Rapid Pyrolysis of SmBa2Cu3O7-δ Films in CSD-MOD Using Extremely-Low-Fluorine Solutions
Abstract
1. Introduction
2. Experimental Details
2.1. Solution and Thin Film Preparation
2.2. Thin Film Characterization
3. Results and Discussion
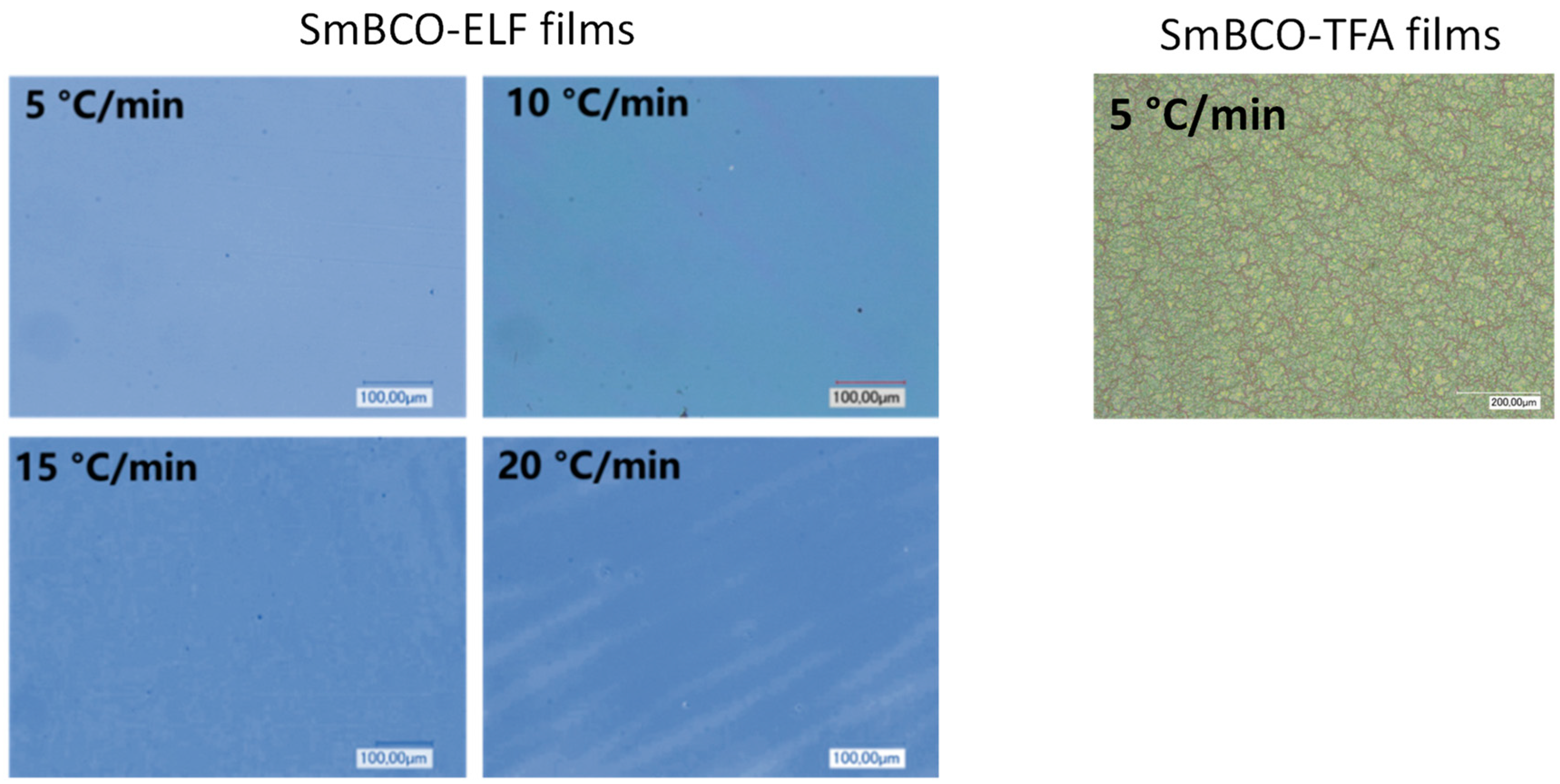
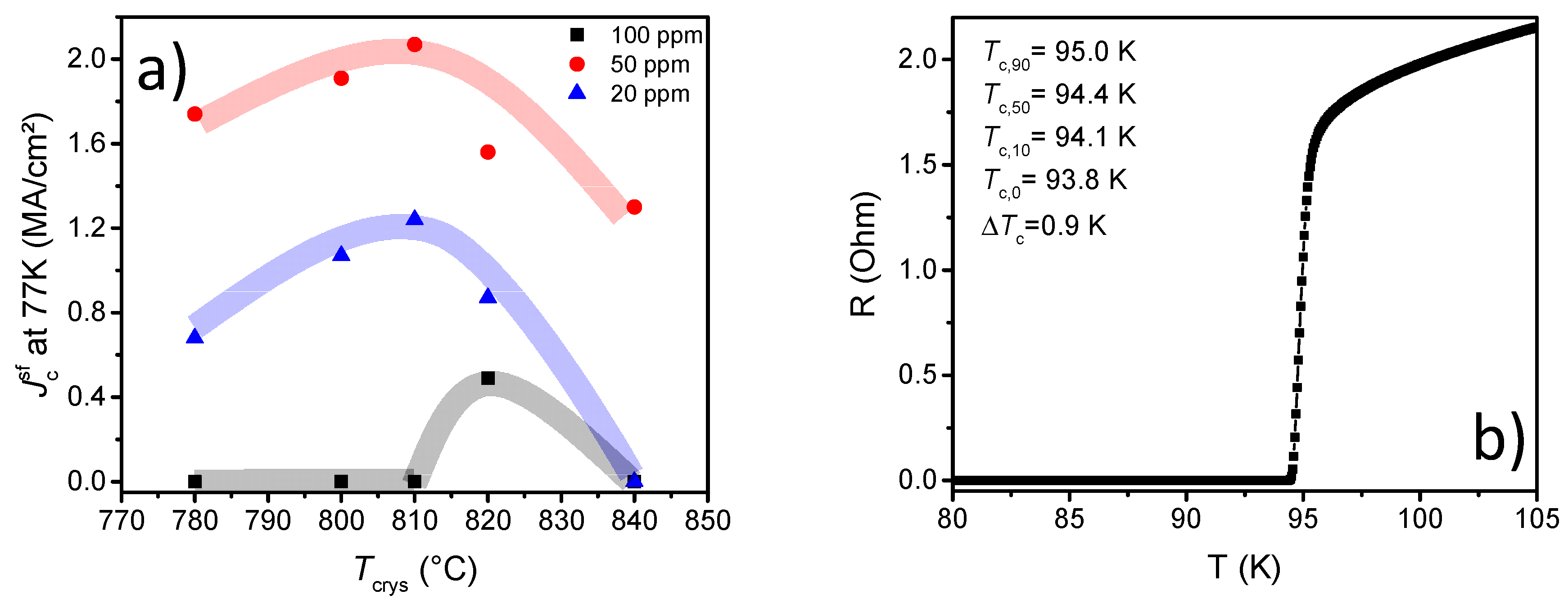
3.1. Influence of the Heating Rate during Pyrolysis
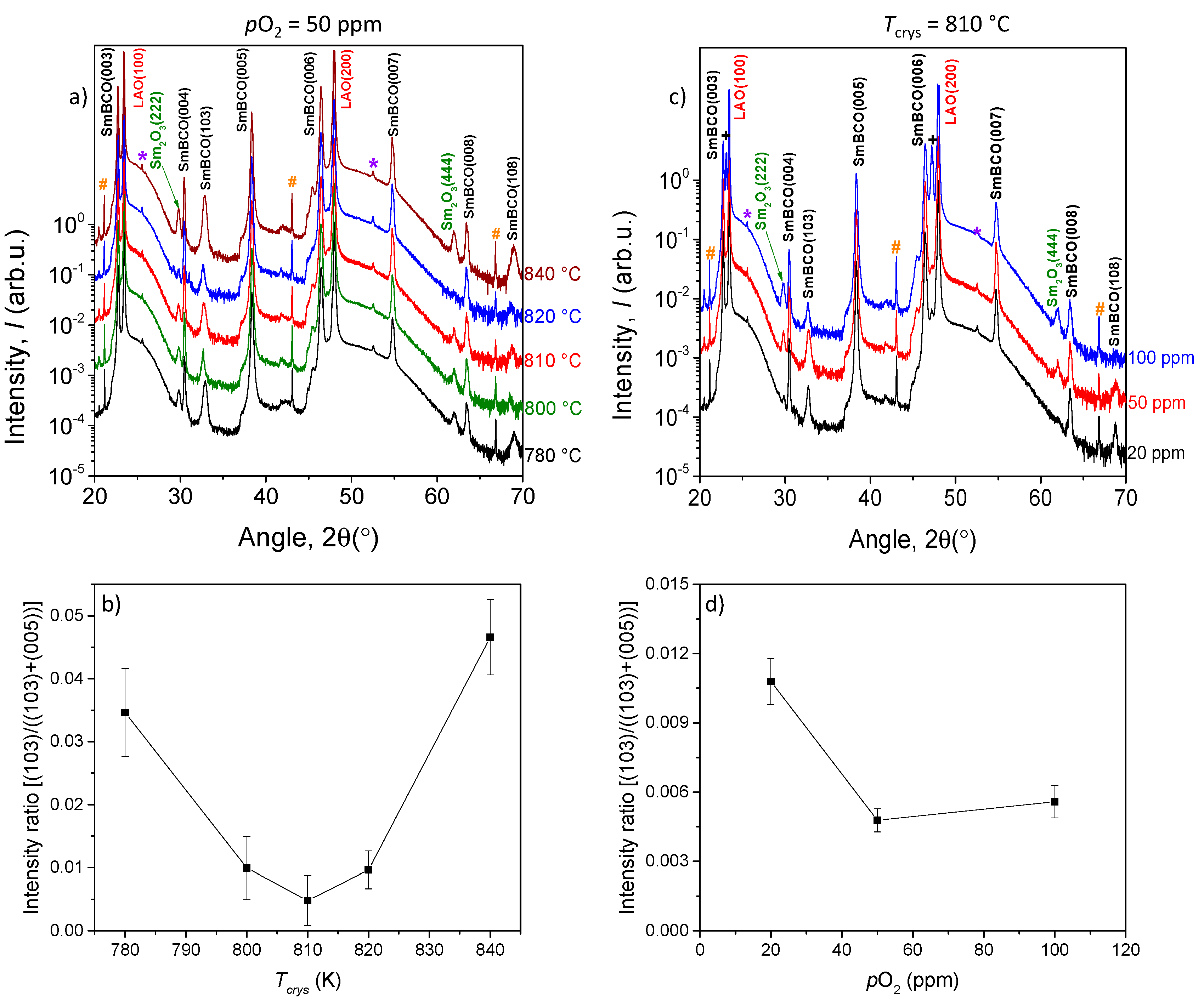
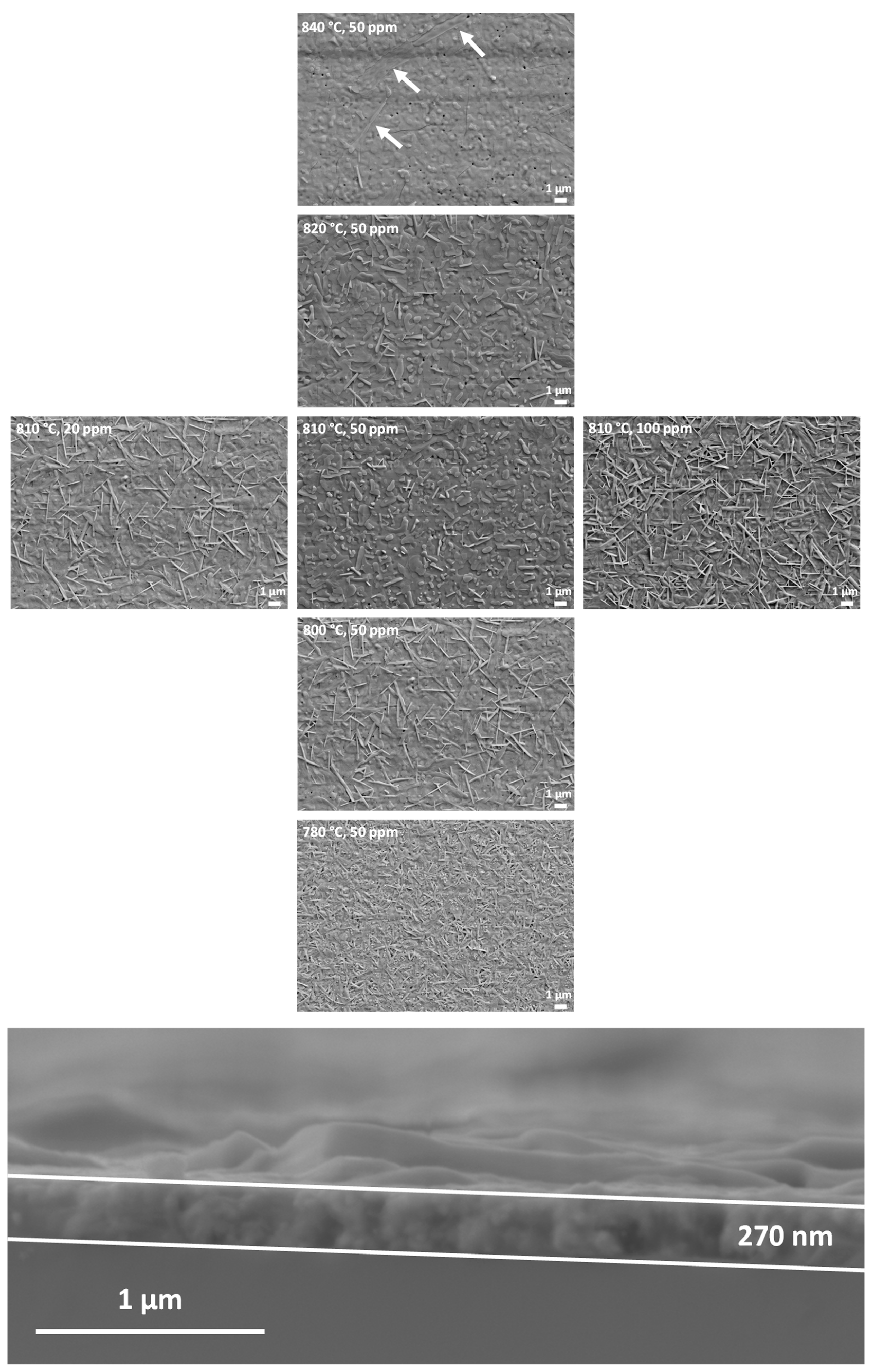
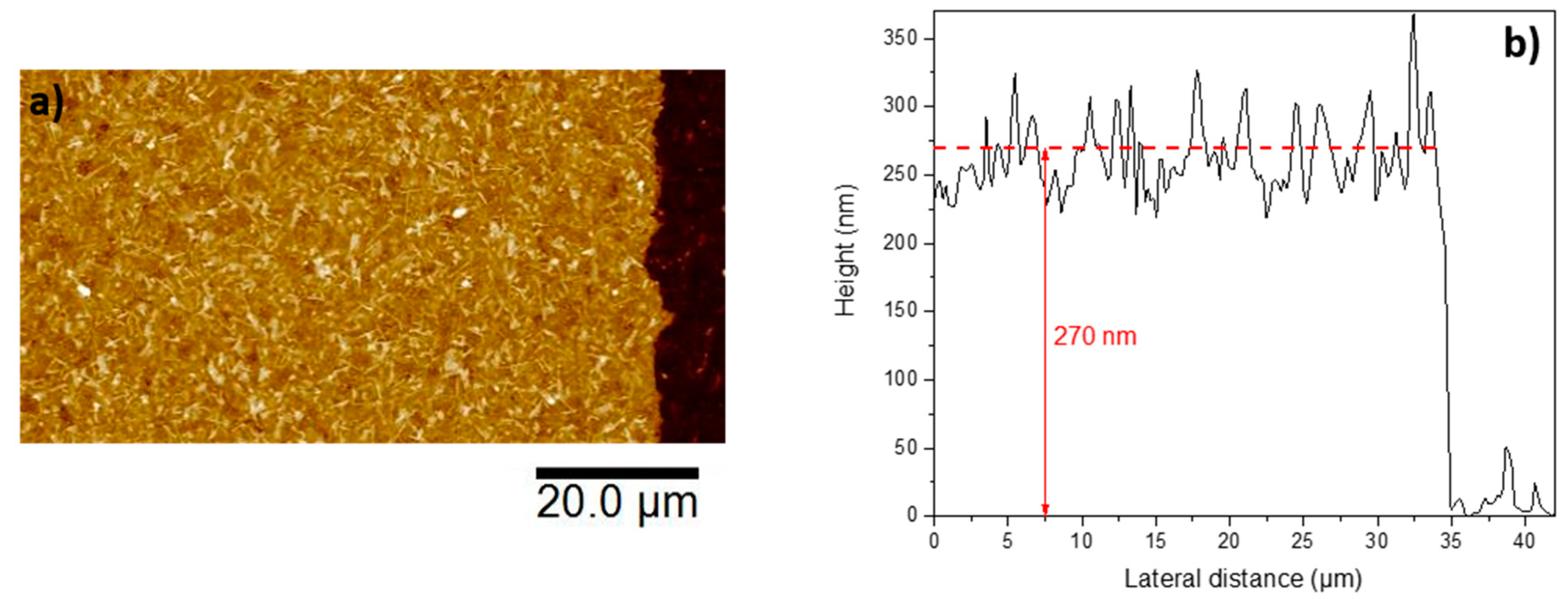
3.2. Optimization of the Growth Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Obradors, X.; Puig, T. Coated conductors for power applications: Materials challenges. Supercond. Sci. Technol. 2014, 27, 044003. [Google Scholar] [CrossRef]
- Van Driessche, I.; Schoofs, B.; Penneman, G.; Bruneel, E. Review of the application of high temperature superconductors in coated conductor development and the measurement of their properties. Measurement 2005, 339–349. [Google Scholar]
- Fernández, L.; Holzapfel, B.; Schindler, F.; de Boer, B.; Attenberger, A.; Hanish, J.; Schultz, L. Influence of the grain boundary network on the critical current of YBa2Cu3O7-δ films grown on biaxially textured metallic substrates. Phys. Rev. B. 2003, 67, 052503. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Ghalsasi, S.; Rusakova, I.; Salama, K. Flux pinning in MOD YBCO films by chemical doping. Supercond. Sci. Technol. 2007, 20, S147–S154. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, B.X.; Song, W.H.; Ma, R.C.; Liu, S.M.; Du, J.J.; Sun, Y.P. Resistivity-temperature characteristics of Y-deficient YBCO thin films derived by TFA-MOD method. Physica C 2004, 415, 79–84. [Google Scholar] [CrossRef]
- Miura, M.; Yoshizumi, M.; Sutoh, Y.; Nakaoka, K.; Miyata, S.; Yamada, Y.; Lzumi, T.; Shiohara, Y.; Goto, T.; Yoshinaka, A.; et al. Introduction of pinning center to enhance Ic under magnetic fields in REBCO coated conductors fabricated by advanced TFA-MOD process. Physica C 2008, 468, 1643–1646. [Google Scholar] [CrossRef]
- Gutierrez, J.; Llordes, A.; Gazquez, J.; Gibert, M.; Roma, N.; Ricart, S.; Pomar, A.; Sandiumenge, F.; Mestres, N.; Puig, T.; et al. Strong isotropic flux pinning in solution-derived YBCO nanocomposite superconductor films. Nat. Mater 2007, 6, 367–373. [Google Scholar] [CrossRef]
- Miura, M.; Maiorov, B.; Baily, S.A.; Haberkorn, N.; Willis, J.O.; Izumi, T.; Shiohara, Y.; Civale, L. Mixed pinning landscape in nanoparticle-introduced YGdBa2Cu3Oy films grown by metal organic deposition. Phys. Rev. B 2011, 83, 184519. [Google Scholar] [CrossRef]
- Dawley, J.T.; Clem, P.; Siegal, M.P.; Tallant, D.R.; Overmyer, D.L. Improving sol-gel YBCO-delta film morphology using high-boiling-point solvents. J. Mater. Res. 2002, 17, 1900–1903. [Google Scholar] [CrossRef]
- Gupta, A.; Jagannathan, R.; Cooper, E.I.; Giess, E.A.; Landman, J.I.; Hussey, B.W. Superconducting oxide films with high transition temperature prepared from metal trifluoroacetate precursors. Appl. Phys. Lett. 1988, 52, 2077–2079. [Google Scholar] [CrossRef]
- Nakaoka, K.; Yoshida, R.; Kimura, K.; Kato, T.; Usui, Y.; Izumi, T.; Shiohara, Y. Another approach for controlling size and distribution of nanoparticles in coated conductors fabricated by the TFA-MOD method. Supercond. Sci. Technol. 2017, 30, 055008. [Google Scholar] [CrossRef]
- Ono, T.; Matsumoto, K.; Osamura, K.; Hirabayashi, I. Crystal growth of YBa2Cu3O7-δ thin films prepared by TFA-MOD method. IEEE Trans. Appl. Supercond. 2003, 13, 2512–2515. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Song, S.-A.; Kim, B.-J.; Park, J.-A.; Kim, H.-J.; Hong, H.-J.; Lee, H.-G.; Jang, S.-H.; Joo, J.; Yoo, J.-M.; et al. Effect of precursor composition on Jc enhancement of YBCO film prepared by TFA-MOD method. Physica C 2006, 445–448, 578–581. [Google Scholar] [CrossRef]
- Chen, H.; Zalamova, K.; Pomar, A.; Granados, X.; Puig, T.; Obradors, X. Growth rate control and solid-gas modeling of TFA-YBa2Cu3O7 thin film processing. Supercond. Sci. Technol. 2010, 23, 034005. [Google Scholar] [CrossRef]
- Araki, T.; Hirabayashi, I. Review of a chemical approach to YBa2Cu3O7−x coated superconductor metalorganic deposition using trifluoroacetates. Supercond. Sci. Technol. 2003, 16, R71–R94. [Google Scholar] [CrossRef]
- Rupich, M.W.; Verebelyi, D.T.; Zhang, W.; Kodenkandath, T.; Li, X.P. Metalorganic Deposition of YBCO Films for Second-Generation High-Temperature Superconductor Wires. MRS Bull. 2004, 29, 572–578. [Google Scholar] [CrossRef]
- Erbe, M.; Hanisch, J.; Freudenberg, T.; Kirchner, A.; Monch, I.; Kaskel, S.; Schultz, L.; Holzapfel, B. Improved REBa2Cu3O7−x (RE=Y, Gd) structure and superconducting properties by addition of acetylacetone in TFA-MOD precursor solutions. J. Mater. Chem. A 2014, 2, 4932–4944. [Google Scholar] [CrossRef]
- Palmer, X.; Pop, C.; Eloussifi, H.; Villarejo, B.; Roura, P.; Farjas, J.; Calleja, A.; Palau, A.; Obradors, X.; Puig, T.; et al. Solution design for low-fluorine trifluoroacetate route to YBa2Cu3O7 films. Supercond. Sci. Technol. 2016, 29, 24002. [Google Scholar] [CrossRef]
- Motoki, T.; Shimoyama, J.; Ogino, H.; Kishio, K.; Roh, J.; Tohei, T.; Ikuhara, Y.; Horri, S.; Doi, T.; Honda, G.; et al. Microstructures and improved Jc–H characteristics of Cl-containing YBCO thin films prepared by the fluorine-free MOD method. Supercond. Sci. Technol. 2016, 29, 015006. [Google Scholar] [CrossRef]
- Wu, W.; Feng, F.; Zhao, Y.; Tang, X.; Xue, Y.R.; Shi, K.; Huang, R.X.; Qu, T.M.; Wang, X.H.; Han, Z.H.; et al. A low-fluorine solution with a 2:1 F/Ba mole ratio for the fabrication of YBCO films. Supercond. Sci. Technol. 2014, 27, 055006. [Google Scholar] [CrossRef]
- Ding, F.Z.; Gu, H.W.; Li, T. The fabrication of YBCO film by metal-organic deposition using terpineol-modified trifluoroacetates. Supercond. Sci. Technol. 2008, 21, 095004. [Google Scholar] [CrossRef]
- Li, M.J.; Yang, W.T.; Shu, G.Q.; Bai, C.Y.; Lu, Y.M.; Guo, Y.Q.; Liu, Z.Y.; Cai, C.B. Controlled-Growth of Film Using Modified Low-Fluorine Chemical Solution Deposition. IEEE Trans. Appl. Supercond. 2015, 25, 6601804. [Google Scholar] [CrossRef]
- Cai, C.; Holzapfel, B.; Hanisch, J.; Schultz, L. Superconductivity suppression and flux-pinning crossover in artificial multilayers of ternary RBa2Cu3O7-δ(R = Gd, Nd, and Eu). Phys. Rev. B 2004, 70, 064504. [Google Scholar] [CrossRef]
- Yamada, Y.; Takahashi, K.; Kobayashi, H.; Konishi, M.; Ibi, A.; Muroga, T.; Miyata, S. Epitaxial nanostructure and defects effective for pinning in Y(RE)Ba2Cu3O7−x coated conductors. Appl. Phys. Lett. 2005, 87, 132502. [Google Scholar] [CrossRef]
- Nomura, K.; Hoshi, S.; Yao, X.; Kakimoto, K.; Nakamura, Y.; Izumi, T.; Shiohara, Y. Preferential growth mechanism of REBa2Cu3Oy (RE = Y, Nd) crystal on MgO substrate by liquid phase epitaxy. J. Mater. Res. 2001, 16, 979–989. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Miura, S.; Awaji, S.; Ichino, Y.; Matsumoto, K.; Izumi, T.; Watanabe, K.; Yoshida, Y. Flux pinning landscape up to 25T in SmBa2Cu3Oy films with BaHfO3 nanorods fabricated by low-temperature growth technique. Supercond. Sci. Technol. 2017, 30, 104004. [Google Scholar] [CrossRef]
- Cayado, P.; Erbe, M.; Kauffmann-Weiss, S.; Buhler, C.; Jung, A.; Hanisch, J.; Holzapfel, B. Large critical current densities and pinning forces in CSD-grown superconducting GdBa2Cu3O7−x-BaHfO3 nanocomposite films. Supercond. Sci. Technol. 2017, 30, 094007. [Google Scholar] [CrossRef]
- Murakami, M.; Sakai, N.; Higuchi, T.; Yoo, S.I. Melt-processed light rare earth element-Ba-Cu-O. Supercond. Sci. Technol. 1996, 9, 1015–1032. [Google Scholar] [CrossRef]
- Yoshida, Y.; Ozaki, T.; Ichino, Y.; Takai, Y.; Matsumoto, K.; Ichinose, A.; Mukaida, M.; Horii, S. Progress in development of advanced PLD process for high Jc REBCO film. Physica C 2008, 468, 1606–1610. [Google Scholar] [CrossRef]
- Wee, S.H.; Goyal, A.; Martin, P.M.; Heatherly, L. High in-field critical current densities in epitaxial NdBa2Cu3O7−δ films on RABiTS by pulsed laser deposition. Supercond. Sci. Technol. 2006, 19, 865–868. [Google Scholar] [CrossRef]
- Andreouli, C.; Tsetsekou, A. Synthesis of HTSC Re(Y)Ba2Cu3Ox powders: The role of ionic radius. Phys. C 1997, 291, 274–286. [Google Scholar] [CrossRef]
- Islam, M.S.; Baetzold, R.C. Atomistic simulation of dopant substitution in YBa2Cu3O7. Phys. Rev. B 1989, 40, 10926–10935. [Google Scholar] [CrossRef] [PubMed]
- Macmanus-Driscoll, J.L. Materials chemistry and thermodynamics of REBa2Cu3O7−x. Adv. Mater. 1997, 9, 457–473. [Google Scholar] [CrossRef]
- Miura, M.; Kato, T.; Yoshizumi, M.; Yamada, Y.; Izumi, T.; Shiohara, Y.; Hirayama, T. Enhancement of Flux Pinning in Y1-xSmxBa1:5Cu3Oy Coated Conductors with Nanoparticles. Appl. Phys. Express 2008, 1, 051701. [Google Scholar] [CrossRef]
- Fuger, R.; Eisterer, M.; Oh, S.S.; Weber, H.W. Superior properties of SmBCO coated conductors at high magnetic fields and elevated temperatures. Physica C 2010, 470, 323–325. [Google Scholar] [CrossRef]
- Shi, Y.H.; Dennis, A.R.; Cardwell, D.A. A new seeding technique for the reliable fabrication of large, SmBCO single grains containing silver using top seeded melt growth. Supercond. Sci. Technol. 2015, 28, 035014. [Google Scholar] [CrossRef]
- Miura, M.; Yoshida, Y.; Ichino, Y.; Ozaki, T.; Takai, Y.; Matsumoto, K.; Ichinose, A.; Horii, S.; Mukaida, M. Dislocation Density and Critical Current Density of Sm1+xBa2-xCu3Oy Films Prepared by Various Fabrication Processes. Jpn. J. Appl Phys. 2006, 45, L701–L704. [Google Scholar] [CrossRef]
- Mitani, A.; Teranishi, R.; Yamada, K.; Mori, N.; Mukaida, M.; Kiss, T.; Inoue, M.; Shiohara, Y.; Izumi, T.; Nakaoka, K.; et al. Effect of fabrication conditions on crystalline of SmBCO films fabricated by TFA-MOD method. Physica C 2008, 468, 1546–1549. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.T.; Yang, X.F.; Wang, M.J.; Zhao, Y. Preparation of High-Performance SmBa2Cu3O7−x Superconducting Films by Chemical Solution Deposition Approach. J. Supercond Nov. Magn. 2014, 27, 1801–1806. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.T.; Zhang, X.; Wang, M.J.; Zhao, Y. Influence of metal cation concentration on structure and performance of SmBa2Cu3O7-z superconducting films by non-fluorine CSD method. Mater. Lett. 2015, 153, 73–76. [Google Scholar] [CrossRef]
- Sun, M.; Liu, W.Q.; Zhou, H.B.; He, K.; Yu, M.; Liu, Z.Y.; Lu, Y.M.; Cai, C.B.; Chen, S.G. La2Zr2O7 Doping Effects on MOD-SmBCO-Coated Conductors. J. Supercond. Nov. Magn. 2018, 31, 3679–3683. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Cayado, P.; Erbe, M.; Jung, A.; Hänisch, J.; Holzapfel, B.; Liu, Z.; Cai, C. Rapid Pyrolysis of SmBa2Cu3O7-δ Films in CSD-MOD Using Extremely-Low-Fluorine Solutions. Coatings 2020, 10, 31. https://doi.org/10.3390/coatings10010031
Li M, Cayado P, Erbe M, Jung A, Hänisch J, Holzapfel B, Liu Z, Cai C. Rapid Pyrolysis of SmBa2Cu3O7-δ Films in CSD-MOD Using Extremely-Low-Fluorine Solutions. Coatings. 2020; 10(1):31. https://doi.org/10.3390/coatings10010031
Chicago/Turabian StyleLi, Minjuan, Pablo Cayado, Manuela Erbe, Alexandra Jung, Jens Hänisch, Bernhard Holzapfel, Zhiyong Liu, and Chuanbing Cai. 2020. "Rapid Pyrolysis of SmBa2Cu3O7-δ Films in CSD-MOD Using Extremely-Low-Fluorine Solutions" Coatings 10, no. 1: 31. https://doi.org/10.3390/coatings10010031
APA StyleLi, M., Cayado, P., Erbe, M., Jung, A., Hänisch, J., Holzapfel, B., Liu, Z., & Cai, C. (2020). Rapid Pyrolysis of SmBa2Cu3O7-δ Films in CSD-MOD Using Extremely-Low-Fluorine Solutions. Coatings, 10(1), 31. https://doi.org/10.3390/coatings10010031





