Point-of-Care C-Reactive Protein Testing to Reduce Antibiotic Prescribing for Respiratory Tract Infections in Primary Care: Systematic Review and Meta-Analysis of Randomised Controlled Trials
Abstract
:1. Introduction
2. Results
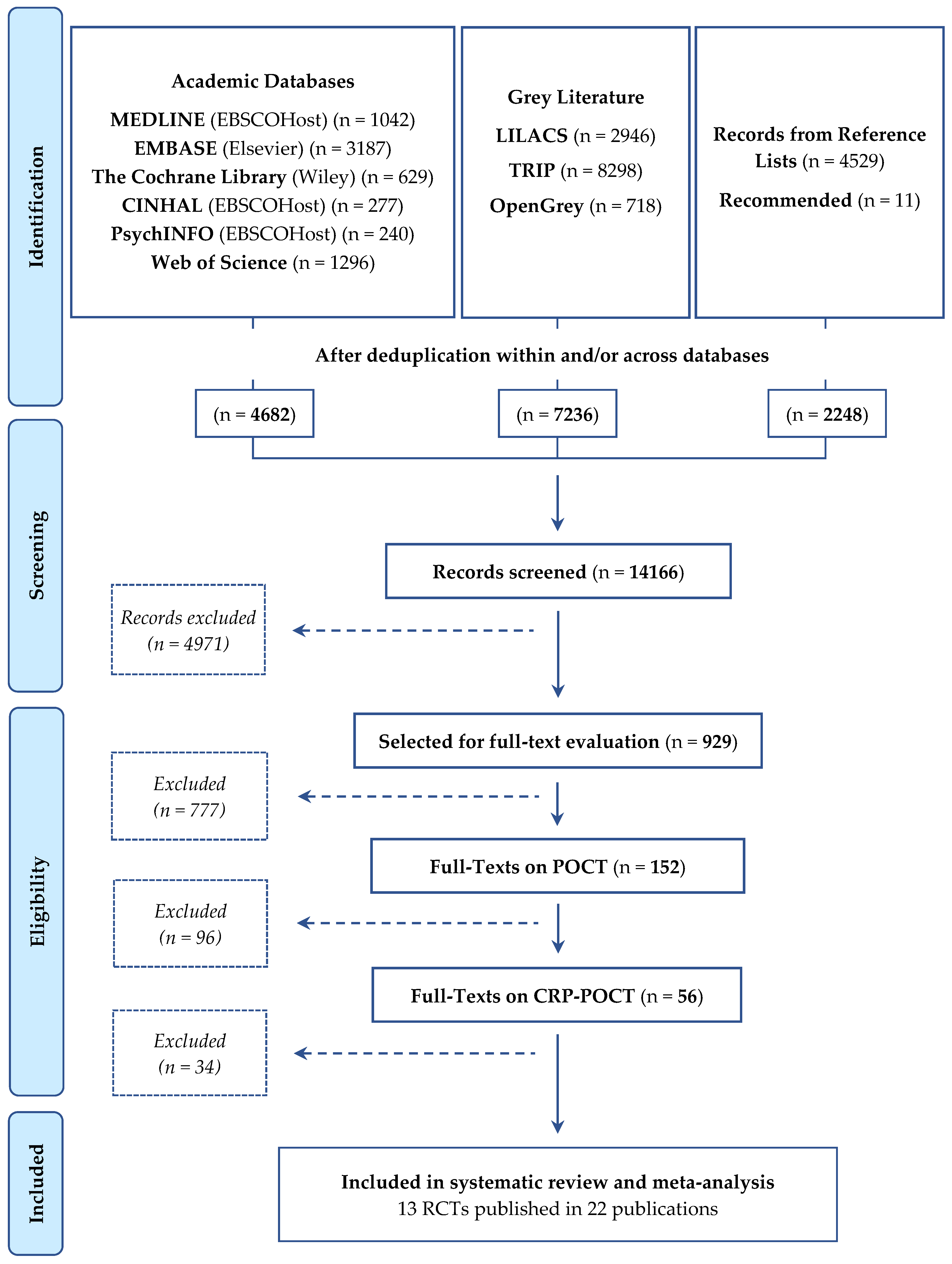
2.1. Identification of Studies
2.2. Study and Population Characteristics
2.3. Intervention Characteristics
2.4. Methodological Quality and Risk of Bias in the Methods of Included Studies
2.5. Effectiveness of the Use of CRP-POCT on (patient) Outcomes
2.5.1. Primary Outcomes
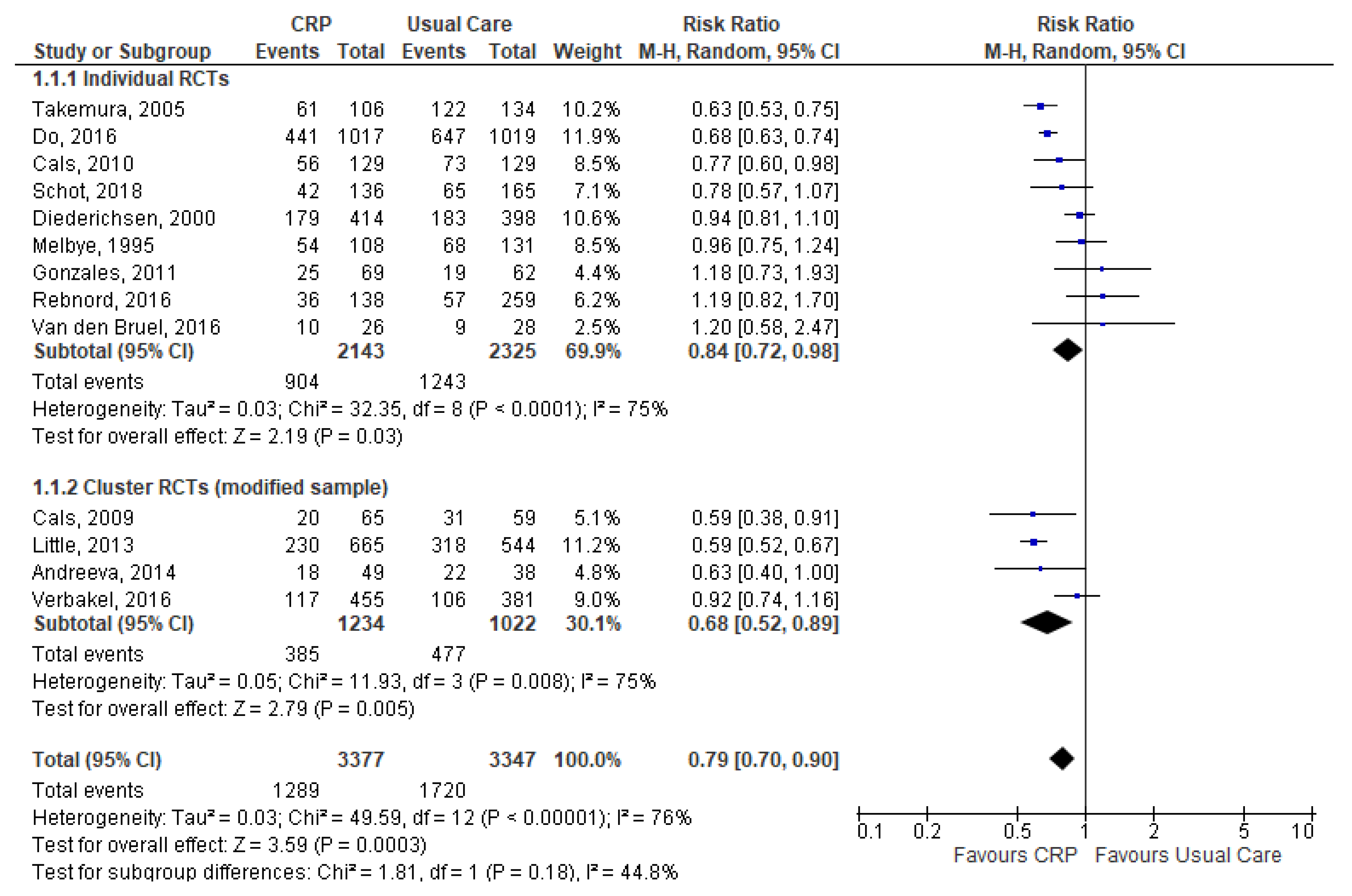
Antibiotic Prescribing Rate at the Index Consultation
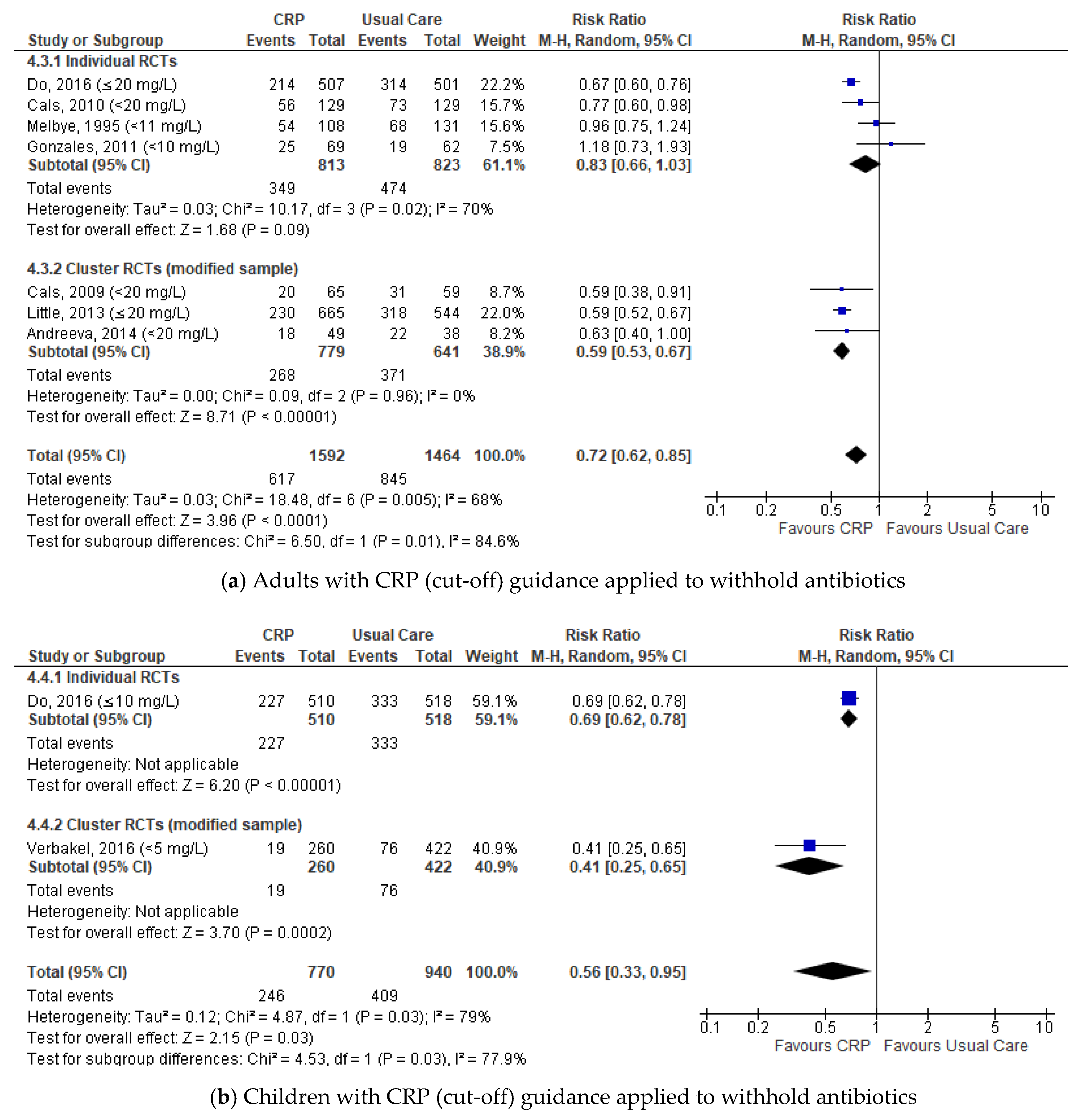
Subgroup Analyses of the Antibiotic Prescribing Rate at the Index Consultation
Antibiotic Prescribing Rate during Patient Follow-Up and Longer-Term Effects
2.5.2. Sensitivity Analysis and Meta-Regression
2.5.3. Secondary Outcomes
Clinical Recovery and Resolution of Symptoms
Visit Duration and Visits at Follow-Up
Re-Consultations and Intention to Re-Consult
Referrals to Secondary Care
Admissions to Hospital and Mortality
Ordering of Investigations
Patient Satisfaction and Patient Enablement
2.5.4. Combined Benefit and Harm Effect of CRP-POCT
2.5.5. Publication Bias
3. Discussion
3.1. Strengths and Limitations of this Review
3.2. Unanswered Questions and Future Research
3.3. Findings in Context with other Interventions and Reviews
4. Literature Review Methods
4.1. Search Strategy
4.2. Eligibility Criteria
4.3. Outcome Assessment
4.4. Selection of Studies and Data Extraction
4.5. Assessment of Methodological Quality and Risk of Bias
4.6. Statistical Analyses and Data Synthesis
4.7. Combined Benefit and Harm Effect of CRP-POCT
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Measuring Outpatient Antibiotic Prescribing. 2020. Available online: https://www.cdc.gov/antibiotic-use/community/programs-measurement/measuring-antibiotic-prescribing.html (accessed on 10 June 2020).
- Fleming-Dutra, K.E.; Hersh, A.L.; Shapiro, D.J.; Bartoces, M.; Enns, E.A.; File, T.M., Jr.; Finkelstein, J.A.; Gerber, J.S.; Hyun, D.Y.; Linder, J.A.; et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. JAMA 2016, 315, 1864–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costelloe, C.; Metcalfe, C.; Lovering, A.; Mant, D.; Hay, A.D. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ 2010, 340, c2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Cars, O.; Högberg, L.D.; Murray, M.; Nordberg, O.; Sivaraman, S.; Lundborg, C.S.; So, A.D.; Tomson, G. Meeting the challenge of antibiotic resistance. BMJ 2008, 337, a1438. [Google Scholar] [CrossRef]
- Smith, S.M.; Fahey, T.; Smucny, J.; Becker, L.A. Antibiotics for acute bronchitis. Cochrane Database Syst. Rev. 2014, 3, CD000245. [Google Scholar]
- Little, P.; Stuart, B.; Moore, M.; Coenen, S.; Butler, C.C.; Godycki-Cwirko, M.; Mierzecki, A.; Chlabicz, S.; Torres, A.; Almirall, J.; et al. Amoxicillin for acute lower-respiratory-tract infection in primary care when pneumonia is not suspected: A 12-country, randomised, placebo-controlled trial. Lancet Infect. Dis. 2013, 13, 123–129. [Google Scholar] [CrossRef]
- Butler, C.C.; Hood, K.; Verheij, T.; Little, P.; Melbye, H.; Nuttall, J.; Kelly, M.J.; Molstad, S.; Godycki-Cwirko, M.; Almirall, J. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: Prospective study in 13 countries. BMJ 2009, 338, B2242. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.; Little, P.; Stokes, T. Antibiotic prescribing for self limiting respiratory tract infections in primary care: Summary of NICE guidance. BMJ 2008, 337, a437. [Google Scholar] [CrossRef]
- Lee, G.C.; Reveles, K.R.; Attridge, R.T.; Lawson, K.A.; Mansi, I.A.; Lewis, J.S.; Frei, C.R. Outpatient antibiotic prescribing in the United States: 2000 to 2010. BMC Med. 2014, 12, 96. [Google Scholar] [CrossRef] [Green Version]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). 2011. Available online: http://www.ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-surveillance-europe-2011.pdf (accessed on 10 June 2020).
- Lode, H. Safety and tolerability of commonly prescribed oral antibiotics for the treatment of respiratory tract infections. Am. J. Med. 2010, 123, S26–S38. [Google Scholar] [CrossRef]
- Kollef, M.H. Broad-spectrum antimicrobials and the treatment of serious bacterial infections: Getting it right up front. Clin. Infect. Dis. 2008, 47 (Suppl. 1), S3–S13. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.Y.; Schiano, T.D. Review article: Drug hepatotoxicity. Aliment Pharmacol. Ther. 2007, 25, 1135–1151. [Google Scholar] [CrossRef] [PubMed]
- Little, P.; Gould, C.; Williamson, I.; Warner, G.; Gantley, M.; Kinmonth, A.L. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: The medicalising effect of prescribing antibiotics. BMJ 1997, 315, 350–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, J. Review on Antimicrobial Resistance Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; Review on Antimicrobial Resistance: London, UK, 2014; Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 10 June 2020).
- Tonkin-Crine, S.K.; Tan, P.S.; van Hecke, O.; Wang, K.; Roberts, N.W.; McCullough, A.; Hansen, M.P.; Butler, C.C.; Del Mar, C.B. Clinician-targeted interventions to influence antibiotic prescribing behaviour for acute respiratory infections in primary care: An overview of systematic reviews. Cochrane Database Syst. Rev. 2017, 9, CD012252. [Google Scholar] [CrossRef] [Green Version]
- Brookes-Howell, L.; Hood, K.; Cooper, L.; Coenen, S.; Little, P.; Verheij, T.; Godycki-Cwirko, M.; Melbye, H.; Krawczyk, J.; Borras-Santos, A.; et al. Clinical influences on antibiotic prescribing decisions for lower respiratory tract infection: A nine country qualitative study of variation in care. BMJ Open 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Van den Bruel, A.; Haj-Hassan, T.; Thompson, M.; Buntinx, F.; Mant, D. Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: A systematic review. Lancet 2010, 375, 834–845. [Google Scholar] [CrossRef]
- Arnold, S.R.; To, T.; McIsaac, W.J.; Wang, E.E. Antibiotic prescribing for upper respiratory tract infection: The importance of diagnostic uncertainty. J. Pediatr. 2005, 146, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Teepe, J.; Broekhuizen, B.D.L.; Loens, K.; Lammens, C.; Ieven, M.; Goossens, H.; Little, P.; Butler, C.C.; Coenen, S.; Godycki-Cwirko, M.; et al. Predicting the presence of bacterial pathogens in the airways of primary care patients with acute cough. CMAJ 2017, 189, E50–E55. [Google Scholar] [CrossRef] [Green Version]
- Falk, G.; Fahey, T. C-reactive protein and community-acquired pneumonia in ambulatory care: Systematic review of diagnostic accuracy studies. Fam. Pract. 2009, 26, 10–21. [Google Scholar] [CrossRef]
- Woodhead, M.; Blasi, F.; Ewig, S.; Garau, J.; Huchon, G.; Ieven, M.; Ortqvist, A.; Schaberg, T.; Torres, A.; van der Heijden, G.; et al. Guidelines for the management of adult lower respiratory tract infections-full version. Clin Microbiol Infect. 2011, 17 (Suppl. 6), E1–E59. [Google Scholar] [CrossRef] [Green Version]
- National Institute for Health and Care Excellence (NICE). Pneumonia: Diagnosis and Management of Community- and Hospital-Acquired Pneumonia in Adults; NICE: London, UK, 2014. [Google Scholar]
- Aabenhus, R.; Jensen, J.U.; Jorgensen, K.J.; Hrobjartsson, A.; Bjerrum, L. Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database Syst. Rev. 2014, 11, CD010130. [Google Scholar] [CrossRef] [PubMed]
- Verbakel, J.Y.; Lee, J.J.; Goyder, C.; Tan, P.S.; Ananthakumar, T.; Turner, P.J.; Hayward, G.; Van den Bruel, A. Impact of point-of-care C reactive protein in ambulatory care: A systematic review and meta-analysis. BMJ Open 2019, 9, e025036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oppong, R.; Smith, R.D.; Little, P.; Verheij, T.; Butler, C.C.; Goossens, H.; Coenen, S.; Jowett, S.; Roberts, T.E.; Achana, F.; et al. Cost-effectiveness of internet-based training for primary care clinicians on antibiotic prescribing for acute respiratory tract infections in Europe. J. Antimicrob Chemother. 2018, 73, 3189–3198. [Google Scholar] [CrossRef] [PubMed]
- Lubell, Y.; Do, N.T.T.; Nguyen, K.V.; Ta, N.T.D.; Tran, N.T.H.; Than, H.M.; Hoang, L.B.; Shrestha, P.; van Doorn, R.H.; Nadjm, B.; et al. C-reactive protein point of care testing in the management of acute respiratory infections in the Vietnamese primary healthcare setting - a cost benefit analysis. Antimicrob Resist Infect. Control. 2018, 7, 119. [Google Scholar] [CrossRef] [Green Version]
- Holmes, E.A.F.; Harris, S.D.; Hughes, A.; Craine, N.; Hughes, D.A. Cost-Effectiveness Analysis of the Use of Point-of-Care C-Reactive Protein Testing to Reduce Antibiotic Prescribing in Primary Care. Antibiotics 2018, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Minnaard, M.C.; van de Pol, A.C.; Hopstaken, R.M.; van Delft, S.; Broekhuizen, B.D.L.; Verheij, T.J.M.; de Wit, N.J. C-reactive protein point-of-care testing and associated antibiotic prescribing. Fam Pract. 2016, 33, 408–413. [Google Scholar] [CrossRef]
- Hughes, A.; Gwyn, L.; Harris, S.; Clarke, C. Evaluating a point-of-care C-reactive protein test to support antibiotic prescribing decisions in a general practice. Clin. Pharmacist. 2016, 8. [Google Scholar]
- Huddy, J.R.; Ni, M.Z.; Barlow, J.; Majeed, A.; Hanna, G.B. Point-of-care C reactive protein for the diagnosis of lower respiratory tract infection in NHS primary care: A qualitative study of barriers and facilitators to adoption. BMJ Open 2016, 6, e009959. [Google Scholar] [CrossRef] [Green Version]
- Hunter, R. Cost-Effectiveness of Point-of-Care C-Reactive Protein Tests for Respiratory Tract Infection in Primary Care in England. Adv. Ther. 2015, 32, 69–85. [Google Scholar] [CrossRef] [Green Version]
- Anthierens, S.; Tonkin-Crine, S.; Cals, J.W.; Coenen, S.; Yardley, L.; Brookes-Howell, L.; Fernandez-Vandellos, P.; Krawczyk, J.; Godycki-Cwirko, M.; Llor, C.; et al. Clinicians’ views and experiences of interventions to enhance the quality of antibiotic prescribing for acute respiratory tract infections. J. Gen. Intern. Med. 2015, 30, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Nijman, R.G.; Moll, H.A.; Smit, F.J.; Gervaix, A.; Weerkamp, F.; Vergouwe, Y.; de Rijke, Y.B.; Oostenbrink, R. C-reactive protein, procalcitonin and the lab-score for detecting serious bacterial infections in febrile children at the emergency department: A prospective observational study. Pediatr. Infect. Dis. J. 2014, 33, e273–e279. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Cots, J.M.; Hernández, S.; Ortega, J.; Arranz, J.; Monedero, M.J.; Alcántara, J.d.D.; Pérez, C.; García, G.; Gómez, M.; et al. Effectiveness of two types of intervention on antibiotic prescribing in respiratory tract infections in Primary Care in Spain. Happy Audit Study. Atencion Primaria. 2014, 46, 492–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llor, C.; Bjerrum, L.; Munck, A.; Cots, J.M.; Hernández, S.; Moragas, A. Access to point-of-care tests reduces the prescription of antibiotics among antibiotic-requesting subjects with respiratory tract infections. Respir. Care 2014, 59, 1918–1923. [Google Scholar] [PubMed] [Green Version]
- Lacroix, L.; Manzano, S.; Vandertuin, L.; Hugon, F.; Galetto-Lacour, A.; Gervaix, A. Impact of the lab-score on antibiotic prescription rate in children with fever without source: A randomized controlled trial. PLoS ONE 2014, 9, e115061. [Google Scholar]
- ClinicalTrials.gov [Internet]. Identifier: NCT04470518. Validation of a Vital Signs and Symptoms Decision Tree and the Effect of a Point-of-care C-Reactive Protein Test, Oxygen Saturation, A Brief Intervention and a Parent Leaflet on Diagnosing, Antibiotic Prescribing Rate and Parental Satisfaction in Acutely Ill Children in Primary Care; National Library of Medicine: Bethesda, MD, USA, 2014. Available online: https://clinicaltrials.gov/ct2/show/NCT02024282?cond=NCT02024282&draw=2&rank=1 (accessed on 8 September 2020).
- Peters, C.M.; Schouwenaars, F.M.; Haagsma, E.; Evenhuis, H.M.; Echteld, M.A. Antibiotic prescribing and C-reactive protein testing for pulmonary infections in patients with intellectual disabilities. Br. J. Gen. Pract. 2013, 63, e326–e330. [Google Scholar] [CrossRef]
- Oppong, R.; Jit, M.; Smith, R.D.; Butler, C.C.; Melbye, H.; Mölstad, S.; Coast, J. Cost-effectiveness of point-of-care C-reactive protein testing to inform antibiotic prescribing decisions. Br. J. Gen. Pract. 2013, 63, e465–e471. [Google Scholar] [CrossRef] [Green Version]
- Llor, C.; Hernández, S.; Cots, J.M.; Bjerrum, L.; González, B.; García, G.; Alcántara, J.d.D.; Guerra, G.; Cid, M.; Gómez, M.; et al. [Physicians with access to point-of-care tests significantly reduce the antibiotic prescription for common cold]. Rev. Esp. Quimioter. 2013, 26, 12–20. [Google Scholar]
- Joshi, A.; Perin, D.P.; Gehle, A.; Nsiah-Kumi, P.A. Feasibility of using C-reactive protein for point-of-care testing. Technol. Health Care 2013, 21, 233–240. [Google Scholar]
- Llor, C.; Cots, J.M.; López-Valcárcel, B.G.; Arranz, J.; García, G.; Ortega, J.; Gómez, M.; Guerra, G.; Monedero, M.J.; Alcántara, J.D.; et al. Interventions to reduce antibiotic prescription for lower respiratory tract infections: Happy Audit study. Eur. Respir. J. 2012, 40, 436–441. [Google Scholar] [CrossRef]
- Llor, C.; Bjerrum, L.; Arranz, J.; García, G.; Cots, J.M.; González López-Valcárcel, B.; Monedero, M.J.; Gómez, M.; Ortega, J.; Guerra, G.; et al. C-reactive protein testing in patients with acute rhinosinusitis leads to a reduction in antibiotic use. Fam. Pract. 2012, 29, 653–658. [Google Scholar] [CrossRef] [Green Version]
- Anthierens, S.; Tonkin-Crine, S.; Douglas, E.; Fernandez-Vandellos, P.; Krawczyk, J.; Llor, C.; Cals, J.W.L.; Francis, N.A.; Yardley, L.; Coenen, S.; et al. General practitioners’ views on the acceptability and applicability of a web-based intervention to reduce antibiotic prescribing for acute cough in multiple European countries: A qualitative study prior to a randomised trial. BMC Fam. Pract. 2012, 13, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavanagh, K.E.; O’Shea, E.; Halloran, R.; Cantillon, P.; Murphy, A.W. A pilot study of the use of near-patient C-Reactive Protein testing in the treatment of adult respiratory tract infections in one Irish general practice. BMC Fam. Pract. 2011, 12, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cals, J.W.L.; Ament, A.J.H.A.; Hood, K.; Butler, C.C.; Hopstaken, R.M.; Wassink, G.F.; Dinant, G.-J. C-reactive protein point of care testing and physician communication skills training for lower respiratory tract infections in general practice: Economic evaluation of a cluster randomized trial. J. Eval. Clin. Pract. 2011, 17, 1059–1069. [Google Scholar] [CrossRef]
- Llor, C.; Sierra, N.; Hernández, S.; Moragas, A.; Hernández, M.; Bayona, C.; Miravitlles, M. Impact of C-reactive protein testing on adherence to thrice-daily antibiotic regimens in patients with lower respiratory tract infection. Prim. Care Respir. J. 2010, 19, 358–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsen, K.A.; Melbye, H.; Kelly, M.J.; Ceynowa, C.; Molstad, S.; Hood, K.; Butler, C.C. Influence of CRP testing and clinical findings on antibiotic prescribing in adults presenting with acute cough in primary care. Scand. J. Prim. Health Care 2010, 28, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Cals, J.W.L.; Chappin, F.H.F.; Hopstaken, R.M.; van Leeuwen, M.E.; Hood, K.; Butler, C.C.; Dinant, G.-J. C-reactive protein point-of-care testing for lower respiratory tract infections: A qualitative evaluation of experiences by GPs. Fam. Pract. 2010, 27, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Rausch, S.; Flammang, M.; Haas, N.; Stein, R.; Tabouring, P.; Delvigne, S.; Holper, D.; Jentges, C.; Pieger, M.; Lieunard, C.; et al. C-reactive protein to initiate or withhold antibiotics in acute respiratory tract infections in adults, in primary care: Review. Bull. Soc. Sci. Med. Grand. Duche Luxemb. 2009, 79–87. [Google Scholar]
- Cals, J.W.L.; Butler, C.C.; Dinant, G.J. ‘Experience talks’: Physician prioritisation of contrasting interventions to optimise management of acute cough in general practice. Implement. Sci. 2009, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Cohen, R.; Lécuyer, A.; Wollner, C.; Deberdt, P.; Thollot, F.; Henriquet, V.; de La Rocque, F. [Evaluation of impact of CRP rapid test in management of febrile children in ambulatory pediatric practice]. Arch Pediatr. 2008, 15, 1126–1132. [Google Scholar] [CrossRef]
- Muszyńska, A.; Steciwko, A.; Horst-Sikorska, W.; Siebert, J.; Mastalerz-Migas, A.; Wawrzyniak, A.; Nitsch-Osuch, A.; Zycińska, K.; Babińska, Z.; Pokorna-Kałwak, D.; et al. Usefulness of rapid CRP tests (NycoCard II® CRP) in everyday work of a family doctor, in the aspect of rationalization of indications for antibiotic therapy in acute infections. Fam. Med. Prim. Care Rev. 2007, 9, 998–1006. [Google Scholar]
- Briel, M.; Young, J.; Tschudi, P.; Hersberger, K.E.; Hugenschmidt, C.; Langewitz, W.; Bucher, H.C. Prevalence and influence of diagnostic tests for acute respiratory tract infections in primary care. Swiss Med. Wkly. 2006, 136, 248–253. [Google Scholar] [PubMed]
- Bjerrum, L.; Gahrn-Hansen, B.; Munck, A.P. [General practitioners who use CRP have a lower antibiotic prescribing rate to patients with sinusitis - secondary publication]. Ugeskr Laeger. 2005, 167, 2775–2777. [Google Scholar] [PubMed]
- Bjerrum, L.; Gahrn-Hansen, B.; Munck, A.P. C-reactive protein measurement in general practice may lead to lower antibiotic prescribing for sinusitis. Br. J. Gen. Pract. 2004, 54, 659–662. [Google Scholar]
- Fagan, M.S. Can the use of antibiotic in the treatment of acute bronchitis be reduced? [Kan bruk av antibiotika ved akutt bronkitt reduseres?]. Tidsskr Nor Laegeforen. 2001, 121, 455–458. [Google Scholar] [PubMed]
- Dahler-Eriksen, B.S.; Lauritzen, T.; Lassen, J.F.; Lund, E.D.; Brandslund, I. Near-patient test for C-reactive protein in general practice: Assessment of clinical, organizational, and economic outcomes. Clin. Chem. 1999, 45, 478–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schot, M.J.; Van den Bruel, A.; Broekhuizen, B.D.; Cals, J.W.; Noteboom, E.A.; Balemans, W.; Hopstaken, R.M.; van Delft, S.; de Wit, N.J.; Verheij, T.J. Point-of-care C-reactive protein to assist in primary care management of children with suspected non-serious lower respiratory tract infection: A randomised controlled trial. BJGP Open 2018, 2, bjgpopen18X101600. [Google Scholar] [CrossRef] [Green Version]
- Verbakel, J.Y.; Lemiengre, M.B.; De Burghgraeve, T.; De Sutter, A.; Aertgeerts, B.; Shinkins, B.; Perera, R.; Mant, D.; Van den Bruel, A.; Buntinx, F. Should all acutely ill children in primary care be tested with point-of-care CRP: A cluster randomised trial. BMC Med. 2016, 14, 131. [Google Scholar] [CrossRef] [Green Version]
- Van den Bruel, A.; Jones, C.; Thompson, M.; Mant, D. C-reactive protein point-of-care testing in acutely ill children: A mixed methods study in primary care. Arch. Dis. Child 2016, 101, 382–385. [Google Scholar] [CrossRef]
- Rebnord, I.K.; Sandvik, H.; Mjelle, A.B.; Hunskaar, S. Out-of-hours antibiotic prescription after screening with C reactive protein: A randomised controlled study. BMJ Open 2016, 6, e011231. [Google Scholar] [CrossRef]
- Do, N.T.; Ta, N.T.; Tran, N.T.; Than, H.M.; Vu, B.T.; Hoang, L.B.; van Doorn, H.R.; Vu, D.T.; Cals, J.W.; Chandna, A.; et al. Point-of-care C-reactive protein testing to reduce inappropriate use of antibiotics for non-severe acute respiratory infections in Vietnamese primary health care: A randomised controlled trial. Lancet Glob Health 2016, 4, e633–e641. [Google Scholar] [CrossRef] [Green Version]
- Andreeva, E.; Melbye, H. Usefulness of C-reactive protein testing in acute cough/respiratory tract infection: An open cluster-randomized clinical trial with C-reactive protein testing in the intervention group. BMC Fam. Pract. 2014, 15, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, P.; Stuart, B.; Francis, N.; Douglas, E.; Tonkin-Crine, S.; Anthierens, S.; Cals, J.W.; Melbye, H.; Santer, M.; Moore, M.; et al. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: A multinational, cluster, randomised, factorial, controlled trial. Lancet 2013, 382, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
- Gonzales, R.; Aagaard, E.M.; Camargo, C.A., Jr.; Ma, O.J.; Plautz, M.; Maselli, J.H.; McCulloch, C.E.; Levin, S.K.; Metlay, J.P. C-reactive protein testing does not decrease antibiotic use for acute cough illness when compared to a clinical algorithm. J. Emerg. Med. 2011, 41, 1–7. [Google Scholar] [CrossRef]
- Cals, J.W.; Schot, M.J.; de Jong, S.A.; Dinant, G.J.; Hopstaken, R.M. Point-of-care C-reactive protein testing and antibiotic prescribing for respiratory tract infections: A randomized controlled trial. Ann. Fam. Med. 2010, 8, 124–133. [Google Scholar] [CrossRef]
- Cals, J.W.; Butler, C.C.; Hopstaken, R.M.; Hood, K.; Dinant, G.J. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: Cluster randomised trial. BMJ 2009, 338, b1374. [Google Scholar] [CrossRef] [Green Version]
- Takemura, Y.; Ebisawa, K.; Kakoi, H.; Saitoh, H.; Kure, H.; Ishida, H.; Kure, M. Antibiotic selection patterns in acutely febrile new outpatients with or without immediate testing for C reactive protein and leucocyte count. J. Clin. Pathol. 2005, 58, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Diederichsen, H.Z.; Skamling, M.; Diederichsen, A.; Grinsted, P.; Antonsen, S.; Petersen, P.H.; Munck, A.P.; Kragstrup, J. Randomised controlled trial of CRP rapid test as a guide to treatment of respiratory infections in general practice. Scand. J. Prim. Health Care 2000, 18, 39–43. [Google Scholar]
- Melbye, H.; Aaraas, I.; Fleten, N.; Kolstrup, N.; Mikalsen, J.I. [The value of C-reactive protein testing in suspected lower respiratory tract infections. A study from general practice on the effect of a rapid test on antibiotic research and course of the disease in adults]. Tidsskr Nor Laegeforen. 1995, 115, 1610–1615. [Google Scholar]
- Lemiengre, M.B.; Verbakel, J.Y.; Colman, R.; Van Roy, K.; De Burghgraeve, T.; Buntinx, F.; Aertgeerts, B.; De Baets, F.; De Sutter, A. Point-of-care CRP matters: Normal CRP levels reduce immediate antibiotic prescribing for acutely ill children in primary care: A cluster randomized controlled trial. Scand. J. Prim. Health Care 2018, 36, 423–436. [Google Scholar] [CrossRef] [Green Version]
- Lemiengre, M.B.; Verbakel, J.Y.; Colman, R.; De Burghgraeve, T.; Buntinx, F.; Aertgeerts, B.; De Baets, F.; De Sutter, A. Reducing inappropriate antibiotic prescribing for children in primary care: A cluster randomised controlled trial of two interventions. Br. J. Gen. Pract. 2018, 68, e204–e210. [Google Scholar] [CrossRef] [Green Version]
- Lemiengre, M.B.; Verbakel, J.Y.; De Burghgraeve, T.; Aertgeerts, B.; De Baets, F.; Buntinx, F.; De Sutter, A. Optimizing antibiotic prescribing for acutely ill children in primary care (ERNIE2 study protocol, part B): A cluster randomized, factorial controlled trial evaluating the effect of a point-of-care C-reactive protein test and a brief intervention combined with written safety net advice. BMC Pediatr. 2014, 14, 246. [Google Scholar]
- Rebnord, I.K.; Sandvik, H.; Mjelle, A.B.; Hunskaar, S. Factors predicting antibiotic prescription and referral to hospital for children with respiratory symptoms: Secondary analysis of a randomised controlled study at out-of-hours services in primary care. BMJ Open 2017, 7, e012992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, P.; Stuart, B.; Francis, N.; Douglas, E.; Tonkin-Crine, S.; Anthierens, S.; Cals, J.W.L.; Melbye, H.; Santer, M.; Moore, M.; et al. Antibiotic Prescribing for Acute Respiratory Tract Infections 12 Months After Communication and CRP Training: A Randomized Trial. Ann. Fam. Med. 2019, 17, 125–132. [Google Scholar] [CrossRef]
- Yardley, L.; Douglas, E.; Anthierens, S.; Tonkin-Crine, S.; O’Reilly, G.; Stuart, B.; Geraghty, A.W.; Arden-Close, E.; van der Velden, A.W.; Goosens, H.; et al. Evaluation of a web-based intervention to reduce antibiotic prescribing for LRTI in six European countries: Quantitative process analysis of the GRACE/INTRO randomised controlled trial. Implement. Sci. 2013, 8, 134. [Google Scholar] [CrossRef] [Green Version]
- Cals, J.W.; de Bock, L.; Beckers, P.J.; Francis, N.A.; Hopstaken, R.M.; Hood, K.; de Bont, E.G.; Butler, C.C.; Dinant, G.J. Enhanced communication skills and C-reactive protein point-of-care testing for respiratory tract infection: 3.5-year follow-up of a cluster randomized trial. Ann. Fam. Med. 2013, 11, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cals, J.W.; Hopstaken, R.M.; Butler, C.C.; Hood, K.; Severens, J.L.; Dinant, G.J. Improving management of patients with acute cough by C-reactive protein point of care testing and communication training (IMPAC3T): Study protocol of a cluster randomised controlled trial. BMC Fam. Pract. 2007, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Diederichsen, H.Z.; Skamling, M.; Diederichsen, A.; Grinsted, P.; Antonsen, S.; Petersen, P.H.; Munck, A.P.; Kragstrup, J. [A randomized controlled trial of the use of CRP rapid test as a guide to treatment of respiratory infections in general practice]. Ugeskr Laeger. 2001, 163, 3784–3787. [Google Scholar]
- VALUE-Dx.eu [Internet]. Platform Randomised Controlled Trial of Point of Care Diagnostics for Enhancing the Quality of aNtibiotic Prescribing for Community Acquired Acute Respiratory Tract Infection (CA-ARTI) in Ambulatory Care in Europe—The PRUDENCE Trial. Available online: https://value-dx.eu/index.php/work-package-4/ (accessed on 8 September 2020).
- ClinicalTrials.gov [Internet]. Identifier: NCT04470518. Impact of Clinical Guidance & Point-of-care CRP in Children: The ARON Project (ARON); National Library of Medicine: Bethesda, MD, USA, 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04470518 (accessed on 8 September 2020).
- Grol, R.; Dalhuijsen, J.; Thomas, S.; Veld, C.i.t.; Rutten, G.; Mokkink, H. Attributes of clinical guidelines that influence use of guidelines in general practice: Observational study. BMJ 1998, 317, 858–861. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.H.; Howick, J.; Roberts, N.W.; Price, C.P.; Heneghan, C.; Plüddemann, A.; Thompson, M. Primary care clinicians’ attitudes towards point-of-care blood testing: A systematic review of qualitative studies. BMC Fam. Pract. 2013, 14, 117. [Google Scholar] [CrossRef] [Green Version]
- Spurling, G.K.; Del Mar, C.B.; Dooley, L.; Foxlee, R.; Farley, R. Delayed antibiotic prescriptions for respiratory infections. Cochrane Database Syst. Rev. 2017, 9, CD004417. [Google Scholar] [CrossRef] [Green Version]
- Van Vugt, S.F.; Broekhuizen, B.D.; Lammens, C.; Zuithoff, N.P.; de Jong, P.A.; Coenen, S.; Ieven, M.; Butler, C.C.; Goossens, H.; Little, P.; et al. Use of serum C reactive protein and procalcitonin concentrations in addition to symptoms and signs to predict pneumonia in patients presenting to primary care with acute cough: Diagnostic study. BMJ 2013, 346, f2450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Chen, R.; Wu, T.; Wei, X.; Guo, A. Association between point-of-care CRP testing and antibiotic prescribing in respiratory tract infections: A systematic review and meta-analysis of primary care studies. Br. J. Gen. Pract. 2013, 63, e787–e794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, M.F.; Paling, F.P.; Hoepelman, A.I.; van der Meer, V.; Oosterheert, J.J. Evaluating the evidence for the implementation of C-reactive protein measurement in adult patients with suspected lower respiratory tract infection in primary care: A systematic review. Fam. Pract. 2012, 29, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Gonzalez, N.A.; Coenen, S.; Plate, A.; Colliers, A.; Rosemann, T.; Senn, O.; Neuner-Jehle, S. The impact of interventions to improve the quality of prescribing and use of antibiotics in primary care patients with respiratory tract infections: A systematic review protocol. BMJ Open 2017, 7, e016253. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Altman, D.G. Principles of and Procedures for Systematic Reviews. In Systematic Reviews in Health Care; BMJ Publishing Group: London, UK, 2008; pp. 23–42. [Google Scholar]
- Higgins, J.P.T.; Green S, E. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0 [updated March 2011]; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Centre for Research in Evidence Based Practice (CREBP). Faculty of Health Sciences and Medicine. In Systematic Reviews Practical Manual; Bond University: Gold Coast, Australia, 2014. [Google Scholar]
- Juni, P.; Witschi, A.; Bloch, R.; Egger, M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999, 282, 1054–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Review Manager (RevMan); Version 5.4; The Nordic Cochrane Centre: Copenhagen, Denmark; The Cochrane Collaboration: London, UK, 2012.
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Lip, G.Y.H.; Marcucci, M.; Thabane, L.; Tian, J.; Levine, M.A. The number needed to treat for net effect (NNTnet) as a metric for measuring combined benefits and harms. J. Clin. Epidemiol. 2020, 125, 100–107. [Google Scholar] [CrossRef]


| Study, Clinical Setting, Facilities and Location | Population | Interventionist and Training in the Intervention | Intervention and Number Randomised at Baseline (N) | Comparator and Number Randomised at Baseline (N) | CRP-POCT Turnaround Time and Manufacturer | CRP (cut-off) Guidance for Interpretation of CRP Levels |
|---|---|---|---|---|---|---|
| Schot, 2018 [61] The Netherlands Individual RCT 28 daytime general practices and 4 OOH services across three different regions in the Netherlands | Children with suspected LRTI presenting with acute cough of <21 days, reported a fever of >38 °C for <5 days Age, mean: 4 (SD 2.1), range: 3 months to 12 years Male, %: 51.5 | GPs’ training in the intervention: n.r. | GP CRP + clinical assessment; N = 136 | Usual Care: treatment decisions based on the clinical assessment as usual with no CRP; N = 165 | ≤4 min Afinion, Alere Technologies AS, Oslo, Norway |
|
| Verbakel, 2016 [62,74,75,76] Belgium Cluster RCT 78 general practices across Flanders | Children with an acute infection lasting a maximum of 5 days at the initial contact Age, mean: 3.87 (SD 4.0), range: 1 month to 16 years Male, %: 52.7 | GPs trained to perform the CRP test. Internal quality control performed according to the manufacturer’s instructions | GP CRP + clinical assessment; N = 1730 infectious episodes in 2773 patients | Usual Care: usual practice + CRP only if at clinical risk and presenting at least one symptom/sign of clinical concern 1; N = 1417 infectious episodes in 2773 patients | ≤4 min Afinion AS100 Analyzer, Alere, USA |
|
| Van den Bruel, 2016 [63] United Kingdom (England) Individual RCT 2 OOH services in Oxfordshire | Children with an acute illness of ≤5 days, fever of ≥38 °C Age, mean: 2.8 (SD 2.8), range: 1 month to 16 years Male, %: 51.5 | Physicians’ training in the intervention: n.r. | Physicians CRP + clinical examination according to usual care + clinical guidance on interpretation of CRP levels; N = 26 | Usual Care: clinical examination according to usual care; N = 28 | 3–4 min Afinion, Alere Technologies |
|
| Rebnord, 2016 [64,77] Norway Individual RCT 4 OOH services and 1 paediatric walk-in emergency hospital facility in Bergen | Children with fever or any respiratory symptoms Age, mean: 2.3 (SD 1.8), range: 0 to 6 years Male, %: 55.7 | NPs trained in the study inclusion criteria and examination procedures, performed a clinical examination and CRP tests for all children before consultation with the doctor | NP CRP pre-tested + NP clinical examination before consultation with doctors + consultation with paediatricians or physicians with an assessment of CRP results; other tests were also available; N = 138 | Usual Care: NP clinical examination with no CRP assistance + clinical assessment by paediatricians or physicians + CRP if necessary, on individual indication; other tests were also available; N = 259 | ≤2 min QuikRead Go, Orion Diagnostica | n.r. |
| Do, 2016 [65] Vietnam Individual RCT 10 primary health-care centres - northern Vietnam (routine, urgent care and hospital referral) within a 60 km radius of Hanoi. Rural sites: outpatient clinics - district general hospital (Ba Vi hospital) 60 km West Hanoi | Children and adults with suspected non-severe acute RTI, with at least one focal and one systemic sign or symptom lasting for less than 2 weeks Age, mean: 21.2 (SD 23.8), range: 1 to 65 years Male, %: 39.9 | Physicians trained to use specific CRP cut-offs with initial workshop and further training during onsite implementation. Training materials: oral presentations and written information leaflets for doctors and health centres; posters and desk reminders with recommended cut-off values for specific age groups | Physician CRP + guidance based on CRP cut-off values adapted for use in children + GPs advised to use their clinical discretion for CRP values between thresholds, and could potentially perform further examinations at their clinical discretion; all patients received a routine medical history examination; N = 1017 | Usual Care: routine practice + use of local treatment guidelines + potential to perform further examinations at the discretion of the treating physician; all patients received a routine medical history examination; N = 1019 | ≤3 min CRP single test kit NycoCard II Reader, Alere Technologies, Norway | General
|
| Andreeva, 2014 [66] Russia Cluster RCT 18 general practices: 9 Arkhangelsk region, 9 Murmansk region | Adults with acute cough/LRTI (acute bronchitis, pneumonia, infectious exacerbations of COPD or asthma), illness of fewer than 28 days duration Age, mean: 50.8 (SD n.r.), range: ≥18 years Male, %: 27.4 | GPs: two vocational training sessions on CRP test, theoretical and practical information, guidelines on the interpretation of CRP, a summary of the literature on RTI and CRP role, and paper cases of patients with different RTIs and different CRP values were discussed | GP CRP + guidance on the interpretation of CRP results +/- accessibility and order of chest radiography (for all patients) and other investigations (e.g., a culture of sputum, spirometry, electrocardiogram) when necessary; N = 8 GP offices, 101 patients | Usual Care: clinical assessment with no CRP +/- chest radiography for all patients and other investigations when necessary; choice of antibiotic therapy regimen left at the discretion of physicians; N = 9 GP offices, 78 patients | ≤5 min Afinion test system, Axis-Shield, Norway |
|
| Little, 2013 [67,78,79] Spain, England, Wales, Poland, Belgium, The Netherlands Individual RCT 111 GP practices from GP networks of at least 2 general practices in the localities of study centres of all 6 countries | Adults with an acute cough lasting up to 28 days, or acute LRTI as the main diagnosis (despite cough not being the most prominent symptom) and acute URTI (sore throat, otitis media, sinusitis, influenza, and coryzal illness) Age, mean: 26.4 (SD 15.0), range: ≥18 years Male, %: 36.7 | GPs: a run-in period of several weeks before data collection to practise using the device, internet training on how to target testing and how to negotiate with the patient about management decisions | GP CRP testing + guidance on the interpretation of CRP testing and prescribing + internet training on how to target testing and how to negotiate with the patient about management decisions 2; N = 1062 | 1) Usual Care: GPs assessed and managed patients according to the practice’s normal procedures; N = 870 | ≤5 min QuikRead CRP kits, Orion Diagnostica, Espoo, Finland |
|
| Gonzales, 2011 [68] United States Individual RCT 1 ED supporting a 3-year emergency medicine residency program located in a large, Midwestern metropolitan city in the United States | Adults with a new cough lasting ≤21 days, at least one other symptom of acute RTI (fever, sore throat, night sweats, body aches, nasal or chest congestion, shortness of breath) Age, mean: 41.2 (SD 12.5), range: ≥18 years Male, %: 32.1 | NPs performed CRP testing; management algorithms placed for doctors in the medical chart. ED visits and house staff received current evidence on CRP levels as adjuncts in the diagnosis of pneumonia (or other antibiotic-responsive illnesses), a 1.5-h educational seminar on evidence-based recommendations for evaluation and treatment of acute cough illness and community-acquired pneumonia for adults | NP CRP + clinical algorithm to guide physicians on the ordering of chest x-ray and on antibiotic treatment for adults with acute cough illness and community-acquired pneumonia + activation of GPs in using the algorithm with a statement (“Please consider using this algorithm in your clinical care decisions, although it should not substitute for your clinical judgment”); N = 69 | Usual Care: no CRP testing + clinical management algorithm to guide recommendations for a chest x-ray and antibiotic treatment for adults with acute cough illness (based on a clinical algorithm for predicting pneumonia) + activation of GPs in using the algorithm with a statement (“Please consider using this algorithm in your clinical care decisions, although it should not substitute for your clinical judgment”); N = 62 | 1 min QuikRead CRP, Orion Corporation, Orion Diagnostica, Espoo, Finland | Low to Intermediate (<30%) probability of Pneumonia = abnormal signs OR abnormal chest examination:
|
| Cals, 2010 [69] The Netherlands Individual RCT 11 family practice centres in the south-eastern Netherlands | Adults with a current episode of LRTI (cough lasting <4 weeks with ≥1 of 4 focal signs and symptoms and at least one systemic sign and symptom) or Rhinosinusitis (episodes lasting <4 weeks with at least one symptom of rhinorrhoea history and blocked nose; and at least one other symptom or sign) Age, mean: 44.3 (SD 13.8), range: ≥18 years Male, %: 30.7 | NPs received CRP device demonstration, did not communicate test result to GP or patient until after the study. GPs were informed about the trial procedure, received a 30-min practice-based seminar on the EB use of CRP, stressing the additional CRP value to rule out serious infection with emphasis on using CRP together with clinical findings, a 4-week run-in period before recruitment to get familiar with CRP devices and interpretation | NP CRP +/- GP clinical assessment and management of antibiotic therapy based on CRP results + decision-making on a management strategy including immediate, delayed or no antibiotics; N = 129 | Usual Care: no CRP testing + antibiotic therapy based on clinical assessment + decision-making on a management strategy including immediate, delayed or no antibiotics; N = 129 | ≤3 min QuikRead CRP analysers, Orion Diagnostica, Espoo, Finland |
|
| Cals, 2009 [70,80,81] The Netherlands Cluster RCT 20 general practices from the South-Eastern part of Noord-Brabant province in the Netherlands including urban and rural areas; GP practices are geographically spread throughout this region | Adults with suspected LRTI, with a cough lasting <4 weeks and with one focal and one systemic symptom Age, mean: 45.4 (SD 8.2), range: ≥18 years Male, %: 38.6 | GPs received a 30-min practice-based guideline on how to use CRP, ruling out a serious infection. Practice nurses received an introduction to technical and practical aspects. Practices received an 8-week-run-in period before recruitment to enable familiarisation with CRP devices and interpretation | GP CRP + guidance on the interpretation of results based on CRP cut-off values with an emphasis on the additional value of CRP in ruling out serious infection + familiarisation with CRP devices and interpretation of results; N = 110 | (1) Usual Care: Dutch guidelines-informed clinical assessment for the diagnosis and management of acute cough, and therapeutic advice for LRTI; practices were informed that they would receive a CRP device and/or communication training after the study period; N = 120 (2) ECST: motivational interviewing built around 11 key tasks 3; practices were informed that they would receive a CRP device and/or the communication training after the study period; N = 84 (3) CRP + ECST; N = 117 | ≤3 min NycoCard II Reader, Axis-Shield, Norway |
|
| Takemura, 2005 [71] Japan Individual RCT 1 general/internal medicine clinic of Nishi-Ohmiya regional/community hospital | Children and adults with a clinically relevant fever of >37.5 °C, and symptoms suspected of infection at the time of or during the week before an initial consultation Age, mean: 34.9 (SD 15.4), range: 8 to 83 Male, %: 55.9 | Physicians’ training in the intervention: n.r. | Advanced testing group: Physician CRP + WBC testing before initial consultation + information on CRP and WBC normal reference levels + if considered necessary, potential to perform urgent testing after history taking and physical examination + results of non-urgent additional or subsequent tests evaluated on patient’s next visit; N = 147 | Usual Care: non-advanced testing group defined as standard management and treatment with no CRP before initial consultation + decision-making on antibiotic management and treatment based on history taking and physical examination + if considered necessary, potential to perform urgent testing after history taking and physical examination; N = 154 | CRP approx. 40–50 min; WBC 10 min CRP multichannel analyser, model TBA-30FR; Toshiba, Saitama City, Japan |
|
| Diederichsen, 2000 [72,82] Denmark Individual RCT 35 General practices in the County of Funen in Denmark | Children and adults with respiratory infections Age, mean: 41 (SD 14.2), range: median 37 (range: 0 to 90) Male, %: 42.8 | GPs discussed, before the start of the study, the trial procedure with the project leader and a product specialist from Nycomed; a supervised test trial was carried out | GP CRP + clinical assessment to guide antibiotics prescribing + information on the normal levels of CRP for antibiotic prescribing but no strict guidelines were given; N = 414 | Usual Care: clinical assessment only; N = 398 | ≤3 min NycoCard CRP Reader, Nycomed, Alere Technologies, Afinion, Norway |
|
| Melbye, 1995 [73] Norway Individual RCT 10 General practices in Northern Norway | Adults with signs of pneumonia, bronchitis and asthma, who presented with symptoms of coughing or heavy breathing, or who had chest pain that was aggravated by coughing or deep inspiration Age, mean: 49.25 (SD 11.6), range: ≥18 years Male, %: 36.9 | GPs’ training in the intervention: n.r. | GP CRP + doctors’ preliminary decision on antibiotic treatment + guide on antibiotic prescribing based on the duration of illness following recommended CRP cut-off values (if a preliminary decision needed to change in light of the CRP results); N = 108 | Usual Care, N = 131 | ≤3 min NycoCard CRP Reader, Nycomed, Alere Technologies, Afinion, Norway | Disease duration 0–24 h
|
| Study (First Author, Publication Year) | Inclusion Criteria | Exclusion Criteria | Primary Outcome(s) | Secondary Outcome(s) | Sample Size and Power | Attrition ≤20% 1ry Outcome (Attrition Bias) | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Clinicians (Performance Bias) | Blinding of Patients (Performance Bias) | Blinding of Outcome Assessors 1ry Outcome (Detection Bias) | Blinding - Outcome Assessors 2ry Outcome (dEtection Bias) | Incomplete Outcome Data 1ry Outcome (Attrition Bias) | Selective Reporting (Reporting Bias) | Participants Comparable at Baseline | Same Length of Follow-Up | Source of Funding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schot, 2018 [61] The Netherlands Individual RCT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |  |  |  |  |  |  |  |  |  |  | Netherlands Organization for HRD; Alere Technologies AS; SALTRO & Star Medical Diagnostic Centre |
| Verbakel, 2016 [62,74,75,76] Belgium Cluster RCT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |  |  |  |  |  |  |  |  |  |  | NIHDI, Research Foundation Flanders, NIHR Diagnostic Evidence Co-operative Oxford |
| Van den Bruel, 2016 [63] United Kingdom (England) Individual RCT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |  |  |  |  |  |  |  |  |  |  | National School for Primary Care Research |
| Rebnord, 2016 [64,77] Norway Individual RCT | ✓ | ✓ | ✓ | ✓ | ✓ |  |  |  |  |  |  |  |  |  |  | Norwegian Research Fund | |
| Do, 2016 [65] Vietnam Individual RCT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |  |  |  |  |  |  |  |  |  |  | Welcome Trust UK and Global Antibiotic Resistance Partnership; Alere Technologies |
| Andreeva, 2014 [66] Russia Cluster RCT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |  |  |  |  |  |  |  |  |  |  | Not reported |
| Little, 2013 [67,78,79] Spain, England, Wales, Poland, Belgium, The Netherlands Cluster RCT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |  |  |  |  |  |  |  |  |  |  | European Commission Framework Programme, NIHR, Research Foundation Flanders |
| Gonzales, 2011 [68] United States Individual RCT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |  |  |  |  |  |  |  |  |  |  | TRIP initiative and agency for HRQ, HSRD Service of the Department of Veterans Affairs |
| Cals, 2010 [69] The Netherlands Individual RCT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |  |  |  |  |  |  |  |  |  |  | Orion Diagnostica Espoo Finland |
| Cals, 2009 [70,80,81] The Netherlands Cluster RCT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |  |  |  |  |  |  |  |  |  |  | Netherlands Organisation for HRD, Wales Office for R&D, NIHSC funded the South East Wales Trial Unit |
| Takemura, 2005 [71] Japan Individual RCT | ✓ | ✓ |  |  |  |  |  |  |  |  |  |  | International Clinical Pathology Centre Tokyo | ||||
| Diederichsen, 2000 [72,82] Denmark Individual RCT | ✓ | ✓ | ✓ | ✓ |  |  |  |  |  |  |  |  |  |  | Not reported | ||
| Melbye, 1995 [73] Norway Individual RCT | ✓ | ✓ | ✓ | ✓ | ✓ |  |  |  |  |  |  |  |  |  |  | Norwegian Research Academy and Nycomed Pharma |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-González, N.A.; Keizer, E.; Plate, A.; Coenen, S.; Valeri, F.; Verbakel, J.Y.J.; Rosemann, T.; Neuner-Jehle, S.; Senn, O. Point-of-Care C-Reactive Protein Testing to Reduce Antibiotic Prescribing for Respiratory Tract Infections in Primary Care: Systematic Review and Meta-Analysis of Randomised Controlled Trials. Antibiotics 2020, 9, 610. https://doi.org/10.3390/antibiotics9090610
Martínez-González NA, Keizer E, Plate A, Coenen S, Valeri F, Verbakel JYJ, Rosemann T, Neuner-Jehle S, Senn O. Point-of-Care C-Reactive Protein Testing to Reduce Antibiotic Prescribing for Respiratory Tract Infections in Primary Care: Systematic Review and Meta-Analysis of Randomised Controlled Trials. Antibiotics. 2020; 9(9):610. https://doi.org/10.3390/antibiotics9090610
Chicago/Turabian StyleMartínez-González, Nahara Anani, Ellen Keizer, Andreas Plate, Samuel Coenen, Fabio Valeri, Jan Yvan Jos Verbakel, Thomas Rosemann, Stefan Neuner-Jehle, and Oliver Senn. 2020. "Point-of-Care C-Reactive Protein Testing to Reduce Antibiotic Prescribing for Respiratory Tract Infections in Primary Care: Systematic Review and Meta-Analysis of Randomised Controlled Trials" Antibiotics 9, no. 9: 610. https://doi.org/10.3390/antibiotics9090610
APA StyleMartínez-González, N. A., Keizer, E., Plate, A., Coenen, S., Valeri, F., Verbakel, J. Y. J., Rosemann, T., Neuner-Jehle, S., & Senn, O. (2020). Point-of-Care C-Reactive Protein Testing to Reduce Antibiotic Prescribing for Respiratory Tract Infections in Primary Care: Systematic Review and Meta-Analysis of Randomised Controlled Trials. Antibiotics, 9(9), 610. https://doi.org/10.3390/antibiotics9090610







