Ultrasonic Sensors-Assisted Corrosion Studies on Surface Coated AlSi9Cu3 Alloy Die Castings
Abstract
1. Introduction
2. Experimental Methods
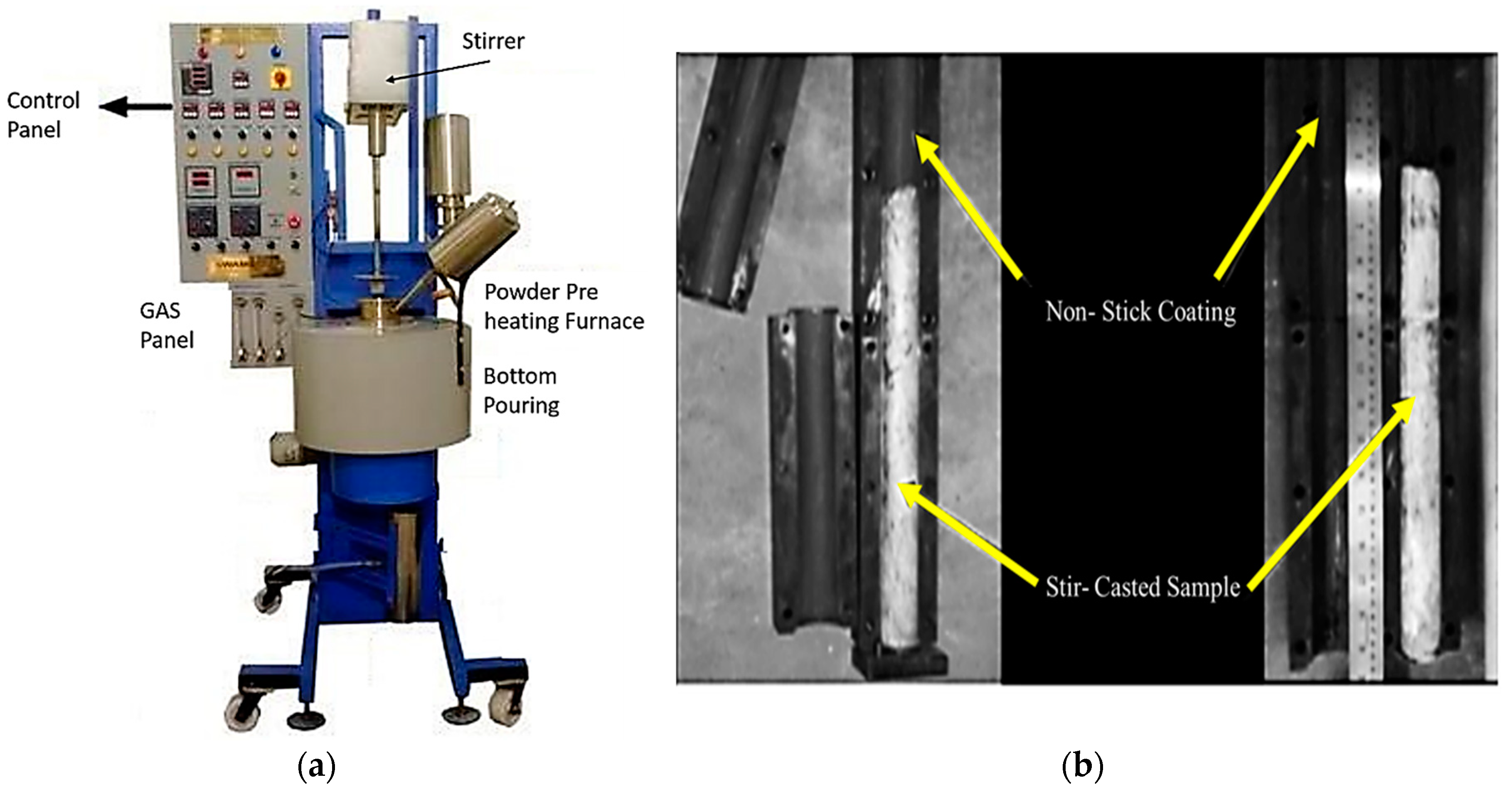
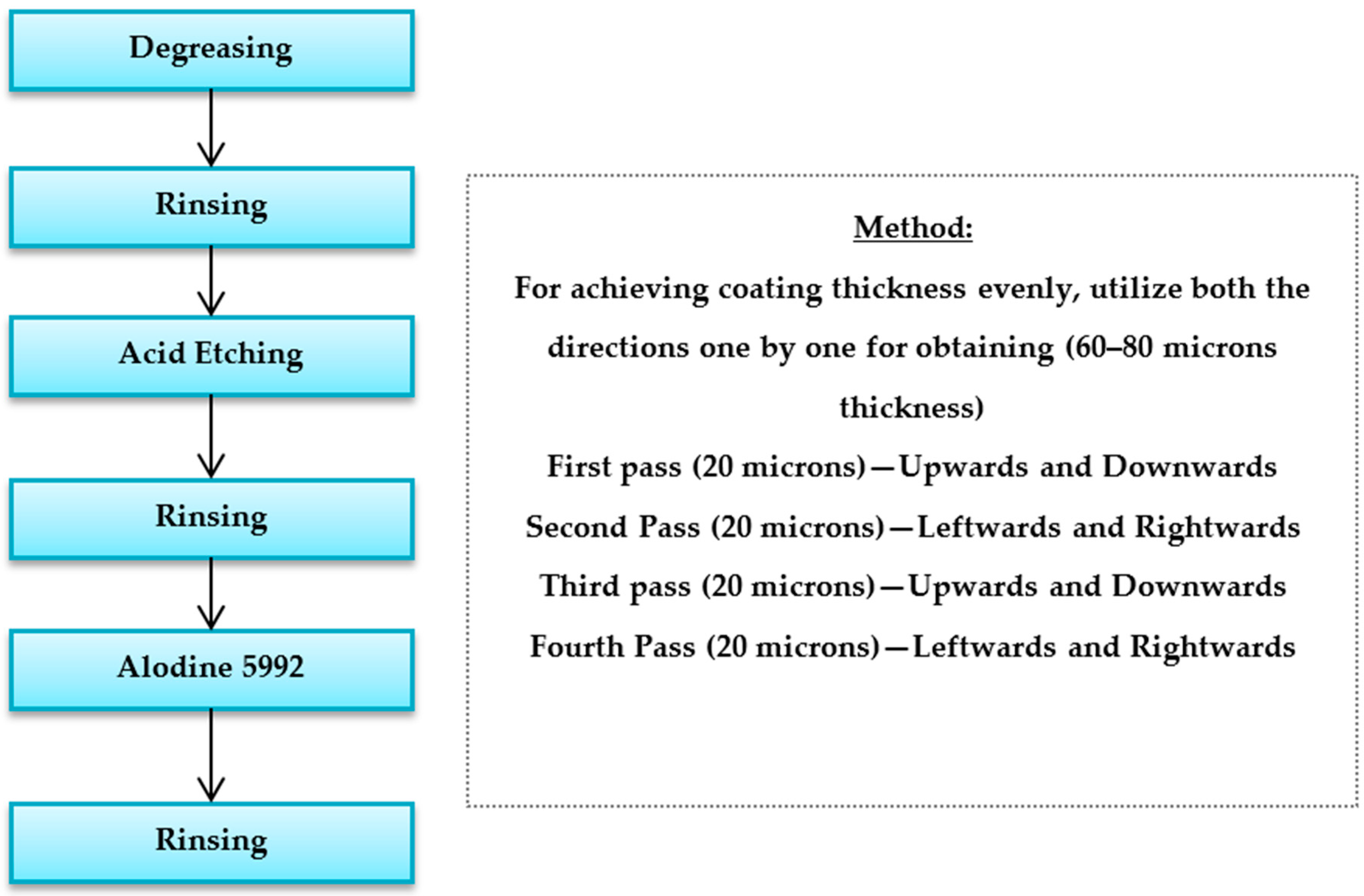
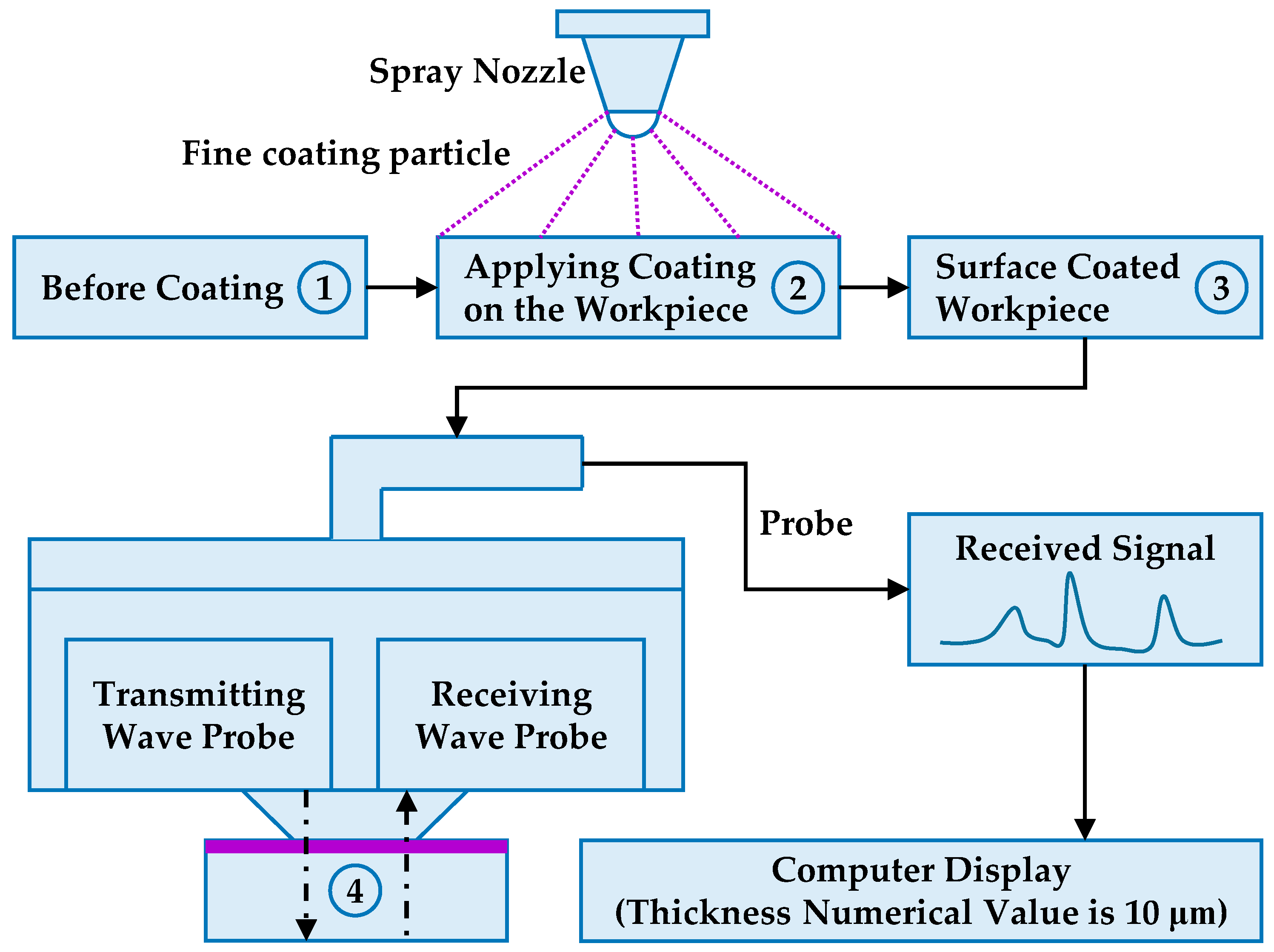
2.1. Preparation of Powder Coating
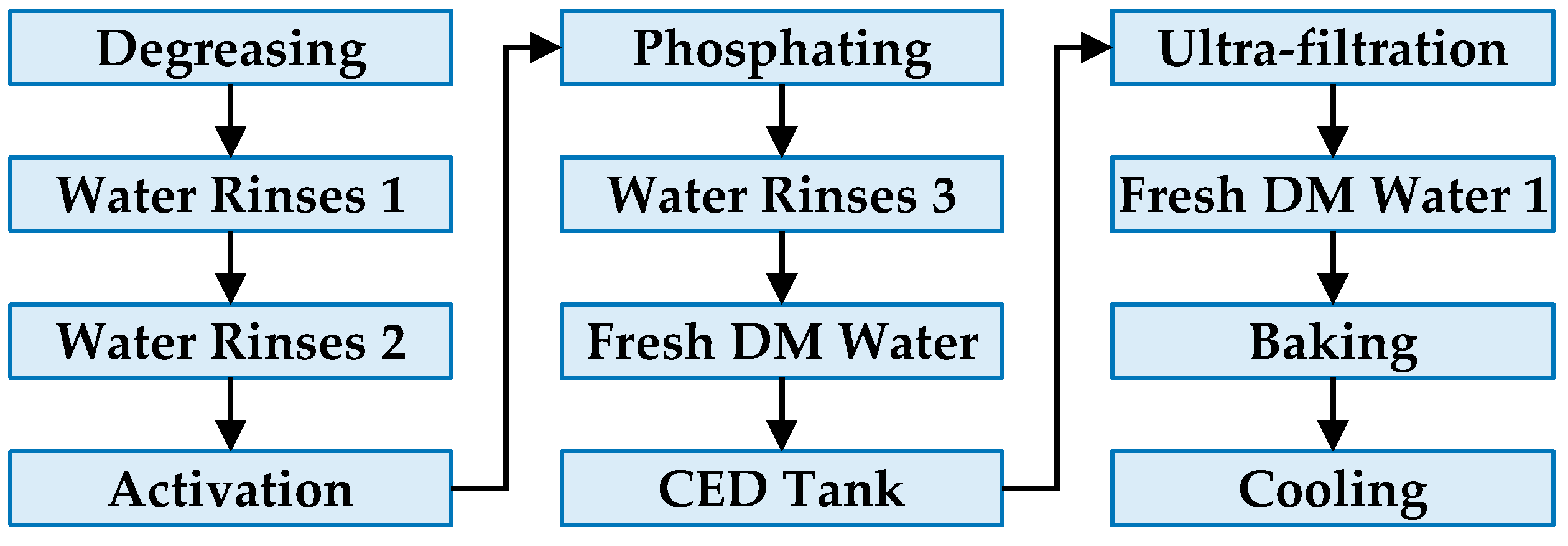
2.2. Preparation of ED Coating
2.3. The Sample Prepared for PD and EIS
3. Results and Discussion
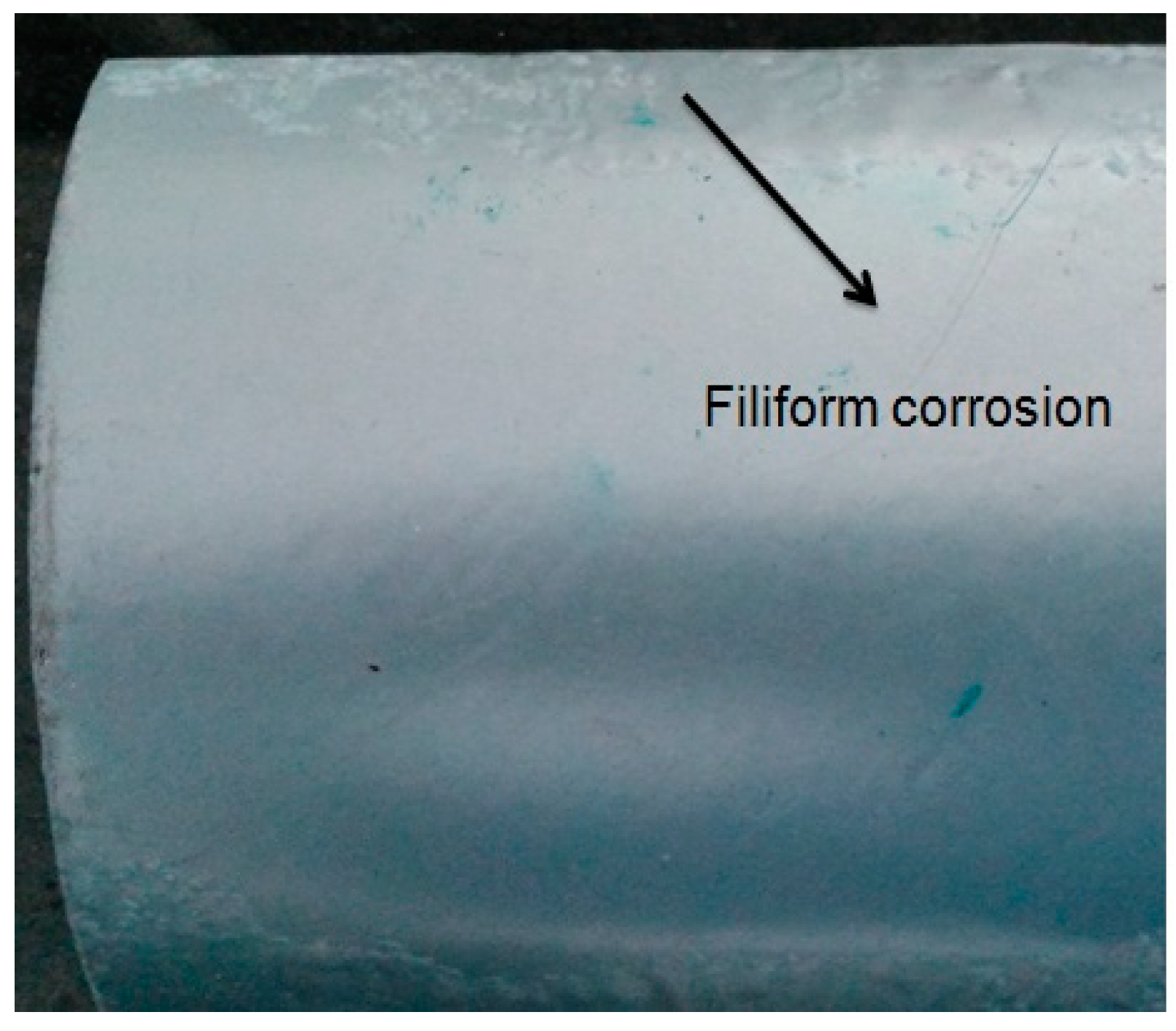
Filiform Corrosion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Johansen, H.D.; Brett, C.M.A.; Motheo, A.D.J. Corrosion protection of aluminium alloy by cerium conversion and conducting polymer duplex coatings. Corros. Sci. 2012, 63, 342–350. [Google Scholar] [CrossRef]
- Baldissera, A.F.; Ferreira, C.A. Coatings based on electronic conducting polymers for corrosion protection of metals. Prog. Org. Coat. 2012, 75, 241–247. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Ortiz-Candelaria, L.; Stone, D.S.; Rammer, D.R. Electrochemical impedance spectroscopy (EIS) as a tool for measuring corrosion of polymer-coated fasteners used in treated wood. For. Prod. J. 2009, 59, 77–82. [Google Scholar]
- Frankel, G.S.; Papavinasam, S.; Berke, N.; Brossia, S.; Dean, S. Electrochemical techniques in corrosion: Status, limitations, and needs. J. ASTM Int. 2008, 5, 101241. [Google Scholar] [CrossRef]
- Kumar, S.A.; Alagar, M.; Mohan, V. Studies on corrosion-resistant behavior of siliconized epoxy interpenetrating coatings over mild steel surface by electrochemical methods. J. Mater. Eng. Perform. 2002, 11, 123–129. [Google Scholar] [CrossRef]
- Mansfeld, F.; Han, L.T.; Lee, C.C.; Zhang, G. Evaluation of corrosion protection by polymer coatings using electrochemical impedance spectroscopy and noise analysis. Electrochim. Acta 1998, 43, 2933–2945. [Google Scholar] [CrossRef]
- Calle, L.; MacDowell, L.G. Corrosion protection of launch infrastructure and flight hardware at the Kennedy Space Center. In Proceedings of the Space Congress, Cape Canaveral, FL, USA, 2 May 2002. [Google Scholar]
- Brickweg, L.J.; Floryancic, B.R.; Sapper, E.D.; Fernando, R.H. Shear-induced 1-D alignment of alumina nanoparticles in coatings. J. Coat. Technol. Res. 2007, 4, 107–110. [Google Scholar] [CrossRef]
- Shabestari, S.G.; Gruzleski, J.E. The effect of solidification condition and chemistry on the formation and morphology of complex intermetallic compounds in aluminium–silicon alloys. Cast Met. 1994, 6, 217–224. [Google Scholar] [CrossRef]
- Gustafsson, G.; Thorvaldsson, T.; Dunlop, G.L. The influence of Fe and Cr on the microstructure of cast Al–Si–Mg alloys. Metall. Trans. A 1986, 17, 45–52. [Google Scholar] [CrossRef]
- Makhlouf, M.M.; Apelian, D.; Wang, L. Microstructures and Properties of Aluminum Die Casting Alloys; USDOE Idaho Operations Office: Idaho Falls, ID, USA, 1998. [Google Scholar] [CrossRef][Green Version]
- Wranglen, G. An Introduction to Corrosion and Protection of Metals, 1st ed.; Springer: Dordrecht, The Netherlands, 1985. [Google Scholar]
- Mansfeld, F.; Bertocci, U. (Eds.) STP727-EB Electrochemical Corrosion Testing; ASTM International: West Conshohocken, PA, USA, 1981. [Google Scholar] [CrossRef]
- Jones, F.N.; Nichols, M.E.; Pappas, S.P. Organic Coatings: Science and Technology, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Hirayama, R.; Haruyama, S. Electrochemical impedance for degraded coated steel having pores. Corrosion 1991, 47, 952–958. [Google Scholar] [CrossRef]
- Rammelt, U.; Reinhard, G. Characterization of active pigments in damage of organic coatings on steel by means of electrochemical impedance spectroscopy. Prog. Org. Coat. 1994, 24, 309–322. [Google Scholar] [CrossRef]
- Mansfeld, F.; Tsai, C.H. Determination of coating deterioration with EIS: I. basic relationships. Corrosion 1991, 47, 958–963. [Google Scholar] [CrossRef]
- Sekine, I. Recent evaluation of corrosion protective paint films by electrochemical methods. Prog. Org. Coat. 1997, 31, 73–80. [Google Scholar] [CrossRef]
- Vereschaka, A.; Volosova, M.; Chigarev, A.; Sitnikov, N.; Ashmarin, A.; Sotova, C.; Bublikov, J.; Lytkin, D. Influence of the thickness of a nanolayer composite coating on values of residual stress and the nature of coating wear. Coatings 2020, 10, 63. [Google Scholar] [CrossRef]
- Ferrari, M.; Benedetti, A.; Cirisano, F. Superhydrophobic coatings from recyclable materials for protection in a real sea environment. Coatings 2019, 9, 303. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, P.; Sytchenko, A.; Sheveyko, A.; Vorotilo, S. Structure and properties of protective coatings deposited by pulsed cathodic arc evaporation in Ar, N2, and C2H4 environments using the TiC–NiCr–Eu2O3 cathode. Coatings 2019, 9, 230. [Google Scholar] [CrossRef]
- Cai, D.; Zhang, D.; Chen, X.; Wu, H.; Wang, M.; Sang, G.; Li, Y. Influences of pH values’ changes on the oxide film of U-0.79 wt.% Ti alloy in aqueous solution—A combined study of traditional electrochemical tests and scanning reference electrode technique. Coatings 2019, 9, 224. [Google Scholar] [CrossRef]
- Su, C.-Y.; Zhou, Q.; Zou, C.-H. Surface deposition on titania in a physiological solution with ultraviolet irradiation in situ and effect of heat treatment. Coatings 2019, 9, 80. [Google Scholar] [CrossRef]
- Yung, T.-Y.; Chen, T.-C.; Tsai, K.-C.; Lu, W.-F.; Huang, J.-Y.; Liu, T.-Y. Thermal spray coatings of Al, ZnAl and inconel 625 alloys on SS304L for anti-saline corrosion. Coatings 2019, 9, 32. [Google Scholar] [CrossRef]
- Katić, J.; Šarić, A.; Despotović, I.; Matijaković, N.; Petković, M.; Petrović, Ž. Bioactive coating on titanium dental implants for improved anticorrosion protection: A combined experimental and theoretical study. Coatings 2019, 9, 612. [Google Scholar] [CrossRef]
- Conradi, M.; Sever, T.; Gregorčič, P.; Kocijan, A. Short- and long-term wettability evolution and corrosion resistance of uncoated and polymer-coated laser-textured steel surface. Coatings 2019, 9, 592. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M.; Bončina, T.; Kek Merl, D. Influence of growth defects on the corrosion resistance of sputter-deposited TiAlN hard coatings. Coatings 2019, 9, 511. [Google Scholar] [CrossRef]
- Monticelli, C.; Fantin, G.; Di Carmine, G.; Zanotto, F.; Balbo, A. Inclusion of 5-mercapto-1-phenyl-tetrazole into β-cyclodextrin for entrapment in silane coatings: An improvement in bronze corrosion protection. Coatings 2019, 9, 508. [Google Scholar] [CrossRef]
- Lahrour, S.; Benmoussat, A.; Bouras, B.; Mansri, A.; Tannouga, L.; Marzorati, S. Glycerin-grafted starch as corrosion inhibitor of C–Mn steel in 1 M HCl solution. Appl. Sci. 2019, 9, 4684. [Google Scholar] [CrossRef]
- Dagdag, O.; Hanbali, G.; Khalaf, B.; Jodeh, S.; El Harfi, A.; Deghles, A. Dual component polymeric epoxy-polyaminoamide based zinc phosphate anticorrosive formulation for 15CDV6 steel. Coatings 2019, 9, 463. [Google Scholar] [CrossRef]
- Shinato, K.W.; Huang, F.; Xue, Y.; Wen, L.; Jin, Y. The protection role of cysteine for Cu–5Zn–5Al–1Sn alloy corrosion in 3.5 wt.% NaCl solution. Appl. Sci. 2019, 9, 3896. [Google Scholar] [CrossRef]
- Lepojević, N.; Šćepan, I.; Glišić, B.; Jenko, M.; Godec, M.; Hočevar, S.; Rudolf, R. Characterisation of NiTi orthodontic archwires surface after the simulation of mechanical loading in CACO2-2 cell culture. Coatings 2019, 9, 440. [Google Scholar] [CrossRef]
- Romasanta, L.J.; D’Alençon, L.; Kirchner, S.; Pradère, C.; Leng, J. Thin coatings of cerium oxide nanoparticles with anti-reflective properties. Appl. Sci. 2019, 9, 3886. [Google Scholar] [CrossRef]
- Seo, Y.; Jung, J.-Y.; Chung, J.; Lee, S. Enhancement of corrosion resistance of aluminum 7075 surface through oil impregnation for subsea application. Appl. Sci. 2019, 9, 3762. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, Y.; Zhang, Z.; Li, K.; Li, Z. Electrochemical impedance spectroscopy investigation on the corrosive behaviour of waterborne silicate micaceous iron oxide coatings in seawater. Coatings 2019, 9, 415. [Google Scholar] [CrossRef]
- D’Elia, M.F.; Magni, M.; Trasatti, S.P.M.; Schweizer, T.B.; Niederberger, M.; Caseri, W. Poly(phenylene methylene)-based coatings for corrosion protection: Replacement of additives by use of copolymers. Appl. Sci. 2019, 9, 3551. [Google Scholar] [CrossRef]
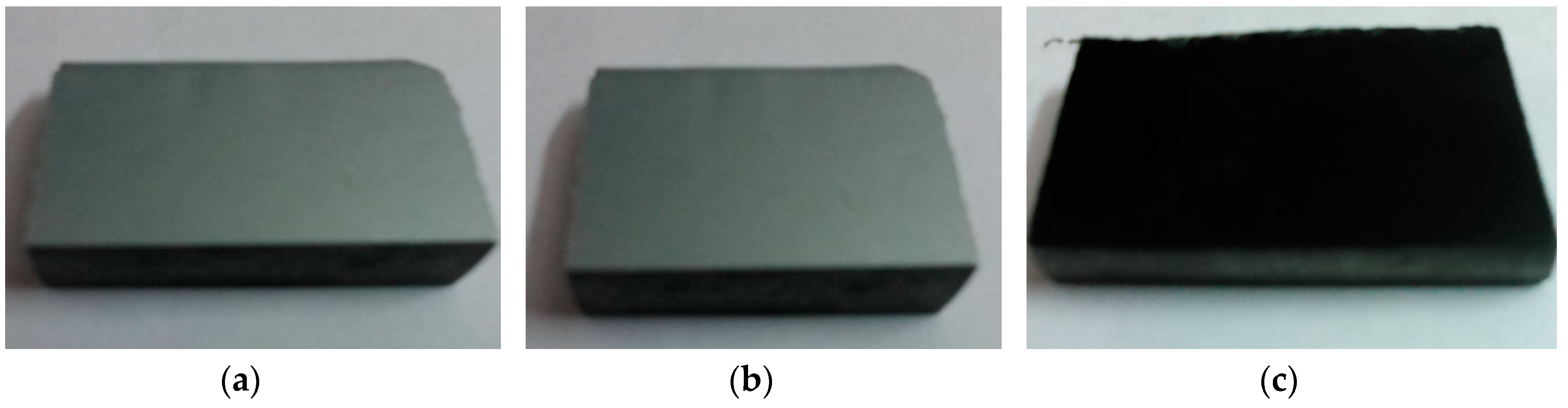
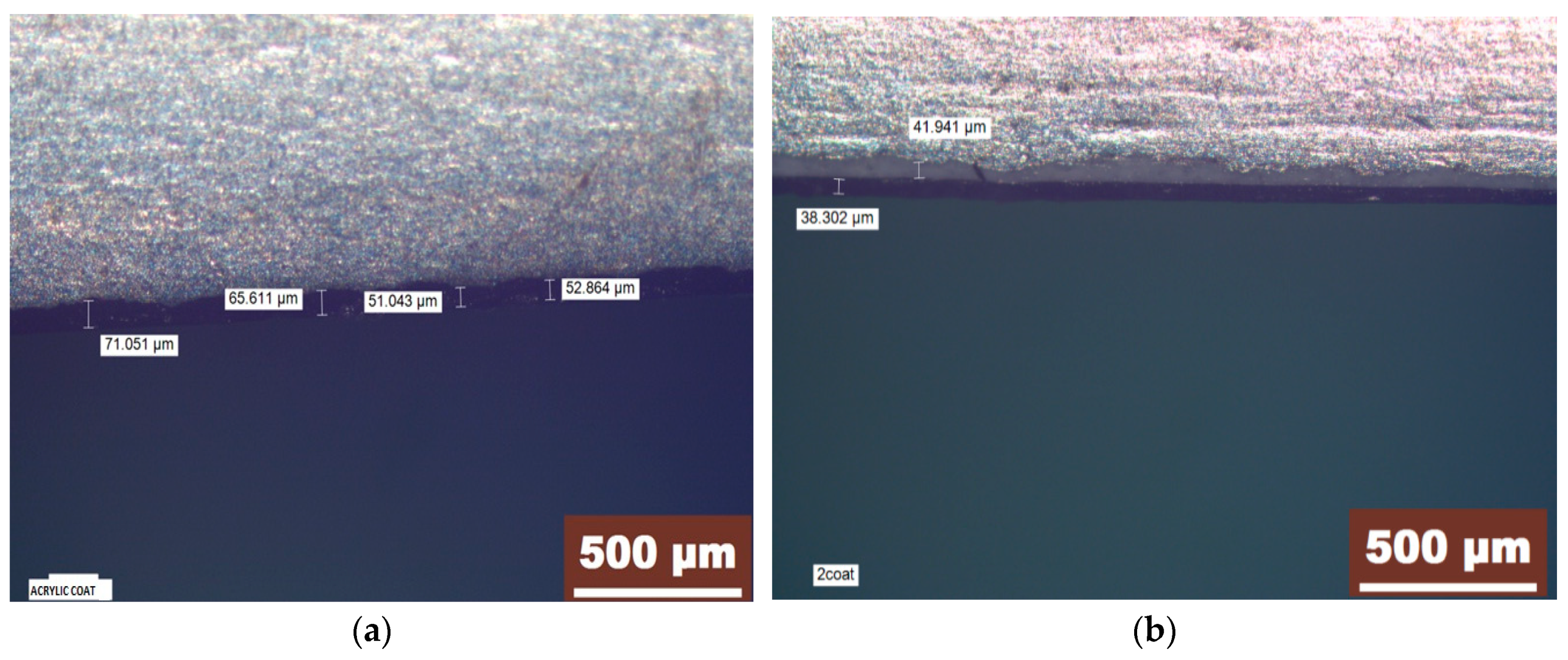
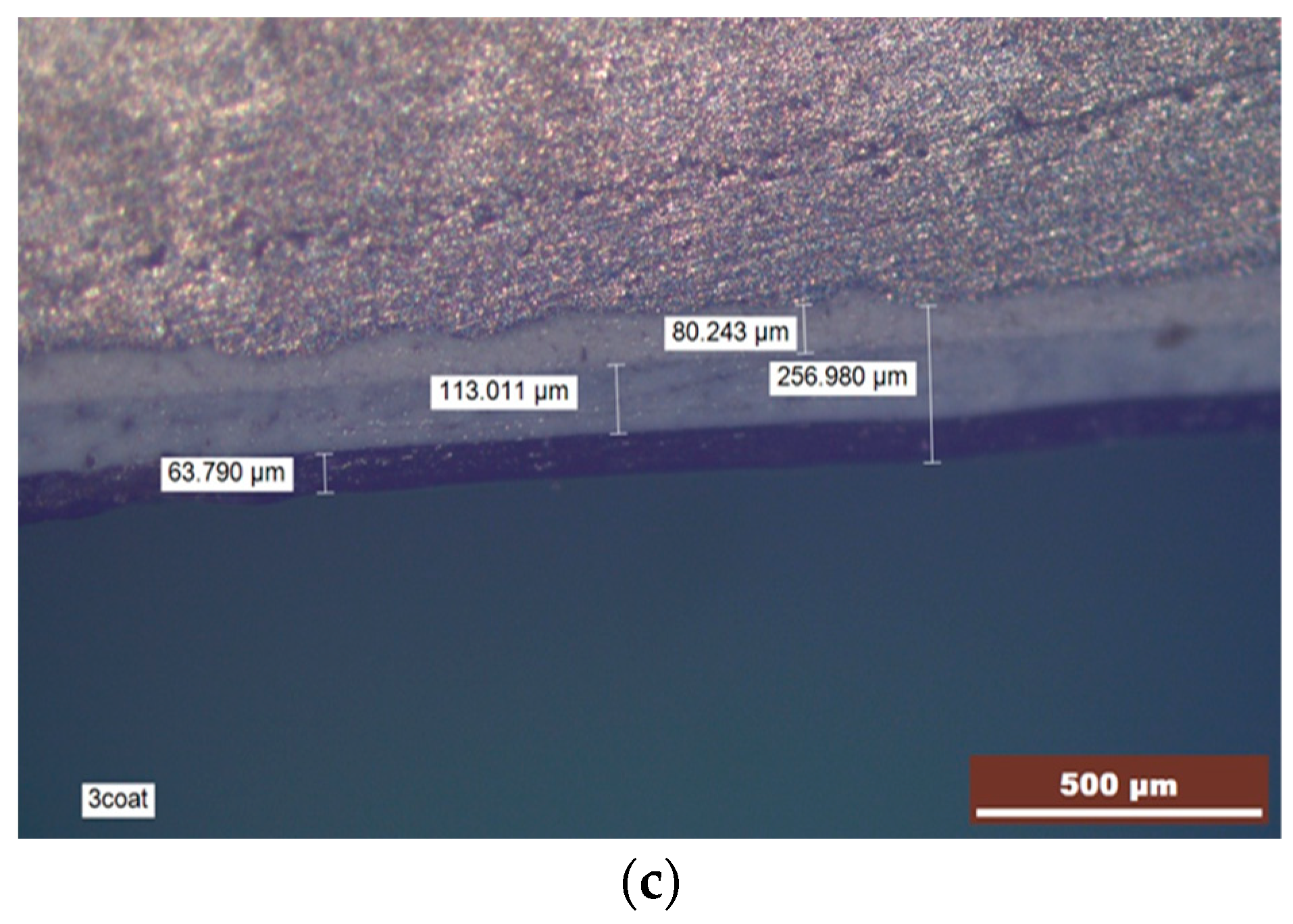




| Element | Si | Cu | Fe | Mn | Ni | Cr | Ti | Pb | Mg | Zn | Sn | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content wt.% | 9 | 3 | 1.3 | 0.55 | 0.55 | 0.15 | 0.25 | 0.35 | 0.55 | 1.2 | 0.15 | Bal. |
| Experimental Sample | βa (mV) | βc (mV) | Icorr (mA/cm2) | Ecorr (mVSCE) | Corrosion Rate (mpy) | Polarization Resistance Rp (kΩ·cm2) |
|---|---|---|---|---|---|---|
| Acrylic paint ED coat | 15.07 | 17.09 | 0.00065 | −736 | 1.18 × 10−4 | 5.3 |
| 2-coat polyester without primer | 23.25 | 16.03 | 0.00072 | −738 | 5.73 × 10−4 | 5.7 |
| 3-coat polyester with epoxy primer | 23.536 | 21.99 | 3.084 × 10−9 | 422.69 | 4.12 × 10−9 | 1.6 |
| Experimental Sample | Rct (Ω·cm2) | Cdl (µF·cm) |
|---|---|---|
| Acrylic paint ED coat | 8.89 × 109 | 1.009 × 10−11 |
| 2-coat polyester without primer | 5.569 × 109 | 3.723 × 10−11 |
| 3-coat polyester with epoxy primer | 2.486 × 109 | 2.694 × 10−11 |
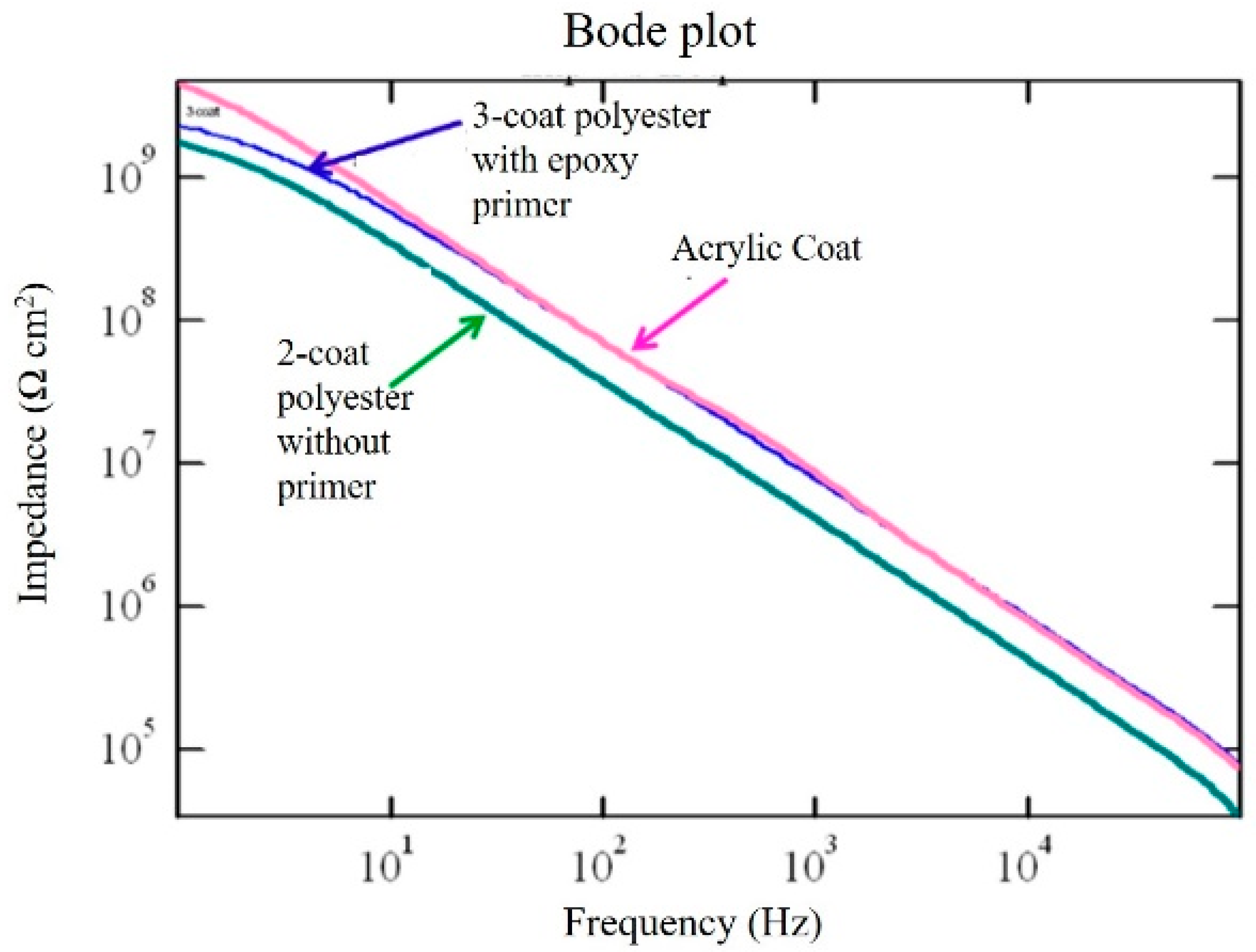
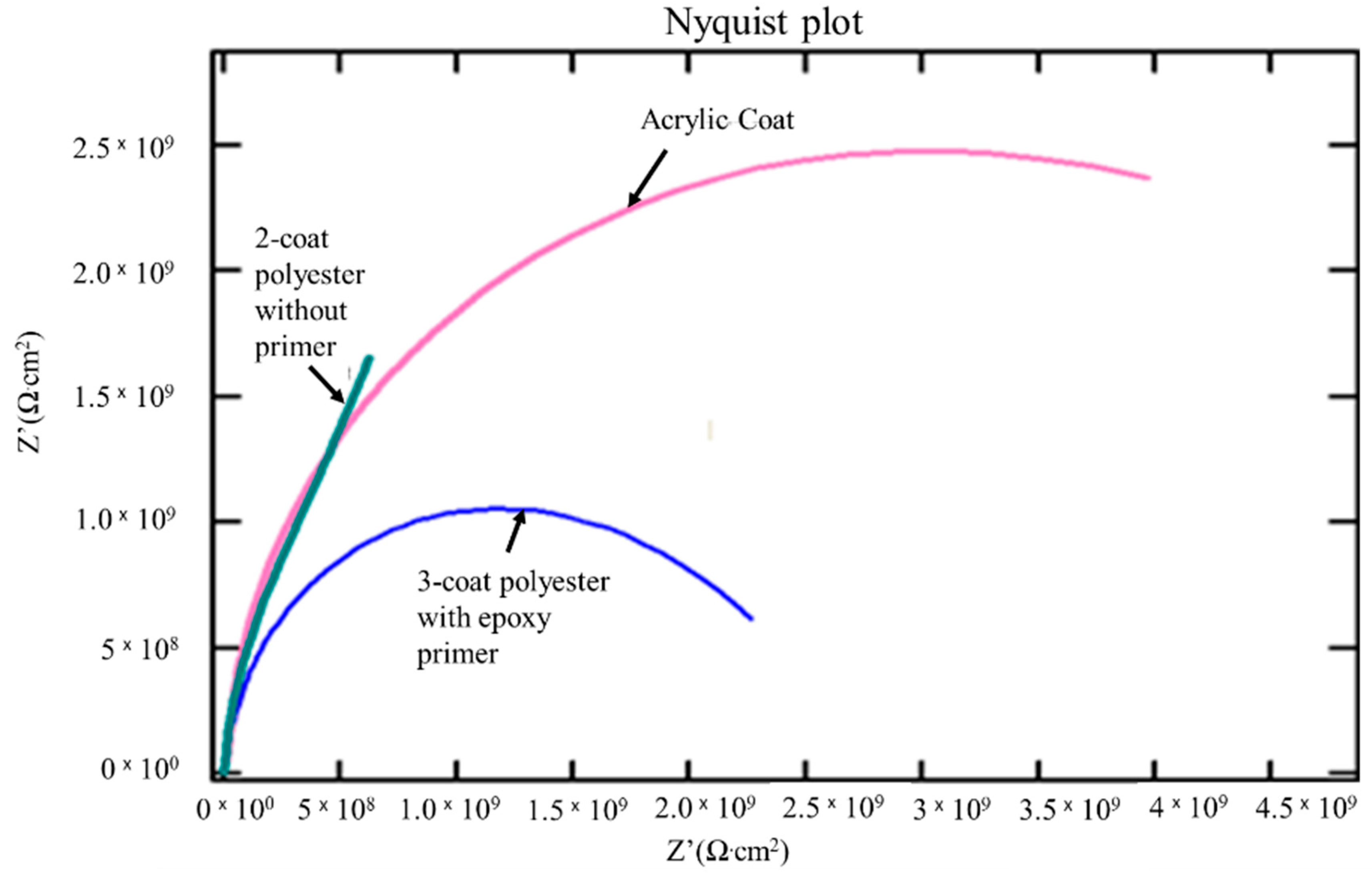
| Experimental Sample | Coating Resistance (Z’ Ω·cm2) |
|---|---|
| Acrylic paint ED coat | 2.50 × 109 |
| 2-coat polyester without primer | 1.35 × 109 |
| 3-coat polyester with epoxy primer | 1.00 × 109 |
| Experimental Sample | Corrosion Rate (mmpy) | |||
|---|---|---|---|---|
| After 24 h | After 48 h | After 72 h | After 96 h | |
| Acrylic paint ED coat | 10.12 | 12.01 | 14.13 | 14.50 |
| 2-coat polyester without primer | 9.43 | 10.31 | 12.14 | 13.13 |
| 3-coat polyester with epoxy primer | 7.21 | 7.51 | 8.00 | 8.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.-C.; Kumaran Selvaraj, S.; Subramanian, M.; Srinivasan, K.; Narayanan, S. Ultrasonic Sensors-Assisted Corrosion Studies on Surface Coated AlSi9Cu3 Alloy Die Castings. Coatings 2020, 10, 85. https://doi.org/10.3390/coatings10010085
Hu Y-C, Kumaran Selvaraj S, Subramanian M, Srinivasan K, Narayanan S. Ultrasonic Sensors-Assisted Corrosion Studies on Surface Coated AlSi9Cu3 Alloy Die Castings. Coatings. 2020; 10(1):85. https://doi.org/10.3390/coatings10010085
Chicago/Turabian StyleHu, Yuh-Chung, Senthil Kumaran Selvaraj, Manivannan Subramanian, Kathiravan Srinivasan, and Srinivasan Narayanan. 2020. "Ultrasonic Sensors-Assisted Corrosion Studies on Surface Coated AlSi9Cu3 Alloy Die Castings" Coatings 10, no. 1: 85. https://doi.org/10.3390/coatings10010085
APA StyleHu, Y.-C., Kumaran Selvaraj, S., Subramanian, M., Srinivasan, K., & Narayanan, S. (2020). Ultrasonic Sensors-Assisted Corrosion Studies on Surface Coated AlSi9Cu3 Alloy Die Castings. Coatings, 10(1), 85. https://doi.org/10.3390/coatings10010085







