The Effects of Bone Morphogenetic Protein-4 on Cellular Viability, Osteogenic Potential, and Global Gene Expression on Gingiva-Derived Stem Cell Spheroids
Abstract
1. Introduction
2. Materials and Methods
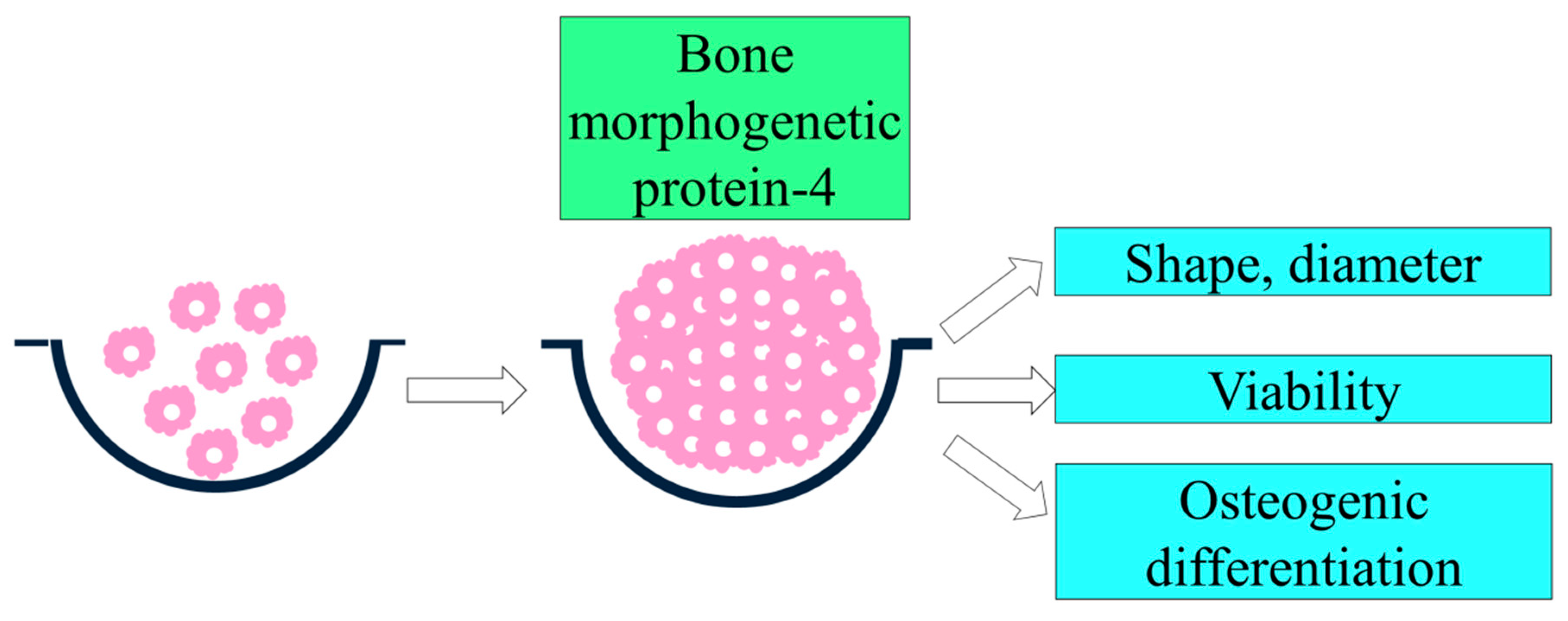
2.1. Formation of Cell Spheroids with Gingiva-Derived Stem Cells
2.2. Evaluation of Cellular Viability
2.3. Evaluation of Osteogenic Differentiation Using Alkaline Phosphatase Activity Assays
2.4. Sequencing of mRNA, Gene Ontology, and Pathway Analysis
2.5. Statistical Analysis
3. Results
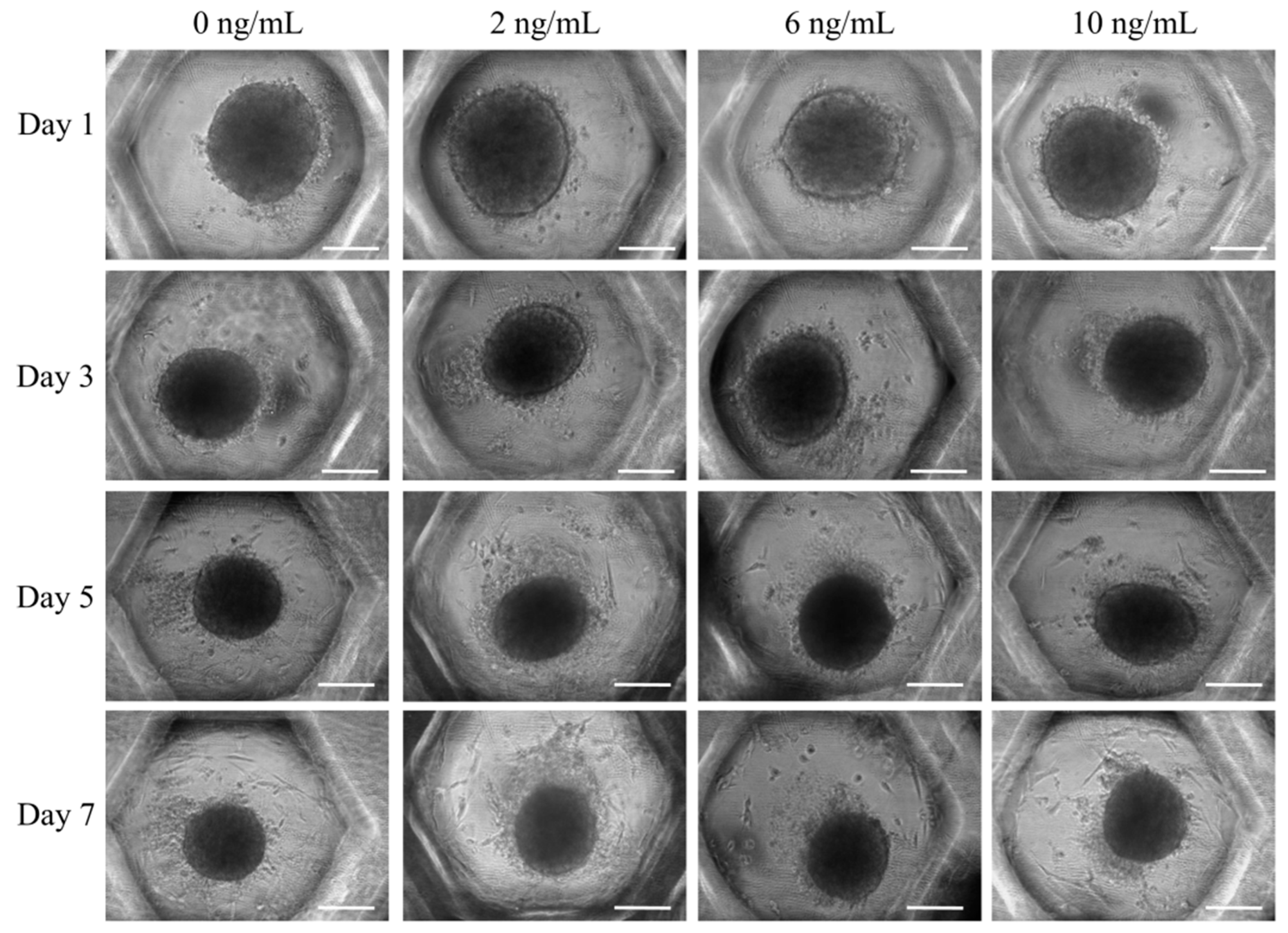
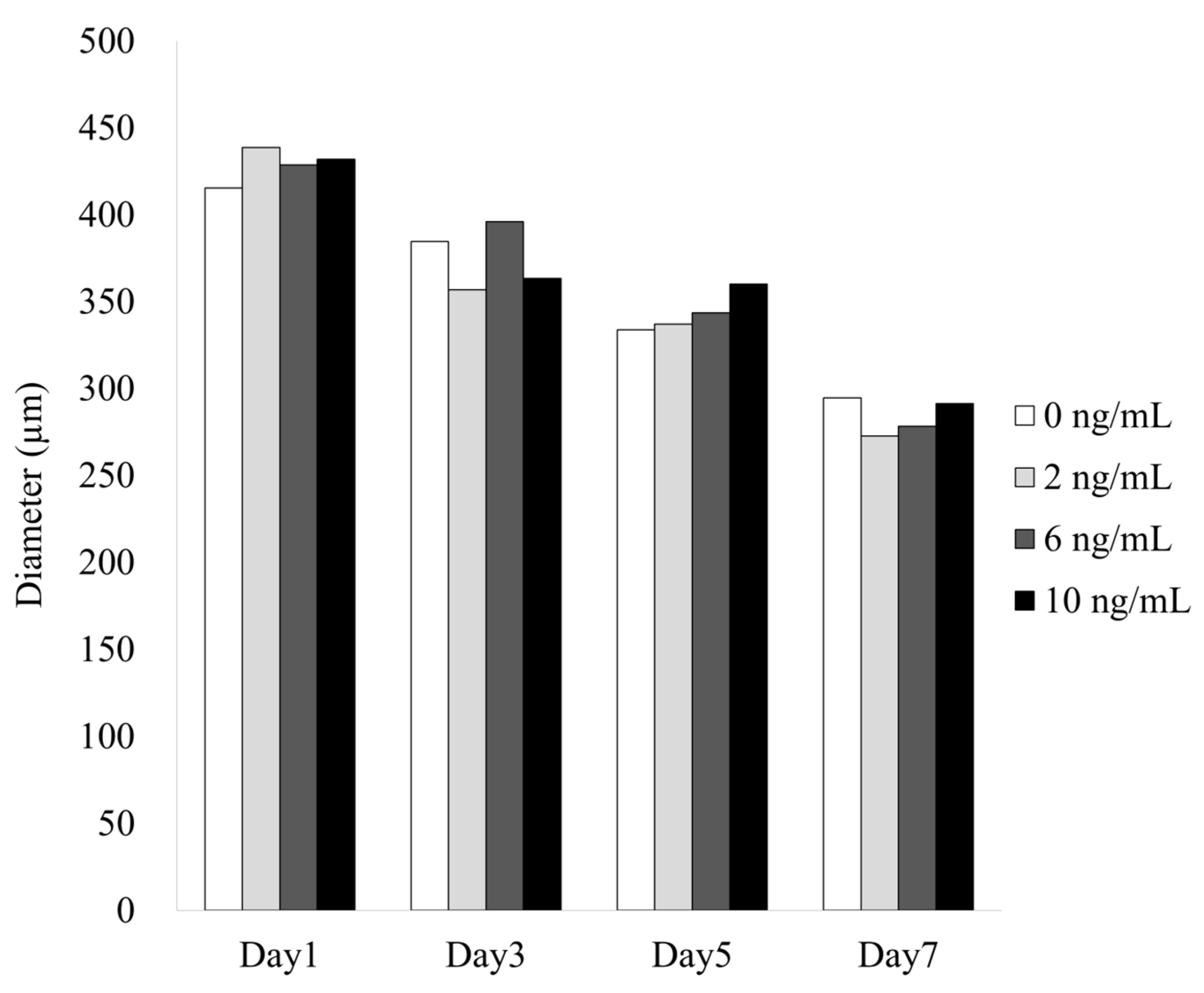
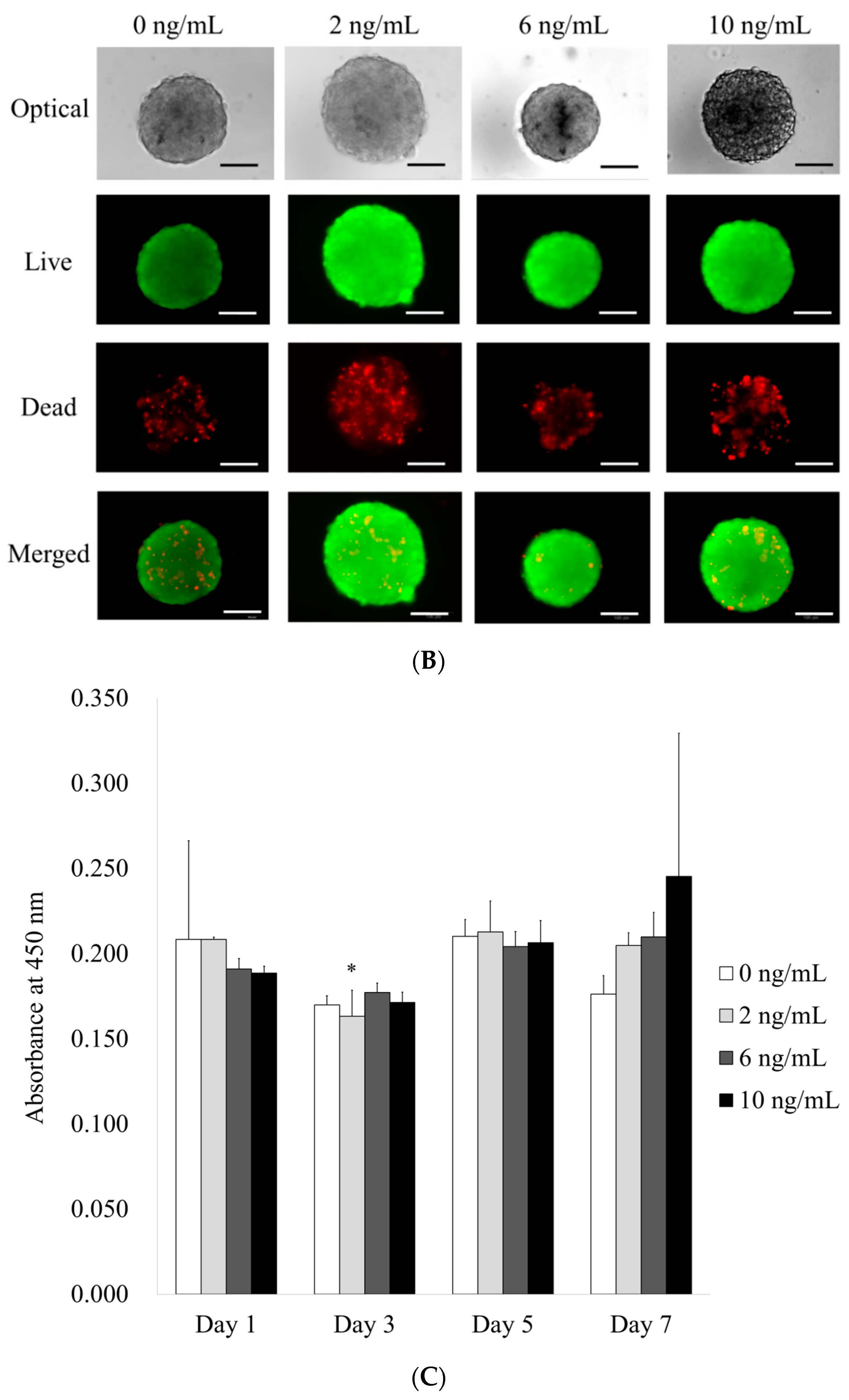
3.1. Formation of Cell Spheroids with Human Gingiva-Derived Stem Cells
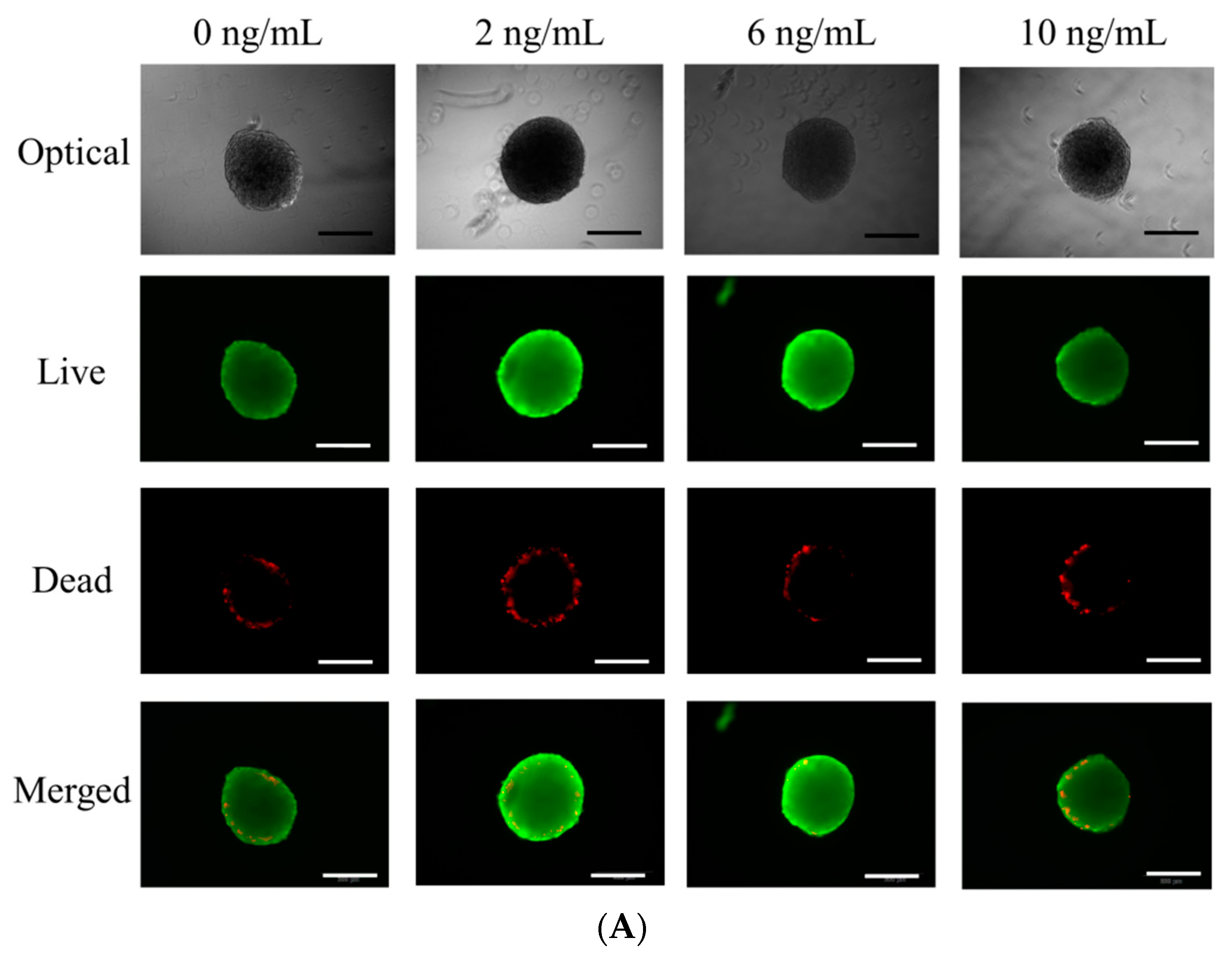
3.2. Determination of Cellular Viability
3.3. Evaluation of Alkaline Phosphatase Activity Assay with the Addition of BMP-4
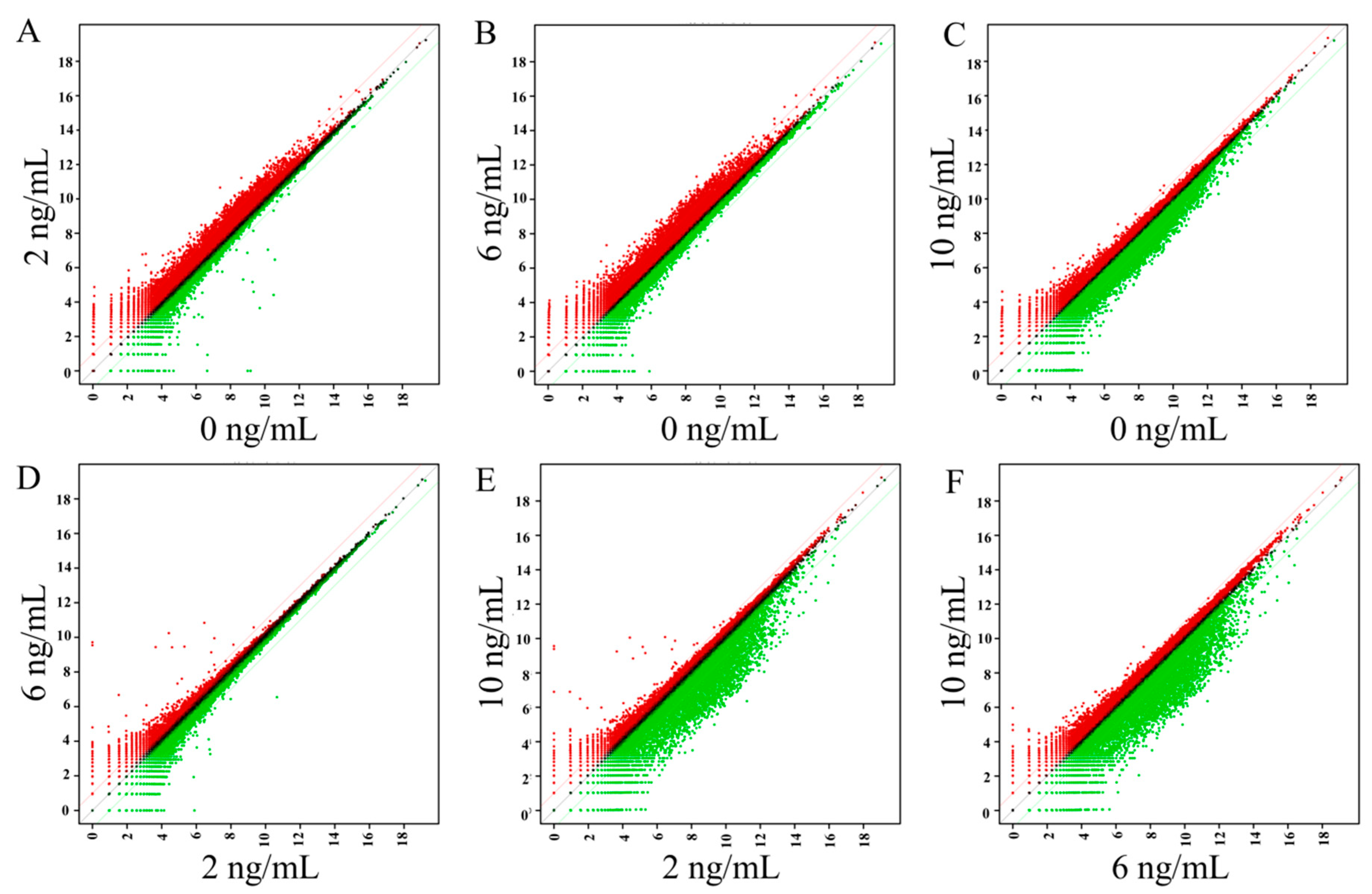
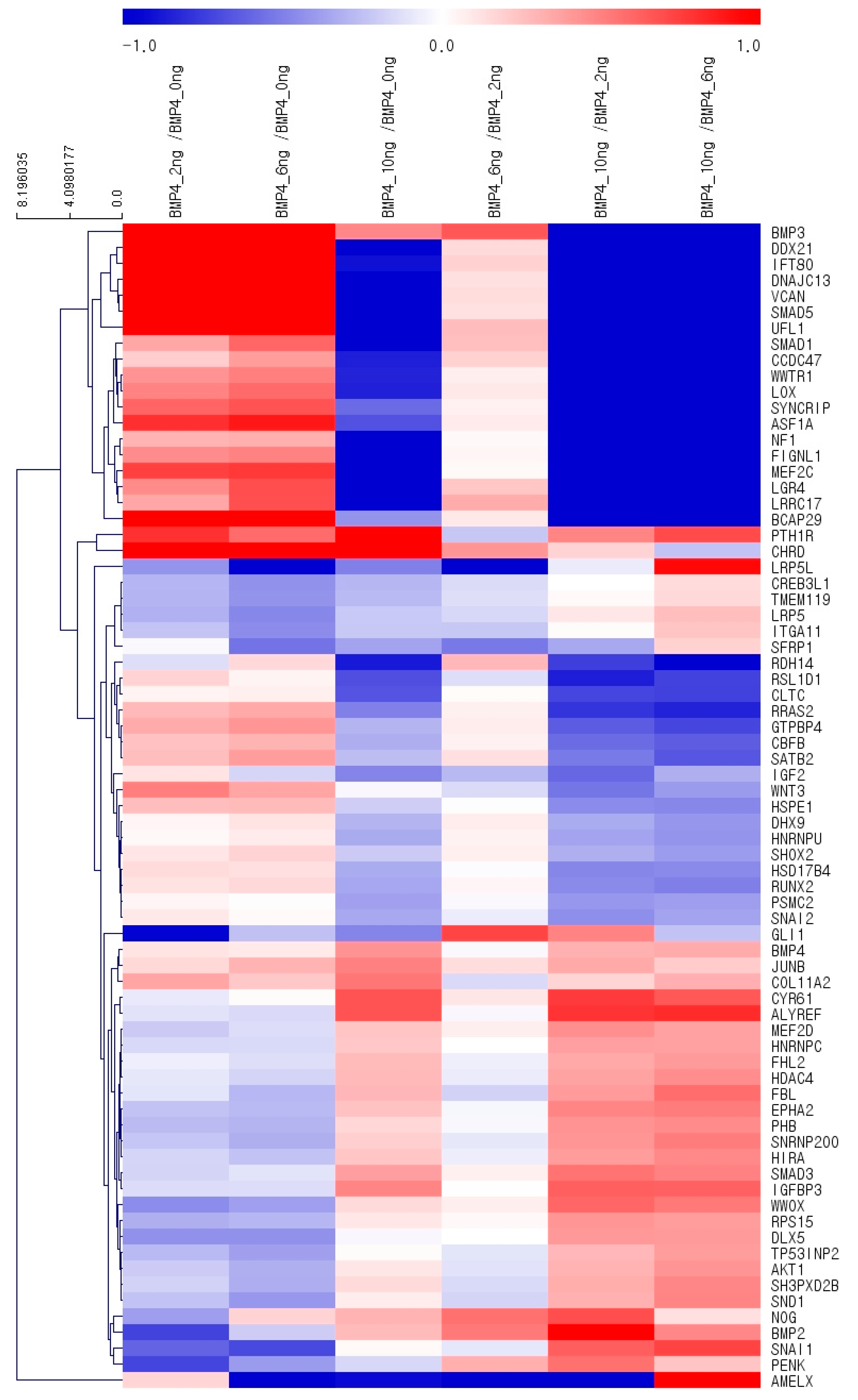
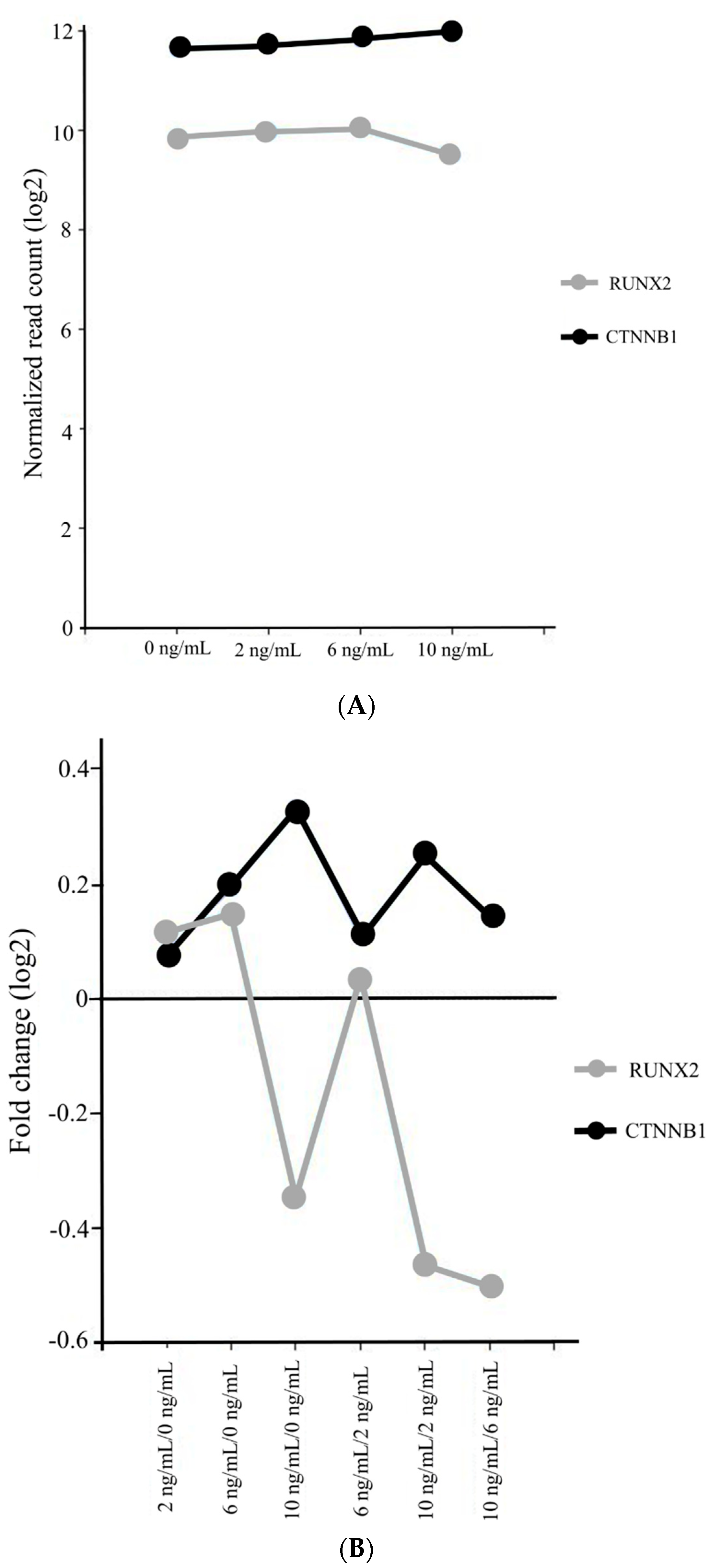
3.4. Gene Ontology
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Castro-Manrreza, M.E.; Montesinos, J.J. Immunoregulation by mesenchymal stem cells: Biological aspects and clinical applications. J. Immunol. Res. 2015, 2015, 394917. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-I.; Yeo, S.-I.; Kim, B.-B.; Ko, Y.; Park, J.-B. Formation of size-controllable spheroids using gingiva-derived stem cells and concave microwells: Morphology and viability tests. Biomed. Rep. 2016, 4, 97–101. [Google Scholar] [CrossRef]
- Lee, S.-I.; Ko, Y.; Park, J.-B. Evaluation of the shape, viability, stemness and osteogenic differentiation of cell spheroids formed from human gingiva-derived stem cells and osteoprecursor cells. Exp. Ther. Med. 2017, 13, 3467–3473. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M.; Driskell, R.R. The therapeutic potential of stem cells. Philos Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Montanez-Sauri, S.I.; Beebe, D.J.; Sung, K.E. Microscale screening systems for 3D cellular microenvironments: Platforms, advances, and challenges. Cell Mol. Life Sci. 2015, 72, 237–249. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.P.; Dean, D.M.; Man, A.J.; Youssef, J.; Ho, D.N.; Rago, A.P.; Lech, M.P.; Morgan, J.R. Scaffold-free three-dimensional cell culture utilizing micromolded nonadhesive hydrogels. Biotechniques 2007, 43, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, Y.; Syková, E.; Kubinová, Š. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res. Ther. 2017, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Han, X.; Wang, S.; Zhang, Y.; Dai, X.; Liu, B.; Liu, L.; Zhao, X. Cell sheets of co-cultured BMP-2-modified bone marrow stromal cells and endothelial progenitor cells accelerate bone regeneration in vitro. Exp. Ther. Med. 2019, 18, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, B.; Moseychuk, O.; Saldanha, S.; King, D.; Julian, J.; Nohe, A. Bone Morphogenetic Proteins: A critical review. Cell Signal 2011, 23, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-β/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef] [PubMed]
- Modica, S.; Wolfrum, C. The dual role of BMP4 in adipogenesis and metabolism. Adipocyte 2017, 6, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wijesekera, O.; Salas, S.J.; Wang, J.Y.; Zhu, M.; Aprhys, C.; Chaichana, K.L.; Chesler, D.A.; Zhang, H.; Smith, C.L.; et al. Mesenchymal stem cells from human fat engineered to secrete BMP4 are nononcogenic, suppress brain cancer, and prolong survival. Clin. Cancer Res. 2014, 20, 2375–2387. [Google Scholar] [CrossRef]
- Kang, Q.; Sun, M.H.; Cheng, H.; Peng, Y.; Montag, A.G.; Deyrup, A.T.; Jiang, W.; Luu, H.H.; Luo, J.; Szatkowski, J.P.; et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004, 11, 1312–1320. [Google Scholar] [CrossRef]
- Gluhak-Heinrich, J.; Guo, D.; Yang, W.; Harris, M.A.; Lichtler, A.; Kream, B.; Zhang, J.; Feng, J.Q.; Smith, L.C.; Dechow, P.; et al. New roles and mechanism of action of BMP4 in postnatal tooth cytodifferentiation. Bone 2010, 46, 1533–1545. [Google Scholar] [CrossRef]
- Stadlinger, B.; Pilling, E.; Mai, R.; Bierbaum, S.; Berhardt, R.; Scharnweber, D.; Eckelt, U. Effect of biological implant surface coatings on bone formation, applying collagen, proteoglycans, glycosaminoglycans and growth factors. J. Mater. Sci. Mater. Med. 2008, 19, 1043–1049. [Google Scholar] [CrossRef]
- Son, J.; Tae, J.Y.; Min, S.K.; Ko, Y.; Park, J.B. Fibroblast growth factor-4 maintains cellular viability while enhancing osteogenic differentiation of stem cell spheroids in part by regulating RUNX2 and BGLAP expression. Exp. Ther. Med. 2020, 20, 2013–2020. [Google Scholar] [CrossRef]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, 80. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Collins, J.R.; Alvord, W.G.; Roayaei, J.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Min, S.K.; Song, Y.; Park, Y.H.; Park, J.B. Bone morphogenetic protein-7 upregulates genes associated with osteoblast differentiation, including collagen I, Sp7 and IBSP in gingiva-derived stem cells. Exp. Ther. Med. 2019, 18, 2867–2876. [Google Scholar] [CrossRef] [PubMed]
- Myllylä, R.M.; Haapasaari, K.M.; Lehenkari, P.; Tuukkanen, J. Bone morphogenetic proteins 4 and 2/7 induce osteogenic differentiation of mouse skin derived fibroblast and dermal papilla cells. Cell Tissue Res. 2014, 355, 463–470. [Google Scholar] [CrossRef]
- Ferreira, C.L.; Abreu, F.A.; Silva, G.A.; Silveira, F.F.; Barreto, L.B.; Paulino Tde, P.; Miziara, M.N.; Alves, J.B. TGF-β1 and BMP-4 carried by liposomes enhance the healing process in alveolar bone. Arch. Oral Biol. 2013, 58, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas-Martínez, L.; Galindo-Moreno, P.; Ávila-Ortiz, G.; Ortega-Oller, I.; Monje, A.; Hernández-Cortés, P.; Aguilar, D.; O’Valle, F. Significance of the immunohistochemical expression of bone morphogenetic protein-4 in bone maturation after maxillary sinus grafting in humans. Clin. Implant Dent. Relat. Res. 2016, 18, 717–724. [Google Scholar] [CrossRef]
- Liang, C.; Sun, R.; Xu, Y.; Geng, W.; Li, J. Effect of the abnormal expression of BMP-4 in the blood of diabetic patients on the osteogenic differentiation potential of alveolar BMSCs and the rescue effect of metformin: A bioinformatics-based study. Biomed. Res. Int. 2020, 2020, 7626215. [Google Scholar] [CrossRef]
- Tang, C.H.; Yang, R.S.; Liou, H.C.; Fu, W.M. Enhancement of fibronectin synthesis and fibrillogenesis by BMP-4 in cultured rat osteoblast. J. Bone Miner Res. 2003, 18, 502–511. [Google Scholar] [CrossRef]
- Kondo, A.; Otsuka, T.; Kuroyanagi, G.; Yamamoto, N.; Matsushima-Nishiwaki, R.; Mizutani, J.; Kozawa, O.; Tokuda, H. Resveratrol inhibits BMP-4-stimulated VEGF synthesis in osteoblasts: Suppression of S6 kinase. Int. J. Mol. Med. 2014, 33, 1013–1018. [Google Scholar] [CrossRef]
- Fujita, K.; Otsuka, T.; Yamamoto, N.; Kainuma, S.; Ohguchi, R.; Kawabata, T.; Sakai, G.; Kuroyanagi, G.; Matsushima-Nishiwaki, R.; Kozawa, O.; et al. (−)-Epigallocatechin gallate but not chlorogenic acid upregulates osteoprotegerin synthesis through regulation of bone morphogenetic protein-4 in osteoblasts. Exp. Ther. Med. 2017, 14, 417–423. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, J.W.; Han, Y.; Lee, K.Y. Synergistic stimulating effect of 2-hydroxymelatonin and BMP-4 on osteogenic differentiation in vitro. Biochem. Biophys. Res. Commun. 2020, 527, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Lavery, K.; Swain, P.; Falb, D.; Alaoui-Ismaili, M.H. BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J. Biol. Chem. 2008, 283, 20948–20958. [Google Scholar] [CrossRef]
- Chang, S.F.; Chang, T.K.; Peng, H.H.; Yeh, Y.T.; Lee, D.Y.; Yeh, C.R.; Zhou, J.; Cheng, C.K.; Chang, C.A.; Chiu, J.J. BMP-4 induction of arrest and differentiation of osteoblast-like cells via p21 CIP1 and p27 KIP1 regulation. Mol. Endocrinol. 2009, 23, 1827–1838. [Google Scholar] [CrossRef] [PubMed]
- van der Sanden, B.; Dhobb, M.; Berger, F.; Wion, D. Optimizing stem cell culture. J. Cell Biochem. 2010, 111, 801–807. [Google Scholar] [CrossRef]
- Kang, S.H.; Park, J.B.; Kim, I.; Lee, W.; Kim, H. Assessment of stem cell viability in the initial healing period in rabbits with a cranial bone defect according to the type and form of scaffold. J. Periodontal Implant Sci. 2019, 49, 258–267. [Google Scholar] [CrossRef]
- Bates, C.; Marino, V.; Fazzalari, N.L.; Bartold, P.M. Soft tissue attachment to titanium implants coated with growth factors. Clin. Implant Dent. Relat. Res. 2013, 15, 53–63. [Google Scholar] [CrossRef]
- Wang, J.; Guo, J.; Liu, J.; Wei, L.; Wu, G. BMP-functionalised coatings to promote osteogenesis for orthopaedic implants. Int. J. Mol. Sci. 2014, 15, 10150–10168. [Google Scholar] [CrossRef]
- Ruan, Y.; Kato, H.; Taguchi, Y.; Yamauchi, N.; Umeda, M. Irradiation by high-intensity red light-emitting diode enhances human bone marrow mesenchymal stem cells osteogenic differentiation and mineralization through Wnt/β-catenin signaling pathway. Lasers Med. Sci. 2020, 25, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; He, H.; Cao, Z.; Fang, Y.; Du, M.; Liu, Z. Regulatory effects of bone morphogenetic protein-4 on tumour necrosis factor-α-suppressed Runx2 and osteoprotegerin expression in cementoblasts. Cell Prolif. 2017, 50, e12344. [Google Scholar] [CrossRef]
- Ayala-Peña, V.B.; Scolaro, L.A.; Santillán, G.E. ATP and UTP stimulate bone morphogenetic protein-2,-4 and -5 gene expression and mineralization by rat primary osteoblasts involving PI3K/AKT pathway. Exp. Cell Res. 2013, 319, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Vega, O.A.; Lucero, C.M.J.; Araya, H.F.; Jerez, S.; Tapia, J.C.; Antonelli, M.; Salazar-Onfray, F.; Las Heras, F.; Thaler, R.; Riester, S.M.; et al. Wnt/β-Catenin Signaling Activates Expression of the Bone-Related Transcription Factor RUNX2 in Select Human Osteosarcoma Cell Types. J. Cell Biochem. 2017, 118, 3662–3674. [Google Scholar] [CrossRef] [PubMed]
- Tae, J.Y.; Lee, H.; Lee, H.; Ko, Y.; Park, J.B. Osteogenic potential of cell spheroids composed of varying ratios of gingiva-derived and bone marrow stem cells using concave microwells. Exp. Ther. Med. 2018, 16, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.; Mirzayans, F.; Berry, F. Foxc1 Expression in Early Osteogenic Differentiation Is Regulated by BMP4-SMAD Activity. J. Cell Biochem. 2016, 117, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Rumiński, S.; Kalaszczyńska, I.; Długosz, A.; Lewandowska-Szumieł, M. Osteogenic differentiation of human adipose-derived stem cells in 3D conditions—Comparison of spheroids and polystyrene scaffolds. Eur. Cell Mater. 2019, 37, 382–401. [Google Scholar] [CrossRef]
- Zhang, J.; He, X.; Chen, X.; Wu, Y.; Dong, L.; Cheng, K.; Lin, J.; Wang, H.; Weng, W. Enhancing osteogenic differentiation of BMSCs on high magnetoelectric response films. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 110970. [Google Scholar] [CrossRef]
- Kim, B.B.; Kim, M.; Park, Y.H.; Ko, Y.; Park, J.B. Short-term application of dexamethasone on stem cells derived from human gingiva reduces the expression of RUNX2 and β-catenin. J. Int. Med. Res. 2017, 45, 993–1006. [Google Scholar] [CrossRef]
- Kramer, I.; Halleux, C.; Keller, H.; Pegurri, M.; Gooi, J.H.; Weber, P.B.; Feng, J.Q.; Bonewald, L.F.; Kneissel, M. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol. Cell Biol. 2010, 30, 3071–3085. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, Y.; Yang, Y.; Liu, Y.; Guo, N.; Wang, L.; Huang, W.; Wu, L.; Sun, D.; Gu, W. β-catenin mediates fluoride-induced aberrant osteoblasts activity and osteogenesis. Environ. Pollut. 2020, 265, 114734. [Google Scholar] [CrossRef]
- Liu, D.; Pavathuparambil Abdul Manaph, N.; Al-Hawwas, M.; Bobrovskaya, L.; Xiong, L.L.; Zhou, X.F. Coating Materials for Neural Stem/Progenitor Cell Culture and Differentiation. Stem Cells Dev. 2020, 29, 463–474. [Google Scholar] [CrossRef]
- Ahmad, T.; Byun, H.; Lee, J.; Madhurakat Perikamana, S.K.; Shin, Y.M.; Kim, E.M.; Shin, H. Stem cell spheroids incorporating fibers coated with adenosine and polydopamine as a modular building blocks for bone tissue engineering. Biomaterials 2020, 230, 119652. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tae, J.-Y.; Park, Y.-H.; Ko, Y.; Park, J.-B. The Effects of Bone Morphogenetic Protein-4 on Cellular Viability, Osteogenic Potential, and Global Gene Expression on Gingiva-Derived Stem Cell Spheroids. Coatings 2020, 10, 1055. https://doi.org/10.3390/coatings10111055
Tae J-Y, Park Y-H, Ko Y, Park J-B. The Effects of Bone Morphogenetic Protein-4 on Cellular Viability, Osteogenic Potential, and Global Gene Expression on Gingiva-Derived Stem Cell Spheroids. Coatings. 2020; 10(11):1055. https://doi.org/10.3390/coatings10111055
Chicago/Turabian StyleTae, Jae-Yong, Yoon-Hee Park, Youngkyung Ko, and Jun-Beom Park. 2020. "The Effects of Bone Morphogenetic Protein-4 on Cellular Viability, Osteogenic Potential, and Global Gene Expression on Gingiva-Derived Stem Cell Spheroids" Coatings 10, no. 11: 1055. https://doi.org/10.3390/coatings10111055
APA StyleTae, J.-Y., Park, Y.-H., Ko, Y., & Park, J.-B. (2020). The Effects of Bone Morphogenetic Protein-4 on Cellular Viability, Osteogenic Potential, and Global Gene Expression on Gingiva-Derived Stem Cell Spheroids. Coatings, 10(11), 1055. https://doi.org/10.3390/coatings10111055





