The Photocatalytical Properties of RGO/TiO2 Coated Fabrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Physicochemical Properties of Coated Fabrics
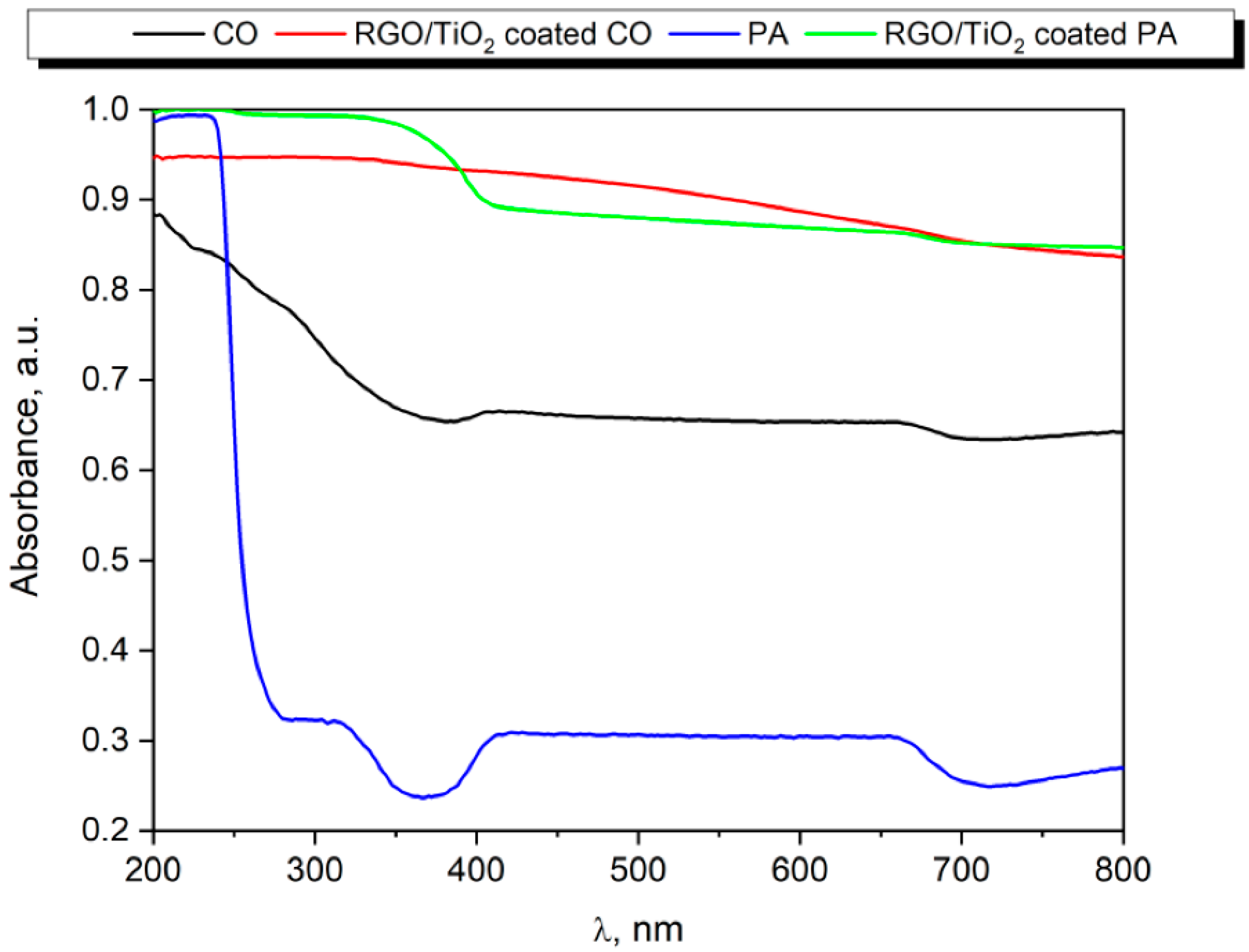
3.1.1. UV–VIS Analysis
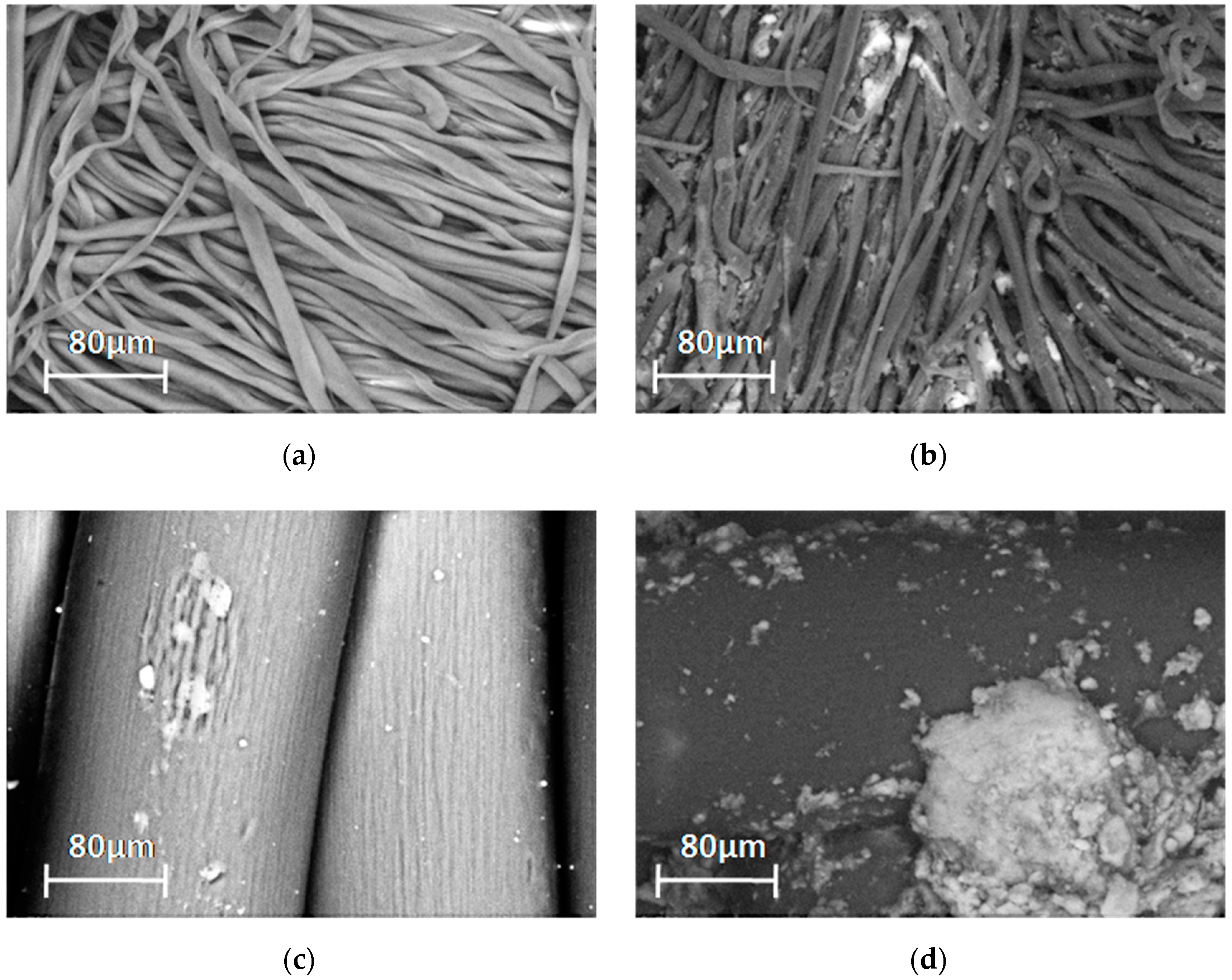
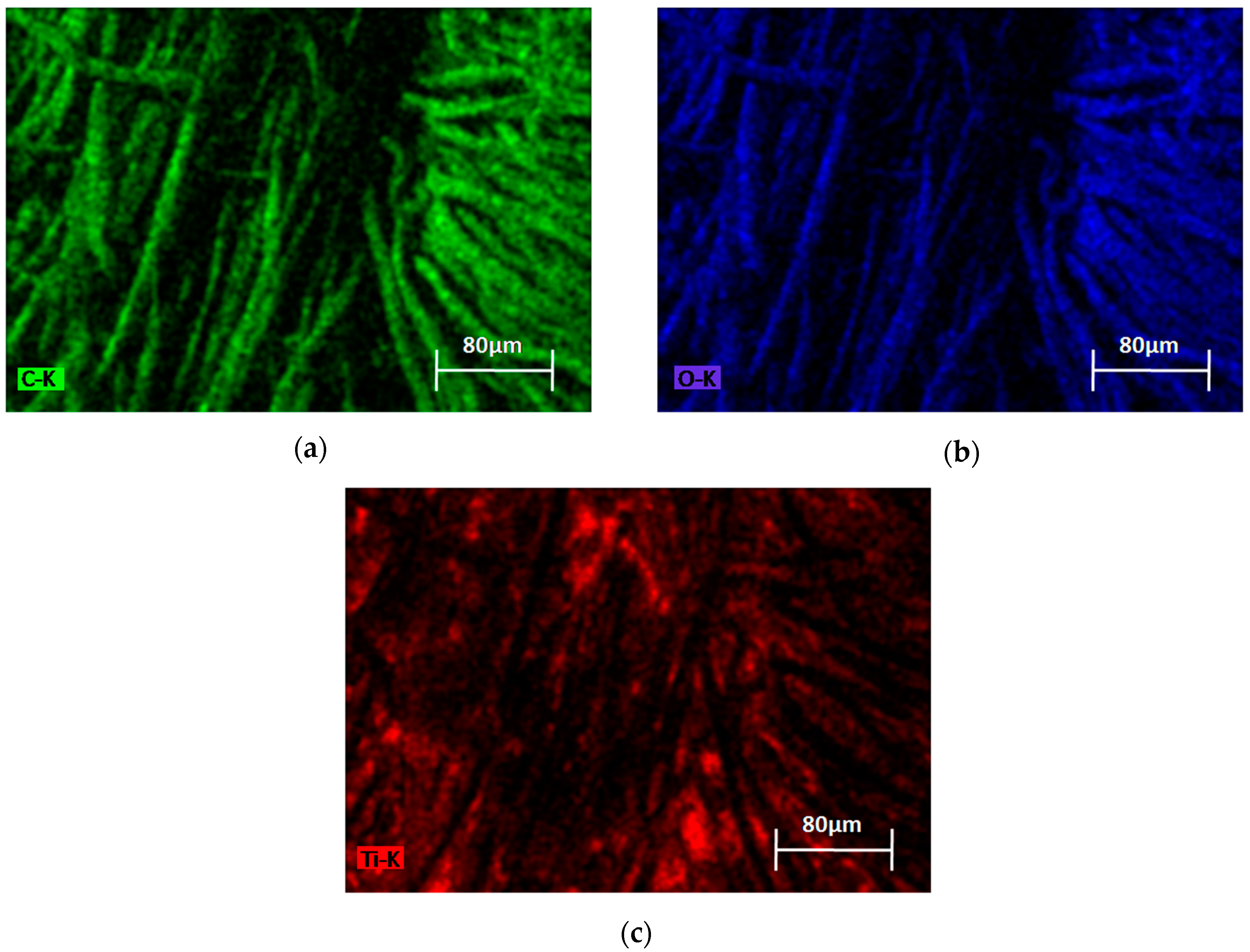
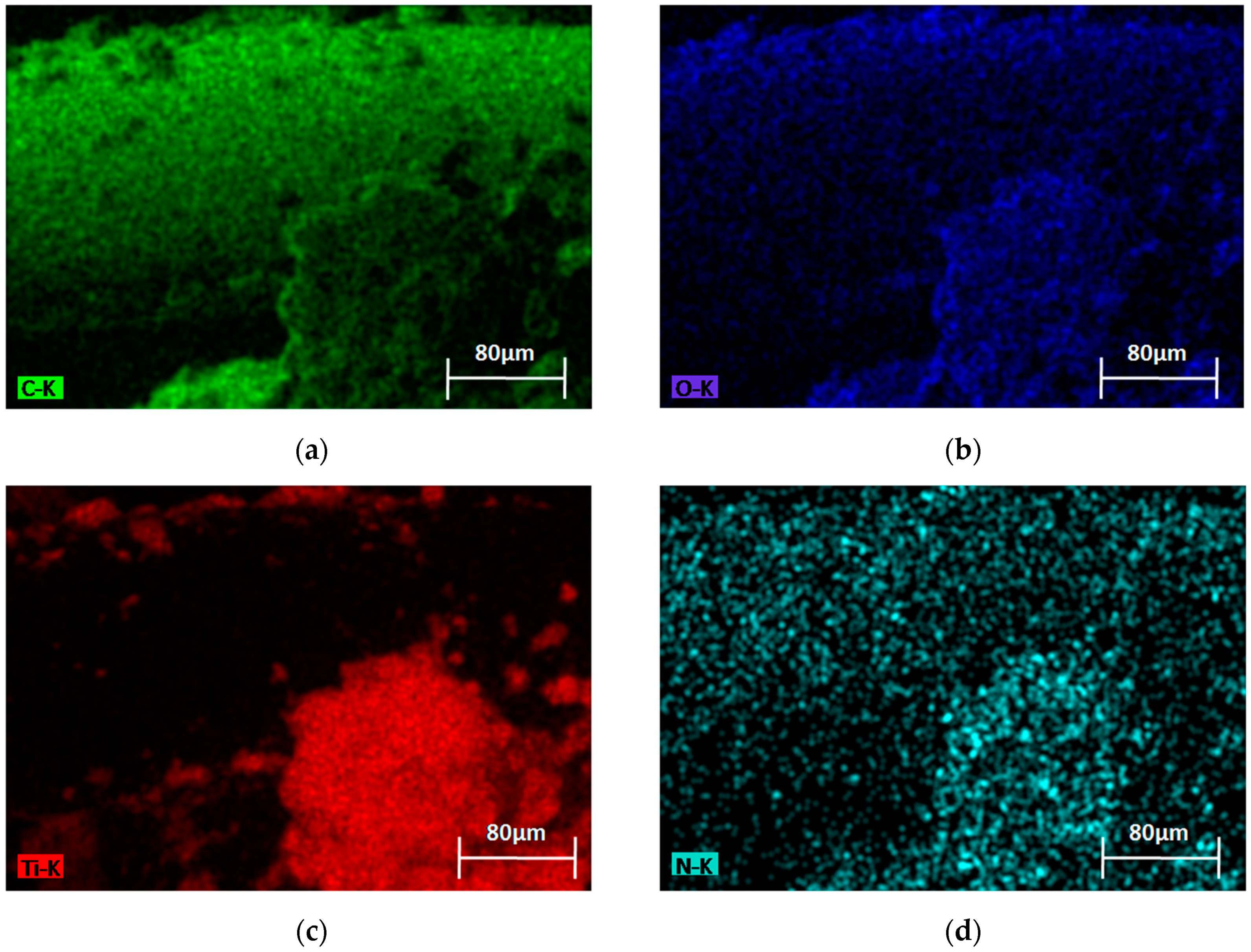
3.1.2. SEM-EDX Analysis
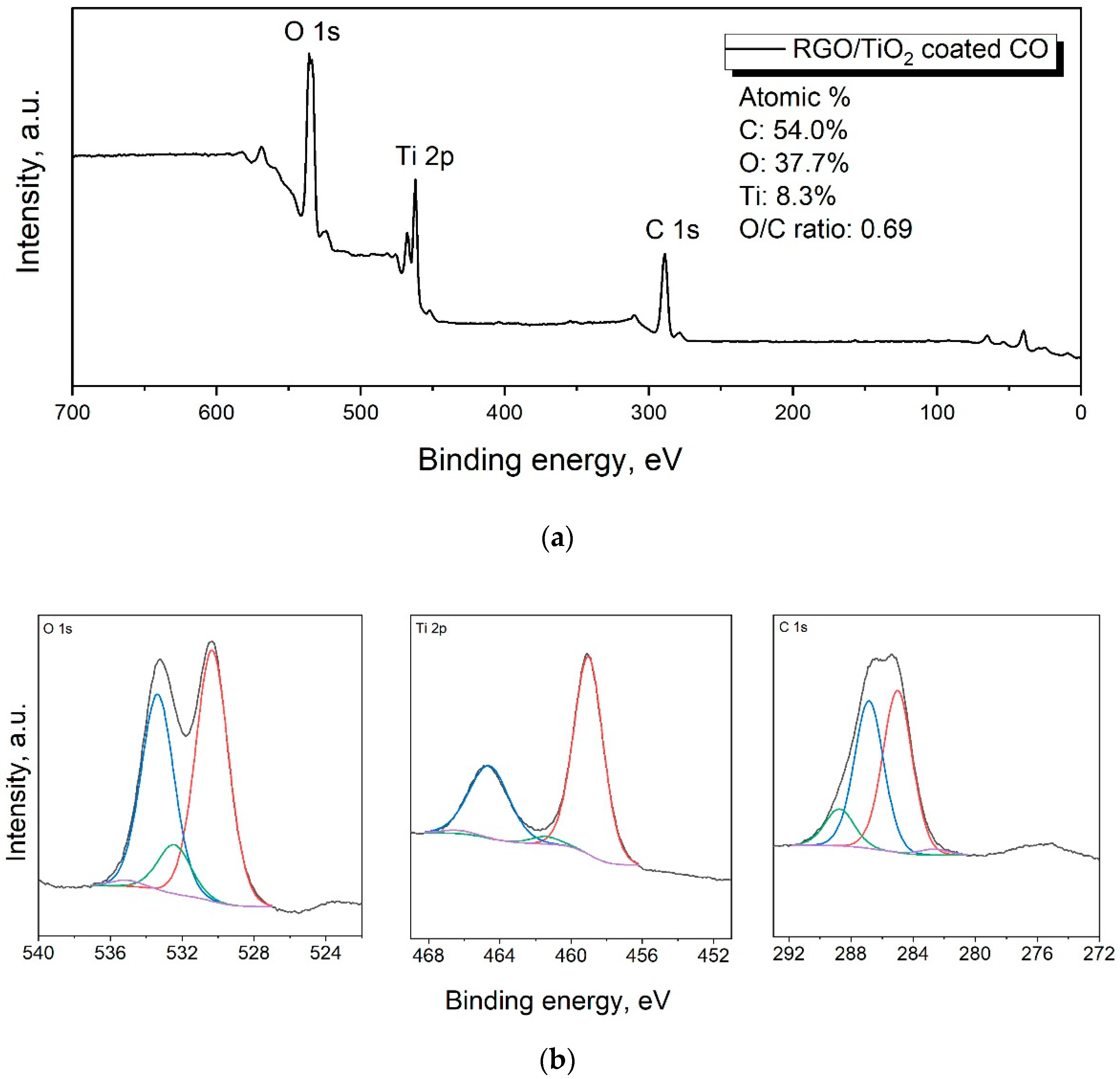
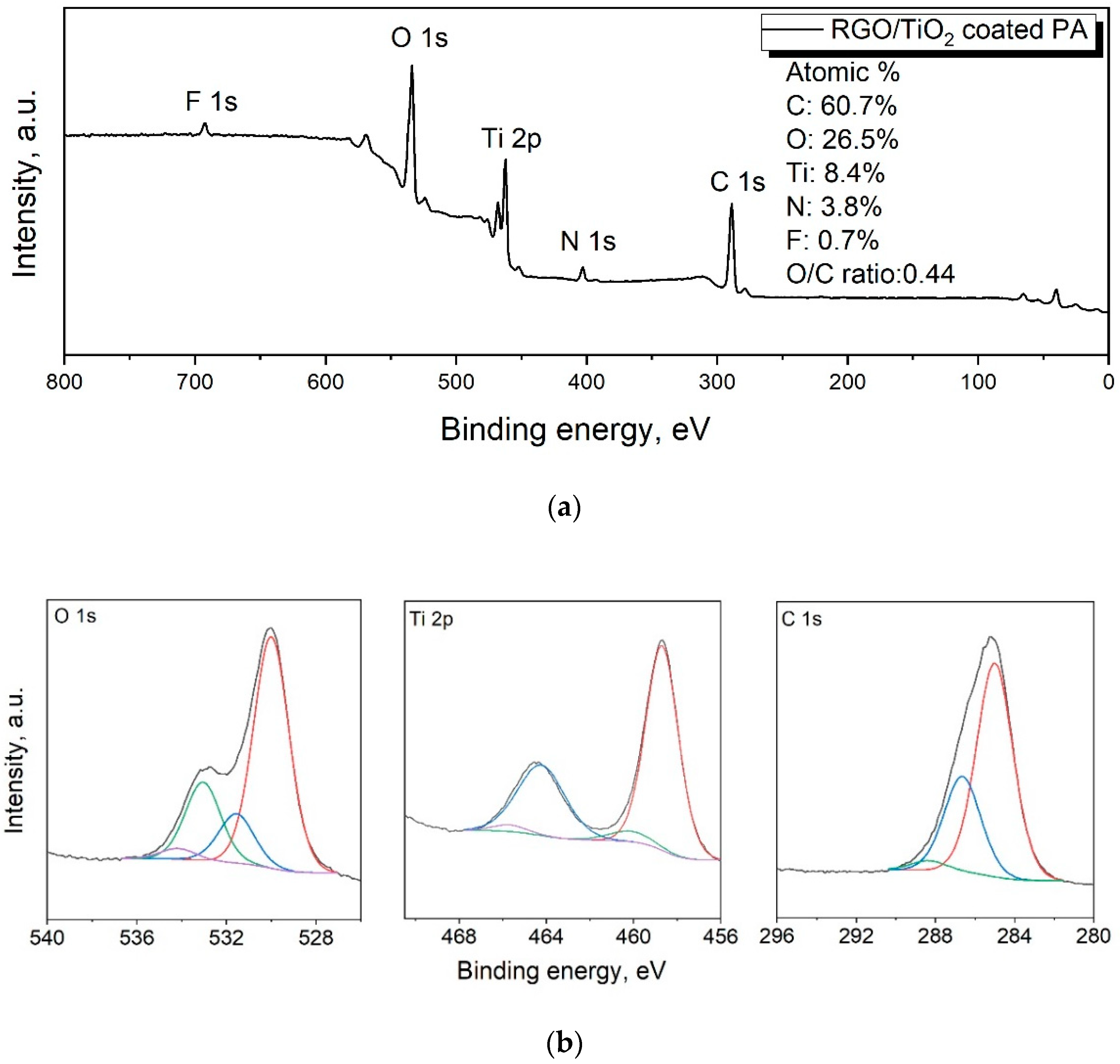
3.1.3. XPS Analysis
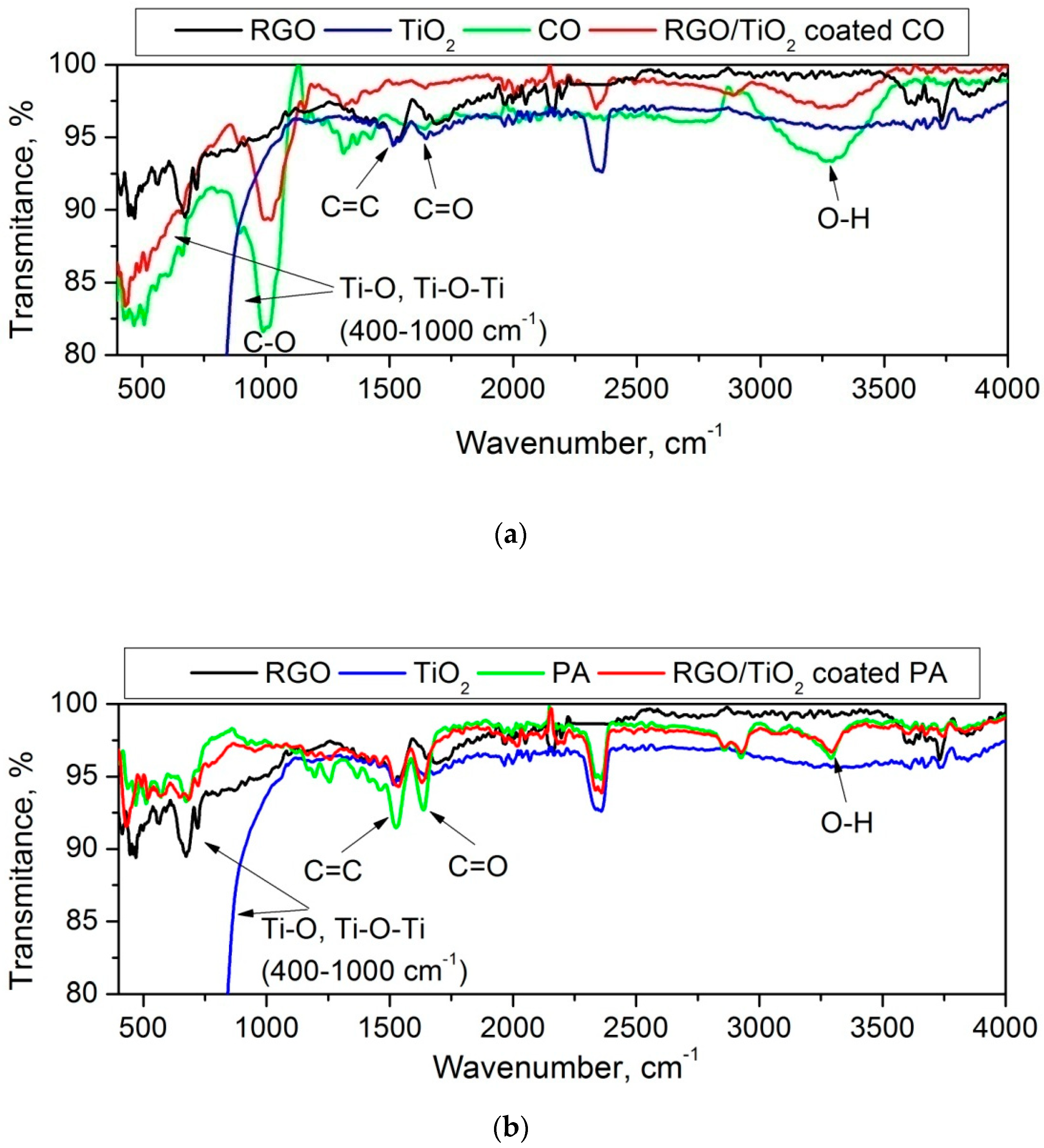
3.1.4. FTIR Analysis
3.1.5. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shateri-Khalilabad, M.; Yazdanshenas, M.E. Fabricating electroconductive cotton textiles using graphene. Carbohydr. Polym. 2013, 96, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Sahito, I.A.; Sun, K.C.; Arbab, A.A.; Qadir, M.B.; Jeong, S.H. Integrating high electrical conductivity and photocatalytic activity in cotton fabric by cationizing for enriched coating of negatively charged graphene oxide. Carbohydr. Polym. 2015, 130, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tian, M.; Qu, L.; Zhu, S.; Han, G. Multifunctional cotton fabrics with graphene/polyurethane coatings with far infrared emission, electrical conductivity, and ultraviolet blocking properties. Carbon 2015, 95, 625–633. [Google Scholar] [CrossRef]
- Cai, G.; Xu, Z.; Yang, M.; Tang, B.; Wang, X. Functionalization of cotton fabrics through thermal reduction of graphene oxide. Appl. Surf. Sci. 2017, 393, 441–448. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Navaneethaiyer, U.; Mohan, R.; Lee, J.; Kim, S.-J. Graphene oxide nanostructures modified multifunctional cotton fabrics. Appl. Nanosci. 2012, 2, 119–126. [Google Scholar] [CrossRef]
- Molina, J.; Fernandes, F.; Fernández, J.; Pastor, M.; Correia, A.; Souto, A.P.; Carneiro, J.O.; Teixeira, V.; Cases, F. Photocatalytic fabrics based on reduced graphene oxide and TiO2 coatings. Mater. Sci. Eng. B 2015, 199, 62–76. [Google Scholar] [CrossRef]
- Karimi, L.; Yazdanshenas, M.E.; Khajavi, R.; Rashidi, A.; Mirjalili, M. Optimizing the photocatalytic properties and the synergistic effects of graphene and nano titanium dioxide immobilized on cotton fabric. Appl. Surf. Sci. 2015, 332, 665–673. [Google Scholar] [CrossRef]
- Hegab, H.M.; El Mekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The controversial antibacterial activity of graphene-based materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- Berendjchi, A.; Khajavi, R.; Yousefi, A.A.; Yazdanshenas, M.E. Improved continuity of reduced graphene oxide on polyester fabric by use of polypyrrole to achieve a highly electro-conductive and flexible substrate. Appl. Surf. Sci. 2016, 363, 264–272. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Z.Q.; Liu, H.Z.; Xie, S.Y.; Wu, J.X.; Jiang, H.Q.; Zhang, J.; Li, L.; Li, J. Laundering durability of photocatalyzed self-cleaning cotton fabric with TiO2 nanoparticles covalently immobilized. ACS Appl. Mater. Interfaces 2013, 5, 3697–3703. [Google Scholar] [CrossRef]
- Some, S.; Shackery, I.; Kim, S.J.; Jun, S.C. Phosphorus-Doped Graphene Oxide Layer as a Highly Efficient Flame Retardant. Chem. Eur. J. 2015, 21, 15480–15485. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, E.; Wang, J.; Kashi, S.; Sun, L.; Wang, X. Advances in photocatalytic self-cleaning, superhydrophobic and electromagnetic interference shielding textile treatments. Adv. Colloid Interfac. Sci. 2020, 277, 102116. [Google Scholar] [CrossRef]
- Li, M.; Yu, Z.; Liu, Q.; Su, L.; Huang, W. Photocatalytic decomposition of perfluorooctanoic acid by noble metallic nanoparticles modified TiO2. Chem. Eng. J. 2016, 286, 232–238. [Google Scholar] [CrossRef]
- Ran, H.; Fan, J.; Zhang, X.; Mao, J.; Shao, G. Enhanced performances of dye-sensitized solar cells based on Au-TiO2 and Ag-TiO2 plasmonic hybrid nanocomposites. Appl. Surf. Sci. 2018, 430, 415–423. [Google Scholar] [CrossRef]
- Ribao, P.; Rivero, M.J.; Ortiz, I. TiO2 structures doped with noble metals and/or graphene oxide to improve the photocatalytic degradation of dichloroacetic acid. Environ. Sci. Pollut. Res. 2017, 24, 12628–12637. [Google Scholar] [CrossRef] [PubMed]
- Iliev, V.; Tomova, D.; Bilyarska, L. Promoting the oxidative removal rate of 2,4-dichlorophenoxyacetic acid on gold-doped WO3/TiO2/reduced graphene oxide photocatalysts under UV light irradiation. J. Photochem. Photobiol. A Chem. 2018, 351, 69–77. [Google Scholar] [CrossRef]
- Moslah, C.; Kandyla, M.; Mousdis, G.A.; Petropoulou, G.; Ksibi, M. Photocatalytic Properties of Titanium Dioxide Thin Films Doped with Noble Metals (Ag, Au, Pd, and Pt). Phys. Status. Solidi. A 2018, 215, 1800023. [Google Scholar] [CrossRef]
- Guan, R.; Zhai, H.; Li, J.; Qi, Y.; Li, M.; Song, M.; Zhao, Z.; Zhang, J.; Wang, D.; Tan, H. Reduced mesoporous TiO2 with Cu2S heterojunction and enhanced hydrogen production without noble metal cocatalyst. Appl. Surf. Sci. 2020, 507, 144772. [Google Scholar] [CrossRef]
- Kumar, P.; Kar, P.; Manuel, A.P.; Zeng, S.; Thakur, U.K.; Alam, K.M.; Zhang, Y.; Kisslinger, R.; Cui, K.; Bernard, G.M. Noble metal free, visible light driven photocatalysis using TiO2 nanotube arrays sensitized by P-doped C3N4 quantum dots. Adv. Optical. Mater. 2020, 8, 1901275. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Raghava Reddy, K.; Raghu, A.V.; Venkata Reddy, C.H. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrog. Energy. 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Faraldos, M.; Bahamonde, A. Environmental applications of titania-graphene photocatalysts. Catal. Today 2017, 285, 13–28. [Google Scholar] [CrossRef]
- Morawski, A.W.; Kusiak-Nejman, E.; Wanag, A.; Kapica-Kozar, J.; Wróbel, R.J.; Ohtani, B.; Aksienionek, M.; Lipińska, L. Photocatalytic degradation of acetic acid in the presence of visible light-active TiO2-reduced graphene oxide photocatalysts. Catal. Today 2017, 280, 108–113. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Design of graphene-based TiO2 photocatalysts-a review. Environ. Sci. Pollut. Res. 2012, 19, 3676–3687. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, H.; Xu, P. Immobilized TiO2-reduced graphene oxide nanocomposites on optical fibers as high performance photocatalysts for degradation of pharmaceuticals. Chem. Eng. J. 2017, 310, 389–398. [Google Scholar] [CrossRef]
- Wu, Y.; Mu, H.; Cao, X.; He, X. Polymer-supported graphene-TiO2 doped with nonmetallic elements with enhanced photocatalytic reaction under visible light. J. Mater. Sci. 2020, 55, 1577–1591. [Google Scholar] [CrossRef]
- Chi Hieu, N.; Mai Lien, T.; Thi Tuong Van, T.; Juang, R.-S. Enhanced removal of various dyes from aqueous solutions by UV and simulated solar photocatalysis over TiO2/ZnO/rGO composites. Sep. Purif. Technol. 2020, 232, 115962. [Google Scholar]
- Kanan, S.; Moyet, M.A.; Arthur, R.B.; Patterson, H.H. Recent advances on TiO2-based photocatalysts toward the degradation of pesticides and major organic pollutants from water bodies. Catal. Rev. 2020, 62, 1–65. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef]
- Pedrosa, M.; Da Silva, E.S.; Pastrana-Martínez, L.M.; Drazic, G.; Falaras, P.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M.T. Hummers’ and Brodie’s graphene oxides as photocatalysts for phenol degradation. J. Colloid. Interface Sci. 2020, 567, 243–255. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Morawski, A.W. TiO2/graphene-based nanocomposites for water treatment: A brief overview of charge carrier transfer, antimicrobial and photocatalytic performance. Appl. Catal. B Environ. 2019, 253, 179–186. [Google Scholar] [CrossRef]
- Chen, L.; Li, N.; Wen, Z.; Zhang, L.; Chen, Q.; Chen, L.; Si, P.; Feng, J.; Li, Y.; Lou, J.; et al. Graphene oxide based membrane intercalated by nanoparticles for high performance nanofiltration application. Chem. Eng. J. 2018, 347, 12–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, X.; Sun, T.; Zhao, X. Calcination of reduced graphene oxide decorated TiO2 composites for recovery and reuse in photocatalytic applications. Ceram. Int. 2017, 43, 1150–1159. [Google Scholar] [CrossRef]
- Ren, H.; Koshy, P.; Chen, W.F.; Qi, S.; Sorrell, C.C. Photocatalytic materials and technologies for air purification. J. Hazard. Mater. 2017, 325, 340–366. [Google Scholar] [CrossRef]
- Xu, D.; Li, L.; He, R.; Qi, L.; Zhang, L.; Cheng, B. Noble metal-free RGO/TiO2 composite nanofiber with enhanced photocatalytic H2-production performance. Appl. Surf. Sci. 2018, 434, 620–625. [Google Scholar] [CrossRef]
- Yaparatne, S.; Tripp, C.P.; Amirbahman, A. Photodegradation of taste and odor compounds in water in the presence of immobilized TiO2-SiO2 photocatalysts. J. Hazard. Mater. 2018, 346, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Padoin, N.; Soares, C. An explicit correlation for optimal TiO2 film thickness in immobilized photocatalytic reaction systems. Chem. Eng. J. 2017, 310, 381–388. [Google Scholar] [CrossRef]
- Lin, L.; Wang, H.; Jiang, W.; Mkaouar, A.R.; Xu, P. Comparison study on photocatalytic oxidation of pharmaceuticals by TiO2-Fe and TiO2-reduced graphene oxide nanocomposites immobilized on optical fibers. J. Hazard. Mater. 2017, 333, 162–168. [Google Scholar] [CrossRef]
- EL-Mekkawi, D.M.; Abdelwahab, N.A.; Mohamed, W.A.A.; Taha, N.A.; Abdel-Mottaleb, M.S.A. Solar photocatalytic treatment of industrial wastewater utilizing recycled polymeric disposals as TiO2 supports. J. Clean. Prod. 2020, 249, 119430. [Google Scholar] [CrossRef]
- Islam, M.T.; Dominguez, A.; Turley, R.S.; Kim, H.; Sultana, K.A.; Shuvo, M.A.I.; Alvarado-Tenorio, B.; Montes, M.O.; Lin, Y.; Gardea-Torresdey, J.; et al. Development of photocatalytic paint based on TiO2 and photopolymer resin for the degradation of organic pollutants in water. Sci. Total. Environ. 2020, 704, 135406. [Google Scholar] [CrossRef]
- De Araujo Scharnberg, A.R.; de Loreto, A.C.; Bender Wermuth, T.; Kopp Alves, A.; Arcaro, S.; Mantey dos Santos, P.A.; de Assis Lawisch Rodriguez, A. Porous ceramic supported TiO2 nanoparticles: Enhanced photocatalytic activity for Rhodamine Biodegradation. Bol. Soc. Esp. Cerám. Vidr. 2020, in press. [Google Scholar] [CrossRef]
- Touhid, S.S.B.; Shawon, M.R.K.; Deb, H.; Khoso, N.A.; Ahmed, A.; Fu, F.; Liu, X.D. Nature inspired rGO-TiO2 micro-flowers on polyester fabric using semicontinuous dyeing method: A binder-free approach towards durable antibacterial performance. Synth. Met. 2020, 261, 116298. [Google Scholar] [CrossRef]
- Grcic, I.; Erjavec, B.; Vrsaljko, D.; Guyon, C.; Tatoulian, M. Influence of plasma surface pretreatment and triaryl methane dye on the photocatalytic performance of TiO2-chitosan coating on textile. Prog. Org. Coat. 2017, 105, 277–285. [Google Scholar] [CrossRef]
- Landi, S., Jr.; Carneiro, J.O.; Fernandes, F.; Parpot, P.; Molina, J.; Cases, F.; Fernández, J.; Santos, J.G.; Soares, G.M.B.; Teixeira, V.; et al. Functionalization of cotton by RGO/TiO2 to enhance photodegradation of Rhodamine B under simulated solar irradiation. Water Air Soil Pollut. 2017, 228, 335. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, T.; Ren, P.; Cao, D.; Lin, Y.; Wang, L.; Zhang, B.; Xiang, X. Doping of chlorine from a neoprene adhesive enhances degradation efficiency of dyes by structured TiO2-coated photocatalytic fabrics. Catal 2020, 10, 69. [Google Scholar] [CrossRef]
- Kiziltas, A.; Nazari, B.; Gardner, D.J.; Bousfield, D.W. Polyamide 6–Cellulose Composites: Effect of cellulose composition on melt rheology and crystallization behavior. Polym. Eng. Sci. 2014, 54, 739–746. [Google Scholar] [CrossRef]
- Freluche, M.; Iliopoulos, I.; Flat, J.J.; Ruzette, A.V.; Leibler, L. Self-organized materials and graft copolymers of polymethyl methacrylate and polyamide-6 obtained by reactive blending. Polym. 2005, 46, 6554–6562. [Google Scholar] [CrossRef]
- Gao, Y.; Ren, X.; Zhang, X.; Chen, C. Environmental fate and risk of ultraviolet- and visible-light transformed graphene oxide: A comparative study. Environ. Pollut. 2019, 251, 821–829. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Fang, X.; Zhang, Z. Reduced graphene oxide dispersed nanofluids with improved photothermal conversion performance for direct absorption solar collectors. Solar Energy Mater. Solar Cells 2017, 163, 125–133. [Google Scholar] [CrossRef]
- Zulan, L.; Zhi, L.; Lan, C.; Sihao, C.; Dayang, W.; Fangyin, D. Reduced Graphene Oxide Coated Silk Fabrics with conductive property for wearable electronic textiles application. Adv. Electron. Mater. 2019, 5, 1800648. [Google Scholar] [CrossRef]
- Abdel-Messih, M.F.; Ahmed, M.A.; El-Sayed, A.S. Photocatalytic decolorization of rhodamine B dye using novel mesoporous SnO2–TiO2 nano mixed oxides prepared by sol–gel method. J. Photochem. Photobiol. A Chem. 2013, 260, 1–8. [Google Scholar] [CrossRef]
- Singh, P.; Sonu Raizada, P.; Sudhaik, A.; Shandilya, P.; Thakur, P.; Agarwal, S.; Kumar Gupta, V. Enhanced photocatalytic activity and stability of AgBr/BiOBr/graphene heterojunction for phenol degradation under visible light. J. Saudi Chem. Soc. 2019, 23, 546–560. [Google Scholar] [CrossRef]
- Staehelin, J.; Hoigne, J. Decomposition of ozone in water in the presence of organic solutes acting as promoters and inhibitors of radical chain reactions. Environ. Sci. Technol. 1985, 19, 1206–1213. [Google Scholar] [CrossRef]
- Wu, C.; Liu, X.; Wei, D.; Fan, J.; Wang, L. Photosonochemical degradation of phenol in water. Wat. Res. 2001, 35, 3927–3933. [Google Scholar] [CrossRef]
- Chun, H.; Yizhong, W.; Hongxiao, T. Destruction of phenol aqueous solution by photocatalysis or direct photolysis. Chemosphere 2000, 41, 1205–1209. [Google Scholar] [CrossRef]
- Kim, T.; Parale, V.G.; Jung, H.; Kim, Y.; Driss, Z.; Driss, D.; Bouabidi, A.; Euchy, S.; Park, H. Facile Synthesis of SnO2 Aerogel/Reduced Graphene Oxide Nanocomposites via in Situ Annealing for the Photocatalytic Degradation of Methyl Orange. Nanomaterials 2019, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Miran, W.; Jang, J.; Sung Lee, D. One-step hydrothermal synthesis of porous 3D reduced graphene oxide/TiO2 aerogel for carbamazepine photodegradation in aqueous solution. Appl. Catal. B Environ. 2017, 203, 85–95. [Google Scholar] [CrossRef]
- Lu, Z.; Zhai, X.; Yi, R.; Li, Z.; Zhang, R.; Wei, Q.; Xing, G.; Lu, G.; Huang, W. Photoluminescence Emission during Photoreduction of Graphene Oxide Sheets as Investigated with Single-Molecule Microscopy. J. Phys. Chem. C 2020, 124, 7914–7921. [Google Scholar] [CrossRef]
- Quezada-Renteria, J.A.; Ania, C.O.; Chazaro-Ruiz, L.F.; Rangel-Mendez, J.R. Influence of protons on reduction degree and defect formation in electrochemically reduced graphene oxide. Carbon 2019, 149, 722–732. [Google Scholar] [CrossRef]
- Hung, Y.; Cheng, C.; Kai Huang, C.; Yang, C. A facile method for batch preparation of electrochemically reduced graphene oxide. Nanomaterials 2019, 9, 376. [Google Scholar] [CrossRef]

| Sample | Mass Percentage, % | |||
|---|---|---|---|---|
| C | O | Ti | N | |
| CO | 46.15 | 53.85 | - | - |
| RGO/TiO2 coated CO | 19.84 | 29.88 | 50.27 | - |
| PA | 74.49 | 13.42 | 0.36 | 11.73 |
| RGO/TiO2 coated PA | 19.96 | 7.19 | 72.67 | 0.19 |
| RGO/TiO2 Coated CO | |||
| Name | Peak BE | Atomic % | Bond |
| Ti2p3 | 459 | 8 | Ti–O (TiO2) |
| Ti2p3 | 461.4 | 0.3 | Ti–O (TiOx) |
| O1s | 530.3 | 18.8 | Ti–O (oxide) |
| O1s | 533.4 | 14.7 | C–O |
| O1s | 532.4 | 3.7 | C=O |
| O1s | 535.1 | 0.5 | CO ads or H2O |
| C1s | 285 | 24.9 | C–C/C–H |
| C1s | 286.8 | 22.6 | C–OH, C–O, C–N |
| C1s | 288.7 | 5.7 | O–C–O, O=C–OH |
| C1s | 282.7 | 0.9 | carbide |
| RGO/TiO2 Coated PA | |||
| Name | Peak BE | Atomic % | Bond |
| Ti2p3 | 458.7 | 7.9 | Ti–O (TiO2) |
| Ti2p3 | 460.2 | 0.5 | Ti–O (TiOx) |
| O1s | 530 | 16.6 | Ti–O (oxide) |
| O1s | 531.6 | 3.5 | C=O |
| O1s | 533 | 5.6 | C–O |
| O1s | 534.2 | 0.8 | CO ads or H2O |
| C1s | 285 | 40.7 | C–C |
| C1s | 286.6 | 18.2 | C–O/C–N |
| C1s | 288.4 | 1.8 | C=O/C–F |
| F1s | 689 | 0.7 | C–F |
| N1s | 399.6 | 3.8 | C–N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olak-Kucharczyk, M.; Szczepańska, G.; Kudzin, M.H.; Pisarek, M. The Photocatalytical Properties of RGO/TiO2 Coated Fabrics. Coatings 2020, 10, 1041. https://doi.org/10.3390/coatings10111041
Olak-Kucharczyk M, Szczepańska G, Kudzin MH, Pisarek M. The Photocatalytical Properties of RGO/TiO2 Coated Fabrics. Coatings. 2020; 10(11):1041. https://doi.org/10.3390/coatings10111041
Chicago/Turabian StyleOlak-Kucharczyk, Magdalena, Grażyna Szczepańska, Marcin H. Kudzin, and Marcin Pisarek. 2020. "The Photocatalytical Properties of RGO/TiO2 Coated Fabrics" Coatings 10, no. 11: 1041. https://doi.org/10.3390/coatings10111041
APA StyleOlak-Kucharczyk, M., Szczepańska, G., Kudzin, M. H., & Pisarek, M. (2020). The Photocatalytical Properties of RGO/TiO2 Coated Fabrics. Coatings, 10(11), 1041. https://doi.org/10.3390/coatings10111041






