. Technol. 2016, 291, 444–451. [Google Scholar] [CrossRef]
- Gariboldi, E.; Rovatti, L.; Lecis, N.; Mondora, L.; Mondora, G.A. Tribological and mechanical behaviour of Cr3C2-NiCr thermally sprayed coatings after prolonged aging. Surf. Coat. Technol. 2016, 305, 83–92. [Google Scholar] [CrossRef]
- Janka, L.; Norpoth, J.; Trache, R.; Thiele, S.; Berger, L.-M. HVOF- and HVAF- sprayed Cr3C2-NiCr coatings deposited from feedstock powders of spherical morphology: Microstructure formation and high-stress abrasive wear resistance up to 800 °C. J. Therm. Spray Technol. 2017, 26, 1720–1731. [Google Scholar] [CrossRef]
- Kamali, R.; Binesh, A.R. The importance of sensitive parameters effect on the combustion in a high velocity oxygen-fuel spray system. Int. J. Heat Mass Transf. 2009, 36, 978–983. [Google Scholar] [CrossRef]
- Guo, X.; Planche, M.P.; Chen, J.; Liao, H. Relationships between in-flight particle characteristics and properties of HVOF sprayed WC-CoCr coatings. J. Mater. Process Technol. 2014, 214, 456–461. [Google Scholar] [CrossRef]
- Souza, V.A.D.; Neville, A. Mechanisms and kinetics of WC-CoCr high velocity oxy-fuel thermal spray coating degradation in corrosive environments. J. Therm. Spray Technol. 2006, 15, 106–117. [Google Scholar] [CrossRef]
- Matthews, S.; James, B. Review of thermal spray coating applications in steel industry: Part 2-zinc pot hardware in the continuous galvanizing line. J. Therm. Spray Technol. 2010, 19, 1277–1286. [Google Scholar] [CrossRef]
- Varis, T.; Suhonen, T.; Ghabchi, A.; Valarezo, A.; Sampath, S.; Liu, X.; Hannula, S.-P. Formation mechanisms, structure, and properties of HVOF-sprayed WC-CoCr coatings: An approach toward process maps. J. Therm. Spray Technol. 2014, 23, 1009–1018. [Google Scholar] [CrossRef]
- Bolelli, G.; Berger, L.-M.; Börner, T.; Koivuluoto, H.; Lusvarghi, L.; Lyphout, C.; Markocsan, N.; Matikainen, V.; Nylén, P.; Sassatelli, P.; et al. Tribology of HVOF- and HVAF-sprayed WC-10Co4Cr hardmetal coatings: A comparative assessment. Surf. Coat. Technol. 2015, 265, 125–144. [Google Scholar] [CrossRef]
- Ang, A.S.M.; Howse, H.; Wade, S.A.; Berndt, C.C. Development of processing windows for HVOF carbide-based coatings. J. Therm. Spray Technol. 2016, 25, 28–35. [Google Scholar] [CrossRef]
- Li, C.J.; Ji, G.C.; Wang, Y.Y.; Sonoya, K. Dominant effect of carbide rebounding on the carbon loss during high velocity oxy-fuel spraying of Cr3C2-NiCr. Thin Solid Films 2002, 419, 137–143. [Google Scholar] [CrossRef]
- Hou, G.; An, Y.; Zhao, X.; Zhou, H.; Chen, J. Effect of alumina dispersion on oxidation behavior as well as friction and wear behavior of HVOF-sprayed CoCrAlYTaCSi coating at elevated temperature up to 1000 °C. Acta Mater. 2015, 95, 164–175. [Google Scholar] [CrossRef]
- Ding, Y.; Hussain, T.; McCartney, D.G. High-temperature oxidation of HVOF thermally sprayed NiCr-Cr3C2 coatings: Microstructure and kinetics. J. Mater. Sci. 2015, 50, 6808–6821. [Google Scholar] [CrossRef]
- Mougin, J.; Le Bihan, T.; Lucazeau, G. High-pressure study of Cr2O3 obtained by high-temperature oxidation by X-ray diffraction and Raman spectroscopy. J. Phys. Chem. Solids 2001, 62, 553–563. [Google Scholar] [CrossRef]
- Shim, S.H.; Duffy, T.S.; Jeanloz, R.; Yoo, C.S.; Iota, V. Raman spectroscopy and X-ray diffraction of phase transitions in Cr2O3 to 61 GPa. Phys. Rev. B 2004, 69, 1124–1133. [Google Scholar] [CrossRef]
- Nakamura, K.; Fujitsuka, M.; Kitajima, M. Disorder-induced line broadening in first order Raman scattering from graphite. Phys. Rev. B 1990, 41, 12260–12263. [Google Scholar] [CrossRef]
- Matthews, S.; Berger, L.-M. Inter-diffusion between thermally sprayed Cr3C2-NiCr coatings and an Alloy 625 substrate during long-term exposure at 500°C, 700°C and 900°C. J. Alloys. Compd. 2019, 770, 1078–1099. [Google Scholar] [CrossRef]
- Wang, H.; Yan, X.; Zhang, H.; Gee, M.; Zhao, C.; Liu, X.; Song, X. Oxidation-dominated wear behaviors of carbide-based cermets: A comparison between WC-WB-Co and Cr3C2-NiCr coatings. Ceram. Int. 2019, 45, 21293–21307. [Google Scholar] [CrossRef]
- Vashishtha, N.; Sapate, S.G.; Sahariah, B.J.; Bagde, P. Microstructural characterization and wear behaviour of high velocity oxy-fuel sprayed Cr3C2-25NiCr coating. Mater. Today Proc. 2018, 5, 17686–17693. [Google Scholar] [CrossRef]
- Matikainen, V.; Bolelli, G.; Koivuluoto, H.; Sassatelli, P.; Lusvarghi, L.; Vuoristo, P. Sliding wear behaviour of HVOF and HVAF sprayed Cr3C2-based coatings. Wear 2017, 388–389, 57–71. [Google Scholar] [CrossRef]
- Liu, X.B.; Liu, H.Q.; Liu, Y.F.; He, X.M.; Sun, C.F.; Wang, M.D.; Yang, H.B.; Qi, L.H. Effects of temperature and normal load on tribological behavior of nickel-based high temperature self-lubricating wear-resistant composite coating. Compos. Part B 2013, 53, 347–354. [Google Scholar] [CrossRef]
- Bolelli, G.; Berger, L.-M.; Börner, T.; Koivuluoto, H.; Matikainen, V.; Lusvarghi, L.; Lyphout, C.; Markocsan, N.; Nylén, P.; Sassatelli, P.; et al. Sliding and abrasive wear behaviour of HVOF-and HVAF-sprayed Cr3C2-NiCr hardmetal coatings. Wear 2016, 358, 32–50. [Google Scholar] [CrossRef]
- Hosterman, B.D.; Farley, J.W.; Johnson, A.L. Spectroscopic study of the vibrational modes of magnesium nickel chromite MgxNi1−xCr2O4. J. Phys. Chem. Solids 2013, 74, 985–990. [Google Scholar] [CrossRef]
- Lin, L.; Li, G.L.; Wang, H.D.; Kang, J.J.; Xu, Z.L.; Wang, H.J. Structure and wear behavior of NiCr-Cr3C2 coatings sprayed by supersonic plasma spraying and high velocity oxy-fuel technologies. Appl. Surf. Sci. 2015, 356, 383–390. [Google Scholar] [CrossRef]
- Hutchings, I.; Shipway, P. Wear by hard particles. In Tribology, 2nd ed.; Butterworth-Heinemann Elsevier Ltd.: Oxford, UK, 2017; pp. 165–166. [Google Scholar]
- Velikanova, T.Y.; Bondar, A.A.; Grytsiv, A.V. The chromium-nickel-carbon (Cr–Ni–C) phase diagram. J. Phase Equilib. 1999, 20, 125–146. [Google Scholar] [CrossRef]
- Poirier, D.; Legoux, J.-G.; Lima, R. Engineering HVOF-sprayed Cr3C2-NiCr coatings: The effect of particle morphology and spraying parameters on the microstructure, properties and high temperature wear performance. J. Therm. Spray Technol. 2013, 22, 280–289. [Google Scholar] [CrossRef]
- Murthy, J.K.N.; Satya Prasad, K.; Gopinath, K.; Venkataraman, B. Characterisation of HVOF sprayed Cr3C2-50(Ni20Cr) coating and the influence of binder properties on solid particle erosion behaviour. Surf. Coat. Technol. 2010, 204, 3975–3985. [Google Scholar] [CrossRef]
- Matthews, S. Development of high carbide dissolution/low carbon loss Cr3C2-NiCr coatings by shrouded plasma spraying. Surf. Coat. Technol. 2014, 258, 886–900. [Google Scholar] [CrossRef]
- Matikainen, V.; Bolelli, G.; Koivuluoto, H.; Honkanen, M.; Vippola, M.; Lusvarghi, L.; Vuoristo, P. A study of Cr3C2-based HVOF- and HVAF-sprayed coatings: Microstructure and carbide retention. J. Therm. Spray Technol. 2017, 26, 1239–1256. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Huang, J.; Ding, C. Mechanical and tribological properties of plasma-sprayed Cr3C2-NiCr, WC-Co, and Cr2O3 coatings. J. Therm. Spray Technol. 1998, 7, 242–246. [Google Scholar]
- Nicholls, J.R.; Wellman, R.G. Oxidative wear. In Handbook of Lubrication and Tribology: Theory and Design; Bruce, R.W., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 1–24. [Google Scholar]
- Liu, Q.; Bai, Y.; Wang, H.D.; Ma, G.Z.; Liu, M.; Chu, C.Y. Microstructural evolution of carbides and its effect on tribological properties of SAPS or HVOF sprayed NiCr-Cr3C2 coatings. J. Alloys Compd. 2019, 803, 730–741. [Google Scholar] [CrossRef]
- Zafar, S.; Sharma, A.K. Microstructure and wear performance of heat-treated WC-12Co microwave clad. Vacuum 2016, 131, 213–222. [Google Scholar] [CrossRef]




| Parameters | Value |
|---|---|
| Fuel | Propane |
| Fuel pressure (kPa) | 579.2 |
| Air pressure (kPa) | 606.8 |
| H2 flow rate (Stard Liter Per Minute, SLPM) | 22 |
| Carrier gas flow rate (N2, SLPM) | 23 |
| Surface speed (mm·s−1) | 2000 |
| Spray distance (mm) | 195 |
| Powder feed rate (g·min−1) | 100–150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhao, X.; Yang, F. Room-Temperature and High-Temperature Wear Behaviors of As-Sprayed and Annealed Cr3C2-25NiCr Coatings Prepared by High Velocity Air-Fuel Spraying. Coatings 2020, 10, 1090. https://doi.org/10.3390/coatings10111090
Liu X, Zhao X, Yang F. Room-Temperature and High-Temperature Wear Behaviors of As-Sprayed and Annealed Cr3C2-25NiCr Coatings Prepared by High Velocity Air-Fuel Spraying. Coatings. 2020; 10(11):1090. https://doi.org/10.3390/coatings10111090
Chicago/Turabian StyleLiu, Xuanzhen, Xiaofeng Zhao, and Fan Yang. 2020. "Room-Temperature and High-Temperature Wear Behaviors of As-Sprayed and Annealed Cr3C2-25NiCr Coatings Prepared by High Velocity Air-Fuel Spraying" Coatings 10, no. 11: 1090. https://doi.org/10.3390/coatings10111090
APA StyleLiu, X., Zhao, X., & Yang, F. (2020). Room-Temperature and High-Temperature Wear Behaviors of As-Sprayed and Annealed Cr3C2-25NiCr Coatings Prepared by High Velocity Air-Fuel Spraying. Coatings, 10(11), 1090. https://doi.org/10.3390/coatings10111090







