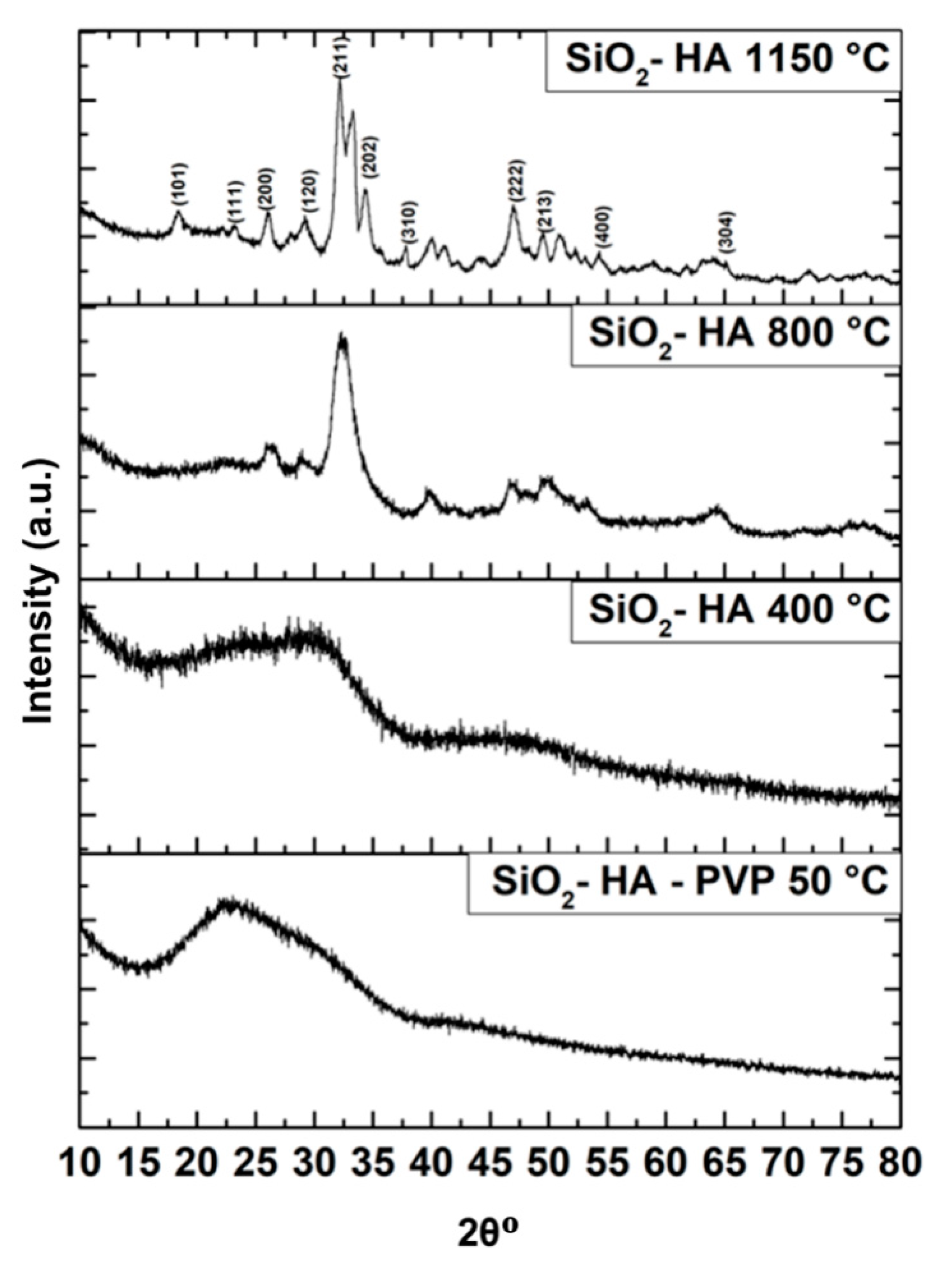
3.1. FTIR Characterization
The infrared absorption spectra of the SiO
2–hydroxyapatite (HA)–PVP fibers are represented in
Figure 1a. There can be several multiple bands corresponding to vibrations from the PVP used for electrospinning. The bands located at 2951 and 1420 cm
−1 correlate to asymmetrical stretching vibrations of methylene (CH
2) and out of plane bending vibrations of the C–H bond, respectively. An intense and narrow band is observed at 1643 cm
−1, characteristic for the C=O bond stretching. According to [
15], the location of this PVP band varies from 1633 to 1644 cm
−1 depending on the molecular weight of the polymer. Likewise, it is reported that, for a weight of 1,300,000 just as the polymer used, the absorption peak should be found at 1633 cm
−1, while there is a possibility in which this maximum is blue-shifted because of the formation of coordination bonds between the oxygen in the carbonyl group and some metal ion such as Ca
2+ in the hydroxyapatite. The band at 1312 cm
−1 belongs to the stretching vibration of C–N in the PVP. In
Figure 1a, it is primarily observed that bands around 3400 and 2950 cm
−1 drastically decrease as the temperature rises due to the progressive decomposition of the polymer and the residual organic matter calcination. The same occurs for the polymer bands at 1643, 1420, 1313, 1167 and 738 cm
−1: they all remain visible at 400 °C. From 200 °C on, the band of Si–O–Si bending turns evident and at 400 °C a band emerges at 821 cm
−1 analogous to the early formation of silanol groups (Si–OH), indicative of chemical instability in the silica, which at current sintering temperature has not yet lost its –OH groups [
1]. At 579 cm
−1 there is a band caused by the phosphate group stretching, more noticeable at 200 and 400 °C. The fibers sintered at 600 °C in
Figure 1b present a well-defined and intense band near 1445 cm
−1 related to the carbonate group. It is appreciated only until the calcium carbonate’s decomposition temperature at 800 °C. The Si–OH band gradually reduces from 600 to 1150 °C, proving the loss of –OH groups from silica. A protruding band at 1046 cm
−1 associated to the ν
3 mode of the phosphate group is found from 600 to 1150 °C. A shoulder at 1083 cm
−1 becomes prominent from 1000 °C on and can be attributed to a contribution from both the phosphate ν
3 mode and the stretching of the O–Si–O bond. Other vibrations corresponding to the ν
2 and ν
4 modes of phosphate are appreciated at 473 and 579 cm
−1, respectively, along all different sintering temperatures, and more clearly at 1150 °C just as
Figure 1b displays.
Du et al. [
16] report three major bands located at 460, 808 and 1090 cm
−1 as part of the characterization of large-scale produced silica fibers by a vapor transport process. The band at 1090 cm
−1 is attributed to the asymmetric stretching mode of the Si–O–Si bond, while bands at 460 and 808 cm
−1 are assigned to the absorption of the symmetric stretching and bending of Si–O–Si. The same bands were also observed in the IR spectrum of pulverized silica. Furthermore, according to the reports from [
17], this confirms the subsistence of bands at 963, 1028 and 1110 cm
−1 for the phosphate group in samples of hydroxyapatite obtained by hydrothermal and growth on silica gels methods, and from [
18], who ascribes the bands at 472, 574, 962, 1046 and 1087 cm
−1 to the modes ν
2, ν
4, ν
1, ν
3 and ν
3, respectively, from a phosphate group in silicon-substituted hydroxyapatite samples, it is inferred that the bands at 943, 1036 and 1083 cm
−1 identified in the IR spectra of the silica–hydroxyapatite coaxial fibers developed in the present investigation belong exclusively to hydroxyapatite. However, the red-shifting of the bands related to the ν
1 and ν
3 modes (1087 cm
−1) and the blue-shifting of the mode ν
3 (1046 cm
−1) can be explained in terms of a substitution of the phosphate groups by silicate within the hydroxyapatite cell. In
Figure 1b it is observed that the IR spectrum for the SiO
2–HA fibers sintered at 1150 °C. A prominent band is present at 1036 cm
−1, and another less intense band appears at 579 cm
−1: both referring to the stretching of the P–O bond from phosphate groups. Those bands found at 870 and 473 cm
−1 correspond to the asymmetric stretching and bending of Si–O–Si, respectively.
3.5. Characterization of the Doped Composite
The silica–hydroxyapatite composite was doped with silver through electrolysis.
Table 1 shows the details of the voltage employed, silver nitrate concentration and the electrodeposition time for each one of the nine samples. Initially, a voltage of 1.5 V and time intervals of 2, 3 and 5 min were used in agreement with the favorable results on silver dendrite obtention reported by Cabello [
22]. As the doped supports did not exhibit the presence of dendrites, a greater voltage and higher silver nitrate concentration were adopted.
As seen in
Figure 5, at a voltage of 1.5 V and a deposition time of 2 min, silver is not homogenously distributed onto the fibers surface. Those regions that contain abundant silver exhibit the barely perceptible growth of irregular cubic crystals, especially in the center and upper right zone of
Figure 5a. However, these structures are fragmented and surrounded by smaller semispherical particles; the ovoid and elongated form of particles favored a nucleation process (
Figure 5b). The silver nanocubes are more easily recognizable as the electrodeposition time. Stacked cubic crystals were detected, as shown in
Figure 5c.
Figure 5d presents the existence of smaller spherical structures growing upon the cubic phases, morphologically alike those particles observed on the 2 min doped support. An increment in the deposition time produced bigger and more defined crystalline arrangements. After 5 min of doping, silver deposits on the fibers acquired more elongated shapes than those in previous supports. There is no trace of nanocubes, but thin needles and rods are distributed all along the fibers’ surface and into deeper regions of the mat (
Figure 5e). It is well known that the morphology of the deposits can be manipulated in function of the applied potential. Liu et al. [
23] registered the achievement of aleatory silver architectures because of a quick nucleation in low reduction potentials, down to −0.9 V vs. Ag/AgCl, producing dendrites since the reduction rate of silver is elevated when the current density is high. By increasing the reduction potential, the later manifestation of nanorods happened. Therefore, congruently to [
24], it is assumed that at 1.5 V nucleation occurs slowly, for the presence of a few cores, their continuous nucleation and the advent of crystals with various sizes are characteristic elements of low-rate nucleation increases.
Figure 6a belongs to the support doped for 2 min at 3.3 V using AgNO
3 10 mM. The manifestation of silver cores along numerous individual fibers, with an enlarged concentration at the cross-region between fused fibers, is noticeable. The settlings present variable shapes, most of them resembling tiny spheres arranged into disperse clusters, like the results obtained after the 1.5 V doping. Some others show planar growth like hexagonal laminated flakes (
Figure 6b). The pores on the fibers are preferential sites for the deposition and accumulation of silver. The microscopy results do not present evidence of significant difference with respect to the growth of silver nanoparticles in samples doped at a lower voltage. Tsai et al. [
25] registered the electrochemical deposition of silver nanoparticles with diameters between 100 and 200 nm onto carbon nanotubes using 10 mM AgNO
3 and a voltage of −0.3 V vs. Ag/AgCl. Analogous studies report the appearance of hexagonal crystals and dendrites isolated on silica–titania-based fibers after two minutes of electrodeposition at 1 V, utilizing a 10 mM AgNO
3 solution [
22]. Hydroxyapatite’s low conductivity, near 5.07 × 10
−10 S cm
−1 according to Suresh et al. [
26], is a determinant factor that could justify the mitigated nucleation of silver in contrast to the silica–titania samples. For a 3 min doping, silver was handsomely deposited on the fibers’ surface in the form of little particles adopting distinct morphologies, predominantly sphere and ovoid shapes, without displaying crystal growth (
Figure 6c). As the magnifications grew, it was possible to observe how those particles agglutinate and become part of a series of disoriented systems. With a doping time of 5 min, the silver deposits in sample F6 exhibited unique morphologically features among all the analyzed cases: as seen in
Figure 6e, multiple cubic crystals with a side length of 303 ± 75 nm were produced and they accumulated on the fiber membrane.
Figure 6f grants a more explicit look to the corner sharpening of some nanocubes, conceding them a nanostar-like appearance, these being preferential sites for the nucleation of new silver cores. It denotes the proliferation of smaller particles on the cubes.
Figure 6e also presents the elongation of one of the silver cores into a 1.1 μm-long needle. At higher magnifications, the subsistence of spherical silver particles is noteworthy, yet the nanocubes remain recognizable.
Micrographs of support F7 in
Figure 7a,b for 3.3 V, 20 mM of AgNO
3 and doped for 2 min do not evidence significative silver accumulations.
Figure 7a, at its central left portion contains signs of growth of some undefined cuboid crystal and many spherical bodies.
Figure 7b shows silver clusters with no evident crystalline growth, composed of a wide variety of spherical nanoparticles. At 3 min of deposition, sample F8 (
Figure 7c) presents increasing cuboid crystals smaller than nanocubes on support F6. At this doping time, crystal growth is clouded by the manifestation of the so-observed spheroid nanoparticles. At higher magnifications, the morphology of the clusters resembles more a group of disordered spheres rather than uniform cubic crystals. At last, 5 min after electrodeposition, the fibers were found remarkably coated by silver. In the first instance, it appeared that the dominant morphology among silver clusters is that of assembled spheroids, but
Figure 7e enables the recognition of a few cuboids growing, far more defined than in samples F7 and F8.
The concentration of silver nitrate does not seem to influence the morphology of the deposited silver nanoparticles as much as the doping time does, but the rise in the concentration does generate more prominent clusters in less time, understanding that conductivity in the electrolyte depends on the concentration of ionic species [
27]. By increasing the concentration of nitrate, the migration of silver ions towards the support is favored, leading to the saturation of certain sites on the fiber mat. Regarding the effect of enlarging the voltage, the cell potential is intrinsically related to a free energy variation, and hence, to the reaction constant for Ag
+:
as represented in Nernst equation. In this case, by increasing both the voltage and the silver nitrate concentration, the reduction rate of silver and the accumulation of nanoparticles on the fibers were favored. The prolongation of the electrodeposition time lapse led to a more ordered crystallization.
From the SEM results, it was concluded that the supports doped for 5 min at 1.5 and 3.3 V employing a 10 mM silver nitrate solution hold the best characteristics for their employment as SERS-active substrates, since the surface plasmon can be tuned between 415 and 623 nm [
28,
29,
30], depending on the architecture of the nanoparticle and especially on the dimensions of the cube or rod. If the plasmon is located nearby the excitation wavelength (532 nm), then it is possible to create a resonance condition between both and make the electromagnetic enhancement prevail for the molecules adsorbed at the metal surface. Likewise, cornered ends and sharp edges become hot spots due to charge gathering. The contribution from electromagnetic enhancement to the final enhancement factor is crucial and depends on the near field properties [
31].
3.7. SEIRAS Activity Evaluation
The SEIRAS analysis generated IR spectra for 1 nM pyridine adsorbed on every support and in each case, it was compared to the spectrum of a 1 mM pyridine solution measured in conditions foreign to the substrate. All samples evidenced an intense band around 3350 cm
−1 corresponding to the C–H bond stretching of pyridine in aqueous systems. The spectrum of 1 mM pyridine also indicates a low band at 1638 cm
−1 along with another, notoriously broader, at 560 cm
−1 attributed to the 8a mode (C–N stretching) and to the ring deformation, respectively. Numerous bands exhibit a shift with respect to the fundamental position of some vibrational modes of pure pyridine reported by Johnson et al. [
33] and Partal-Ureña et al. [
34]. The band shift can be associated to the interaction of the analyte with silver.
Figure 9 shows the spectra obtained during the essays with supports F1–F3. The characteristic pyridine bands observed in the spectrum of the 1 mM pyridine solution are clearly appreciable on the spectra of 1 nM pyridine placed on the silica–hydroxyapatite–silver supports. On the 2 min doped support there is an important band at 1024 cm
−1 due to ν
3 vibrational mode of the phosphate group in the hydroxyapatite of the substrate. Bands at 983, 874 and 559 cm
−1 (see
Table 2), which are assigned to the ν
3 mode of silicate, the Si–O–Si bond’s asymmetrical stretching and the ν
4 mode of phosphate, become attenuated as the electrodeposition time rises, indicating that those modes could be affected by electromagnetic enhancement because of the proximity of the fibers to the silver nanoparticles. It is imperative to point that several bands between 1080 and 1003 cm
−1 that belong to the pyridine could possibly be overshadowed by the phosphate modes from the support, particularly the band at 1020 cm
−1 attributed to transition 12. The bands at 992 and 943 cm
−1 may owe their magnitude to a combined contribution from the phosphate modes and pyridine (see
Table 2).
The enhancement was defined depending on the area beneath the curve of the main bands, through the expression in Equation (1):
Equation (1): enhancement factor (FA) determination, ISEIRAS being the intensity of a band, expressed in terms of its area, obtained the analyte of concentration CSEIRAS on the substrate; IFTIR corresponds to the intensity of the same band for the analyte in conditions foreign to the substrate at a concentration CFTIR.
By SEIRAS, enhancement factors up to 1.93 × 10
6 for a band at 711 cm
−1, related to the C–H stretching, and 1.16 × 10
6 for the band at 1639 cm
−1, concerning the C–N stretching in pyridine, were both originated on the 3 min doped support. In that same device appears a low intensity shoulder at 1468 cm
−1 because of the enhancement of pyridine’s mode 19a, which corresponds to the stretching of C–N and C–C. The latter vibration mode mitigates in the spectrum of the 5 min doped support and the band at 1406 cm
−1 for the C–C stretching intensifies. Infrared spectra of the pyridine set on the support series doped at 3.3 V with 10 mM silver nitrate (F4–F7) are shown in
Figure 10. A more pronounced tendency for the bands at the 1100–500 cm
−1 region in comparison to the series doped at 1.5 V is observed, peculiarly in the band at 1046 cm
−1, manifesting the presence of silica and hydroxyapatite vibrational modes. Only the 2 min doped support presented an enhancement for the 8a and 11 modes localized at 1638 and 713 cm
−1, with factors of 1.32 × 10
6 and 1.49 × 10
6, respectively. In addition, all three substrates exhibited a broad and low-intensity band around 1466 cm
−1, generated by transition 19a, and a shoulder close to 1450 cm
−1, due to pyridine’s mode 19b (see
Table 2). Li et al. [
35] solely report the bands 1603, 1445 and 1447 cm
−1 during a SEIRAS essay with pyridine adsorbed on a silver electrode. They also pointed a band-shifting for mode 19b from 1437 to 1447 cm
−1, comparable to the deviation in the spectra on
Figure 10, indicating a coordination bond between silver and the nitrogen-free electron pair.
Figure 11 highlights a more uniform behavior for all the bands in every support. A probable cause is the high silver accumulation generated by doping with 20 mM silver nitrate, which was demonstrated to minimize the morphological cluster variation among distinct time intervals and thus lead to the aggregation of spherical nanoparticles, as observed in the micrographs.
Table 2 summarizes the major bands found in the IR spectra of the F7–F9 series and the vibrations they belong to. A broadening for the C–H stretching band at 3362 cm
−1 occurs as the electrodeposition time increases. The bands at 1638, 1541, 1456 and 708 cm
−1 related to their respective pyridine transitions 8a, 8b, 19b and 11 are noticeable in all three supports. The 2 min doped support displays the highest enhancement for the bands at 1541 cm
−1, owning a factor of 1.09 × 10
6, and 708 cm
−1, with a factor of 1.34 × 10
6. Bands at 1019 and 558 cm
−1 corresponding to the phosphate modes ν
3 and ν
4, and at 983, 860 and 788 cm
−1, related to the ν
3, asymmetric stretching and v1 silicate transitions are present on the three spectra, presenting an amplitude increment in the 3 min support graph, suggesting the enhancement of such modes or even pyridine modes that help the enlargement of these bands.
The variation in the modes enhanced amidst different supports is caused because of the fact that a determined adsorbed pyridine orientation over the metal nanoparticle favors the coupling of the localized electric fields to specific vibrational transitions, and is consistent with the presence of diverse morphologies including spheres, cubes and rods or needles observed in the supports doped for different time intervals. The amplification effect originates from the excitation of the electrons at the surface of metal nanoarrays due to their interaction with the electromagnetic field of the incident beam. The resonance between the plasmon at the metal and the excitation radiation generates localized electric fields that, thanks to their proximity to the adsorbed analyte, couple with the vibrational transitions of pyridine and magnify its spectral signal [
36,
37].
As displayed in
Table 3, the top enhancement factor was 2.01 × 10
6 for the band at 3335 cm
−1 in the F4 support, doped with 10 mM silver nitrate at 3.3 V for 2 min. The same substrate presented the enhancement of the bands at 1639 and 711 cm
−1. Here, the enhancement effect is assigned mainly to the hot spots on the surface of the fibers generated by silver nanospheres and flakes distributed along the fibers, as appreciated in micrographs. Support F2 also presented the enhancement of these three main bands. In this case, enhancement is linked to the presence of nanocubes gathered over the substrate, whose pointy features and sharpened edges favor charge accumulation and the advent of hot spots [
38]. Despite support F6 showed to have the higher number of cubic nanostructures and defined crystals compiled, its infrared enhancement turned poor in comparison, because of circumstantial factors such as the absence of highly doped regions, and the consequence of non-uniform metal clusters’ distribution. The rest of the supports demonstrated the enhancement of at least one band. Supports F5 and F9 did not show apparent amplification of any characteristic pyridine band.
Table 3 indicates how all enhanced vibrational transitions can be classified into modes with A
1 or B
1 symmetry [
39]
. The amplification of A1 modes by supports F2, F4 and F6 suggests molecular orientation perpendicular to the metal surface, typically referred to as “end-on” orientation, which hints the formation of a bond via the lone electronic pair of nitrogen with silver [
40].
3.8. SERS Activity Evaluation
The SERS activity of all supports was evaluated by comparing the Raman spectrum of 1 mM pyridine tested in conditions foreign to the silica–hydroxyapatite–silver substrate with the spectrum of 1 nM pyridine placed upon the supports doped at different time intervals (2, 3 and 5 min).
Figure 12 shows the SERS spectra of 1 nM pyridine evaluated on the series of supports doped at 1.5 V (F1–F3). Several enhanced bands can be, as described in
Table 4. Bands at 586 and 1097 cm
−1 on the 1 mM pyridine spectrum are highlighted: they belong to the 6a and 18a modes, respectively, which represent the distortion and stretching of the aromatic ring. As observed, support F3, doped for 5 min, exhibits a considerably superior enhancement of these bands with respect to the other supports, as well as for those bands located at 963, 1340 and 1593 cm
−1, attributed to the modes 5, 14 and 18a, related to the C–H wagging, stretching and in-plane aromatic ring bending, and C–N/C=N stretching. Only the bands for the modes 6a and 5 suffered a slight red-shifting in comparison to the normal Raman vibration modes [
34], while the transitions correlated to the ring bending and stretching experienced a blue shifting. Bindhu et al. [
41] says that the shift of the ring stretching modes suggests that the pyridine adopted an end-on orientation on the surface of silver. They also pointed out that a displacement of Raman normal modes occurs because of the modification of the adsorbate’s structure caused by the overlapping of its molecular orbitals with the metal orbitals.
Figure 13 shows the SERS spectra obtained from 1 nM pyridine on supports F4–F6. A clear band sharpening in the region from 500 to 1600 cm
−1 is especially visible on the spectra from the 2 and 3 min doped supports. The spectrum of support F4, deposited for 2 min, shows prominence in the bands at 595, 960, 1082, 1372 and 1595 cm
−1, assigned to the pyridine modes 6a, 5, 18a, 14 and 8a. In contrast, the spectrum of the 3 min doped support rises with a superior resolution degree for several bands and less spectral noise. The transitions 11, 10a, 12, 15, 3, 14 and 8b, localized at 719, 846, 1041, 1174, 1260, 1372 and 1539 cm
−1 are acutely identified. Support F6, doped for 5 min, did not evidence the amplification of the vibrational modes 6a and 18a observed on the spectrum of 1 mM pyridine, respectively, located at 586 and 1097 cm
−1. Nonetheless, the bands at 1202, 1485 and 1611 cm
−1 for vibrations 9a, 19a and 8a are sufficient. The other vibration modes presented in
Figure 13 are enlisted in
Table 4. Although the pyridine molecule could adopt a parallel orientation to the adsorbent surface, interacting through the electrons in the aromatic ring, it is well known that the formation of an N–Ag bond, perpendicular to the silver face, is the geometric configuration most energetically stable [
42]. This is the case of pyridine adsorbed upon the supports of this series, considering that the bands enhanced by supports F4 and F6 relate to stretching transitions of C–C, C–N and C=N, and nearly all modes in support F5 are attributed to in-plane bending, stretching and aromatic ring breathing vibrations. It is worth noting that modes 3 and 10a seen in the 3 min doped substrate are equivalent to C–C and C–N stretching, as well as C–H bending, both out of the plane. Out-of-plane vibrations indicate a planar pyridine orientation and the possible development of a multilayer [
40,
41].
Figure 14 is shown next, presenting the SERS spectra of pyridine put on the supports doped with 20 mM silver nitrate at 3.3 V (F7–F9). The spectrum of the 2 min support is highly active, emphasizing the bands at 747, 844, 938, 1174 and 1428 cm
−1 referent to the modes 4, 10a, 10b, 15 and 19b, respectively. The 3 min doped support spectrum discloses the enhancement of the bands at 572, 952, 1336, 1571 and 1607 cm
−1 for the pyridine modes 6a, 10b, 14, 8b and 8a. The spectral strength decays drastically once the electrodeposition time reaches 5 min. Substrate F9 owns defined but low intensity bands compared to its precursors at 860, 1031, 1135, 1386 and 1608 cm
−1 for vibrational modes 10a, 12, 15, 14 and 8a (see
Table 4). As a result of the preferential enhancement of modes 10b (C–H in-plane wagging) and 15 (C–C–H bending) from support F7, and of modes 8a (C–C stretching), 12 (aromatic ring breathing) and 14 (C–C–H in-plane bending) from F9, a perpendicular orientation to the silver nanoparticle surface is suggested for adsorbed pyridine. The existence of an intense band linked to the out-of-plane stretching (mode 8b) in the spectrum of support F8 indicates the combination of various orientation types for pyridine, involving different bond angles to silver [
33,
43,
44,
45]. All supports presented SERS activity. When the radiation interacts with metal nanoparticles smaller than the incident wavelength, a displacement in the electronic density occurs and the particle polarizes, turning into a dipolar antenna that emits light. Surface plasmons are excited when the excitation wavelength is resonant with the plasmon absorption profile in the nanoparticle. Consequently, a strong electromagnetic field is induced and the Raman modes of a molecule nearby the metal surface are drastically intensified [
46].
Figure 15 and
Table 5 show the behavior of the calculated four bands, at 1264, 1355, 1480 and 1567 cm
−1, as a function of silver electrodeposition time for each device. It is necessary to underline that an inverse performance is shared for all bands among the supports doped with 10 mM silver nitrate and 1.5 V, and those doped with a 20 mM silver nitrate and 3.3 V: in the first case, the area climbs with a positive slope as the doping interval increases, with a maximum at 5 min, while in the second case the calculated area reaches its maximum earlier, at 2 min of electrodeposition; from there, it drops until 5 min, where amplification is practically null. On the other hand, the area of bands at 1480 cm
−1 (
Figure 15b) and 1567 cm
−1 (
Figure 15d) for the series doped with 10 mM silver nitrate and 3.3 V finds its top value after 3 min of doping and later it descends significantly. The area of the bands at 1264 (
Figure 15a) and 1355 (
Figure 15c) from this same series maximizes at 2 min and after that it radically decreases after 5 min of doping. Such behavior leads to note that at minor voltages the silver reduction rate on the fibers is low, and at short time intervals, nucleation is supported and hence producing a sequential growth by silver cores stacking.
Accordingly, at early doping stages the enhancement of the electromagnetic field over precise spherical nanoparticles, as the microscopy analysis points out, is subtle compared to the response generated once the silver nanostructures accumulates in the form of rods and needles. Although the voltage intensification to 3.3 V suggests an elevated silver crystallization rate, allowing the observation of nanocubes growth, the employment of an elevated nitrate concentration and the prolongation of doping time resulted in the amassing of larger deposits over the substrate, which inhibits the generation of localized hot spots. The enhancement factor (EF) for each selected band was calculated in an analogous way to the SEIRAS analysis, using Equation (1).
Table 6 shows details for the factors for the main four bands on the 1 nM pyridine spectrum resulting from the essays carried out with each one of the nine experimental supports. As appreciated, the highest enhancement factor was 3.46 × 10
8, obtained from support F3, doped for 5 min with 10 mM silver nitrate at 1.5 V, for the band at 1567 cm
−1, however all the bands on this substrate presented a factor in the order of 10
8. The degree of amplification in support F3 is primarily attributed to the presence of silver nanorods and needles over the fibers. The Raman amplification effect due to nanorods has been already tested [
47], obtaining enhancement factors up to 1.44 × 10
8 in essays with 1.6 × 10
−7 M Nile blue in the presence of nanorods synthesized by silver seeds growth aid by Cetyl Trimethyl Ammonium Bromide (CTAB). The overlaying of the transversal and longitudinal plasmon bands constitutes the basis of electromagnetic enhancement on nanorods, so that the top SERS activity expresses in perpendicular direction to the major axis [
48].
Silver nanocubes are considered as excellent scaffold for enhanced Raman spectroscopy. Zeng et al. [
49] detailed a SERS test using elongated silver cubes (nanobars), made by seed growth assisted by growing control agents and 1, 4-benzenedithiol, obtaining an EF of 8.6 × 10
4; Tegegne et al. [
50] performed the synthesis of silver nanocubes with a side length of 50 nm by the polyol route, applied to the detection of deoxynivalenol, where they registered a maximum EF of 1.82 × 10
7, mentioning how a previous polydopamine coating increased the analyte’s adsorption capacity and therefore its signal magnification, pointing to the relevance of the chemical enhancement; Kundu et al. [
30] used silver nanocubes with a side length of 335 ± 5 nm, obtained by the reaction of silver nitrate with the sodium salt of poly (styrene sulfonate) in the presence of gold seeds, and achieved an EF of 1.04 × 10
5 for a band of 1 mM blue methylene. They highlight size as a decisive factor above the nanoparticles’ shape and architecture by comparing the EF obtained for the same band adsorbed on 9 nm diameter nanospheres, resulting two orders of magnitude higher than the factor exhibited by nanocubes. Bhattacharjee et al. [
28] stated an EF of 7.52 × 10
9 for 4-mercaptobenzoic acid with silver nanocubes with a gold core and described how the close proximity between the excitation wavelength and the surface plasmon band on the nanocubes contributed to such elevated enhancement. Finally, Ben-Jaber et al. [
51] informed the enhancement factors of 1.19 × 10
11 for Rhodamine-6G and 9.26 × 10
10 for cyclotrimethylenetrinitramine in tests with 153 nm-long silver nanocubes. The polyol technique is frequently used for the synthesis of silver nanocubes [
52]. Zhang et al. [
53] developed the synthesis of silver nanocubes with a side length between 30 and 700 nm by a modified polyol method, using silver trifluoroacetate as a precursor; Sun and Xia [
54] synthesized 175 ± 13 nm-long nanocubes working with ethylene glycol at high temperature; Chang et al. [
55] employed this polyol technique to make 70–80 nm-long nanocubes, and Huang et al. [
56] produced 2 nm-long nanocubes by UV irradiation.
From the two mechanisms responsible for the Raman enhancement, charge transfer, also called chemical interaction, depends on the interaction between the analyte molecule and the metal surface, and provides only in a factor of 10–100 to the final amplification, whereas the electromagnetic enhancement contributes in a factor of 10
10. Electromagnetic enhancement is influenced in turn by the close field properties and is subject to the electric field distribution on the nanoparticle. The main reason of why pointy architectures and sharp-edged features, such as nanocubes, produce amplification spots is because they promote the plasmon red-shifting by its oscillation rate attenuation, which follows the charge separation by the accumulation of electrons on those spikey structures [
29,
31]. In the present research, the interaction involving the pyridine and the silver nanoparticles is flagrant, as most of the observed transitions are shifted with regard to the normal Raman modes, so it is recognized that the existence of a charge transfer contribution aids the final spectral response. Concerning the factors implied in the electromagnetic enhancement, it is considered that the incisive features, for instance the pointed corners and prominent borders from the cubic morphology of silver settlings, decidedly supported the intensity raise of the pyridine Raman modes. The size of those nanocubes could have inhibited the obtention of higher enhancement factors. Moskovits [
57] determines that, for coinage metals, the optimal size of the SERS responsible nanoparticle is from 10 to 100 nm, lower than the incident wavelength but larger than the conduction electron’s path.
The SERS activity of the substrates with outstanding performance (F3, F4 and F7) was tested again with a 10
−4 M crystal violet solution (C
24H
28N
3Cl). The Raman spectrum of the analyte of interest is shown in
Figure 16. Nine of its key vibrational modes are significant.
Table 7 approaches with clearness the location and nature of those vibrations. It is also noticed that the baseline of the spectrum presents an acute slope due to the high degree of fluorescence. Background fluorescence emerges as spectral noise and is generated because the emission band of crystal violet reaches its maximum at about 575 nm, very close to the excitation wavelength from the laser used for the SERS essay at 532 nm [
58]. A comparison between the SERS spectra from the tested supports is outlined in
Figure 17. There, no evident difference was noticed between the spectrum of the sample in support F7 and the crystal violet spectrum by itself. Both share a comparable slope, indicating that fluorescence still prevails upon the substrate doped with an elevated concentration of silver nitrate. It was deduced that the great nanoparticle clusters, mainly spheroids, do not sustain an electric field intensity suitable for the magnification of Raman signals, neither an unimpeded surface for the adsorption of crystal violet. Alternatively, fluorescence disappears in supports F3 and F4, though the intensity of the primary bands results insignificant compared to the Raman spectrum of the sample.
Table 7 indicates the enhancement factors gained for the bands of crystal violet. As seen, all factors are less than 1, which is translated into a seemingly null amplification.
The SERS substrate with less sensitivity to crystal violet is marked. The phenomenon may be due to a lower interaction of the violet crystal with silver at this wavelength, Chadha et al. [
59]. In 2013, they demonstrated that the SERS spectra of CV adsorbed on Ag and Au nanoparticles increase in intensity with increasing laser excitation wavelengths of 532 and 660 nm. Even when none of the supports exhibited an increase in the area on the bands studied, supports F3 and F4 indeed displayed more defined spectra for crystal violet. From the 31 vibrational transitions for crystal violet studied in detail by Cañamares et al. [
60], at least 15 are clearly identifiable on the spectra of F3 and F4 (
Figure 18) while only nine are visible on the spectrum of the analyte placed out of the supports. A reason that would explain these low enhancement factors is the significant contribution of fluorescence to the band’s amplitude, as denoted by the elevated intensity count in the spectrum plot of
Figure 16, an issue that aided the Raman bands to be superior to the SERS bands. In that sense, the Raman enhancement effect has proved to weaken fluorescence [
61]. Additionally, the strength of the Raman dispersion varies according to the adsorption angle of the analyte to the substrate surface; in this case, the analyte molecules own three phenyl rings bonded to a central carbonium [
62]. The sensitivity increment provided by F3 and F4 is owed to the piercing features of the nanoarchitecture of the supports and to a favorable analyte–substrate interaction. High SERS yields have been demonstrated in studies carried out with the nanoarrangements produced by this approach, primarily for nanocubes with a side length around 100 nm. The amount and form of silver particles electrodeposited on the fibers modify the number of hot spots in the substrate, causing the substrate to have a greater amplification effect.