Evidence of the Practice of Self-Medication with Antibiotics among the Lay Public in Low- and Middle-Income Countries: A Scoping Review
Abstract
:1. Introduction
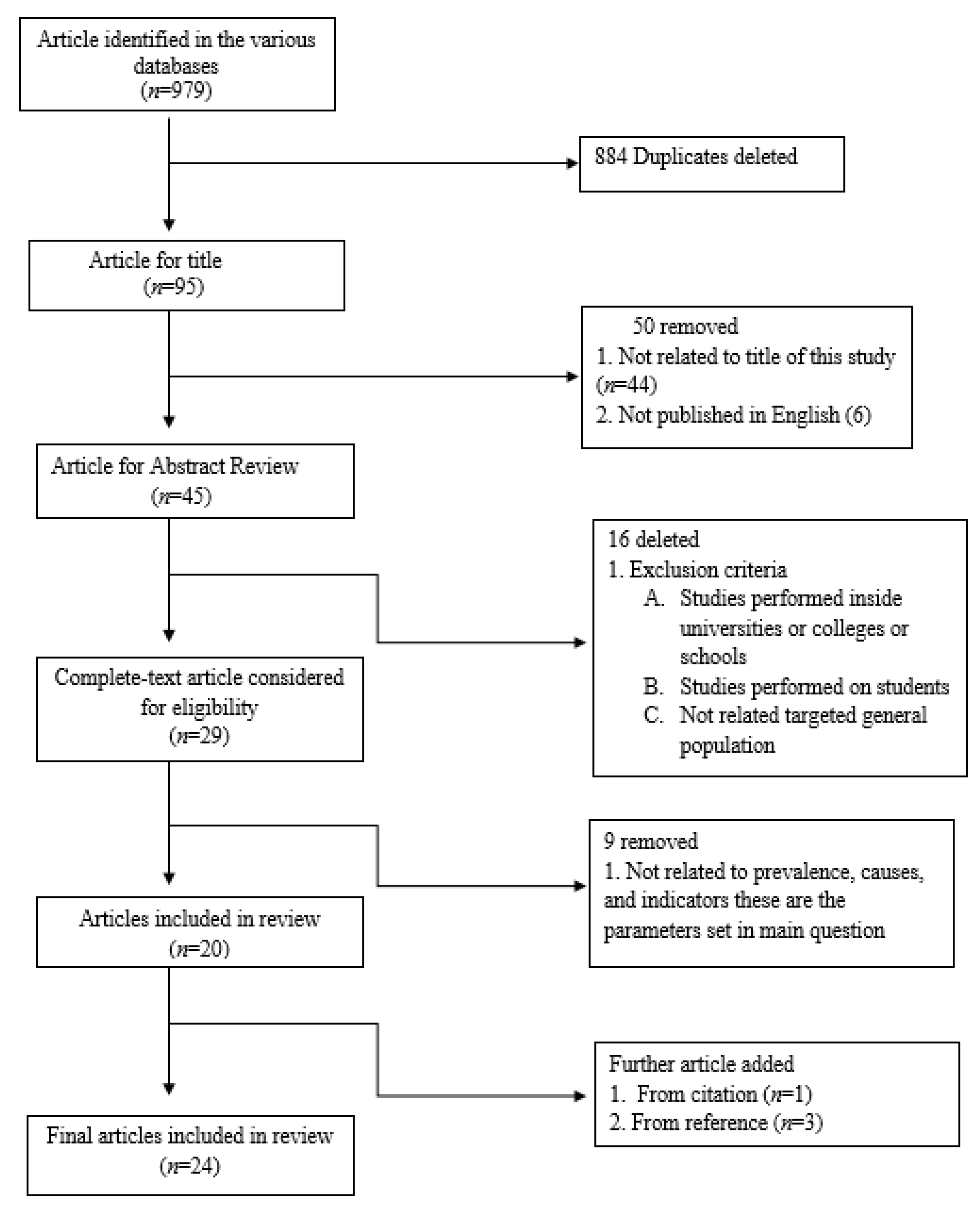
2. Methodology
2.1. Identifying the Research Question
- What is the prevalence of SMA?
- What are the common causes of self-medication with antibiotics?
- What are the common patterns associated with self-medication with antibiotics?
- What are the sociodemographic factors associated with self-medication with antibiotics?
- What are the predictors for self-medication with antibiotics?
2.2. Inclusion and Exclusion Criteria
2.3. Relevant Literature Identification
2.4. Screening and Selection of Relevant Literature
2.5. Charting, Collating and Summarizing the Results
3. Results
3.1. Characteristics of Included Studies
3.2. Prevalence of Self-Medication with Antibiotics
3.3. The Distribution Pattern of the Most Commonly Used Antibiotics for SMA
3.4. Factors Associated with SMA
3.5. Common Indications Related to SMA
3.6. Information on and Source of Antibiotics for Self-Medication
4. Discussion
Main Findings
5. Conclusions
6. Future Recommendation
7. Strength and Limitation
Author Contributions
Funding
Conflicts of Interest
References
- Levy, S.B. Antibiotic resistance-the problem intensifies. Adv. Drug Deliv. Rev. 2005, 57, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance Fact Sheet February. Available online: http://www.who.int/world-health-day/2011 (accessed on 1 January 2019).
- Wolff, M.J. Use and misuse of antibiotics in Latin America. Clin. Infect. Dis. 1993, 17, S346–S351. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Albericio, F. Antibiotic resistance: From the bench to patients. Antibiotics 2019, 3, 129. [Google Scholar] [CrossRef] [Green Version]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J.J.C.I.D. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alp, E.; Damani, N. Healthcare-associated infections in intensive care units: Epidemiology and in control in low-to-middle income countries. Infect. Dev. Ctries. 2015, 9, 1040–1045. [Google Scholar] [CrossRef] [Green Version]
- Jakovljevic, M.; Jurisevic, M.; Mouselli, S. Antibiotic resistance in Syria: A local problem turns into a global threat. Front. Public Health 2018, 6, 212. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Guidelines for the Regulatory Assessment of Medicinal Products for Use in Self Medication. Available online: http://apps.who.int/medicinedocs/en/d/Js2218e/ (accessed on 10 January 2019).
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M.; Group, E.P. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Belongia, E.A.; Naimi, T.S.; Gale, C.M.; Besser, R.E. Antibiotic use and upper respiratory infections: A survey of knowledge, attitudes, and experience in Wisconsin and Minnesota. Prev. Med. 2002, 34, 346–352. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance. Fact Sheet Revised January. Available online: http://www.who.int/mediacentre/factsheets/fs194/en/ (accessed on 2 January 2019).
- Khan, S.J.; Amanullah, K.S.; Shah, N. Selfmedication with antibiotics in Urban areas of Peshawar. Gomal J. Med. Sci. 2011, 9, 1–4. [Google Scholar]
- Bilal, M.; Haseeb, A.; Khan, M.H.; Arshad, M.H.; Ladak, A.A.; Niazi, S.K.; Musharraf, M.D.; Manji, A.A.-K. Self-medication with antibiotics among people dwelling in rural areas of Sindh. J. Clin. Diagn. Res. JCDR 2016, 10, OC08. [Google Scholar] [CrossRef]
- Abdulraheem, I.; Adegboye, A.; Fatiregun, A. Self-medication with antibiotics: Empirical evidence from a Nigerian rural population. Br. J. Pharm. Res. 2016, 11, 1–13. [Google Scholar] [CrossRef]
- Oxford Learners Dicitionary Dictionary. Available online: https://www.oxfordlearnersdictionaries.com/definition/english/layperson?q=layperson (accessed on 24 June 2020).
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. 2005, 8, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Review of Interventions, 2nd ed.; The Cochrane Collaboration; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Collaboration, C. What Is a Systematic Review? Available online: https://consumers.cochrane.org/what-systematic-review (accessed on 10 January 2019).
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, A.L.; Hillier, K.; Ataya, R.; Thabet, P.; Whelan, A.M.; O’Reilly, C.; Gardner, D. A scoping review of community pharmacists and patients at risk of suicide. Can. Pharm. J. 2017, 150, 366–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, M.T.; Rajić, A.; Greig, J.D.; Sargeant, J.M.; Papadopoulos, A.; McEwen, S.A. A scoping review of scoping reviews: Advancing the approach and enhancing the consistency. Res. Synth. Methods 2014, 5, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Brien, S.E.; Lorenzetti, D.L.; Lewis, S.; Kennedy, J.; Ghali, W.A. Overview of a formal scoping review on health system report cards. Implement. Sci. 2010, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Dimova, R.; Dimitrova, D.; Semerdjieva, M.; Doikov, I. Patient Attitudes and Patterns of Self-Medication with Antibiotics–A Cross-Sectional Study in Bulgaria. Maced. J. Med. Sci. 2014, 7, 655–661. [Google Scholar] [CrossRef] [Green Version]
- Biswas, M.; Roy, M.N.; Manik, M.I.N.; Hossain, M.S.; Tapu, S.T.A.; Moniruzzaman, M.; Sultana, S. Self medicated antibiotics in Bangladesh: A cross-sectional health survey conducted in the Rajshahi City. BMC Public Health 2014, 14, 847. [Google Scholar] [CrossRef] [Green Version]
- Skliros, E.; Merkouris, P.; Papazafiropoulou, A.; Gikas, A.; Matzouranis, G.; Papafragos, C.; Tsakanikas, I.; Zarbala, I.; Vasibosis, A.; Stamataki, P. Self-medication with antibiotics in rural population in Greece: A cross-sectional multicenter study. BMC Fam. Pract. 2010, 11, 58. [Google Scholar] [CrossRef] [Green Version]
- Widayati, A.; Suryawati, S.; de Crespigny, C.; Hiller, J.E. Self medication with antibiotics in Yogyakarta City Indonesia: A cross sectional population-based survey. BMC Res. Notes 2011, 4, 491. [Google Scholar] [CrossRef] [Green Version]
- Ramalhinho, I.; Cordeiro, C.; Cavaco, A.; Cabrita, J. Assessing determinants of self-medication with antibiotics among Portuguese people in the Algarve Region. Int. J. Clin. Pharm. Net. 2014, 36, 1039–1047. [Google Scholar] [CrossRef]
- Al Rasheed, A.; Yagoub, U.; Alkhashan, H.; Abdelhay, O.; Alawwad, A.; Al Aboud, A.; Al Battal, S. Prevalence and predictors of self-medication with antibiotics in Al Wazarat Health Center, Riyadh City, KSA. BioMed Res. Int. 2016, 2016, 3916874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alghadeer, S.; Aljuaydi, K.; Babelghaith, S.; Alhammad, A.; Alarifi, M.N. Self-medication with antibiotics in Saudi Arabia. Saudi Pharm. J. 2018, 5, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Yousif, M.; Abubaker, I. Prevalence, determinants and practices of self-medication with antibiotics–a population based survey in Taif, Kingdom of Saudi Aarabiaksa. Age 2015, 172, 57.50. [Google Scholar]
- Abasaeed, A.; Vlcek, J.; Abuelkhair, M.; Kubena, A. Self-medication with antibiotics by the community of Abu Dhabi Emirate, United Arab Emirates. J. Infect. Dev. Ctries. 2009, 3, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Cheaito, L.; Azizi, S.; Saleh, N.; Salameh, P. Assessment of self-medication in population buying antibiotics in pharmacies: A pilot study from Beirut and its suburbs. Int. J. Public Health 2014, 59, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Ngu, R.; Feteh, V.; Kika, B.; Ayeah, C.; Chifor, T.; Njim, T.; Fankem, A.; Yengo, F. Prevalence and Determinants of Antibiotic Self-Medication among Adult Patients with Respiratory Tract Infections in the Mboppi Baptist Hospital, Douala, Cameroon: A Cross-Sectional Study. Diseases 2018, 6, 49. [Google Scholar] [CrossRef] [Green Version]
- Landers, T.F.; Ferng, Y.h.; McLoughlin, J.W.; Barrett, A.E.; Larson, E. Antibiotic identification, use, and self-medication for respiratory illnesses among urban Latinos. J. Am. Acad. Nurse Prac. 2010, 22, 488–495. [Google Scholar] [CrossRef]
- Ramay, B.M.; Lambour, P.; Cerón, A. Comparing antibiotic self-medication in two socio-economic groups in Guatemala City: A descriptive cross-sectional study. BMC Pharmacol. Toxicol. 2015, 16, 11. [Google Scholar] [CrossRef] [Green Version]
- Moise, K.; Bernard, J.J.; Henrys, J.H. Evaluation of antibiotic self-medication among outpatients of the state university hospital of Port-Au-Prince, Haiti: A cross-sectional study. Pan Afr. Med. J. 2017, 28. [Google Scholar] [CrossRef]
- Muras, M.; Krajewski, J.; Nocun, M.; Godycki-Cwirko, M. A survey of patient behaviours and beliefs regarding antibiotic self-medication for respiratory tract infections in Poland. Arch. Med. Sci. AMS 2013, 9, 854. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, M.N.; Durukan, E.; Ilhan, S.Ö.; Aksakal, F.N.; Özkan, S.; Bumin, M.A. Self-medication with antibiotics: Questionnaire survey among primary care center attendants. Pharmacoepidemiol. Drug Saf. 2009, 18, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, M.A.; Amin, H.S.; Al-Qahtani, A.A.; Alshahrani, A.M.; Alghamdi, H.A.; Althwayee, M.S.; Alzahrani, A.A. Self-medication with antibiotics in a primary care setting in King Khalid University Hospital, Riyadh, Saudi Arabia. J. Fam. Community Med. 2018, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Sawair, F.A.; Baqain, Z.H.; Karaky, A.A.; Eid, R.A. Assessment of self-medication of antibiotics in a Jordanian population. Med. Princ. Pract. 2009, 18, 21–25. [Google Scholar] [CrossRef]
- Scicluna, E.A.; Borg, M.A.; Gür, D.; Rasslan, O.; Taher, I.; Redjeb, S.B.; Elnassar, Z.; Bagatzouni, D.P.; Daoud, Z. Self-medication with antibiotics in the ambulatory care setting within the Euro-Mediterranean region; results from the ARMed project. J. Infect. Public Health 2009, 2, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Nazir, S.; Azim, M. Assessment of antibiotic self-medication practice among public in the northwestern region of Pakistan. Eur. J. Hosp. Pharm. 2016, 4, 200–203. [Google Scholar] [CrossRef]
- Bianca anghel, I.; Crăciun, C. Self-medication with over-the-counter drugs and antibiotics in romanian consumers: A qualitative study. Cogn. Brain Behav. 2013, 17, 215. [Google Scholar]
- Bennadi, D. Self-medication: A current challenge. J. Basic Clin. Pharm. 2013, 5, 19–23. [Google Scholar] [CrossRef]
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef] [Green Version]
- Jakovljevic, M.; Jakab, M.; Gerdtham, U.; McDaid, D.; Ogura, S.; Varavikova, E.; Merrick, J.; Adany, R.; Okunade, A.; Getzen, T. Comparative financing analysis and political economy of noncommunicable diseases. J. Med. Econ. 2019, 22, 722–727. [Google Scholar] [CrossRef]
- Radyowijati, A.; Haak, H. Improving antibiotic use in low-income countries: An overview of evidence on determinants. Soc. Sci. Med. 2003, 57, 733–744. [Google Scholar] [CrossRef]
- Morgan, D.J.; Okeke, I.N.; Laxminarayan, R.; Perencevich, E.N.; Weisenberg, S. Non-prescription antimicrobial use worldwide: A systematic review. Lancet Infect. Dis. 2011, 11, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Nickerson, E.K.; Hongsuwan, M.; Limmathurotsakul, D.; Wuthiekanun, V.; Shah, K.R.; Srisomang, P.; Mahavanakul, W.; Wacharaprechasgul, T.; Fowler Jr, V.G.; West, T.E. Staphylococcus aureus bacteraemia in a tropical setting: Patient outcome and impact of antibiotic resistance. PLoS ONE 2009, 4, e4308. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The World Medicines Situation. World Health Organization: Geneva, Switzerland. Available online: http://www.who.int/medicines/areas/policy/world_medicines_situation/WMS_ch6_wPricing_v6.pdf (accessed on 14 March 2019).
- Okeke, I.N.; Klugman, K.P.; Bhutta, Z.A.; Duse, A.G.; Jenkins, P.; O’Brien, T.F.; Pablos-Mendez, A.; Laxminarayan, R. Antimicrobial resistance in developing countries. Part II: Strategies for containment. Lancet Infect. Dis. 2005, 5, 568–580. [Google Scholar] [CrossRef]
- Chang, F.R.; Trivedi, P.K. Economics of self-medication: Theory and evidence. Health Econ. 2003, 9, 721–739. [Google Scholar] [CrossRef]
- Ibrahim, E.H.; Sherman, G.; Ward, S.; Fraser, V.J.; Kollef, M.H. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000, 118, 146–155. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Community-Based Surveillance of Antimicrobial Use and Resistance in Resource-Constrained Settings: Report on Five Pilot Projects. Available online: http://www.who.int/medicines/publications/community_based_may09.pdf (accessed on 20 March 2019).
- Hughes, C.M.; McElnay, J.C.; Fleming, G.F. Benefits and risks of self medication. Drug Saf. 2001, 24, 1027–1037. [Google Scholar] [CrossRef]
- Gajdács, M.J.M. The concept of an ideal antibiotic: Implications for drug design. Molecules 2019, 24, 892. [Google Scholar] [CrossRef] [Green Version]
| Author (Year), Country | Aim | Sample Size | Study Design, Data Collection, Instrument and Setting | Conclusion | Results |
|---|---|---|---|---|---|
| Dimova et al. (2014) Bulgaria [23] | To examine the attitudes and SM patterns that associated with the utilization of antibiotics between the Bulgarian general population and also their determinants. | Patients (n = 1044) Male (n = 482) Female (n = 562) | Cross-sectional and observational study design; survey performed through mail (closed and open-ended questions) | High prevalence of SMA due to sociodemographic factors. | The observed SMA rate was 43%. The younger, female, students and employees tended to have a much higher SM rate. The most consistent patterns that associated with the SM practice were fever (22.0%), discomfort when urinating (8.2%) and sore throat and cough (12.7%). |
| Biswas et al. (2014) Bangladesh [24] | The aims and objective of the current study was to explore the prevalence of SMA for treating various illnesses by the peoples | Patients: (n = 1300) | Cross-sectional survey (self-completed questionnaire) public places | SMA is a common problem among the Bangladesh population. Authority must take strict action to control the distribution and sale of antibiotics to decrease the incidence of misuse. | 347 (26.6%) experienced SMA and the highest percentage of SMA was (50.4%) metronidazole. The key reasons for the SMA were past experience (45.8%) and recommendations from others (28.2%). While the primary reasons behind SMA were food poisoning and diarrhea (36.0%), toothache (9.2%), fever, cough and cold (28.2%), infection (12.9%), acne (4.3%), irritable bowel syndrome (3.4%), throat and ear pain (2.3%). |
| Cheaito et al. (2014) Beirut [32] | This study was performed to evaluate SMA in the overall population and its correlated factors. | Participants: (n = 319) Male (n = 143) Female (n = 176) | Cross-sectional: (face-to-face interview using standardized questionnaire) Pharmacies | SMA was a frequent issue in the Beirut area. Authorities must endorse interventions to minimize antibiotic misuse. | The prevalence of SMA was 42% and the pharmacists were the primary source of buying antibiotics (18.8%). SMA was most commonly associated with sore throat symptoms and the most frequently used antibiotic was amoxicillin. |
| Ngu et al. (2018) Cameroon [33] | The objectives of this study were to evaluate the incidence of SMA and recognize the factors that contribute to SM among adult patients with respiratory tract illness in Cameroon. | Participants (n = 308) Male (n = 138) Female (170) | Cross-sectional: (self-completed questionnaire) Hospital | Outcomes revealed that SMA for respiratory tract infections (RTIs) is a widespread practice that needs to be addressed properly. | The prevalence of SMA among individuals with RTIs was 41.9%. Individuals with a history of pulmonary tuberculosis (TB) were considerably less likely do SMA. The most consistent way to obtain antibiotic for SM was pharmacies (62%). Cotrimoxazole (38.8%) and Amoxicillin (26.4%) were the most frequently used antibiotics. |
| Landers et al. (2010) Columbia [34] | The objective of this study was to find out the level of antibiotics utilization meant for upper respiratory tract infections. | Participants (n = 100) | Cross-sectional: self-completed questionnaire (door-to-door survey) | Researchers suggested that there is a need to address social and cultural contexts to improve antibiotic usage among the general public. | Overall, participants 34% using antibiotics for upper respiratory tract infection (URT). Sevety-three percent of women in this study reported that at least one member of the household used antibiotics during the past season. Among adult individuals, SMA was reported 67.2%, however only 2.4% among children. |
| Skliros et al. (2010) Greece [25] | To evaluate prevalence Self-SMA without prescription. | Participants (n = 1139) Male (n = 545) Female (n = 595) | Cross-sectional: self-completed questionnaires. Carried out in 6 rural health centers in southern Greece. | According to researchers a higher percentage of the rural adult population was consuming antibiotics without any proper prescription. | Total (76.2%) participants self-medicated, while 508 (66.6%) buy medicine from pharmacies. The most commonly used antibiotic was amoxicillin (18.3%), then cefaclor (9.7%), after that cefuroxime (7.9%), finally cefprozil (4.7%) and ciprofloxacin (2.3%). While most frequent reasons for SMA were highest for fever (41.2%), common cold (32.0%) and lowest for sore throat (20.6%). |
| Ramay et al. (2015) Guatemala [35] | To explore and compare the magnitude of SMA and the characteristics among socioeconomic communities | Participants (n = 418) Male (n = 126) Female (n = 292) | Cross-sectional: (descriptive study) Pharmacies | Researchers recommended that there is a need for interventional programs for the sale of antibiotics and also for interventional programs for pharmacists to play effective roles in antibiotic use. | The higher percentage of SMA was reported in the suburban area 79% and while 77% in the city area. In both places, amoxicillin was the most commonly bought antibiotic for SM. Flu-like symptoms were the most common in the Suburban (33%) and, while in metropolitan areas pain and fever (32%) were the most common indication for SMA. |
| Moise et al. (2017) Haiti [36] | To evaluate the prevalence of SMA, Factor associated with it and knowledge to examine SMA in Haiti. | Participants (n = 200) Male (n = 76) Female (n = 124) | Cross-sectional: (face-to-face interview using standardized open-ended and close-ended questionnaire) Hospital | SMA is a common practice among the Haitian population and it is essential to improve public awareness about the dangers practice of SMA and enforce the current law to minimize the consumption of over-the-counter antibiotics. | Among the study sample, 45.5% practiced SMA and It was less prevalent among individuals having good educational background (23.1%). While key reasons for SMA were vaginal itching and mild infection symptoms (44.4%, 28.6%). amoxicillin was reported highest used antibiotic at 67.0%. |
| Widayati et al. (2011) Indonesia [26] | The aim of study was to evaluate SMA prevalence rates and patterns associated with it. | participants (n = 559) Male (n = 219) Female (n = 259) | Cross-sectional: population-based survey (closed-ended self-completed questionnaire). Public places | Researchers identified that the key reason behind SMA was the past experience and a higher percentage of SMA was noted among males. | The prevalence rate among the population was 7.3% and around (6%) doing SMA. while 7% of participants was used prescription-only antibiotics; Amoxicillin (77%) was the commonly used antibiotic and 64% bought medicine from pharmacies. Headache, fever, common cold, toothache, cough, itching, sore throat was the most common indication for antibiotics use. |
| Sawair et al. (2009) Jordan [40] | To determine the extent of SMA among the Jordanian general public. | Patients (n = 477) Male (n = 220) Female (n = 257) | Quantitative (face-to-face interview using a standardized questionnaire) Dental clinics | SMA is apparently common among Jordanians and there is a need to design antibiotic educational campaigns to educate the peoples about their unwanted effects. | The rate of SMA among the general public was 40.7% and peoples with low income and aged between 36–55 years mostly do SMA. Antibiotics were mainly utilized for dental infection, common colds, sore throats and pharmacies were the primary sources (53.6% cases). While Amoxicillin (37.6%) was a commonly used antibiotic. |
| Scicluna et al. (2009) Euro-Mediterranean Countries [41] | The aim of study was to evaluate and identify SMA rates inside Mediterranean countries. | Participants (n = 1705) | Quantitative (short structured interviews using questionnaire) Hospitals and private clinics | Researchers found that in the southern and eastern region of Mediterranean countries non-prescribed antibiotic consumption was much higher inside ambulatory care. | In Cyprus, the SM rate was 19.1%, while in Lebanon it was 37% and in Jordan 70.7%. URTs symptoms was the most consistent known reasons for SMA. A total of 48.4% of the peoples responded that they have leftover antibiotics in their homes. |
| Abdulraheem et al. 2016 Nigeria [14] | To estimate the prevalence of SMA in a sample of the rural population and to evaluate sociodemographic aspects linked to this practice. | Participants (n = 1150) Adults (n = 602) Adults Parents (n = 548) | Cross-sectional: (self-completed questionnaire) Primary health care center | SMA is a critical problem in Nigeria and needs substantial attention. | The prevalence of SMA was 82.2% and the antibiotics most regularly used for SMA were ampicillin (20.3%), second, sulfamethoxazole/ trimethoprim mixture (14.2%), third, metronidazole (13.9%) and tetracycline (13.1%). A cough with mucus (30.1%), sore throat (23.7%), fever (20.7%), dysuria (10.6%) skin sepsis (7.5%) and vaginal discharge (7.4%) were the most typical reasons for SMA. |
| Nazir et al. (2016) Pakistan [42] | To assess various factors that lead to SMA, which may cause AMR and cause a hindering effect in healthcare. | Participants (n = 527) | Cross-sectional: (standardized questionnaire) Public places | A need for public health policy through drug regulatory authorities, public education campaigns about AMR and appropriate utilization of antibiotics were highlighted. | SMA was reported by 26% participants, with an increased prevalence in males compared to females (48% vs 38%, respectively). The primary reason for SMA was the previous encounter with the same antibiotic (68%). The mostly used antibiotics was amoxicillin-clavulanate (40%) and the main indications for self-medicine were sore throat (29%) and flu (24%). Out Of the 527 respondents, just 20% were alert to AMR. |
| Khan et al. (2011) Pakistan [12] | To evaluate the SMA rate among the study was the urban general public and to identify the factors associated with this practice. | Participants: (n = 744) Male (n = 350) Female (n = 394) | Quantitative Descriptive study Private clinics | Researchers concluded that healthcare facilities in Peshawar are good and the general public also has easy access to them, despite that people’s utilization of non-prescription antibiotics. | The prevalence towards SMA was (69%) and the antibiotics most regularly used at the first place were amoxicillin/clavulanic acid (45%), then ciprofloxacin (31%), after that sulfamethoxazole/trimethoprim (18%) and finally place clarithromycin (5%). The main indications for SMA were fever (70%), then sore throat (22%) and common cold (8%). |
| Bilal et al. (2016) Pakistan [13] | The study was aimed to explore the prevalence rate and practice towards SMA between general public livening in rural regions of Sindh province. | Participants (n = 400) Male (n = 263) Female (n = 137) | Cross-sectional: (Self-completed; close-end questionnaire) Hospital | The SMA rate was higher in rural regions of Sindh. The government must enforce stricter laws to control this practice. Lastly, by providing cost-effective treatment to the public sector, SMA can be considerably decreased. | 325 rural dwellers reported usage SMA during the past 6 months period with a prevalence rate of 81.25%. The mostly bought antibiotics were amoxicillin (52.0%) accompanied by tetracycline (16.9%), then ciprofloxacin (14.8), after that co-trimoxazole (11.4%) and ampicillin (8.3%) finally place. Common indications for SMA was flu (60.0%) and (88.0%) also reported that economic reasons for SMA. |
| Muras et al. (2013) Poland [37] | The purpose of this study was to find out the prevalence rate for SMA intended for RTIs and also identified factors associated with this practice. | Participants (n = 891) Male (n = 304) Female (n = 587) | Cross-sectional: (self-completed questionnaire) Family medicine Clinics | SMA for RTI was frequent among the general population and according to researchers, this maybe because of the people’s opinion that the drugs treat almost all infections. | Overall, 41.4% of participants reported SMA for respiratory tract infection (RTI) and the second most common indication reported for SMA was influenza or flu-like disease (43.9%). The primary sources for antibiotics was drugs kept at home obtained from previous prescriptions (73.7%). While (13.5%) bought antibiotics from the pharmacy or got it from friends or their family members (12.7%). |
| Ramalhinho et al. (2014) Portugal [27] | To evaluate the prevalence of SMA and measure the predictive factors connected with such SM. | Participants (n = 1192) Male (581) Female (611) | Cross-sectional: a population-based survey. (self-completed questionnaire) Public places | SMA was greater among older males than young adults. Researchers also reported that gender and age were key determinants for SMA. | 218 respondents (18.9%) admitted that they previously took antibiotics without a prescription and 267 (23%) participants noted that they were keeping leftover antibiotics in their homes. The factors that affect SMA rate were age; in particular 18–34 years and in the 50–64; while these practices less seen in 44–49 age groups. |
| Bianca et al. (2013) Romania [43] | The purpose of this study was to explore factors that responsible for OTC purchasing of antibiotics and SMA in Romania. | Participants: (n = 20) In-depth interview (n = 10) | In-depth qualitative face-to-face interview; (snowball sampling technique) | This study highlighted that population generally dissatisfied with their medical situations and the healthcare program. | The participants that self-medicated mentioned kidney and urinary infections as the main indication. Antibiotics used for these diseases were amoxicillin, cephalexin and norfloxacin. Flu and cold, treated by over-the-counter medicines and antibiotics used were amoxicillin, cephalexin and ampicillin. |
| Yousif et al. (2015) Saudi Arabia [30] | Exploring the prevalence of SMA and also determine its determinants. | Participants (n = 400) Male (n = 228) Female (n = 291) | Cross–sectional: Population-based survey. (Close-ended self-completed questionnaire) Public places | SMA was prevalent and there is a need for general public education campaigns along with stringent strategies to decrease or restrict OTC sales of antibiotics inside community pharmacies. | Almost 315 (80.6%) individuals accepted that they are doing SMA and 235 (74.6%) participants practiced it due to easy availability. Around 123 (39.0%) Participants doing SMA and they thought they treat infectious successfully with the help of antibiotics. while 146 (46.3%) individuals were not sure about this and 46 (14.6%) responded: “they cannot”. |
| Alghadeer et al. (2018) Saudi Arabia [29] | This research aimed to find out the prevalence rate and common indication for SMA. | Respondents (n = 1264) Male (n = 345) Female (n = 919) | Cross-sectional: An online survey using the snowball technique. (self-completed questionnaire) | The high prevalence rate for SMA demands to take significant actions by authorities to implement strict laws to prevent the sale of antibiotics without a proper prescription. | Overall, 34% of participants consumed antibiotics without a prescription and around 81.3% of individuals recognized that it could be unsafe. The common antibiotics utilized were amoxicillin/clavulanic acid (45.1%) in the first place, amoxicillin alone (39.9%), then azithromycin (16.8%), after that cefuroxime (9.7%) and cephalexin (5.7%) finally place. The most typical reasons for antibiotic were tonsillitis (76.7%). The main source of SMA were drugs obtained earlier through a doctor’s prescription (36.6%). |
| Al Rasheed et al. (2016) Saudi Arabia [28] | To determine the common predictors and prevalence towards SMA. | Patients: (n = 681) Male (n = 523) Female (158) | Cross-sectional: (closed and open–ended questionnaire) Hospital | High prevalence rate towards SMA was noted and the researchers suggested that education campaigns are required for appropriate antibiotic utilization. | The prevalence rate for SMA was 78.7% and amoxicillin was the most utilized antibiotic with the overall prevalence rate of (22.3%). Advice from friends on SMA and pharmacy proximity to individuals were the most common predictors for SMA. |
| Al-Qahtani et al. (2018) Saudi Arabia [39] | To determine the prevalence of SMA and factors related to this behavior | Participant (n = 519) Male (n = 255) Female (n = 294) | Cross-sectional: (Self-administered questionnaire) Hospital | A high prevalence rate toward SMA was noted among the lay population. Researchers suggested that by using media awareness campaigns, authorities can decrease SMA and the adverse events related to it. | SMA prevalence rate was 40.8% and elder people were mostly associated with SMA. While the most common disease was RTIs (73.2%) and most of the peoples obtained antibiotics from pharmacies (82.8%). Past good experience with SMA was the main reason for continuous SM (67.2%) |
| Ilhan et al. (2009) Turkey [38] | The purpose to find out the frequency and know the reasons for SMA. | Participants (n = 2696) | Face-to-face interview (using standardized questionnaire) Primary health care center | Researchers indicated the necessity of strict laws to regulate antibiotic sales and also identified the need for education policies in the community to reduce inappropriate use of antibiotics. | The most common reasons for SMA were sore throat (59.6%), then fever (46.2%) and finally cough (40.0%). Other common indications were rheumatism, exhaustion and dental infection. According to age ranges, the most prevalent age group was 40–49 with 23% doing SMA and the least rate noted around 11.8% among 60–69 years old. |
| Abasaeed et al. (2009) United Arab Emirates [31] | The main purpose was to evaluate the prevalence of SMA. | Participants: (n = 860) Male (n = 566) Female (294) | Cross-sectional: population-based survey (self-completed questionnaire) Public places | The outcomes indicated that SMA is a consistent issue in the general public of Abu Dhabi. Interventions are required to cut-down the improper use of antibiotics. | In both genders overall, 485 (56%) admitted that they consumed antibiotics during the past year. Education level has a significant impact on SMA, and amoxicillin was the antibiotic most commonly used (46.3%). The common reason for SMA were influenza, toothache, ear, upper respiratory and gastrointestinal infection. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslam, A.; Gajdács, M.; Zin, C.S.; Ab Rahman, N.S.; Ahmed, S.I.; Zafar, M.Z.; Jamshed, S. Evidence of the Practice of Self-Medication with Antibiotics among the Lay Public in Low- and Middle-Income Countries: A Scoping Review. Antibiotics 2020, 9, 597. https://doi.org/10.3390/antibiotics9090597
Aslam A, Gajdács M, Zin CS, Ab Rahman NS, Ahmed SI, Zafar MZ, Jamshed S. Evidence of the Practice of Self-Medication with Antibiotics among the Lay Public in Low- and Middle-Income Countries: A Scoping Review. Antibiotics. 2020; 9(9):597. https://doi.org/10.3390/antibiotics9090597
Chicago/Turabian StyleAslam, Adeel, Márió Gajdács, Che Suraya Zin, Norny Syafinaz Ab Rahman, Syed Imran Ahmed, Muhammad Zeeshan Zafar, and Shazia Jamshed. 2020. "Evidence of the Practice of Self-Medication with Antibiotics among the Lay Public in Low- and Middle-Income Countries: A Scoping Review" Antibiotics 9, no. 9: 597. https://doi.org/10.3390/antibiotics9090597
APA StyleAslam, A., Gajdács, M., Zin, C. S., Ab Rahman, N. S., Ahmed, S. I., Zafar, M. Z., & Jamshed, S. (2020). Evidence of the Practice of Self-Medication with Antibiotics among the Lay Public in Low- and Middle-Income Countries: A Scoping Review. Antibiotics, 9(9), 597. https://doi.org/10.3390/antibiotics9090597







