Epitaxial Silver Films Morphology and Optical Properties Evolution over Two Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Thin Films
2.2. Over-Time Characterization
3. Results
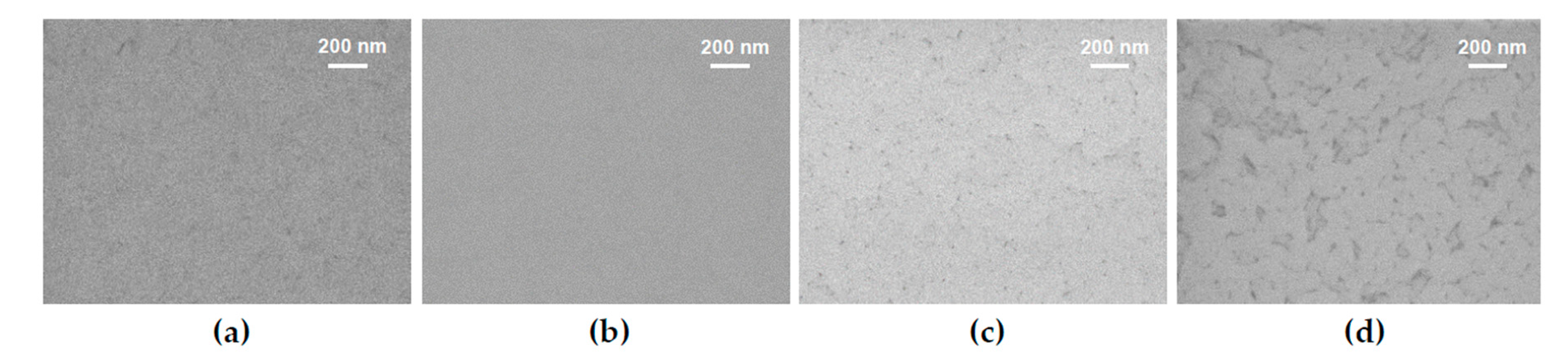
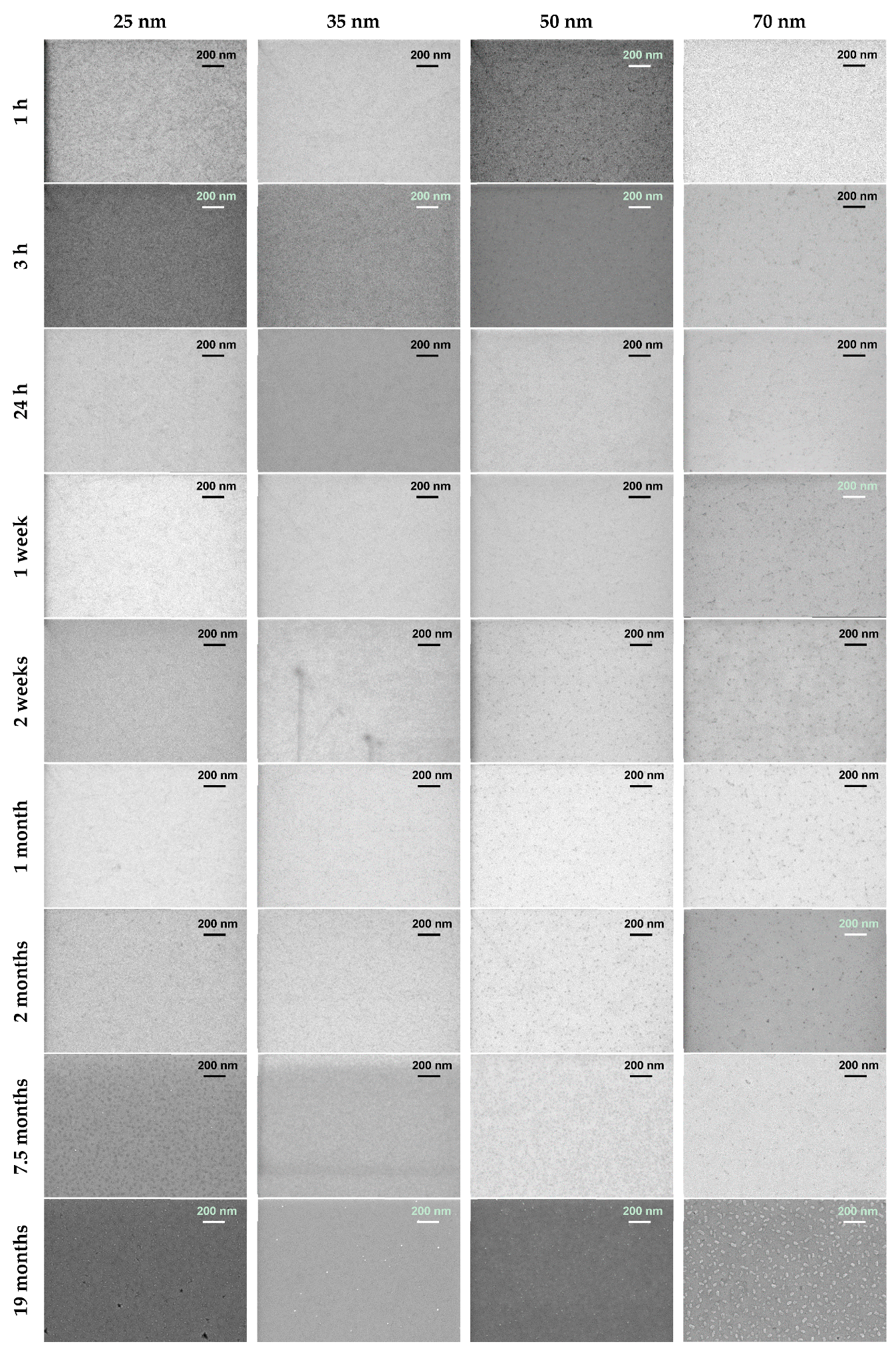
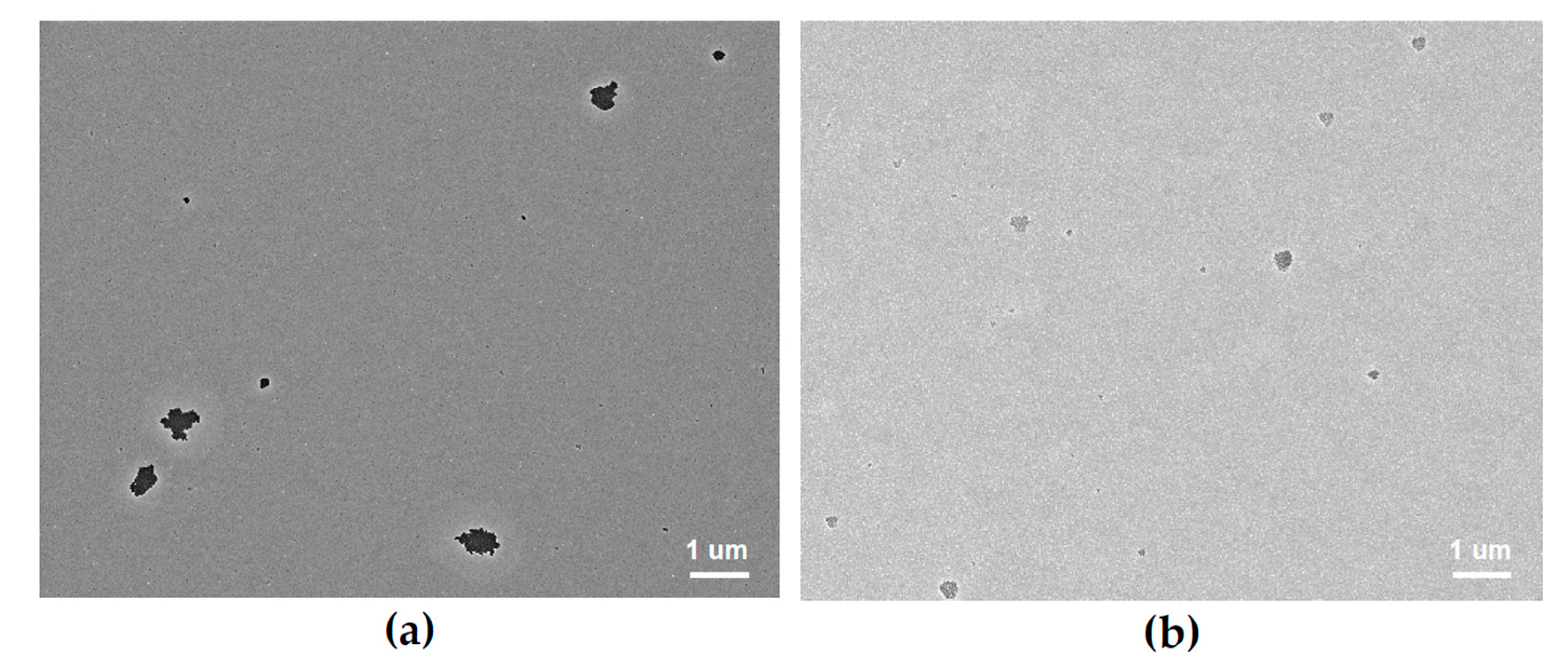
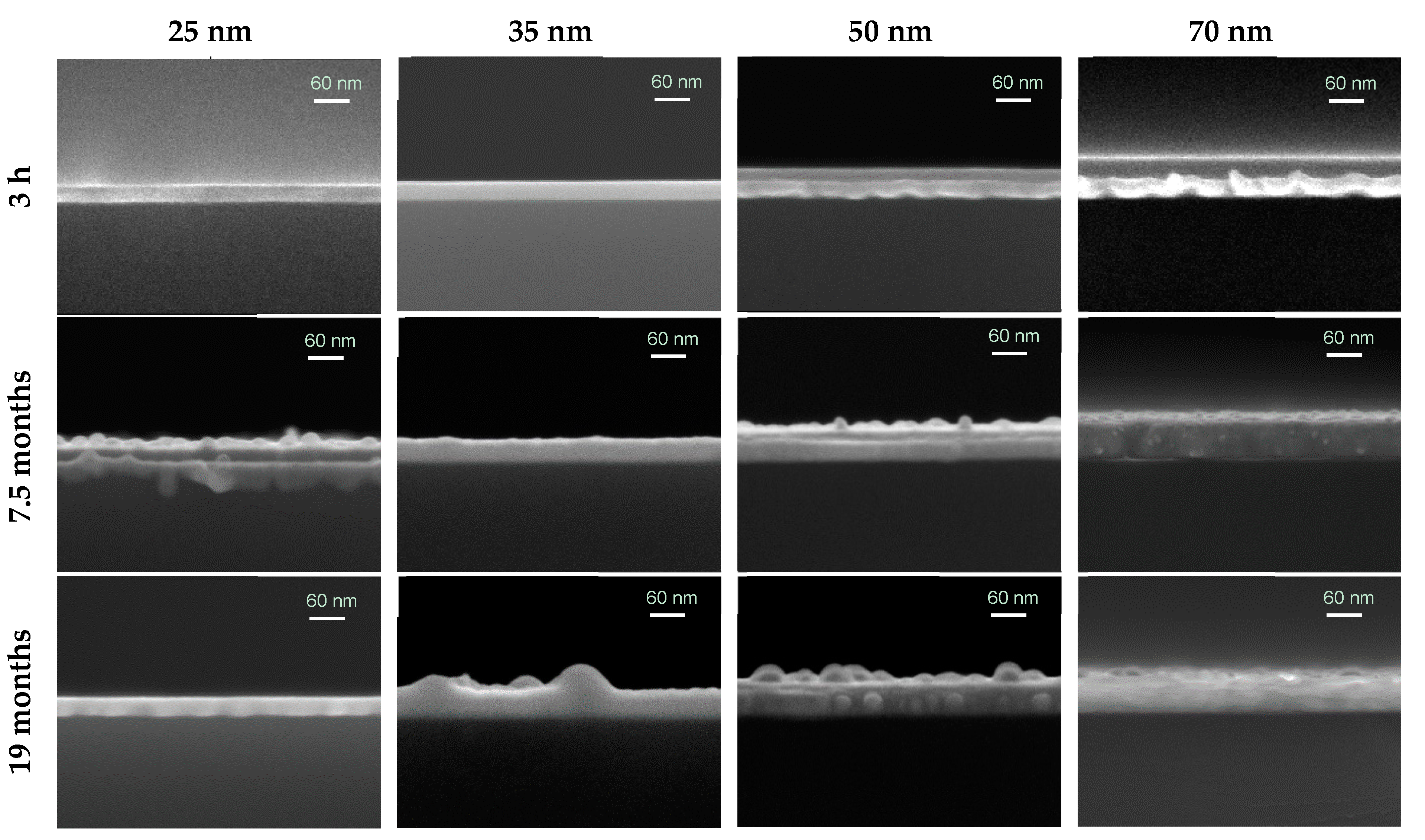
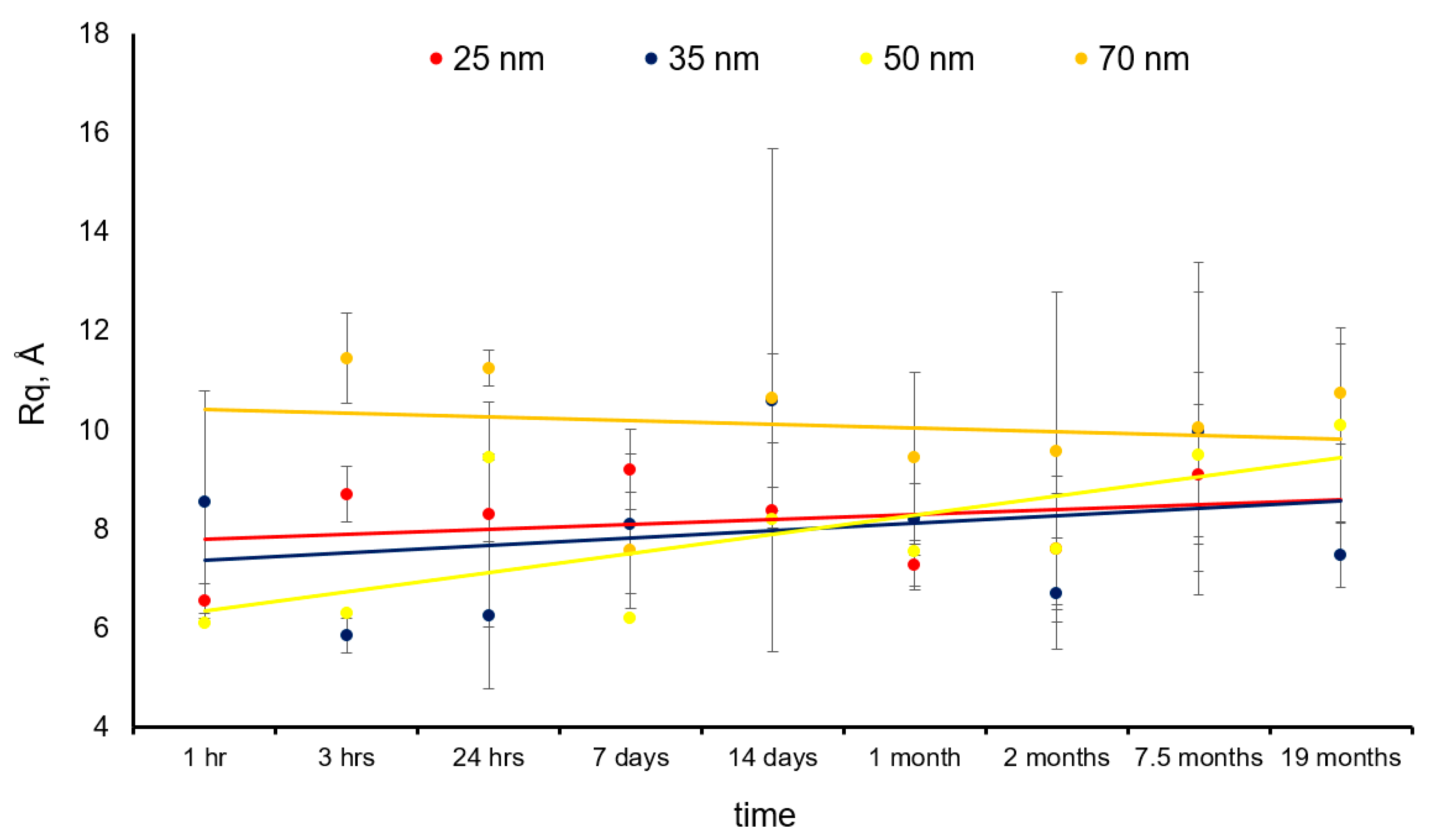
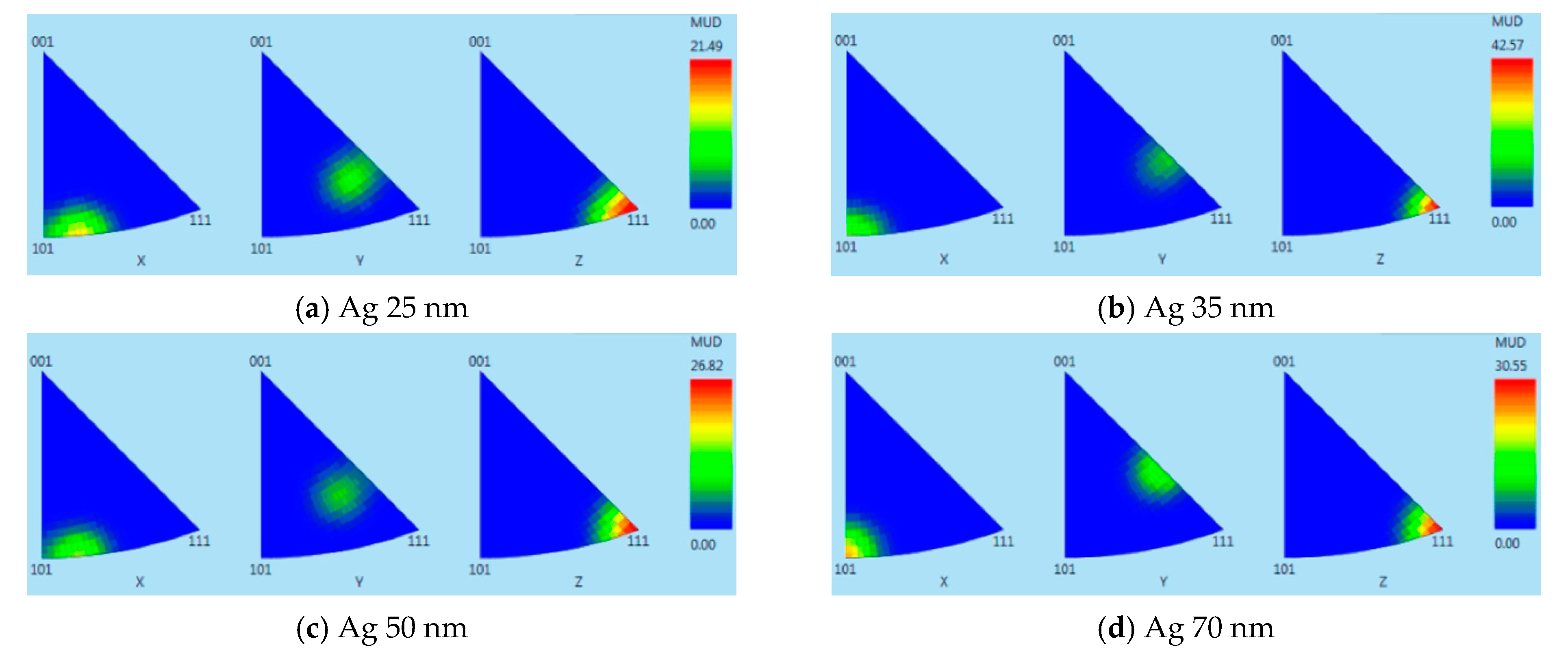
3.1. Thin Film Surface Structure
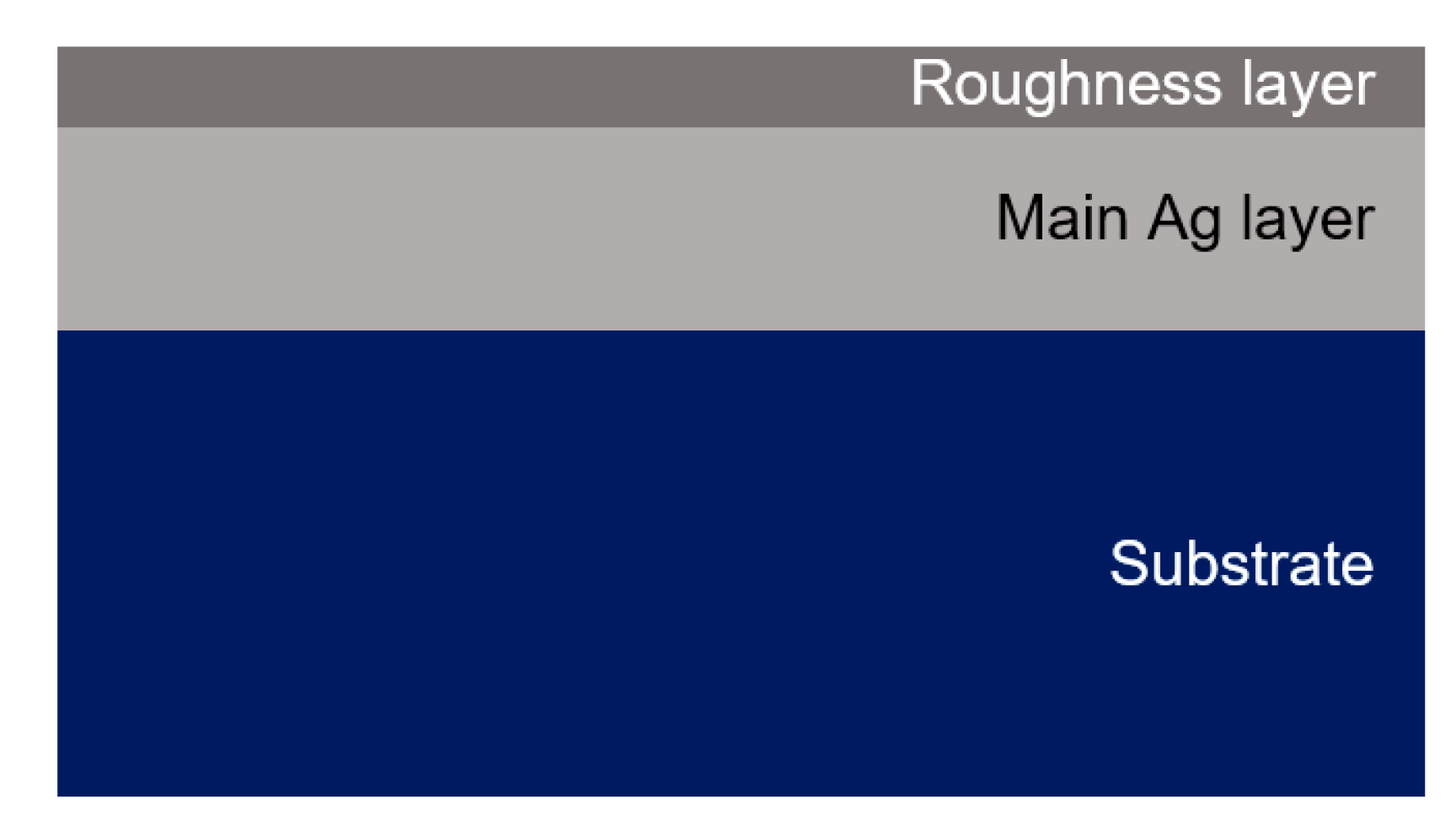
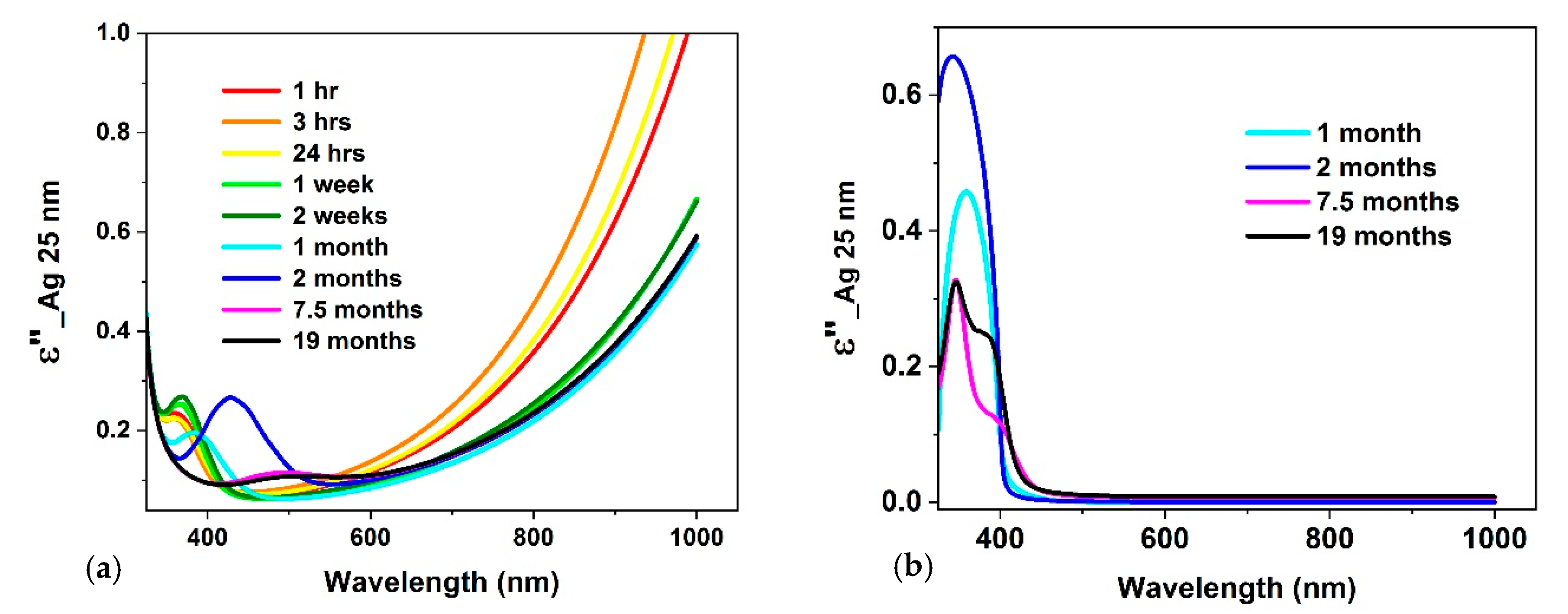
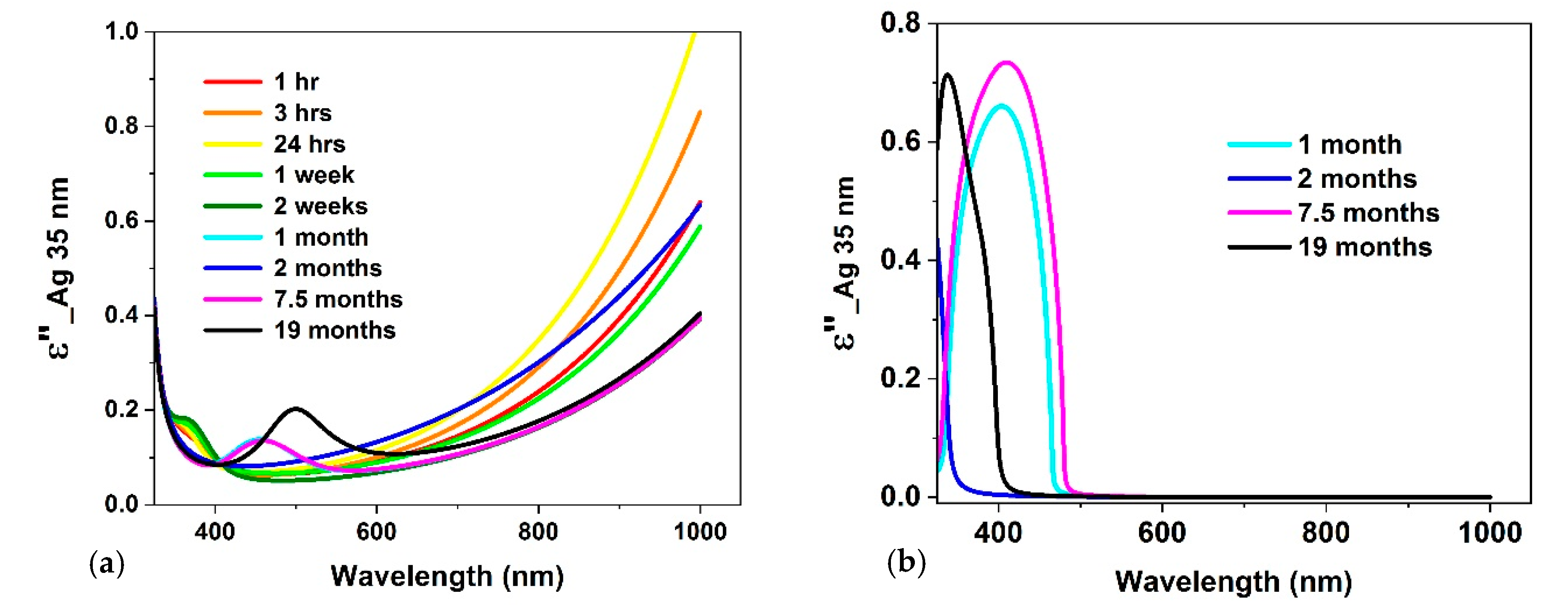
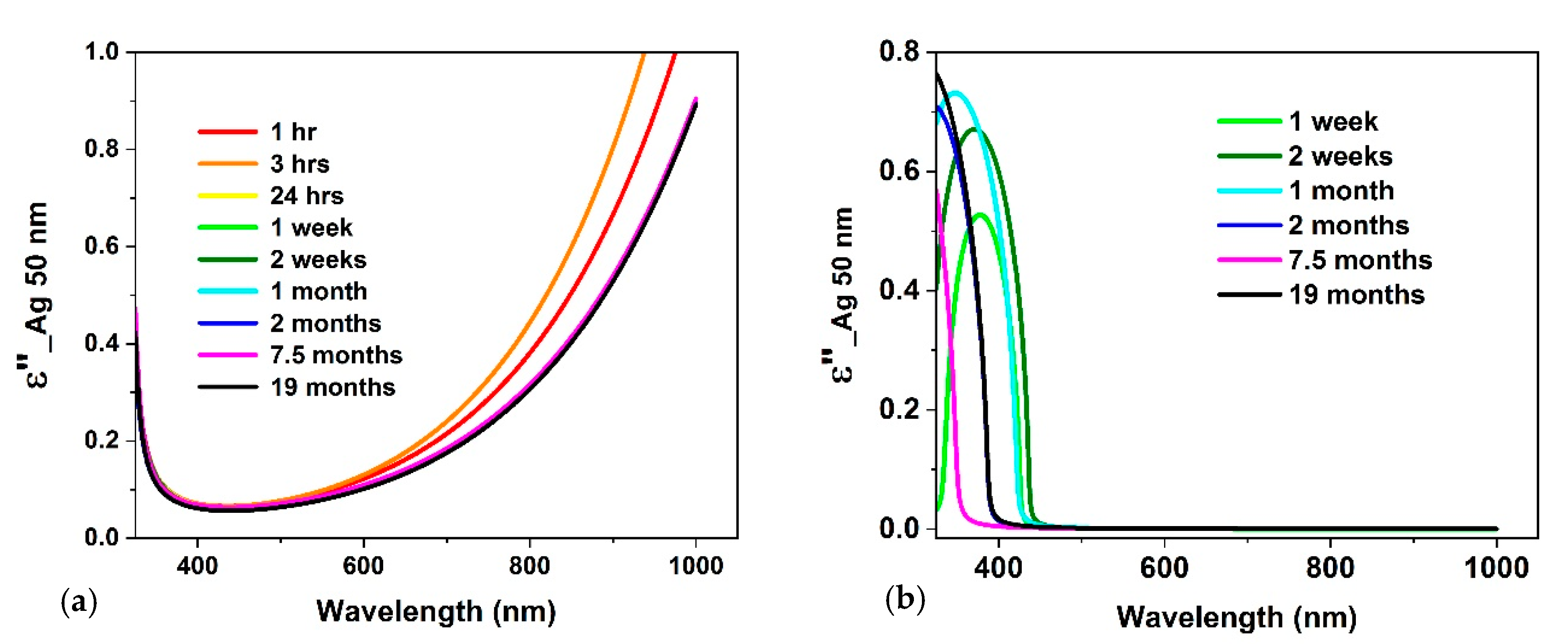
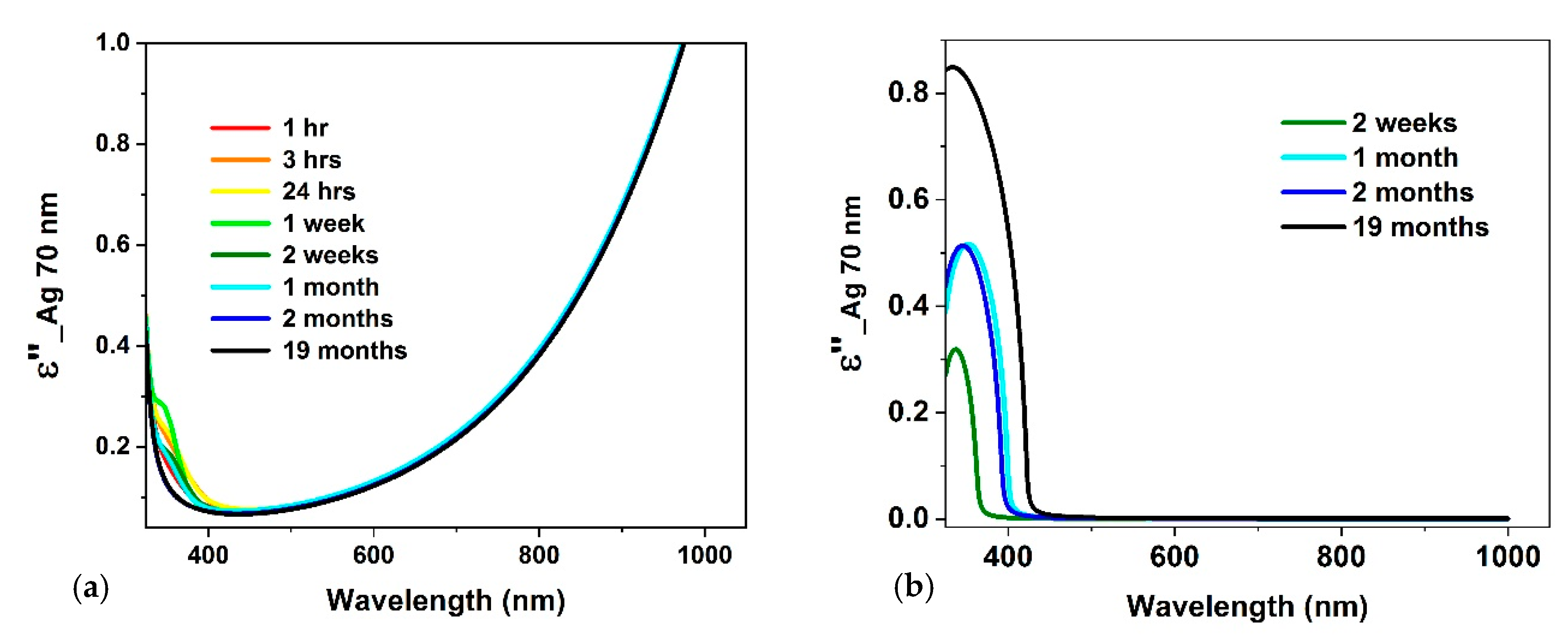
3.2. Optical Constants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baburin, A.S.; Merzlikin, A.M.; Baryshev, A.V.; Ryzhikov, I.A.; Panfilov, Y.V.; Rodionov, I.A. Silver-based plasmonics: Golden material platform and application challenges. Opt. Mater. Express 2019, 9, 611–642. [Google Scholar] [CrossRef]
- West, P.R.; Ishii, S.; Naik, G.V.; Emani, N.K.; Shalaev, V.M.; Boltasseva, A. Searching for better plasmonic materials. Laser Photonics Rev. 2010, 4, 795–808. [Google Scholar] [CrossRef]
- Yankovskii, G.M.; Komarov, A.V.; Puz’ko, R.S.; Baryshev, A.V.; Afanas’ev, K.N.; Boginskaya, I.A.; Bykov, I.V.; Merzlikin, A.M.; Rodionov, I.A.; Ryzhikov, I.A. Structural and optical properties of single and bilayer silver and gold films. Phys. Solid State 2016, 58, 2503–2510. [Google Scholar] [CrossRef]
- Haffner, C.; Chelladurai, D.; Fedoryshyn, Y.; Josten, A.; Baeuerle, B.; Heni, W.; Watanabe, T.; Cui, T.; Cheng, B.; Saha, S.; et al. Low-loss plasmon-assisted electro-optic modulator. Nature 2018, 556, 483–486. [Google Scholar] [CrossRef]
- Ma, R.M.; Yin, X.B.; Oulton, R.F.; Sorger, V.J.; Zhang, X. Multiplexed and Electrically Modulated Plasmon Laser Circuit. Nano Lett. 2012, 12, 5396–5402. [Google Scholar] [CrossRef] [PubMed]
- Danqing, W.; Weijia, W.; Knudson, M.P.; Schatz, G.C.; Odom, T.W. Structural Engineering in Plasmon Nanolasers. Chem. Rev. 2018, 118, 2865–2881. [Google Scholar]
- Nahata, A.; Linke, R.A.; Ishi, T.; Ohashi, K. Enhanced nonlinear optical conversion from a periodically nanostructured metal film. Opt. Lett. 2003, 28, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Eisenbraum, E.T.; Klaver, A.; Patel, Z.; Nuesca, G.; Kaloyeros, A.E. Low temperature metalorganic chemical vapor deposition of conformal silver coatings for applications in high aspect ratio structures. J. Vac. Sci. Technol. B 2001, 19, 585. [Google Scholar] [CrossRef]
- Baburin, A.S.; Kalmykov, A.S.; Kirtaev, R.V.; Negrov, D.V.; Moskalev, D.O.; Ryzhikov, I.A.; Melentiev, P.N.; Rodionov, I.A.; Balykin, V.I. Toward a theoretically limited SPP propagation length above two hundred microns on an ultra-smooth silver surface. Opt. Mater. Express 2018, 8, 3254–3261. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Baburin, A.S.; Rizhikov, I.A.; Trofimov, I.V.; Philippov, I.A.; Gabidulin, A.R.; Dobronosova, A.A.; Vinogradov, A.P.; Zverev, A.V.; Ivanov, A.I.; et al. Mass production compatible fabrication techniques of single-crystalline silver metamaterials and plasmonics devices. In Proceedings of the Metamaterials, Metadevices, and Metasystems 2017, San Diego, CA, USA, 6–10 August 2017. [Google Scholar]
- Melentiev, P.; Kalmykov, A.; Gritchenko, A.; Afanasiev, A.; Balykin, V.; Baburin, A.S.; Ryzhova, E.; Filippov, I.; Rodionov, I.A.; Nechepurenko, I.A.; et al. Plasmonic nanolaser for intracavity spectroscopy and sensorics. Appl. Phys. Lett. 2017, 111, 213104. [Google Scholar] [CrossRef]
- Baburin, A.S.; Ivanov, A.I.; Trofimov, I.V.; Dobronosova, A.A.; Melentiev, P.N.; Balykin, V.I.; Moskalev, D.O.; Pishchimova, A.A.; Ganieva, L.A.; Ryzhikov, I.A.; et al. Highly directional plasmonic nanolaser based on high-performance noble metal film photonic crystal. In Proceedings of the Nanophotonics VII. International Society for Optics and Photonics, Strasbourg, France, 22–26 April 2018; p. 10672. [Google Scholar]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Baburin, A.S.; Gritchenko, A.S.; Orlikovsky, N.A.; Dobronosova, A.A.; Rodionov, I.A.; Balykin, V.I.; Melentiev, P.N. State-of-the-art plasmonic crystals for molecules fluorescence detection. Opt. Mater. Express 2019, 9, 1173–1179. [Google Scholar] [CrossRef]
- Lagarkov, A.; Boginskaya, I.; Bykov, I.; Budashov, I.; Ivanov, A.; Kurochkin, I.; Ryzhikov, I.; Rodionov, I.; Sedova, M.; Zverev, A.; et al. Light localization and SERS in tip-shaped silicon metasurface. Opt. Express 2017, 25, 17021–17038. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.M.; Zhou, L.; Su, X.R.; Xiao, S.; Liu, S.D.; Wang, Q.Q. Illuminating Dark Plasmons of Silver Nanoantenna Rings to Enhance Exciton-Plasmon Interactions. Adv. Funct. Mater. 2009, 19, 298–303. [Google Scholar] [CrossRef]
- Bogdanov, S.I.; Shalaginov, M.Y.; Lagutchev, A.S.; Chiang, C.C.; Shah, D.; Baburin, A.S.; Ryzhikov, I.A.; Rodionov, I.A.; Kildishev, A.V.; Boltasseva, A.; et al. Ultrabright room-temperature sub-nanosecond emission from single nitrogen-vacancy centers coupled to nanopatch antennas. Nano Lett. 2018, 18, 4837–4844. [Google Scholar] [CrossRef]
- High, A.A.; Devlin, R.C.; Dibos, A.; Polking, M.; Wild, D.S.; Perczel, J.; de Leon, N.P.; Lukin, M.D.; Park, H. Visible-frequency hyperbolic metasurface. Nature 2015, 522, 192–196. [Google Scholar] [CrossRef]
- Nepal, D.; Park, K.; Vaia, R.A. High-Yield Assembly of Soluble and Stable Gold Nanorod Pairs for High-Temperature Plasmonics. Small 2012, 8, 1013–1020. [Google Scholar] [CrossRef]
- Park, J.H.; Ambwani, P.; Manno, M.; Lindquist, N.C.; Nagpal, P.; Oh, S.H.; Leighton, C.; Norris, D.J. Single-crystalline silver films for plasmonics. Adv. Mater. 2010, 24, 3988–3992. [Google Scholar] [CrossRef]
- Breslin, C.B.; Macdonald, D.D. The influence of Uv light on the dissolution and passive behavior of copper-containing alloys in chloride solution. Electrochim. Acta 1998, 44, 643–651. [Google Scholar] [CrossRef]
- Burleigh, T.D.; Ruhe, C.; Forsyth, J. Photo-corrosion of different metals during long-term exposure to ultraviolet light. Corros. Sci. 2003, 59, 774–779. [Google Scholar] [CrossRef]
- Keast, V.J.; Myles, T.A.; Shahcheraghi, N.; Cortie, M.B. Corrosion processes of triangular silver nanoparticles compared to bulk silver. J. Nanopart. Res. 2016, 18, 45. [Google Scholar] [CrossRef]
- Oates, T.W.H.; Losurdo, M.; Noda, S.; Hinrichs, K. The effect of atmospheric tarnishing on the optical and structural properties of silver nanoparticles. J. Phys. D Appl. Phys. 2013, 46, 145308. [Google Scholar] [CrossRef]
- Águas, H.; Silva, R.J.C.; Viegas, M.; Pereira, L.; Fortunato, E.; Martins, R. Study of environmental degradation of silver surface. Phys. Status Solidi C 2008, 5, 1215–1218. [Google Scholar] [CrossRef]
- Trautmann, S.; Dathe, A.; Csáki, A.; Thiele, M.; Müller, R.; Fritzsche, W.; Stranik, O. Time-Resolved Study of Site-Specific Corrosion in a Single Crystalline Silver Nanoparticle. Nanoscale Res. Lett. 2019, 14, 240. [Google Scholar] [CrossRef] [PubMed]
- Sanders, C.E.; Zhang, C.; Kellogg, G.L.; Shih, C.K. Role of thermal processes in dewetting of epitaxial Ag(111) film on Si(111). Surf. Sci. 2014, 630, 168–173. [Google Scholar] [CrossRef]
- Wang, X.; Santschi, C.; Martin, O.J.F. Strong Improvement of Long-Term Chemical and Thermal Stability of Plasmonic Silver Nanoantennas and Films. Small 2017, 13, 1700044. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Alford, T.L.; Allee, D.R. Thickness Dependence on the Thermal Stability of Silver Thin Films. Appl. Phys. Lett. 2002, 81, 4287–4289. [Google Scholar] [CrossRef]
- Thompson, C.V. Solid-State Dewetting of Thin Films. Annu. Rev. Mater. Res. 2012, 42, 399–434. [Google Scholar] [CrossRef]
- Chi, L.; Bassim, N. Ag Thin Film Dewetting Prevention by Ion Implantation. Adv. Mater. Interfaces 2019, 6, 1900108. [Google Scholar] [CrossRef]
- Ciesielski, A.; Skowronski, L.; Trzcinski, M.; Szoplik, T. Controlling the optical parameters of self-assembled silver films with wetting layers and annealing. Appl. Surf. Sci. 2017, 421, 349–356. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Baburin, A.S.; Gabidullin, A.R.; Maklakov, S.S.; Peters, S.; Ryzhikov, I.A.; Andriyash, A.V. Quantum Engineering of Atomically Smooth Single Crystalline Silver Films. Sci. Rep. 2019, 9, 12232. [Google Scholar] [CrossRef] [PubMed]
- Brendel, R.; Bormann, D. An infrared dielectric function model for amorphous solids. J. Appl. Phys. 1992, 71, 1–6. [Google Scholar] [CrossRef]
- Bruggeman, D.A.G. The calculation of various physical constants of heterogeneous substances. I. The dielectric constants and conductivities of mixtures composed of isotropic substances. Ann. Phys. 1935, 23, 636–664. [Google Scholar] [CrossRef]
- Li, X.D.; Chen, T.P.; Liu, Y.; Leong, K.C. Influence of localized surface plasmon resonance and free electrons on the optical properties of ultrathin Au films: A study of the aggregation effect. Opt. Express 2014, 22, 5124–5132. [Google Scholar] [CrossRef] [PubMed]
- Abermann, R. Measurements of the intrinsic stress in thin metal films. Vacuum 1990, 41, 1279–1282. [Google Scholar] [CrossRef]
- Chason, E.; Guduru, P.R. Tutorial: Understanding residual stress in polycrystalline thin films through real-time measurements and physical models. J. Appl. Phys. 2016, 119, 191101. [Google Scholar] [CrossRef]
- Leib, J.; Mo¨nig, R.; Thompson, V. Direct Evidence for Effects of Grain Structure on Reversible Compressive Deposition Stresses in Polycrystalline Gold Films. Phys. Rev. Lett. 2009, 102, 256101. [Google Scholar] [CrossRef]
- Nix, W.D.; Clemens, B.M. Crystallite coalescence: A mechanism for intrinsic tensile stresses in thin films. J. Mater. Res. 1999, 14, 3467–3473. [Google Scholar] [CrossRef]
- Spaepen, F. Interfaces and stresses in thin films. Acta Mater. 2000, 48, 31–42. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baburin, A.S.; Ivanov, A.I.; Lotkov, E.S.; Sorokina, O.S.; Boginskaya, I.A.; Sergeev, E.V.; Buzaverov, K.A.; Konstantinova, T.G.; Moskalev, D.O.; Issabayeva, Z.; et al. Epitaxial Silver Films Morphology and Optical Properties Evolution over Two Years. Coatings 2020, 10, 911. https://doi.org/10.3390/coatings10100911
Baburin AS, Ivanov AI, Lotkov ES, Sorokina OS, Boginskaya IA, Sergeev EV, Buzaverov KA, Konstantinova TG, Moskalev DO, Issabayeva Z, et al. Epitaxial Silver Films Morphology and Optical Properties Evolution over Two Years. Coatings. 2020; 10(10):911. https://doi.org/10.3390/coatings10100911
Chicago/Turabian StyleBaburin, Aleksandr S., Anton I. Ivanov, Evgeniy S. Lotkov, Olga S. Sorokina, Irina A. Boginskaya, Evgeniy V. Sergeev, Kirill A. Buzaverov, Tatiana G. Konstantinova, Dmitriy O. Moskalev, Zhamila Issabayeva, and et al. 2020. "Epitaxial Silver Films Morphology and Optical Properties Evolution over Two Years" Coatings 10, no. 10: 911. https://doi.org/10.3390/coatings10100911
APA StyleBaburin, A. S., Ivanov, A. I., Lotkov, E. S., Sorokina, O. S., Boginskaya, I. A., Sergeev, E. V., Buzaverov, K. A., Konstantinova, T. G., Moskalev, D. O., Issabayeva, Z., Ryzhikov, I. A., & Rodionov, I. A. (2020). Epitaxial Silver Films Morphology and Optical Properties Evolution over Two Years. Coatings, 10(10), 911. https://doi.org/10.3390/coatings10100911




