Aluminum Nitride Nanofilms by Atomic Layer Deposition Using Alternative Precursors Hydrazinium Chloride and Triisobutylaluminum
Abstract
:1. Introduction
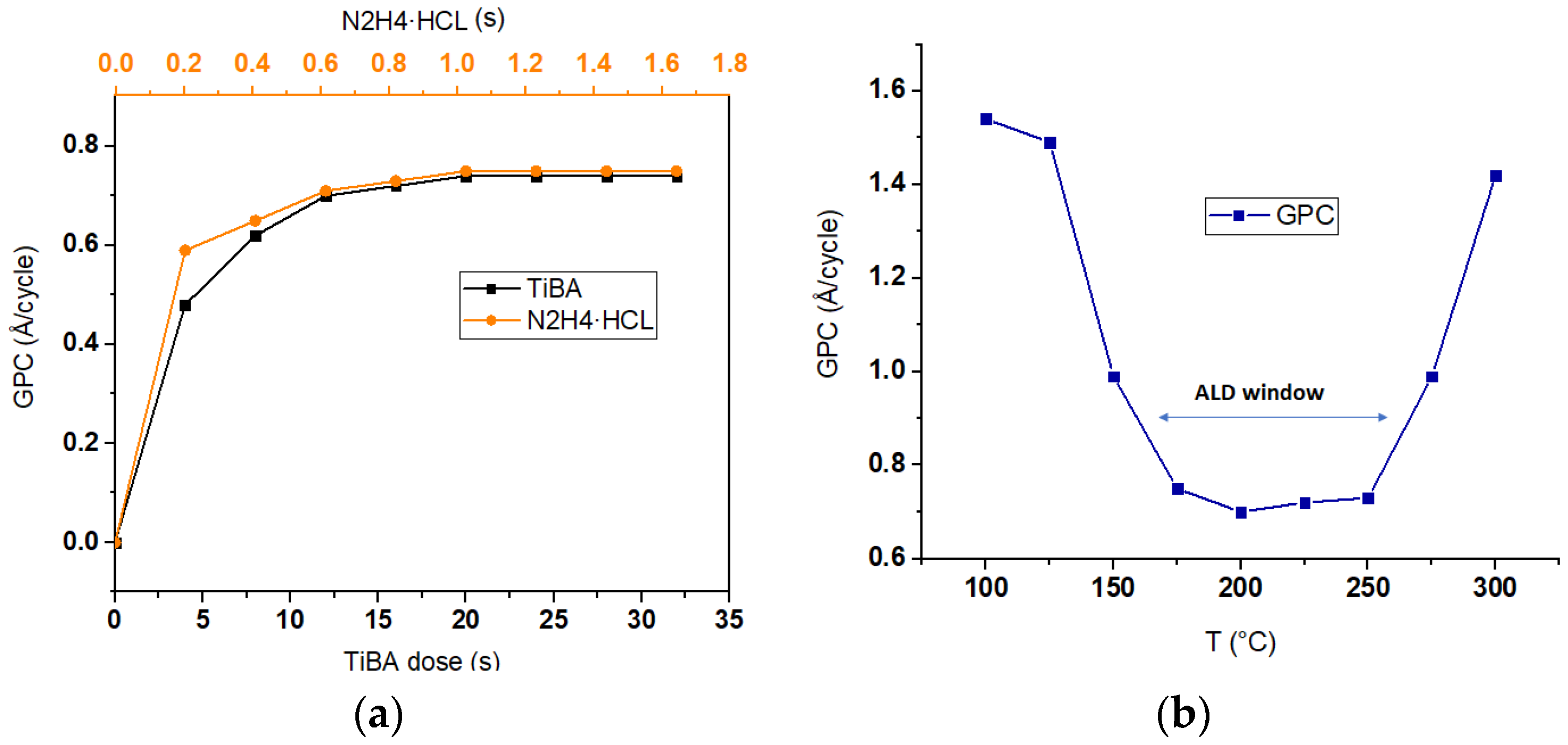
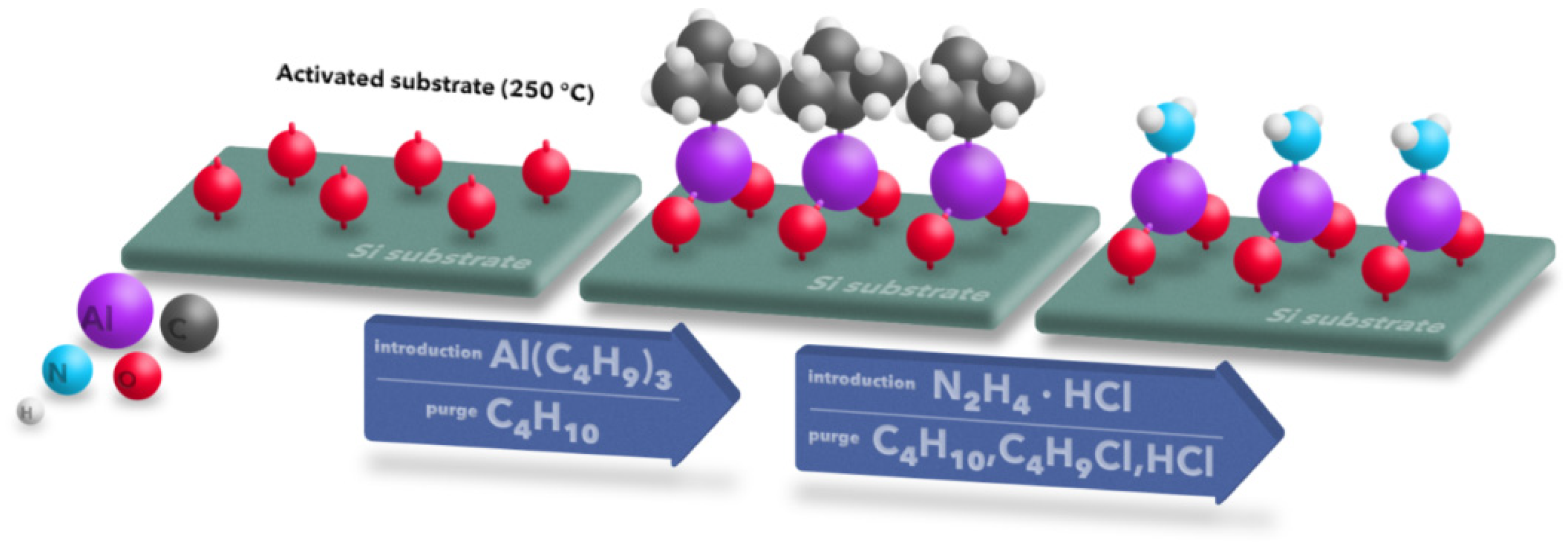
2. Experimental Detail
3. Experimental Data
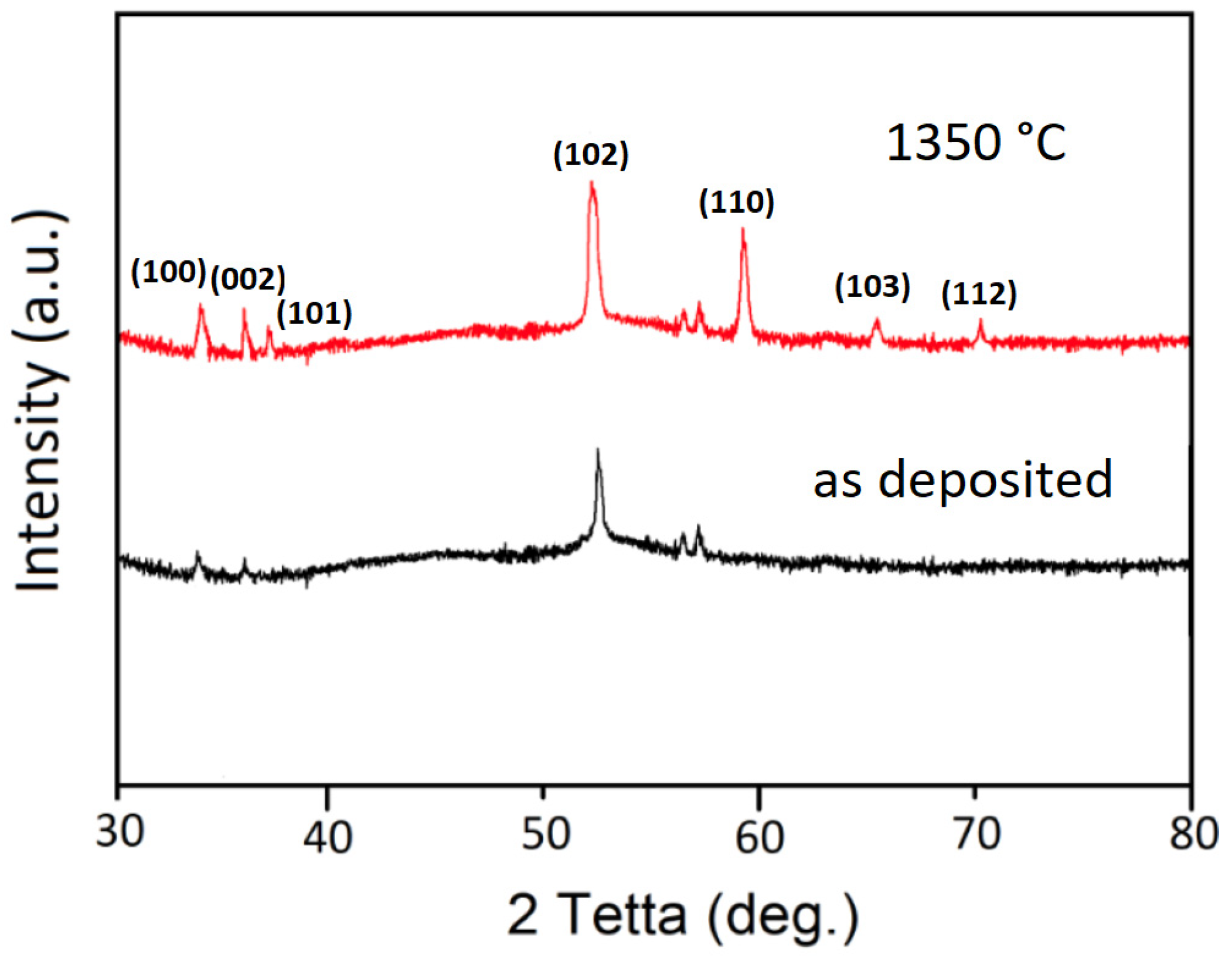
3.1. Atomic Force Microscopy (AFM) and X-ray Diffraction (XRD) Data
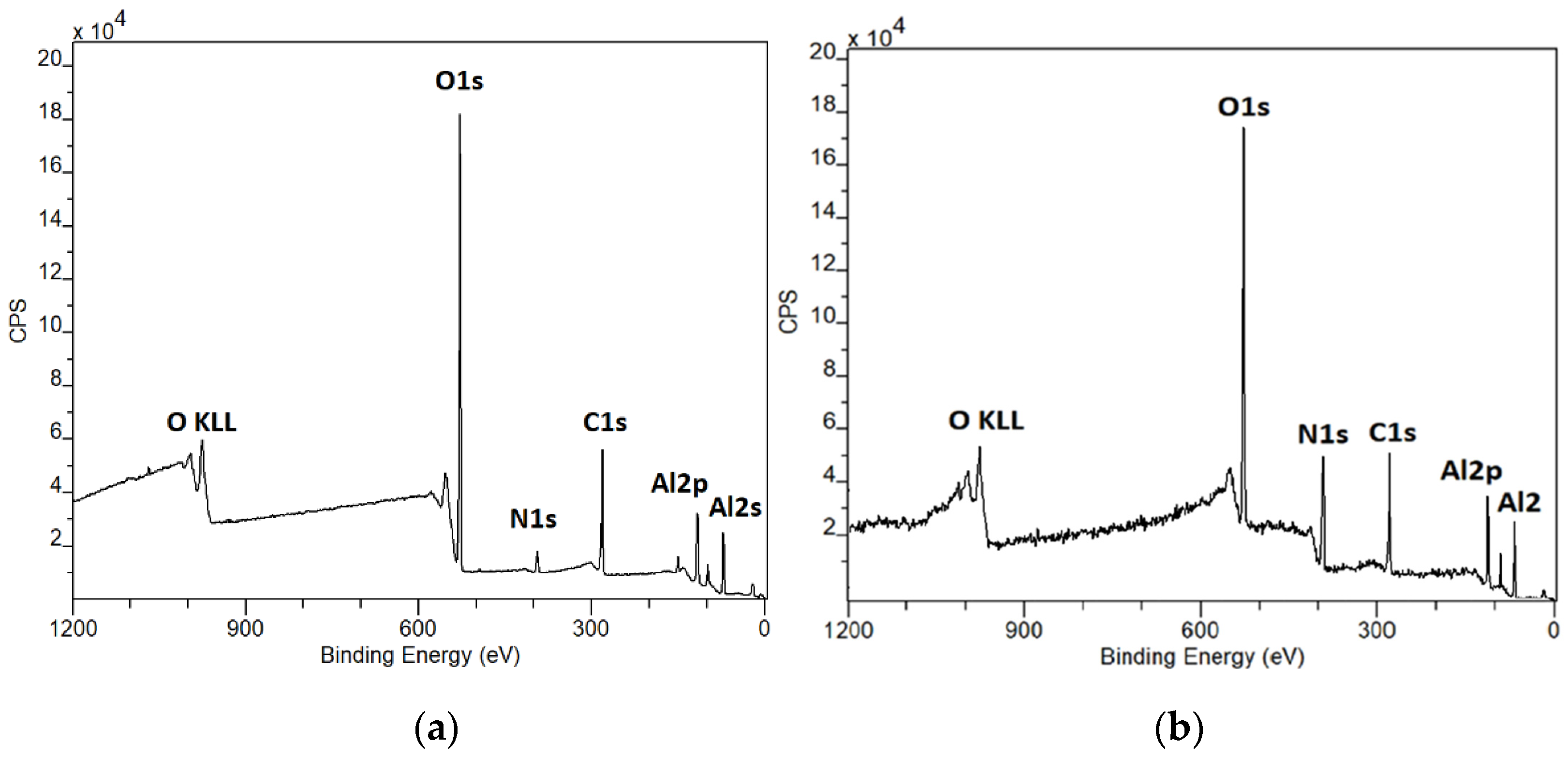
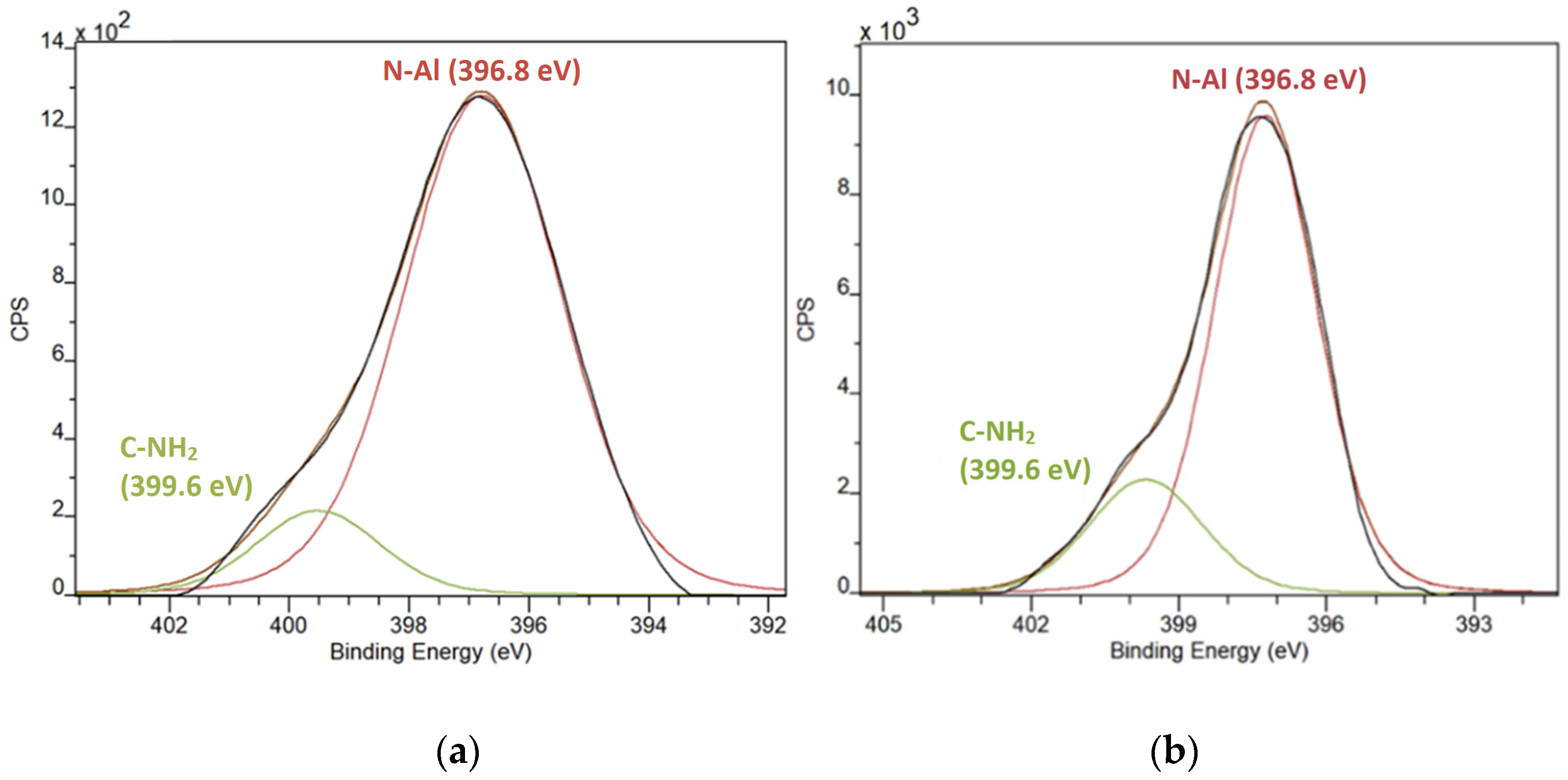
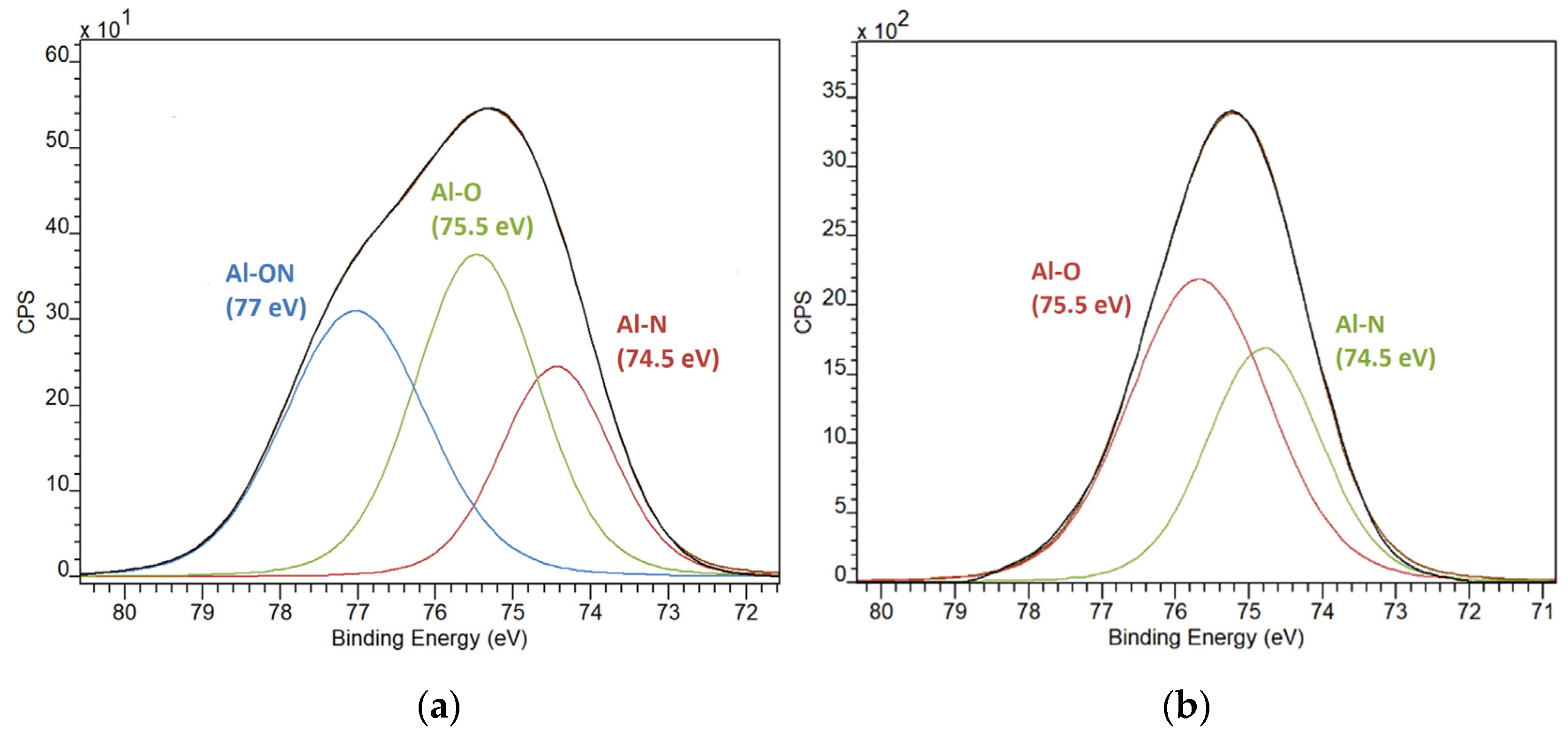
3.2. X-ray Photoelectron Spectroscopy (XPS) Data
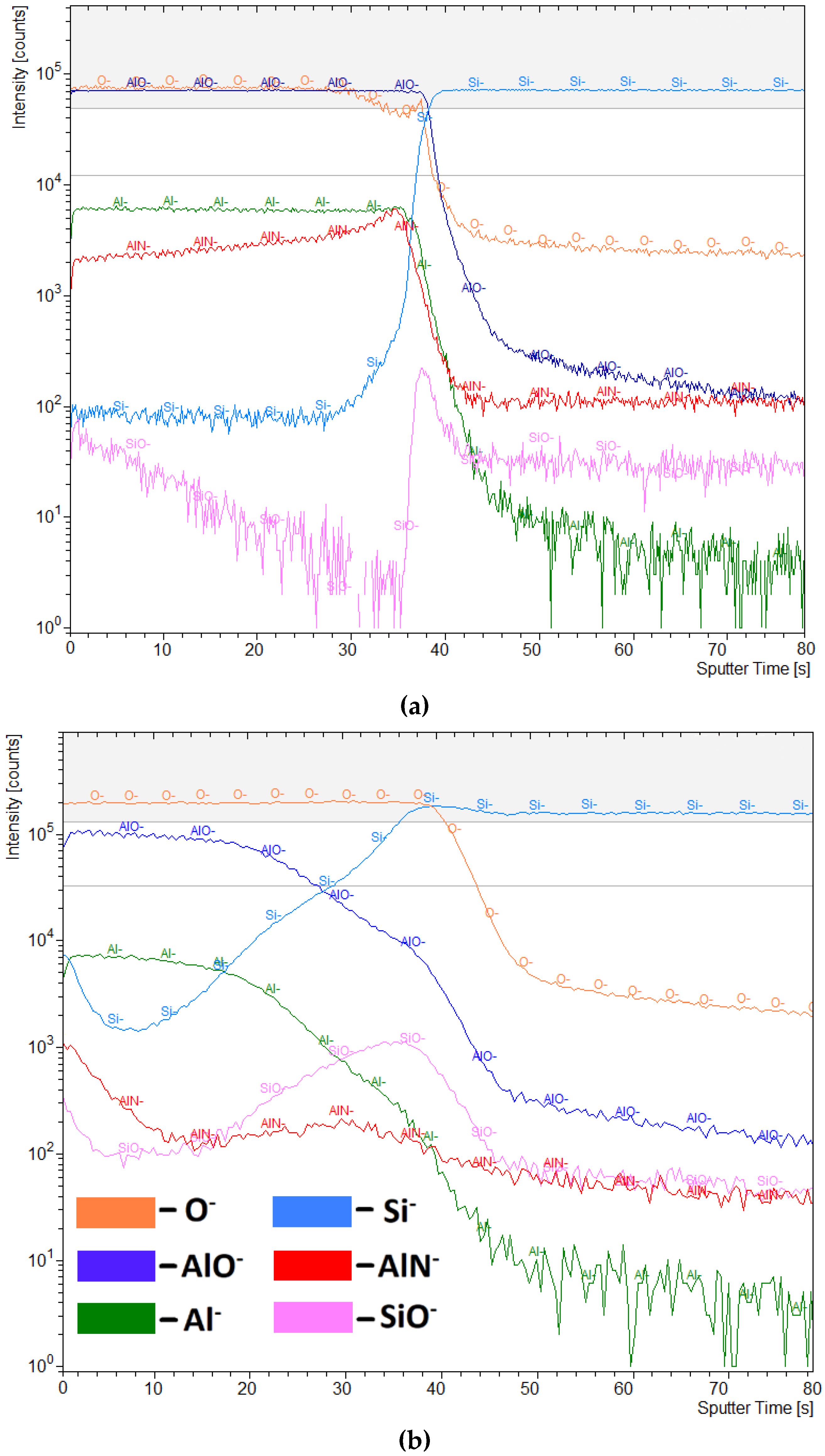
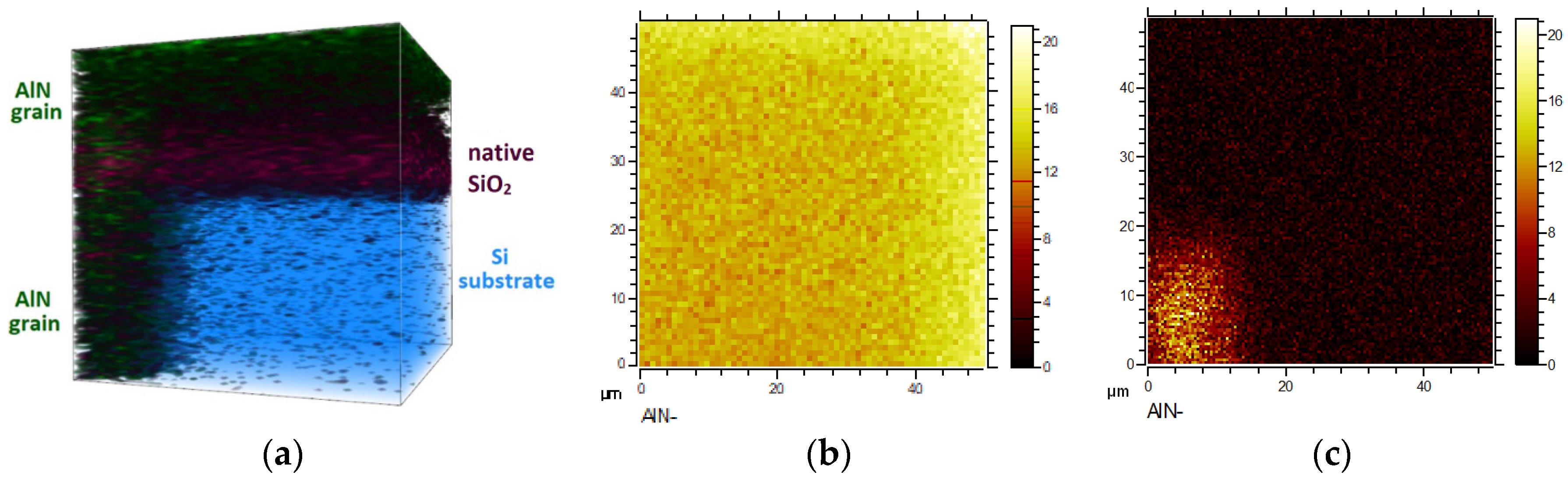
3.3. Secondary-Ion Mass Spectrometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nieminen, M.; Lehto, S.; Niinistö, L. Atomic layer epitaxy growth of AlN thin films. J. Mater. Chem. 2001, 11, 3148–3153. [Google Scholar] [CrossRef]
- Van Bui, H.; Wiggers, F.B.; Gupta, A.; Nguyen, M.D.; Aarnink, A.A.I.; De Jong, M.P.; Kovalgin, A.Y. Initial growth, refractive index, and crystallinity of thermal and plasma-enhanced atomic layer deposition AlN films. J. Vac. Sci. Technol. A Vac. Surf. Film 2015, 33, 01A111. [Google Scholar] [CrossRef]
- Tarala, V.A.; Altakhov, A.S.; Martens, Y.A.; Lisitsyn, S.V. Growing aluminum nitride films by Plasma-Enhanced Atomic. Inorg Mater. 2015, 51, 728–735. [Google Scholar] [CrossRef]
- Andrei, A.; Krupa, K.; Jozwik, M.; Delobelle, V.; Hirsinger, L.; Gorecki, C.; Nieradko, L.; Meunier, C. AlN as an actuation material for MEMS applications. The case of AlN driven multilayered cantilevers. Sens. Actuators A Phys. 2008, 141, 565–576. [Google Scholar] [CrossRef]
- Giordano, C.; Ingrosso, I.; Todaro, M.; Maruccio, G.; De Guido, S.; Cingolani, R.; Passaseo, A.; De Vittorio, M. AlN on polysilicon piezoelectric cantilevers for sensors/actuators. Microelectron. Eng. 2009, 86, 1204–1207. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Yang, H.; Wu, H.; Zhou, J.; Hu, L. Bipolar resistive switching properties of AlN films deposited by plasma-enhanced atomic layer deposition. Appl. Surf. Sci. 2014, 315, 110–115. [Google Scholar] [CrossRef]
- Găluşcă, D.G.; Perju, M.C.; Nejneru, C.; Nergiş, D.D.B.; E Lăzărescu, I. Aluminum coating influence on nitride layer performance deposited by MO-CVD in fluidized bed on austenitic stainless steel substrate. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374. [Google Scholar] [CrossRef] [Green Version]
- Czok, G.S.; Werther, J. Liquid spray vs. gaseous precursor injection—Its influence on the performance of particle coating by CVD in the fluidized bed. Powder Technol. 2006, 162, 100–110. [Google Scholar] [CrossRef]
- Meng, J.P.; Liu, X.P.; Fu, Z.Q.; Wang, X.J.; Hao, L. Thermal stability of AlN films prepared by ion beam assisted deposition. Appl. Surf. Sci. 2015, 347, 109–115. [Google Scholar] [CrossRef]
- Jose, F.; Ramaseshan, R.; Dash, S.; Bera, S.; Tyagi, A.K.; Raj, B. Response of magnetron sputtered AlN films to controlled atmosphere annealing. J. Phys. D Appl. Phys. 2010, 43, 075304. [Google Scholar] [CrossRef]
- Ma, D.; Liu, H.; Deng, Q.; Yang, W.; Silins, K.; Huang, N.; Leng, Y. Optimal target sputtering mode for aluminum nitride thin film deposition by high power pulsed magnetron sputtering. Vacuum 2019, 160, 410–417. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Ozgit, C.; Donmez, I.; Alevli, M.; Biyikli, N.; Ozgit-Akgun, C. Self-Limiting low-temperature growth of crystalline AlN thin films by plasma-enhanced atomic layer deposition. Thin Solid Films 2012, 520, 2750–2755. [Google Scholar] [CrossRef] [Green Version]
- Goerke, S.; Ziegler, M.; Ihring, A.; Dellith, J.; Undisz, A.; Diegel, M.; Anders, S.; Huebner, U.; Rettenmayr, M.; Meyer, H.G. Atomic layer deposition of AlN for thin membranes using trimethylaluminum and H2/N2 plasma. Appl. Surf. Sci. 2015, 338, 35–41. [Google Scholar] [CrossRef]
- Sadeghpour, S.; Ceyssens, F.; Puers, R. Crystalline growth of AlN thin films by atomic layer deposition. J. Phys. Conf. Ser. 2016, 757, 6–11. [Google Scholar] [CrossRef]
- Shih, H.Y.; Lee, W.H.; Kao, W.C.; Chuang, Y.C.; Lin, R.M.; Lin, H.C.; Shiojiri, M.; Chen, M.-J. Low-Temperature atomic layer epitaxy of AlN ultrathin films by layer-by-layer, in-situ atomic layer annealing. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Motamedi, P.; Cadien, K. XPS analysis of AlN thin films deposited by plasma enhanced atomic layer deposition. Appl. Surf. Sci. 2014, 315, 104–109. [Google Scholar] [CrossRef]
- Rontu, V.; Sippola, P.; Broas, M.; Ross, G.; Sajavaara, T.; Lipsanen, H.; Paulasto-Kröckel, M.; Franssila, S. Atomic layer deposition of AlN from AlCl3 using NH3 and Ar/NH3 plasma. J. Vac. Sci. Technol. A Vac. Surf. Film 2018, 36, 021508. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhu, Z.; Härkönen, K.; Salmi, E. Batch processing of aluminum nitride by atomic layer deposition from AlCl3 and NH3. J. Vac. Sci. Technol. A 2019, 37, 020925. [Google Scholar] [CrossRef]
- Abdulagatov, A.I.; Ramazanov, S.; Dallaev, R.S.; Murliev, E.K.; Palchaev, D.; Rabadanov, M.K.; Abdulagatov, I.M. Atomic layer deposition of aluminum nitride using tris(diethylamido)aluminum and hydrazine or ammonia. Russ. Microelectron. 2018, 47, 118–130. [Google Scholar] [CrossRef]
- Jones, A.C.; Hitchman, M.L. Overview of Chemical Vapor Deposition; Chemical Vapor Deposition: Precursors, Processes and Applications; Jones, A.C., Hitchman, M.L., Eds.; Royal Society of Chemistry: London, UK, 2009; pp. 327–328. [Google Scholar]
- Kermagoret, A.; Kerber, R.N.; Conley, M.P.; Callens, E.; Florian, P.; Massiot, D.; Copéret, C.; Delbecq, F.; Rozanska, X.; Sautet, P. Triisobutylaluminum: Bulkier and yet more reactive towards silica surfaces than triethyl or trimethylaluminum. Dalton Trans. 2013, 42, 12681–12687. [Google Scholar] [CrossRef] [PubMed]
- Cellier, P.P.; Spindler, J.-F.; Taillefer, M.; Cristau, H.-J. Pd/C-catalyzed room-temperature hydrodehalogenation of aryl halides with hydrazine hydrochloride. Tetrahedron Lett. 2003, 44, 7191–7195. [Google Scholar] [CrossRef]
- Xiao, S.; Suzuki, R.; Miyake, H.; Harada, S.; Ujihara, T. Improvement mechanism of sputtered AlN films by high-temperature annealing. J. Cryst. Growth 2018, 502, 41–44. [Google Scholar] [CrossRef]
- Cao, D.; Cheng, X.; Xie, Y.-H.; Zheng, L.; Wang, Z.; Yu, X.; Wang, J.; Shen, D.; Yu, Y. Effects of rapid thermal annealing on the properties of AlN films deposited by PEALD on AlGaN/GaN heterostructures. RSC Adv. 2015, 5, 37881–37886. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Peng, M.; Hou, C.; He, Y.; Li, M.; Zheng, X. PEALD-Grown Crystalline AlN Films on Si (100) with Sharp Interface and Good Uniformity. Nanoscale Res. Lett. 2017, 12, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ozgit-Akgun, C.; Goldenberg, E.; Okyay, A.K.; Biyikli, N. Hollow cathode plasma-assisted atomic layer deposition of crystalline AlN, GaN and AlxGa1−xN thin films at low temperatures. J. Mater. Chem. C 2014, 2, 2123–2136. [Google Scholar] [CrossRef] [Green Version]
- Motamedi, P.; Cadien, K. Structural and optical characterization of low-temperature ALD crystalline AlN. J. Cryst. Growth 2015, 421, 45–52. [Google Scholar] [CrossRef]
- Kim, H.; Kim, N.D.; An, S.C.; Yoon, H.J.; Choi, B.J. Improved interfacial properties of thermal atomic layer deposited AlN on GaN. Vacuum 2019, 159, 379–381. [Google Scholar] [CrossRef]
- Rosenberger, L.; Baird, R.; McCullen, E.; Auner, G.; Shreve, G. XPS analysis of aluminum nitride films deposited by plasma source molecular beam epitaxy. Surf. Interface Anal. 2008, 40, 1254–1261. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Hsu, J.-C.; Ding, Y.; Wang, P.W. Optical properties of high transmittance aluminum oxynitride thin films for spectral range from near ultraviolet to visible. Opt. Rev. 2009, 16, 400–403. [Google Scholar] [CrossRef]
- Morgan, D.J. Imaging XPS for industrial applications. J. Electron Spectrosc. Relat. Phenom. 2018, 231, 109–117. [Google Scholar] [CrossRef]
- Reddy, N.; Bera, P.; Reddy, V.R.; Sridhara, N.; Dey, A.; Anandan, C.; Sharma, A.K. XPS study of sputtered alumina thin films. Ceram. Int. 2014, 40, 11099–11107. [Google Scholar] [CrossRef]
- Chen, D.; Xu, D.; Wang, J.; Zhang, Y. Investigation of chemical etching of AlN film with different textures by X-ray photoelectron spectroscopy. J. Phys. D Appl. Phys. 2008, 41. [Google Scholar] [CrossRef]
- Radwan, A.B.; Shakoor, R.A. Aluminum nitride (AlN) reinforced electrodeposited Ni–B nanocomposite coatings. Ceram. Int. 2020, 46, 9863–9871. [Google Scholar] [CrossRef]
- Graniel, O.; Kempiński, M.; Jancelewicz, M.; Zaleski, K.; Jurga, S.; Smyntyna, V. Structural and XPS characterization of ALD Al2O3 coated porous silicon. Vacuum 2015, 113, 52–58. [Google Scholar] [CrossRef]
- Zhang, T.; Park, J.Y.; Huang, W.; Somorjai, G.A. Influence of reaction with XeF2 on surface adhesion of Al and Al2O3 surfaces. Appl. Phys. Lett. 2008, 93. [Google Scholar] [CrossRef]
- Wang, P.W.; Hsu, J.-C.; Lin, Y.-H.; Chen, H.-L. Structural investigation of high-transmittance aluminum oxynitride films deposited by ion beam sputtering. Surf. Interface Anal. 2010, 43, 1089–1094. [Google Scholar] [CrossRef]
- Wang, C.C.; Chiu, M.C.; Shiao, M.H.; Shieu, F.S. Characterization of AlN Thin Films Prepared by unbalanced magnetron sputtering. J. Electrochem. Soc. 2004, 151, 252–256. [Google Scholar] [CrossRef]
- Garcia-Mendez, M.; Morales-Rodríguez, S.; Shaji, S.; Krishnan, B.; Bartolo-Pérez, P. Structural properties of ALN films with oxygen content deposited by reactive magnetron sputtering: XRD and XPS characterization. Surf. Rev. Lett. 2011, 18, 23–31. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, P.; Fu, R.K.; Liu, W.; Lin, C.; Chu, P.K. AlN thin films fabricated by ultra-high vacuum electron-beam evaporation with ammonia for silicon-on-insulator application. Appl. Surf. Sci. 2005, 239, 327–334. [Google Scholar] [CrossRef]
- Oikawa, H.; Akiyama, R.; Kanazawa, K.; Kuroda, S.; Harayama, I.; Nagashima, K.; Sekiba, D.; Ashizawa, Y.; Tsukamoto, A.; Nakagawa, K.; et al. Deposition and characterization of amorphous aluminum nitride thin films for a gate insulator. Thin Solid Films 2015, 574, 110–114. [Google Scholar] [CrossRef]
- Shimizu, W.; Nakamura, S.; Sato, T.; Murakami, Y. Creation of high-refractive-index amorphous titanium oxide thin films from low-fractal-dimension polymeric precursors synthesized by a sol-gel technique with a hydrazine monohydrochloride catalyst. Langmuir 2012, 28, 12245–12255. [Google Scholar] [CrossRef] [PubMed]
- Cullis, C.F.; Yates, J.G. Reaction of carbon with nitrogen. Trans. Faraday Soc. 1964, 60, 141–148. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dallaev, R.; Sobola, D.; Tofel, P.; Škvarenina, Ľ.; Sedlák, P. Aluminum Nitride Nanofilms by Atomic Layer Deposition Using Alternative Precursors Hydrazinium Chloride and Triisobutylaluminum. Coatings 2020, 10, 954. https://doi.org/10.3390/coatings10100954
Dallaev R, Sobola D, Tofel P, Škvarenina Ľ, Sedlák P. Aluminum Nitride Nanofilms by Atomic Layer Deposition Using Alternative Precursors Hydrazinium Chloride and Triisobutylaluminum. Coatings. 2020; 10(10):954. https://doi.org/10.3390/coatings10100954
Chicago/Turabian StyleDallaev, Rashid, Dinara Sobola, Pavel Tofel, Ľubomir Škvarenina, and Petr Sedlák. 2020. "Aluminum Nitride Nanofilms by Atomic Layer Deposition Using Alternative Precursors Hydrazinium Chloride and Triisobutylaluminum" Coatings 10, no. 10: 954. https://doi.org/10.3390/coatings10100954
APA StyleDallaev, R., Sobola, D., Tofel, P., Škvarenina, Ľ., & Sedlák, P. (2020). Aluminum Nitride Nanofilms by Atomic Layer Deposition Using Alternative Precursors Hydrazinium Chloride and Triisobutylaluminum. Coatings, 10(10), 954. https://doi.org/10.3390/coatings10100954






