Engineering Biomaterials to Guide Heart Cells for Matured Cardiac Tissue
Abstract
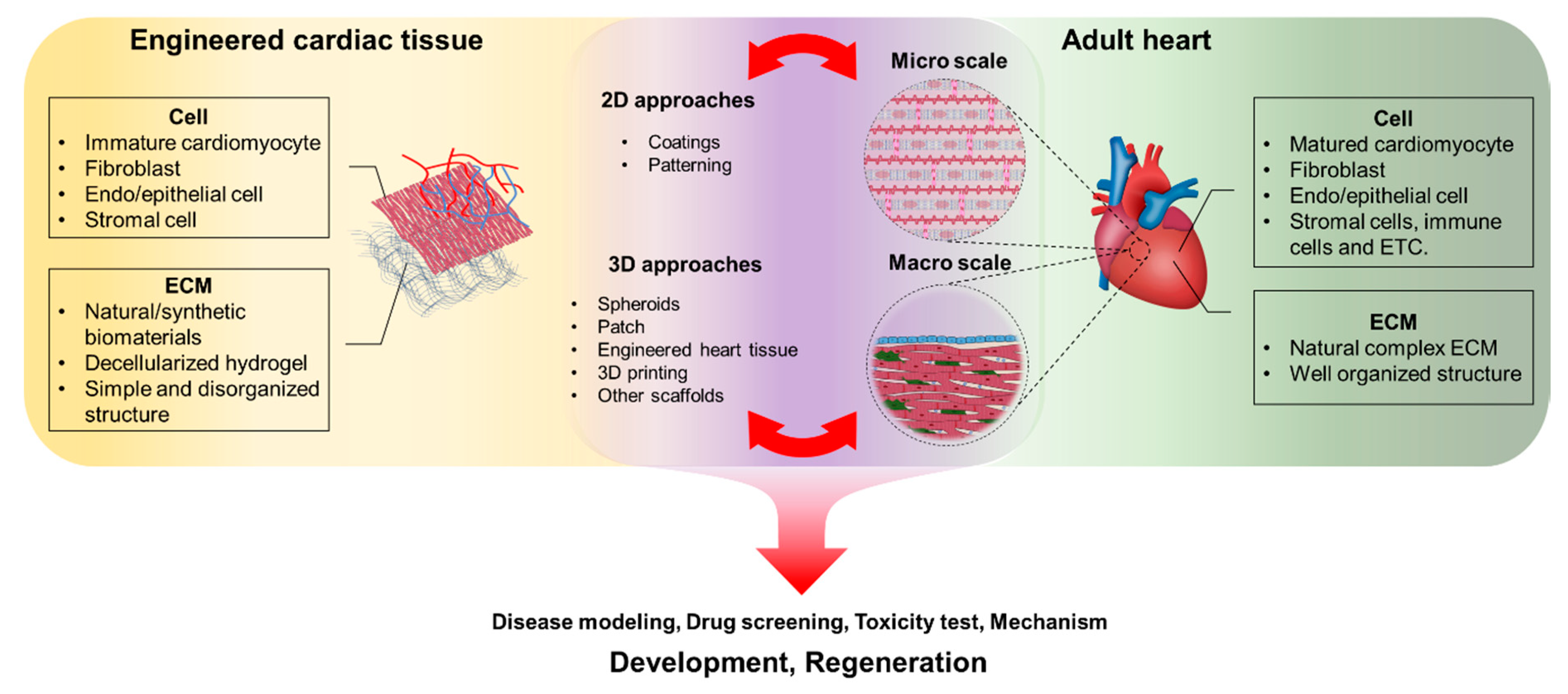
:1. Introduction
2. Materials for Cardiac Tissue Engineering
2.1. Natural Materials
2.1.1. Collagen
2.1.2. Fibrin
2.1.3. Fibronectin
2.1.4. Matrigel
2.1.5. Decellularized ECM
2.2. Synthetic Materials
2.2.1. Polycaprolactone (PCL)
2.2.2. Polylactide (PLA)
2.2.3. Poly(Ethylene Glycol) (PEG)
3. Approaches for Cardiac Tissue Preparation
3.1. Two-Dimensional (2D) Culture
3.1.1. Coatings
3.1.2. Patterning
3.2. Three-Dimensional (3D) Culture
3.2.1. Spheroids
3.2.2. Cardiac Patches
3.2.3. Rod-Shaped Engineered Heart Tissues
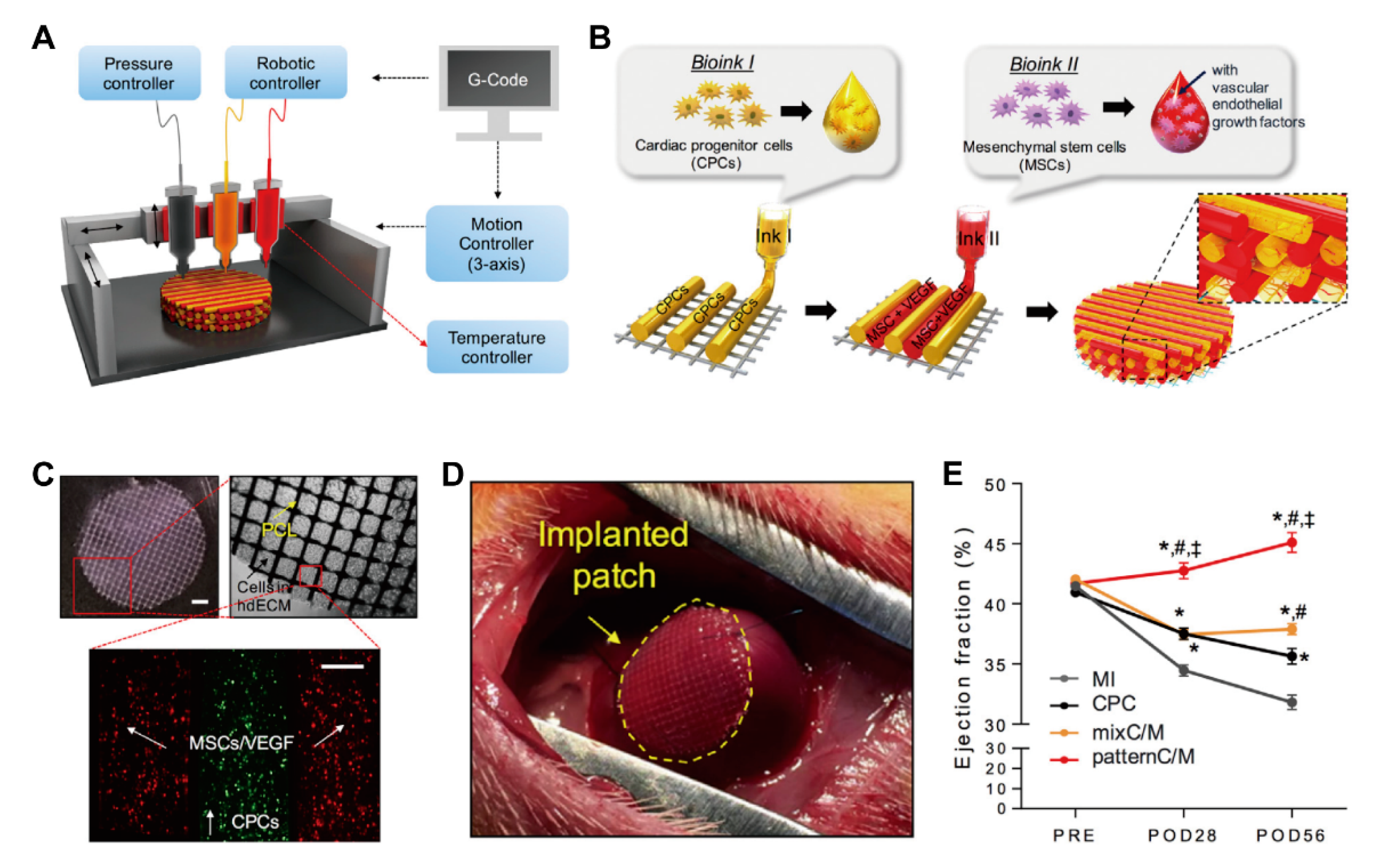
3.2.4. 3D Bioprinting for Cardiac Tissue Preparation
3.2.5. Other Scaffold Systems
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Swinehart, I.T.; Badylak, S.F. Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis. Dev. Dyn 2016, 245, 351–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Chin, I.L.; Hool, L.; Choi, Y.S. A Review of in vitro Platforms for Understanding Cardiomyocyte Mechanobiology. Front. Bioeng. Biotechnol. 2019, 7, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.; Quinn, K.P.; Georgakoudi, I.; Black, L.D., 3rd. Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro. Acta Biomater. 2014, 10, 194–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Wagner, J.U.G.; Pham, M.D.; Nicin, L.; Hammer, M.; Bottermann, K.; Yuan, T.; Sharma, R.; John, D.; Muhly-Reinholz, M.; Tombor, L.; et al. Dissection of heterocellular cross-talk in vascularized cardiac tissue mimetics. J. Mol. Cell. Cardiol. 2020, 138, 269–282. [Google Scholar] [CrossRef] [Green Version]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018, 556, 239–243. [Google Scholar] [CrossRef]
- van Meer, B.J.; Tertoolen, L.G.; Mummery, C.L. Concise Review: Measuring Physiological Responses of Human Pluripotent Stem Cell Derived Cardiomyocytes to Drugs and Disease. Stem Cells 2016, 34, 2008–2015. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.; Jung, D.J.; Choi, S.-C.; Lim, D.-S.; Kim, J.-H.; Jeoung, G.S.; Kim, J.; Park, Y. Multidimensional assembly using layer-by-layer deposition for synchronized cardiac macro tissues. RSC Adv. 2020, 10, 18806–18815. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Young, J.L.; Kretchmer, K.; Ondeck, M.G.; Zambon, A.C.; Engler, A.J. Mechanosensitive kinases regulate stiffness-induced cardiomyocyte maturation. Sci. Rep. 2014, 4, 6425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, S.; Miyagawa, S.; Fukushima, S.; Kawamura, T.; Kashiyama, N.; Ohashi, F.; Toyofuku, T.; Toda, K.; Sawa, Y. Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes by Soluble Factors from Human Mesenchymal Stem Cells. Mol. Ther. 2018, 26, 2681–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.L.; Engler, A.J. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 2011, 32, 1002–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kapalczynska, M.; Kolenda, T.; Przybyla, W.; Zajaczkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Blizniak, R.; Luczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Kamei, K.I.; Koyama, Y.; Tokunaga, Y.; Mashimo, Y.; Yoshioka, M.; Fockenberg, C.; Mosbergen, R.; Korn, O.; Wells, C.; Chen, Y. Characterization of Phenotypic and Transcriptional Differences in Human Pluripotent Stem Cells under 2D and 3D Culture Conditions. Adv. Healthc. Mater. 2016, 5, 2951–2958. [Google Scholar] [CrossRef]
- Beauchamp, P.; Jackson, C.B.; Ozhathil, L.C.; Agarkova, I.; Galindo, C.L.; Sawyer, D.B.; Suter, T.M.; Zuppinger, C. 3D Co-culture of hiPSC-Derived Cardiomyocytes with Cardiac Fibroblasts Improves Tissue-Like Features of Cardiac Spheroids. Front. Mol. Biosci. 2020, 7, 14. [Google Scholar] [CrossRef]
- Eder, A.; Vollert, I.; Hansen, A.; Eschenhagen, T. Human engineered heart tissue as a model system for drug testing. Adv. Drug Deliv. Rev. 2016, 96, 214–224. [Google Scholar] [CrossRef]
- Paolo Capparè, G.T.; Sberna, M.T.; Panina-Bordignon, P. The emerging role of stem cells in regenerative dentistry. Curr. Gene Ther. 2020, 20. [Google Scholar] [CrossRef]
- Tete, G.; Cappare, P.; Gherlone, E. New Application of Osteogenic Differentiation from HiPS Stem Cells for Evaluating the Osteogenic Potential of Nanomaterials in Dentistry. Int. J. Environ. Res. Public Health 2020, 17, 1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosales, A.M.; Anseth, K.S. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aszodi, A.; Legate, K.R.; Nakchbandi, I.; Fassler, R. What mouse mutants teach us about extracellular matrix function. Annu. Rev. Cell Dev. Biol. 2006, 22, 591–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [Green Version]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [Green Version]
- Chevallay, B.; Herbage, D. Collagen-based biomaterials as 3D scaffold for cell cultures_applications for tissue engineering and gene therapy. Med Biol. Eng. Comput. 2000, 38, 211–218. [Google Scholar] [CrossRef]
- Ben Shoham, A.; Rot, C.; Stern, T.; Krief, S.; Akiva, A.; Dadosh, T.; Sabany, H.; Lu, Y.; Kadler, K.E.; Zelzer, E. Deposition of collagen type I onto skeletal endothelium reveals a new role for blood vessels in regulating bone morphology. Development 2016, 143, 3933–3943. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.L.; Jiao, K.; Niu, L.N.; Jiao, Y.; Song, Q.; Shen, L.J.; Tay, F.R.; Chen, J.H. Intrafibrillar silicified collagen scaffold modulates monocyte to promote cell homing, angiogenesis and bone regeneration. Biomaterials 2017, 113, 203–216. [Google Scholar] [CrossRef]
- Rhee, S.; Puetzer, J.L.; Mason, B.N.; Reinhart-King, C.A.; Bonassar, L.J. 3D Bioprinting of Spatially Heterogeneous Collagen Constructs for Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2016, 2, 1800–1805. [Google Scholar] [CrossRef]
- Meng, Q.; An, S.; Damion, R.A.; Jin, Z.; Wilcox, R.; Fisher, J.; Jones, A. The effect of collagen fibril orientation on the biphasic mechanics of articular cartilage. J. Mech. Behav. Biomed. Mater. 2017, 65, 439–453. [Google Scholar] [CrossRef] [Green Version]
- Sawadkar, P.; Sibbons, P.; Ahmed, T.; Bozec, L.; Mudera, V. Engineering of a Functional Tendon Using Collagen As a Natural Polymer. ACS Biomater. Sci. Eng. 2019, 5, 5218–5228. [Google Scholar] [CrossRef]
- Yang, S.; Shi, X.; Li, X.; Wang, J.; Wang, Y.; Luo, Y. Oriented collagen fiber membranes formed through counter-rotating extrusion and their application in tendon regeneration. Biomaterials 2019, 207, 61–75. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M.G.; Wei, J.M.; Choi, S.; Goerger, J.P.; Zipfel, W.; Fischbach, C. Collagen Fiber Orientation Regulates 3D Vascular Network Formation and Alignment. ACS Biomater. Sci. Eng. 2018, 4, 2967–2976. [Google Scholar] [CrossRef]
- Sherman, V.R.; Tang, Y.; Zhao, S.; Yang, W.; Meyers, M.A. Structural characterization and viscoelastic constitutive modeling of skin. Acta Biomater. 2017, 53, 460–469. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Bozkurt, A.; Boecker, A.; Tank, J.; Altinova, H.; Deumens, R.; Dabhi, C.; Tolba, R.; Weis, J.; Brook, G.A.; Pallua, N.; et al. Efficient bridging of 20 mm rat sciatic nerve lesions with a longitudinally micro-structured collagen scaffold. Biomaterials 2016, 75, 112–122. [Google Scholar] [CrossRef]
- Saeki, M.; Tanaka, K.; Imatani, J.; Okamoto, H.; Watanabe, K.; Nakamura, T.; Gotani, H.; Ohi, H.; Nakamura, R.; Hirata, H. Efficacy and safety of novel collagen conduits filled with collagen filaments to treat patients with peripheral nerve injury: A multicenter, controlled, open-label clinical trial. Injury 2018, 49, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef]
- Yang, W.; Meyers, M.A.; Ritchie, R.O. Structural architectures with toughening mechanisms in Nature: A review of the materials science of Type-I collagenous materials. Prog. Mater. Sci. 2019, 103, 425–483. [Google Scholar] [CrossRef]
- Buehler, M.J. Nature designs tough collagen: Explaining the nanostructure of collagen fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 12285–12290. [Google Scholar] [CrossRef] [Green Version]
- Wolf, K.; Alexander, S.; Schacht, V.; Coussens, L.M.; von Andrian, U.H.; van Rheenen, J.; Deryugina, E.; Friedl, P. Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 2009, 20, 931–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiti, M.C.; Dolmans, M.M.; Donnez, J.; Amorim, C.A. Fibrin in Reproductive Tissue Engineering: A Review on Its Application as a Biomaterial for Fertility Preservation. Ann. Biomed. Eng. 2017, 45, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Mosesson, M.W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 2005, 3, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ma, L.; Zhou, J.; Mao, Z.; Gao, C.; Shen, J. Fabrication and physical and biological properties of fibrin gel derived from human plasma. Biomed. Mater. 2008, 3, 015001. [Google Scholar] [CrossRef] [Green Version]
- Zollinger, A.J.; Smith, M.L. Fibronectin, the extracellular glue. Matrix Biol. 2017, 60, 27–37. [Google Scholar] [CrossRef]
- Singh, P.; Carraher, C.; Schwarzbauer, J.E. Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 2010, 26, 397–419. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Schwarzbauer, J.E. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005, 24, 389–399. [Google Scholar] [CrossRef]
- Shahbazi, M.N.; Scialdone, A.; Skorupska, N.; Weberling, A.; Recher, G.; Zhu, M.; Jedrusik, A.; Devito, L.G.; Noli, L.; Macaulay, I.C.; et al. Pluripotent state transitions coordinate morphogenesis in mouse and human embryos. Nature 2017, 552, 239–243. [Google Scholar] [CrossRef]
- Uemura, M.; Refaat, M.M.; Shinoyama, M.; Hayashi, H.; Hashimoto, N.; Takahashi, J. Matrigel supports survival and neuronal differentiation of grafted embryonic stem cell-derived neural precursor cells. J. Neurosci. Res. 2010, 88, 542–551. [Google Scholar] [CrossRef]
- Wang, J.; Chu, R.; Ni, N.; Nan, G. The effect of Matrigel as scaffold material for neural stem cell transplantation for treating spinal cord injury. Sci. Rep. 2020, 10, 2576. [Google Scholar] [CrossRef] [PubMed]
- Benton, G.; Kleinman, H.K.; George, J.; Arnaoutova, I. Multiple uses of basement membrane-like matrix (BME/Matrigel) in vitro and in vivo with cancer cells. Int. J. Cancer 2011, 128, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Fridman, R.; Benton, G.; Aranoutova, I.; Kleinman, H.K.; Bonfil, R.D. Increased initiation and growth of tumor cell lines, cancer stem cells and biopsy material in mice using basement membrane matrix protein (Cultrex or Matrigel) co-injection. Nat. Protoc. 2012, 7, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Inokuma, M.S.; Denham, J.; Golds, K.; Kundu, P.; Gold, J.D.; Carpenter, M.K. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 2001, 19, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H.K.; Koblinski, J. Matrigel: From discovery and ECM mimicry to assays and models for cancer research. Adv. Drug Deliv. Rev. 2014, 79, 3–18. [Google Scholar] [CrossRef]
- Fischbach, C.; Chen, R.; Matsumoto, T.; Schmelzle, T.; Brugge, J.S.; Polverini, P.J.; Mooney, D.J. Engineering tumors with 3D scaffolds. Nat. Methods 2007, 4, 855–860. [Google Scholar] [CrossRef]
- Poincloux, R.; Collin, O.; Lizarraga, F.; Romao, M.; Debray, M.; Piel, M.; Chavrier, P. Contractility of the cell rear drives invasion of breast tumor cells in 3D Matrigel. Proc. Natl. Acad. Sci. USA 2011, 108, 1943–1948. [Google Scholar] [CrossRef] [Green Version]
- Sieveking, D.P.; Buckle, A.; Celermajer, D.S.; Ng, M.K. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: Insights from a novel human angiogenesis assay. J. Am. Coll. Cardiol. 2008, 51, 660–668. [Google Scholar] [CrossRef]
- Mousseau, Y.; Mollard, S.; Qiu, H.; Richard, L.; Cazal, R.; Nizou, A.; Vedrenne, N.; Remi, S.; Baaj, Y.; Fourcade, L.; et al. In vitro 3D angiogenesis assay in egg white matrix: Comparison to Matrigel, compatibility to various species, and suitability for drug testing. Lab. Investig. 2014, 94, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Ott, H.C. Organ engineering based on decellularized matrix scaffolds. Trends Mol. Med. 2011, 17, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Hoshiba, T.; Lu, H.; Kawazoe, N.; Chen, G. Decellularized matrices for tissue engineering. Expert Opin. Biol. Ther. 2010, 10, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Abedalwafa, M.; Wang, F.; Wang, L.; Li, C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: A review. Rev. Adv. Mater. Sci. 2013, 34, 123–140. [Google Scholar]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Shah Mohammadi, M.; Bureau, M.N.; Nazhat, S.N. Polylactic acid (PLA) biomedical foams for tissue engineering. In Biomedical Foams for Tissue Engineering Applications; Woodhead Publishing: Cambridge, UK, 2014; pp. 313–334. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Wang, M.; Yuan, D.; He, C. Amphiphilic conetworks and gels physically cross-linked via stereocomplexation of polylactide. Langmuir 2013, 29, 14307–14313. [Google Scholar] [CrossRef]
- Wolf, M.T.; Dearth, C.L.; Sonnenberg, S.B.; Loboa, E.G.; Badylak, S.F. Naturally derived and synthetic scaffolds for skeletal muscle reconstruction. Adv. Drug Deliv. Rev. 2015, 84, 208–221. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.C.; Anseth, K.S. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Reid, B.; Gibson, M.; Singh, A.; Taube, J.; Furlong, C.; Murcia, M.; Elisseeff, J. PEG hydrogel degradation and the role of the surrounding tissue environment. J. Tissue Eng. Regen. Med. 2015, 9, 315–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Sun, Z.; Foskett, A.; Trzeciakowski, J.P.; Meininger, G.A.; Muthuchamy, M. Cardiomyocyte contractile status is associated with differences in fibronectin and integrin interactions. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H2071–H2081. [Google Scholar] [CrossRef] [Green Version]
- Deitch, S.; Gao, B.Z.; Dean, D. Effect of matrix on cardiomyocyte viscoelastic properties in 2D culture. Mol. Cell. Biomech. 2012, 9, 227–249. [Google Scholar] [PubMed]
- Vanwinkle, W.B.; Snuggs, M.B.; Buja, L.M. Cardiogel: A biosynthetic extracellular matrix for cardiomyocyte culture. In Vitro Cell. Dev. Biol. 1996, 32, 478–485. [Google Scholar] [CrossRef]
- Baghalishahi, M.; Efthekhar-Vaghefi, S.H.; Piryaei, A.; Nematolahi-Mahani, S.N.; Mollaei, H.R.; Sadeghi, Y. Cardiac extracellular matrix hydrogel together with or without inducer cocktail improves human adipose tissue-derived stem cells differentiation into cardiomyocyte-like cells. Biochem. Biophys. Res. Commun. 2018, 502, 215–225. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement membrane matrix with biological activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef]
- Melkoumian, Z.; Weber, J.L.; Weber, D.M.; Fadeev, A.G.; Zhou, Y.; Dolley-Sonneville, P.; Yang, J.; Qiu, L.; Priest, C.A.; Shogbon, C.; et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat. Biotechnol. 2010, 28, 606–610. [Google Scholar] [CrossRef]
- Alvarez-Paino, M.; Amer, M.H.; Nasir, A.; Cuzzucoli Crucitti, V.; Thorpe, J.; Burroughs, L.; Needham, D.; Denning, C.; Alexander, M.R.; Alexander, C.; et al. Polymer Microparticles with Defined Surface Chemistry and Topography Mediate the Formation of Stem Cell Aggregates and Cardiomyocyte Function. ACS Appl. Mater. Interfaces 2019, 11, 34560–34574. [Google Scholar] [CrossRef]
- Cui, Z.; Ni, N.C.; Wu, J.; Du, G.Q.; He, S.; Yau, T.M.; Weisel, R.D.; Sung, H.W.; Li, R.K. Polypyrrole-chitosan conductive biomaterial synchronizes cardiomyocyte contraction and improves myocardial electrical impulse propagation. Theranostics 2018, 8, 2752–2764. [Google Scholar] [CrossRef] [PubMed]
- Nakane, T.; Masumoto, H.; Tinney, J.P.; Yuan, F.; Kowalski, W.J.; Ye, F.; LeBlanc, A.J.; Sakata, R.; Yamashita, J.K.; Keller, B.B. Impact of Cell Composition and Geometry on Human Induced Pluripotent Stem Cells-Derived Engineered Cardiac Tissue. Sci. Rep. 2017, 7, 45641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Kim, P.; Song, I.; Cha, J.M.; Lee, S.H.; Kim, B.; Suh, K.Y. Guided three-dimensional growth of functional cardiomyocytes on polyethylene glycol nanostructure. Langmuir 2006, 22, 5419–5426. [Google Scholar] [CrossRef]
- Carson, D.; Hnilova, M.; Yang, X.; Nemeth, C.L.; Tsui, J.H.; Smith, A.S.; Jiao, A.; Regnier, M.; Murry, C.E.; Tamerler, C.; et al. Nanotopography-Induced Structural Anisotropy and Sarcomere Development in Human Cardiomyocytes Derived from Induced Pluripotent Stem Cells. ACS Appl. Mater. Interfaces 2016, 8, 21923–21932. [Google Scholar] [CrossRef] [Green Version]
- Salick, M.R.; Napiwocki, B.N.; Sha, J.; Knight, G.T.; Chindhy, S.A.; Kamp, T.J.; Ashton, R.S.; Crone, W.C. Micropattern width dependent sarcomere development in human ESC-derived cardiomyocytes. Biomaterials 2014, 35, 4454–4464. [Google Scholar] [CrossRef] [Green Version]
- Abadi, P.P.; Garbern, J.C.; Behzadi, S.; Hill, M.J.; Tresback, J.S.; Heydari, T.; Ejtehadi, M.R.; Ahmed, N.; Copley, E.; Aghaverdi, H.; et al. Engineering of Mature Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Using Substrates with Multiscale Topography. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Xu, B.; Magli, A.; Anugrah, Y.; Koester, S.J.; Perlingeiro, R.C.R.; Shen, W. Nanotopography-responsive myotube alignment and orientation as a sensitive phenotypic biomarker for Duchenne Muscular Dystrophy. Biomaterials 2018, 183, 54–66. [Google Scholar] [CrossRef]
- Achilli, T.M.; Meyer, J.; Morgan, J.R. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin. Biol. Ther. 2012, 12, 1347–1360. [Google Scholar] [CrossRef] [Green Version]
- Pontes Soares, C.; Midlej, V.; de Oliveira, M.E.; Benchimol, M.; Costa, M.L.; Mermelstein, C. 2D and 3D-organized cardiac cells shows differences in cellular morphology, adhesion junctions, presence of myofibrils and protein expression. PLoS ONE 2012, 7, e38147. [Google Scholar] [CrossRef] [Green Version]
- Zuppinger, C. 3D culture for cardiac cells. Biochim. Biophys. Acta 2016, 1863, 1873–1881. [Google Scholar] [CrossRef]
- Polonchuk, L.; Chabria, M.; Badi, L.; Hoflack, J.C.; Figtree, G.; Davies, M.J.; Gentile, C. Cardiac spheroids as promising in vitro models to study the human heart microenvironment. Sci. Rep. 2017, 7, 7005. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Richards, D.; Xu, R.; Stewart-Clark, S.; Mani, S.K.; Borg, T.K.; Menick, D.R.; Tian, B.; Mei, Y. Silicon nanowire-induced maturation of cardiomyocytes derived from human induced pluripotent stem cells. Nano Lett. 2015, 15, 2765–2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.; Richards, D.; Coyle, R.C.; Yao, J.; Xu, R.; Gou, W.; Wang, H.; Menick, D.R.; Tian, B.; Mei, Y. Cell number per spheroid and electrical conductivity of nanowires influence the function of silicon nanowired human cardiac spheroids. Acta Biomater. 2017, 51, 495–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tallawi, M.; Rosellini, E.; Barbani, N.; Cascone, M.G.; Rai, R.; Saint-Pierre, G.; Boccaccini, A.R. Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. J. R. Soc. Interface 2015, 12, 20150254. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, R.; Krishnan, U.M.; Sethuraman, S. Living cardiac patch: The elixir for cardiac regeneration. Expert Opin. Biol. Ther. 2012, 12, 1623–1640. [Google Scholar] [CrossRef]
- Zhang, D.; Shadrin, I.Y.; Lam, J.; Xian, H.Q.; Snodgrass, H.R.; Bursac, N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 2013, 34, 5813–5820. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.; Choi, S.C.; Lim, D.S.; Kim, J.H.; Kim, J.; Park, Y. Modulating cardiomyocyte and fibroblast interaction using layer-by-layer deposition facilitates synchronisation of cardiac macro tissues. Soft Matter 2020, 16, 428–434. [Google Scholar] [CrossRef]
- Kitsara, M.; Joanne, P.; Boitard, S.E.; Ben Dhiab, I.; Poinard, B.; Menasché, P.; Gagnieu, C.; Forest, P.; Agbulut, O.; Chen, Y. Fabrication of cardiac patch by using electrospun collagen fibers. Microelectron. Eng. 2015, 144, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Pok, S.; Myers, J.D.; Madihally, S.V.; Jacot, J.G. A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater. 2013, 9, 5630–5642. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Jiang, J.; Hua, W.; Darabi, A.; Song, X.; Song, C.; Zhong, W.; Xing, M.M.Q.; Qiu, X. Mussel-Inspired Conductive Cryogel as Cardiac Tissue Patch to Repair Myocardial Infarction by Migration of Conductive Nanoparticles. Adv. Funct. Mater. 2016, 26, 4293–4305. [Google Scholar] [CrossRef]
- Navaei, A.; Saini, H.; Christenson, W.; Sullivan, R.T.; Ros, R.; Nikkhah, M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016, 41, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Eder, A.; Bonstrup, M.; Flato, M.; Mewe, M.; Schaaf, S.; Aksehirlioglu, B.; Schwoerer, A.P.; Uebeler, J.; Eschenhagen, T. Development of a drug screening platform based on engineered heart tissue. Circ. Res. 2010, 107, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tulloch, N.L.; Muskheli, V.; Razumova, M.V.; Korte, F.S.; Regnier, M.; Hauch, K.D.; Pabon, L.; Reinecke, H.; Murry, C.E. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ. Res. 2011, 109, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Bargehr, J.; Ong, L.P.; Colzani, M.; Davaapil, H.; Hofsteen, P.; Bhandari, S.; Gambardella, L.; Le Novere, N.; Iyer, D.; Sampaziotis, F.; et al. Epicardial cells derived from human embryonic stem cells augment cardiomyocyte-driven heart regeneration. Nat. Biotechnol. 2019, 37, 895–906. [Google Scholar] [CrossRef]
- Mannhardt, I.; Breckwoldt, K.; Letuffe-Breniere, D.; Schaaf, S.; Schulz, H.; Neuber, C.; Benzin, A.; Werner, T.; Eder, A.; Schulze, T.; et al. Human Engineered Heart Tissue: Analysis of Contractile Force. Stem Cell Rep. 2016, 7, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Lemme, M.; Ulmer, B.M.; Lemoine, M.D.; Zech, A.T.L.; Flenner, F.; Ravens, U.; Reichenspurner, H.; Rol-Garcia, M.; Smith, G.; Hansen, A.; et al. Atrial-like Engineered Heart Tissue: An in vitro Model of the Human Atrium. Stem Cell Rep. 2018, 11, 1378–1390. [Google Scholar] [CrossRef] [Green Version]
- Maffioletti, S.M.; Sarcar, S.; Henderson, A.B.H.; Mannhardt, I.; Pinton, L.; Moyle, L.A.; Steele-Stallard, H.; Cappellari, O.; Wells, K.E.; Ferrari, G.; et al. Three-Dimensional Human iPSC-Derived Artificial Skeletal Muscles Model Muscular Dystrophies and Enable Multilineage Tissue Engineering. Cell Rep. 2018, 23, 899–908. [Google Scholar] [CrossRef] [Green Version]
- Cohn, R.; Thakar, K.; Lowe, A.; Ladha, F.A.; Pettinato, A.M.; Romano, R.; Meredith, E.; Chen, Y.S.; Atamanuk, K.; Huey, B.D.; et al. A Contraction Stress Model of Hypertrophic Cardiomyopathy due to Sarcomere Mutations. Stem Cell Rep. 2019, 12, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, M.L.; Werner, T.R.; Becker, B.; Eschenhagen, T.; Hirt, M.N. A magnetics-based approach for fine-tuning afterload in engineered heart tissues. ACS Biomater. Sci. Eng. 2019, 5, 3663–3675. [Google Scholar] [CrossRef]
- Tijore, A.; Irvine, S.A.; Sarig, U.; Mhaisalkar, P.; Baisane, V.; Venkatraman, S. Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel. Biofabrication 2018, 10, 025003. [Google Scholar] [CrossRef]
- Lind, J.U.; Busbee, T.A.; Valentine, A.D.; Pasqualini, F.S.; Yuan, H.; Yadid, M.; Park, S.J.; Kotikian, A.; Nesmith, A.P.; Campbell, P.H.; et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater. 2017, 16, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, R.; Doevendans, P.A.; Metz, C.H.; Alblas, J.; Messina, E.; Giacomello, A.; Sluijter, J.P. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 2012, 33, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, R.; Feyen, D.A.; Verhage, V.; Slaats, R.; Messina, E.; Christman, K.L.; Giacomello, A.; Doevendans, P.A.; Sluijter, J.P. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 2015, 61, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Park, H.J.; Kim, S.W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lee, S.J.; Cheng, H.J.; Yoo, J.J.; Atala, A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018, 70, 48–56. [Google Scholar] [CrossRef]
- Ho, C.M.; Mishra, A.; Lin, P.T.; Ng, S.H.; Yeong, W.Y.; Kim, Y.J.; Yoon, Y.J. 3D Printed Polycaprolactone Carbon Nanotube Composite Scaffolds for Cardiac Tissue Engineering. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef]
- Zhu, K.; Shin, S.R.; van Kempen, T.; Li, Y.C.; Ponraj, V.; Nasajpour, A.; Mandla, S.; Hu, N.; Liu, X.; Leijten, J.; et al. Gold Nanocomposite Bioink for Printing 3D Cardiac Constructs. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Lei, Q.; He, J.; Li, D. Electrohydrodynamic 3D printing of layer-specifically oriented, multiscale conductive scaffolds for cardiac tissue engineering. Nanoscale 2019, 11, 15195–15205. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.; Yang, B.; Li, R.-K. Application of Biomaterials in Cardiac Repair and Regeneration. Engineering 2016, 2, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Gorain, B.; Choudhury, H.; Pandey, M.; Kesharwani, P.; Abeer, M.M.; Tekade, R.K.; Hussain, Z. Carbon nanotube scaffolds as emerging nanoplatform for myocardial tissue regeneration: A review of recent developments and therapeutic implications. Biomed. Pharmacother. 2018, 104, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Pok, S.; Vitale, F.; Eichmann, S.L.; Benavides, O.M.; Pasquali, M.; Jacot, J.G. Biocompatible carbon nanotube-chitosan scaffold matching the electrical conductivity of the heart. ACS Nano 2014, 8, 9822–9832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Eng, G.; Caridade, S.G.; Mano, J.F.; Reis, R.L.; Vunjak-Novakovic, G. Electrically conductive chitosan/carbon scaffolds for cardiac tissue engineering. Biomacromolecules 2014, 15, 635–643. [Google Scholar] [CrossRef]
- Wu, F.; Gao, A.; Liu, J.; Shen, Y.; Xu, P.; Meng, J.; Wen, T.; Xu, L.; Xu, H. High Modulus Conductive Hydrogels Enhance In Vitro Maturation and Contractile Function of Primary Cardiomyocytes for Uses in Drug Screening. Adv. Healthc. Mater. 2018, 7, e1800990. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Prabhakaran, M.P.; Kai, D.; Ramakrishna, S. Human cardiomyocyte interaction with electrospun fibrinogen/gelatin nanofibers for myocardial regeneration. J. Biomater. Sci. Polym. Ed. 2013, 24, 1660–1675. [Google Scholar] [CrossRef]
- Kai, D.; Prabhakaran, M.P.; Jin, G.; Ramakrishna, S. Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 379–386. [Google Scholar] [CrossRef]
- Ding, M.; Andersson, H.; Martinsson, S.; Sabirsh, A.; Jonebring, A.; Wang, Q.D.; Plowright, A.T.; Drowley, L. Aligned nanofiber scaffolds improve functionality of cardiomyocytes differentiated from human induced pluripotent stem cell-derived cardiac progenitor cells. Sci. Rep. 2020, 10, 13575. [Google Scholar] [CrossRef]
- Hussain, A.; Collins, G.; Yip, D.; Cho, C.H. Functional 3-D cardiac co-culture model using bioactive chitosan nanofiber scaffolds. Biotechnol. Bioeng. 2013, 110, 637–647. [Google Scholar] [CrossRef]














| Source | Scaffold | Type | Advantage | Reference |
|---|---|---|---|---|
| Natural | Fibronectin | 2D surface coating | -Improved cell attachment, actin filaments development and cell stiffness | [75,76] |
| Natural | Fibroblast-derived ECM | 2D surface coating | -Improved cell adhesion, cytoskeleton development and contractility | [77] |
| Natural | Decellularized ECM | 2D surface coating | -Improved cardiac cell differentiation | [78] |
| Synthetic | Peptide-acrylate surface (PAS) | 2D surface coating | -Chemically defined and xeno-free materials | [80] |
| Synthetic | PLA with poly(poly(ethylene glycol) methacrylate) and poly[N-(3-aminopropyl)methacrylamide] | 2D surface coating | -Increased cell attachment and contractility | [81] |
| Synthetic/Natural | poly(ethylene glycol) diacrylate with thiolated-hyaluronic acid (HA) | 2D surface coating | -Increased stiffness of hydrogels and cell maturation | [14] |
| Synthetic/Natural | Conductive polypyrrole (PPY) with chitosan (CHI) | 2D surface coating | -Increased Ca2+ transient and contractility | [82] |
| Synthetic | PEG with RGD peptide | Surface patterning | -Improved cell organization and structural development | [84,85] |
| Synthetic/Natural | poly(ethylene glycol) with Matrigel and fibronectin | Surface patterning | -Improved cell alignment | [86] |
| Synthetic | Electrically conductive silicon nanowires (e-SiNW) | Spheroids | -Improved synchronized beating and structural maturation | [93,94] |
| Natural | Fibrin gel | Patterned patch | -Improved sarcomere development, maturation, and synchronization | [97] |
| Natural | Collagen type I | Patterned patch | -Improved synchronized beating and maturation | [9,98] |
| Natural | Atelocollagen | Fibrous patch | -Improved biocompatibility | [99] |
| Synthetic/Natural | PCL in gelatin-chitosan hydrogel | Patch | -Improved mechanical properties | [100] |
| Synthetic/Natural | PPY nanoparticles, gelatin-methacrylate, poly(ethylene glycol) diacrylate | Patch | -Improved synchronized beating and maturation | [101] |
| Synthetic/Natural | Gold nanorod-incorporated gelatin methacrylate | Patch | -Improved cell adhesion, viability, metabolic activity, and maturation | [102] |
| Natural | Collagen | Rod-shaped EHT | -Improved myofibrillogenesis and sarcomeric banding | [104,105] |
| Natural | Fibrin gel | Rod-shaped EHT | -Improved sarcomere organization and number of mitochondria | [106] |
| Natural | Alginate hydrogel | Bio ink | -Improved cell viability and expression of cardiac transcription factor | [113] |
| Natural | Hyaluronic acid/gelatin | Bio ink | -Improved cardiac differentiation | [114] |
| Natural | Decellularized ECM | Bio ink | -Improved alignment and complex structure | [116] |
| Synthetic | PCL with carbon nanotube | Bio ink | -Improved mechanical properties | [117] |
| Synthetic | Gold nanorods-GelMA | Bio ink | -Increased cell adhesion and synchronized contraction | [118] |
| Synthetic | PCL with PEDOT:PSS-PEO conductive fibers | Bio ink | -Improved conductivity, cell adhesion and synchronized beating | [119] |
| Natural | Decellularized hydrogel with gelatin | Bio ink | -Increased formation of a complex structure | [120] |
| Synthetic/Natural | Carbon nanotube with gelatin-chitosan hydrogel | 3D scaffold | -Improved electrical coupling and synchronized beating | [123] |
| Synthetic/Natural | Chitosan-carbon composite | 3D scaffold | -Improved electrical properties and porosity | [125] |
| Synthetic | Polyvinyl alcohol with carbon fibers | 3D scaffold | -Improved conductivity and elastic modulus | [126] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, Y.; Park, Y.; Kim, J. Engineering Biomaterials to Guide Heart Cells for Matured Cardiac Tissue. Coatings 2020, 10, 925. https://doi.org/10.3390/coatings10100925
Jang Y, Park Y, Kim J. Engineering Biomaterials to Guide Heart Cells for Matured Cardiac Tissue. Coatings. 2020; 10(10):925. https://doi.org/10.3390/coatings10100925
Chicago/Turabian StyleJang, Yongjun, Yongdoo Park, and Jongseong Kim. 2020. "Engineering Biomaterials to Guide Heart Cells for Matured Cardiac Tissue" Coatings 10, no. 10: 925. https://doi.org/10.3390/coatings10100925
APA StyleJang, Y., Park, Y., & Kim, J. (2020). Engineering Biomaterials to Guide Heart Cells for Matured Cardiac Tissue. Coatings, 10(10), 925. https://doi.org/10.3390/coatings10100925







