Figainin 1, a Novel Amphibian Skin Peptide with Antimicrobial and Antiproliferative Properties
Abstract
:1. Introduction
2. Material and Methods
2.1. Obtention of Skin Secretion
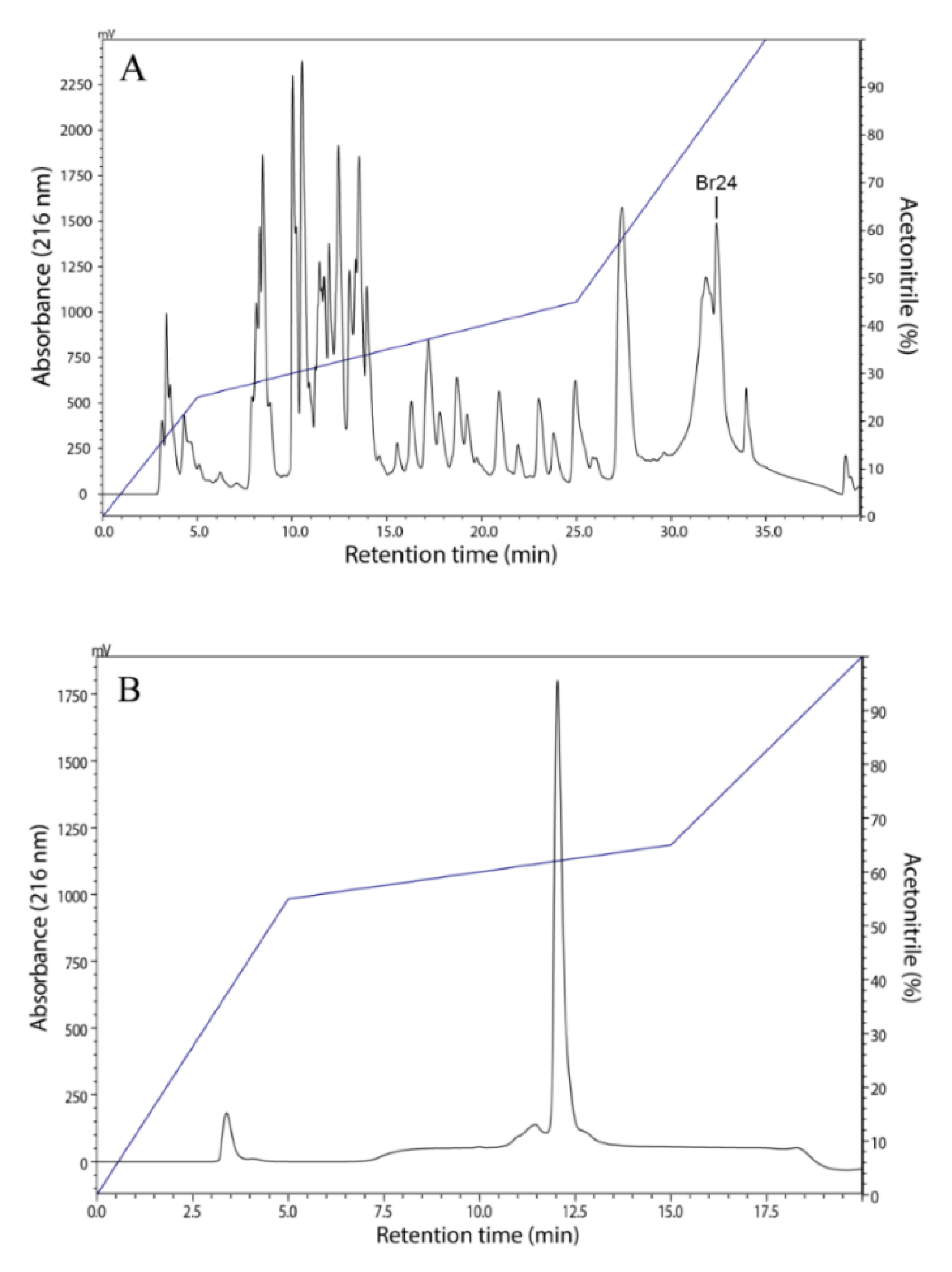
2.2. Peptide Purification
2.3. Peptide Quantification
2.4. Structural Characterization
2.4.1. MALDI-TOF MS Analysis and N-Terminal Chemical Sequencing
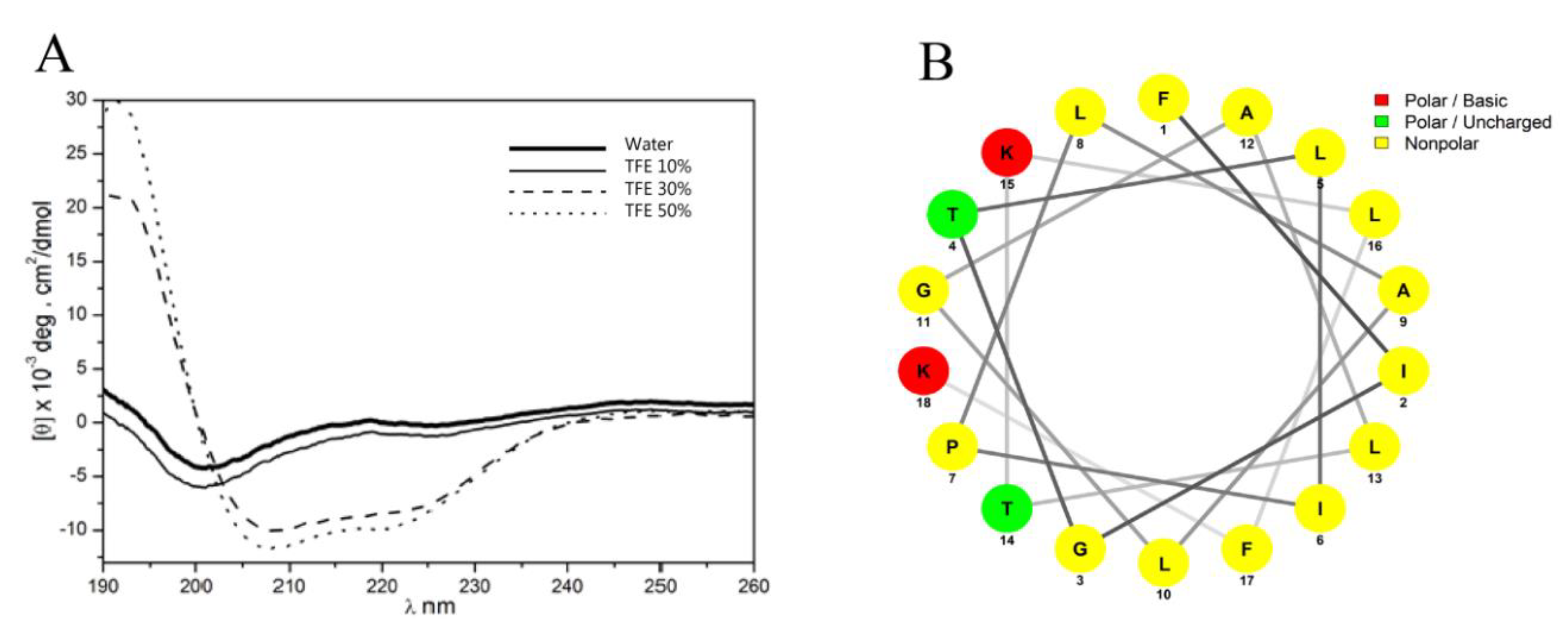
2.4.2. Secondary Structure Analysis by Circular Dichroism
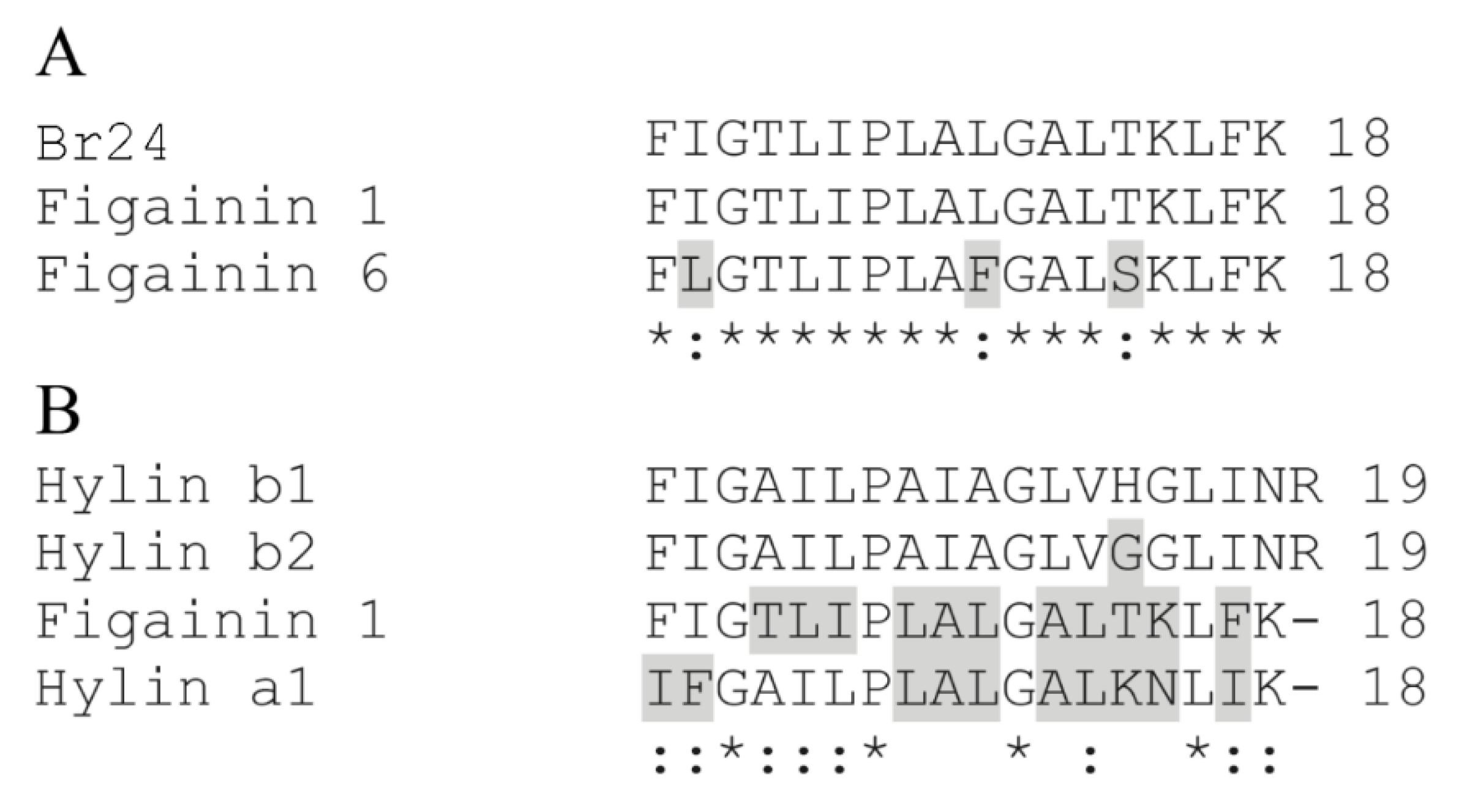
2.4.3. Bioinformatics Analysis
2.5. Antibacterial and Antifungal Assays
2.6. Anti-Epimastigote Activity against Trypanosoma cruzi
2.7. Hemolytic Assay
2.8. Antiproliferative Assay
3. Results
3.1. Isolation, Identification and Structural Characterization of Figainin 1
3.2. Antimicrobial and Antitrypanosomal Activity
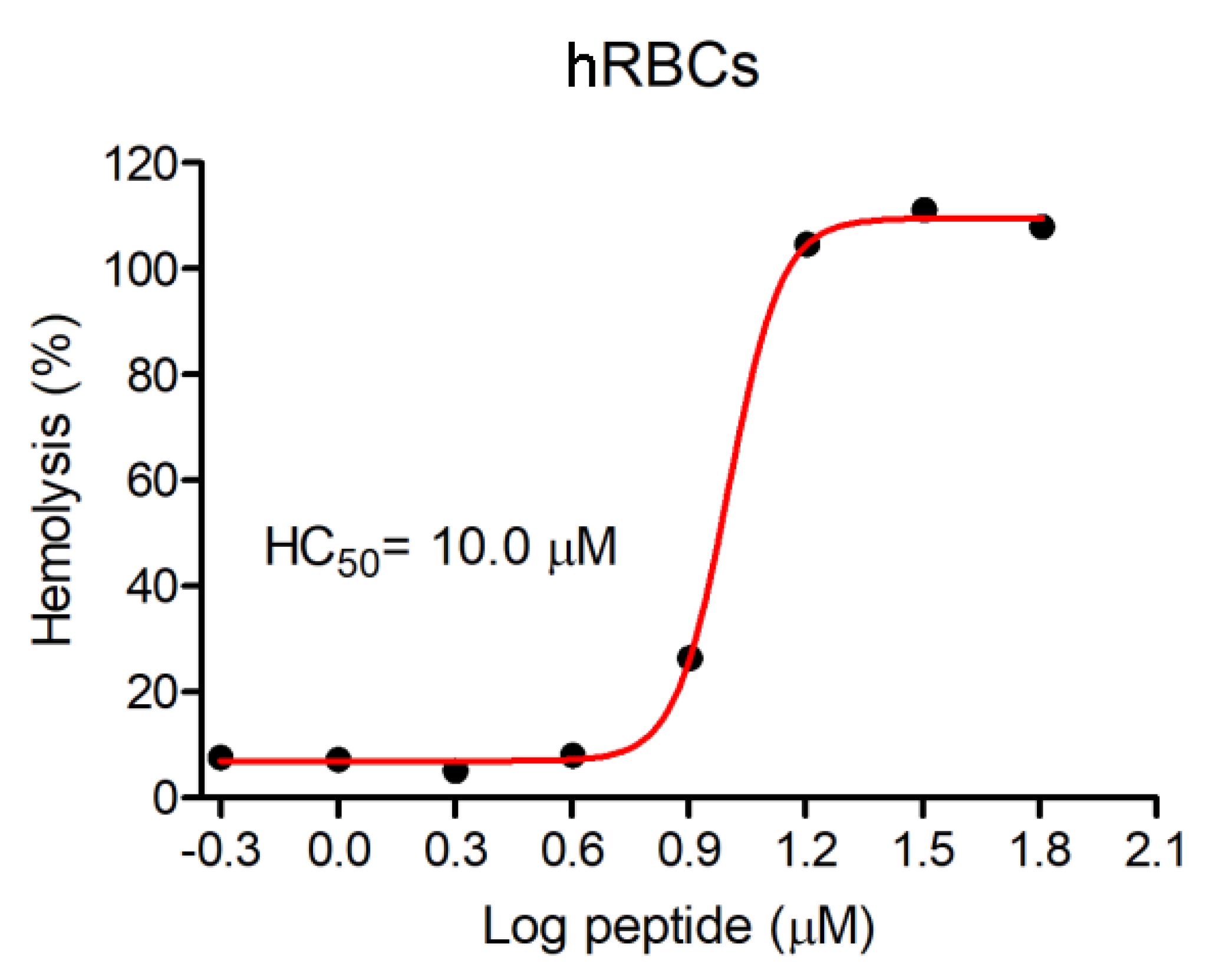
3.3. Hemolytic Activity
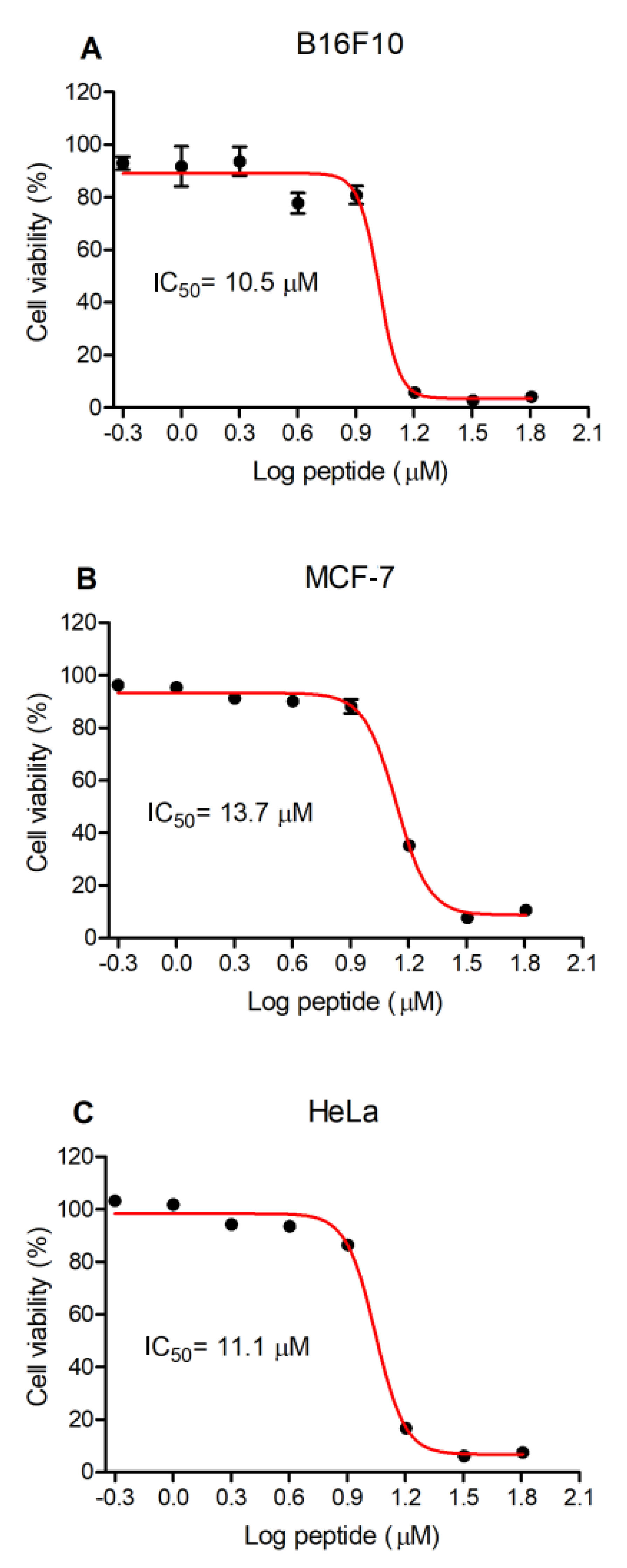
3.4. Antiproliferative Activity of Figainin 1
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, X.; Lai, R. The chemistry and biological activities of peptides from amphibian skin secretions. Chem. Rev. 2015, 115, 1760–1846. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.T. The natural history of amphibian skin secretions, their normal functioning and potential medical applications. Biol. Rev. Camb. Philos. Soc. 1997, 72, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.C.C.; Fontes, W.; Sebben, A.; Castro, M.S. Antimicrobial peptides from anurans skin secretions. Protein Pept. Lett. 2003, 10, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Park, S.J.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides: Therapeutic potentials. Expert Rev. Anti Infect. Ther. 2014, 12, 1477–1486. [Google Scholar] [CrossRef]
- Hancock, R.E.; Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Zaiou, M. Multifunctional antimicrobial peptides: Therapeutic targets in several human diseases. J. Mol. Med. 2007, 85, 317–329. [Google Scholar] [CrossRef]
- Mwangi, J.; Hao, X.; Lai, R.; Zhang, Z.Y. Antimicrobial peptides: New hope in the war against multidrug resistance. Zool. Res. 2019, 40, 488–505. [Google Scholar] [CrossRef]
- Dubois, A. The nomenclatural status of Hysaplesia, Hylaplesia, Dendrobates and related nomina (Amphibia, Anura), with general comments on zoological nomenclature and its governance, as well as on taxonomic databases and websites. Bionomina 2017, 11, 1–48. [Google Scholar] [CrossRef]
- Magalhães, B.S.; Melo, J.A.; Leite, J.R.S.; Silva, L.P.; Prates, M.V.; Vinecky, F.; Barbosa, E.A.; Verly, R.M.; Mehta, A.; Nicoli, J.R. Post-secretory events alter the peptide content of the skin secretion of Hypsiboas raniceps. Biochem. Biophys. Res. Commun. 2008, 377, 1057–1061. [Google Scholar] [CrossRef] [Green Version]
- Siano, A.; Húmpola, M.V.; de Oliveira, E.; Albericio, F.; Simonetta, A.C.; Lajmanovich, R.; Tonarelli, G.G. Antimicrobial peptides from skin secretions of Hypsiboas pulchellus (Anura: Hylidae). J. Nat. Prod. 2014, 77, 831–841. [Google Scholar] [CrossRef]
- Almeida, R.A.; Gordo, M.; Silva, F.M.A.; Araújo, R.C.; Ramada, M.H.S.; Abrão, F.Y.; Costa, T.O.G.; Koolen, H.H.F.; Souza, A.D.L.; Bloch, C., Jr. Cinerascetins, new peptides from Hypsiboas cinerascens: MALDI LIFT-TOF-MS/MS de novo sequence and imaging analysis. J. Braz. Chem. Soc. 2015, 26, 2290–2297. [Google Scholar]
- Nacif-Marçal, L.; Pereira, G.R.; Abranches, M.V.; Costa, N.C.S.; Cardoso, S.A.; Honda, E.R.; de Paula, S.O.; Feio, R.N.; Oliveira, L.L. Identification and characterization of an antimicrobial peptide of Hypsiboas semilineatus (Spix, 1824) (Amphibia, Hylidae). Toxicon 2015, 99, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, M.S.; Matsushita, R.H.; Sebben, A.; Sousa, M.V.; Fontes, W. Hylins: Bombinins H structurally related peptides from the skin secretion of the Brazilian tree-frog Hyla biobeba. Protein Pept. Lett. 2005, 12, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.S.; Ferreira, T.C.G.; Cilli, E.M.; Crusca, E., Jr.; Mendes-Giannini, M.J.S.; Sebben, A.; Ricart, C.A.O.; Sousa, M.V.; Fontes, W. Hylin a1, the first cytolytic peptide isolated from the arboreal South American frog Hypsiboas albopunctatus (“spotted treefrog”). Peptides 2009, 30, 291–296. [Google Scholar] [CrossRef]
- Aitken, A.; Learmonth, M.P. Protein determination by UV absorption. In The Protein Protocols Handbook, 2nd ed.; Walker, J.M., Ed.; Springer Protocols Handbooks; Humana Press: Totowa, NJ, USA, 2002; pp. 3–6. [Google Scholar]
- Hunt, D.F.; Yates, J.R.; Shabanowitz, J.; Winston, S.; Hauer, C.R. Protein sequencing by tandem mass spectrometry. Proc. Natl. Acad. Sci. USA 1986, 83, 6233–6237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenfield, N.J.; Fasman, G.D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 1969, 8, 4108–4116. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2015, 44, D1087–D1093. [Google Scholar] [CrossRef] [Green Version]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Bjellqvist, B.; Hughes, G.J.; Pasquali, C.; Paquet, N.; Ravier, F.; Sanchez, J.C.; Frutiger, S.; Hochstrasser, D. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 1993, 14, 1023–1031. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Springer Protocols Handbooks; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Mol, A.R.; Castro, M.S.; Fontes, W. NetWheels: A web application to create high quality peptide helical wheel and net projections. BioRxiv 2018, 416347. [Google Scholar] [CrossRef] [Green Version]
- Libério, M.S.; Joanitti, G.A.; Azevedo, R.B.; Cilli, E.M.; Zanotta, L.C.; Nascimento, A.C.; Sousa, M.V.; Júnior, O.R.P.; Fontes, W.; Castro, M.S. Anti-proliferative and cytotoxic activity of pentadactylin isolated from Leptodactylus labyrinthicus on melanoma cells. Amino Acids 2011, 40, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.A.; Campos, P.F.; Andrade, A.C.; Bloch, C., Jr. Response of Hypsiboas raniceps to Abiotic and Biotic Stresses: Gene Expression and MALDI-Mass Spectrometry Imaging Analysis of Skin Peptides. Unpublished; 2017. Available online: https://www.ncbi.nlm.nih.gov/protein/1346344964 (accessed on 2 June 2020).
- Conlon, J.M. Structural diversity and species distribution of host-defense peptides in frog skin secretions. Cell Mol. Life Sci. 2011, 68, 2303–2315. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Pieper, D.H. Tackling threats and future problems of multidrug-resistant bacteria. Curr. Top. Microbiol. Immunol. 2016, 398, 3–33. [Google Scholar]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef]
- Zasloff, M. Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Huang, J.; Chen, Y. Alpha-helical cationic antimicrobial peptides: Relationships of structure and function. Protein Cell 2010, 1, 143–152. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, K. Control of cell selectivity of antimicrobial peptides. Biochim. Biophys. Acta 2009, 1788, 1687–1692. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Feng, Q.; Yan, Q.; Hao, X.; Chen, Y. Alpha-helical cationic anticancer peptides: A promising candidate for novel anticancer drugs. Mini Rev. Med. Chem. 2015, 15, 73–81. [Google Scholar] [CrossRef]
- Dathe, M.; Wieprecht, T. Structural features of helical antimicrobial peptides: Their potential to modulate activity on model membranes and biological cells. Biochim. Biophys. Acta 1999, 1462, 71–87. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, A.B.; Costa, F.J.; Pires, O.R.; Fontes, W.; Castro, M.S. The amazing world of peptide engineering: The example of antimicrobial peptides from frogs and their analogues. Protein Pept. Lett. 2016, 23, 722–737. [Google Scholar] [CrossRef]
- Chen, Y.; Guarnieri, M.T.; Vasil, A.I.; Vasil, M.L.; Mant, C.T.; Hodges, R.S. Role of peptide hydrophobicity in the mechanism of action of α-helical antimicrobial peptides. Antimicrob. Agents Chemother. 2007, 51, 1398–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G. Post-translational modifications of natural antimicrobial peptides and strategies for peptide engineering. Curr. Biotechnol. 2012, 1, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Shyla, G.; Vineethkumar, T.V.; Arun, V.; Divya, M.P.; Thomas, S.; George, S. Functional characterization of two novel peptides and their analogs identifed from the skin secretion of Indosylvirana aurantiaca, an endemic frog species of Western Ghats, India. Chemoecology 2019, 29, 179–187. [Google Scholar] [CrossRef]
- Mura, M.; Wang, J.; Zhou, Y.; Pinna, M.; Zvelindovsky, A.V.; Dennison, S.R.; Phoenix, D.A. The effect of amidation on the behaviour of antimicrobial peptides. Eur. Biophys. J. 2016, 45, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Dennison, S.R.; Mura, M.; Harris, F.; Morton, L.H.G.; Zvelindovsky, A.; Phoenix, D.A. The role of C-terminal amidation in the membrane interactions of the anionic antimicrobial peptide, maximin H5. Biochim. Biophys. Acta 2015, 1848, 1111–1118. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.V.R.; Souza, B.M.; Cabrera, M.P.S.; Dias, N.B.; Gomes, P.C.; Ruggiero Neto, J.; Stabeli, R.G.; Palma, M.S. The effects of the C-terminal amidation of mastoparans on their biological actions and interactions with membrane-mimetic systems. Biochim. Biophys. Acta 2014, 1838, 2357–2368. [Google Scholar] [CrossRef] [Green Version]
- Blondelle, S.E.; Lohner, K.; Aguilar, M. Lipid-induced conformation and lipid-binding properties of cytolytic and antimicrobial peptides: Determination and biological specificity. Biochim. Biophys. Acta 1999, 1462, 89–108. [Google Scholar] [CrossRef] [Green Version]
- Viitala, J.; Järnefelt, J. The red cell surface revisited. Trends Biochem. Sci. 1985, 10, 392–395. [Google Scholar] [CrossRef]
- Oelkrug, C.; Hartke, M.; Schubert, A. Mode of action of anticancer peptides (ACPs) from amphibian origin. Anticancer Res. 2015, 35, 635–643. [Google Scholar] [PubMed]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta 2008, 1778, 357–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandreschi, S.; Piras, A.M.; Batoni, G.; Chiellini, F. Perspectives on polymeric nanostructures for the therapeutic application of antimicrobial peptides. Nanomedicine 2016, 11, 1729–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deslouches, B.; Di, Y.P. Antimicrobial peptides with selective antitumor mechanisms: Prospect for anticancer applications. Oncotarget 2017, 8, 46635–46651. [Google Scholar] [CrossRef] [Green Version]
- Piotrowska, U.; Sobczak, M.; Oledzka, E. Current state of a dual behaviour of antimicrobial peptides -Therapeutic agents and promising delivery vectors. Chem. Biol. Drug Des. 2017, 90, 1079–1093. [Google Scholar] [CrossRef]
- Wang, C.; Yang, C.; Chen, Y.C.; Ma, L.; Huang, K. Rational design of hybrid peptides: A novel drug design approach. Curr. Med. Sci. 2019, 39, 349–355. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Beuerman, R.W.; Dua, H.S.; Lakshminarayanan, R.; Mohammed, I. Strategies in translating the therapeutic potentials of host defense peptides. Front. Immunol. 2020, 11, 983. [Google Scholar] [CrossRef]
- Lee, J.K.; Seo, C.H.; Luchian, T.; Park, Y. Antimicrobial peptide CMA3 derived from the CA-MA hybrid peptide: Antibacterial and anti-inflammatory activities with low cytotoxicity and mechanism of action in Escherichia coli. Antimicrob. Agents Chemother. 2015, 60, 495–506. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Zhang, Y.; Shan, Y.; Wang, J.; Liu, F.; Liu, H.; Xing, G.; Lei, J.; Zhou, J. A pH-dependent antibacterial peptide release nano-system blocks tumor growth in vivo without toxicity. Sci. Rep. 2017, 7, 11242. [Google Scholar] [CrossRef] [Green Version]
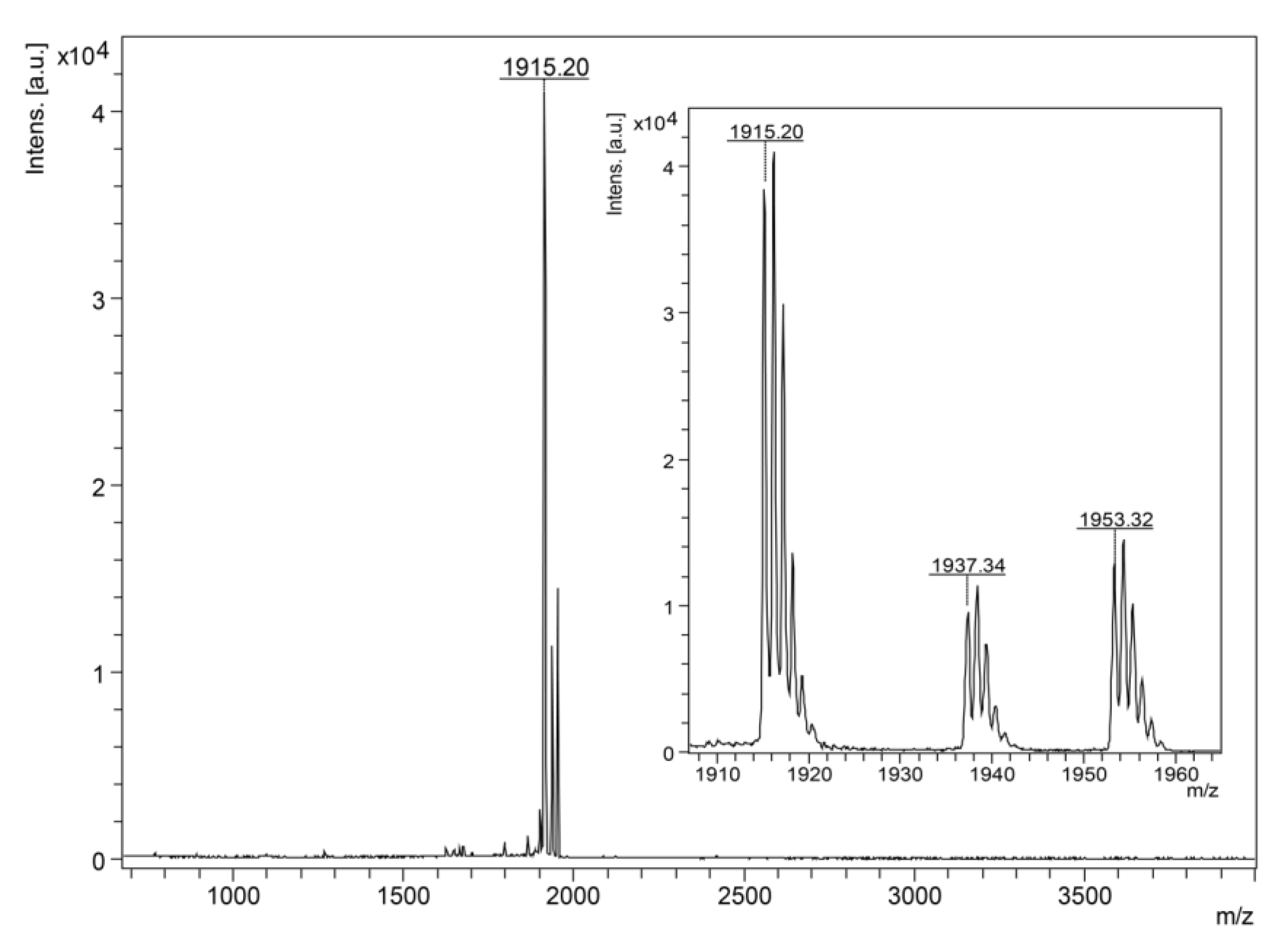
| Peptide | MM (calc) a (Da) | MM (obs) b (Da) | Net Charge | Hydrophobic Ratio (%) | GRAVY |
|---|---|---|---|---|---|
| Figainin 1 | 1915.19 | 1914.20 | +3 | 61 | 1.46 |
| Figainin 1 | ||
|---|---|---|
| Microorganisms | µM | g/L |
| Gram-positive bacteria (MIC) | ||
| E. faecalis (ATCC 29212) | 8 | 0.015 |
| S. aureus (ATCC 25923) | 4 | 0.008 |
| S. epidermidis (ATCC 12228) | 2 | 0.004 |
| E. casseliflavus (ATCC 700327) | 16 | 0.030 |
| Gram-negative bacteria (MIC) | ||
| E. coli (ATCC 25922) | 16 | 0.030 |
| P. aeruginosa (ATCC 27853) | NA a | NA a |
| K. pneumoniae (ATCC 13883) | 4 | 0.008 |
| Yeast (MIC) | ||
| C. albicans (ATCC 90028) | NA a | NA a |
| C. parapsilosis (ATCC 22019) | NA a | NA a |
| Trypanosoma epimastigotes (IC50) T. cruzi | 15.9 | 0.030 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, C.J.C.; Magalhães, A.C.M.; dos Santos Júnior, A.C.M.; Ricart, C.A.O.; Lima, B.D.; Álvares, A.d.C.M.; Freitas, S.M.d.; Pires, O.R., Jr.; Fontes, W.; Castro, M.S. Figainin 1, a Novel Amphibian Skin Peptide with Antimicrobial and Antiproliferative Properties. Antibiotics 2020, 9, 625. https://doi.org/10.3390/antibiotics9090625
Santana CJC, Magalhães ACM, dos Santos Júnior ACM, Ricart CAO, Lima BD, Álvares AdCM, Freitas SMd, Pires OR Jr., Fontes W, Castro MS. Figainin 1, a Novel Amphibian Skin Peptide with Antimicrobial and Antiproliferative Properties. Antibiotics. 2020; 9(9):625. https://doi.org/10.3390/antibiotics9090625
Chicago/Turabian StyleSantana, Carlos José Correia, Ana Carolina Martins Magalhães, Agenor C. M. dos Santos Júnior, Carlos André Ornelas Ricart, Beatriz D. Lima, Alice da Cunha Morales Álvares, Sonia Maria de Freitas, Osmindo Rodrigues Pires, Jr., Wagner Fontes, and Mariana S. Castro. 2020. "Figainin 1, a Novel Amphibian Skin Peptide with Antimicrobial and Antiproliferative Properties" Antibiotics 9, no. 9: 625. https://doi.org/10.3390/antibiotics9090625
APA StyleSantana, C. J. C., Magalhães, A. C. M., dos Santos Júnior, A. C. M., Ricart, C. A. O., Lima, B. D., Álvares, A. d. C. M., Freitas, S. M. d., Pires, O. R., Jr., Fontes, W., & Castro, M. S. (2020). Figainin 1, a Novel Amphibian Skin Peptide with Antimicrobial and Antiproliferative Properties. Antibiotics, 9(9), 625. https://doi.org/10.3390/antibiotics9090625







