Synergistic Effects of Bacteriocin from Lactobacillus panis C-M2 Combined with Dielectric Barrier Discharged Non-Thermal Plasma (DBD-NTP) on Morganella sp. in Aquatic Foods
Abstract
:1. Introduction
2. Results
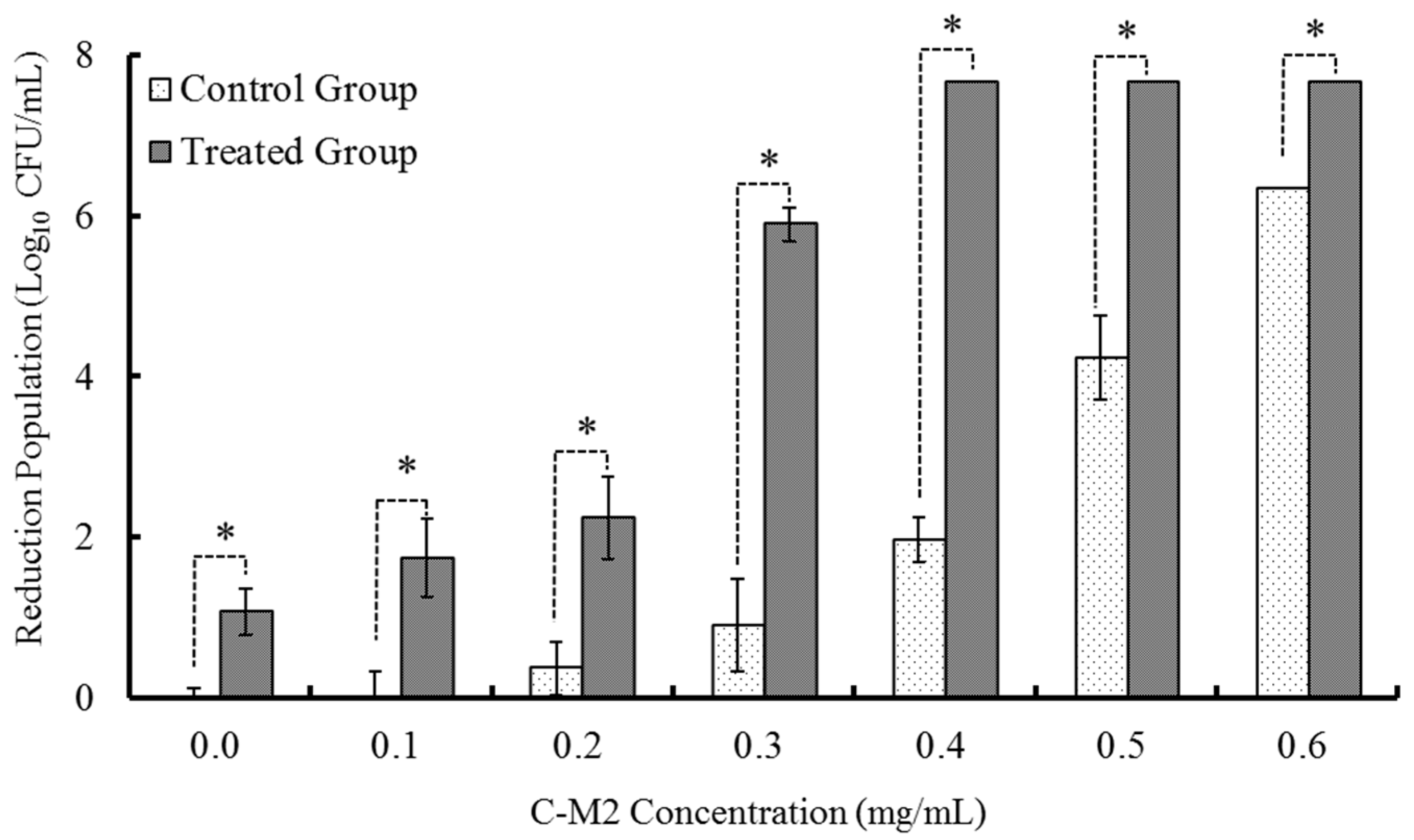
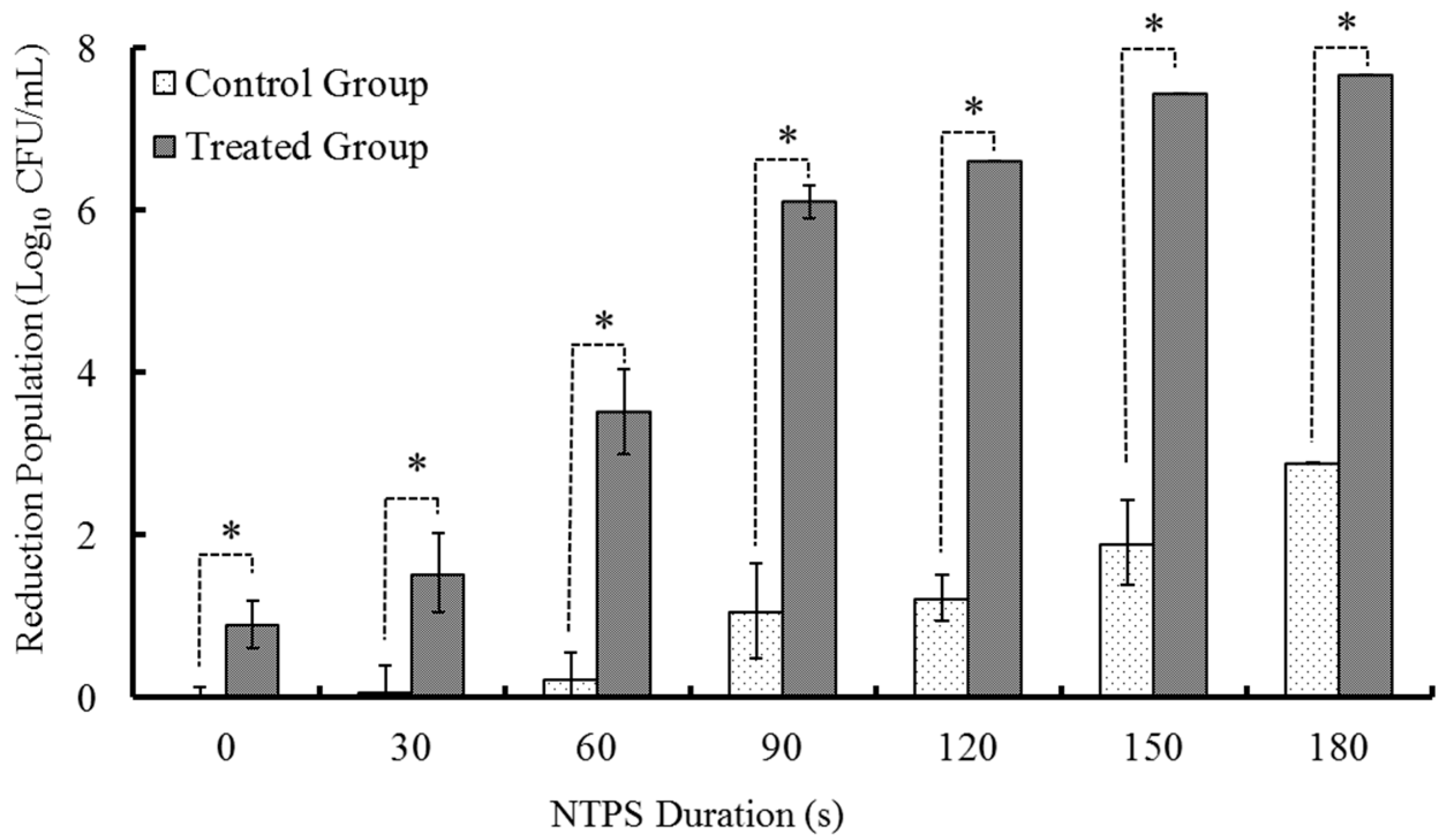
2.1. Effects of C-M2 and NTPS on the Growth Inhibition of Morganella sp. wf-1
2.1.1. Treatments with Different Concentrations of C-M2
2.1.2. Treatments with Different times of CSP
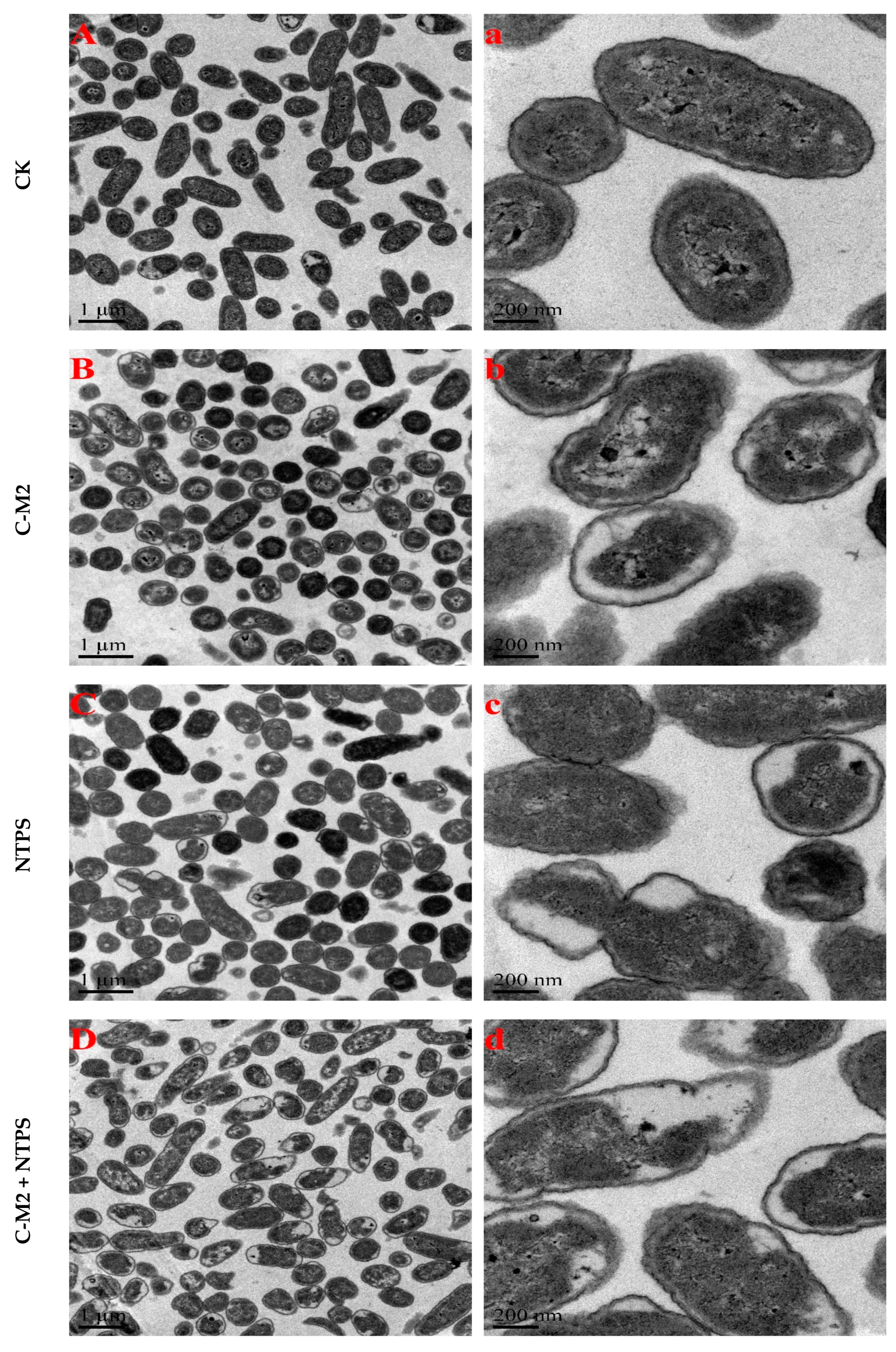
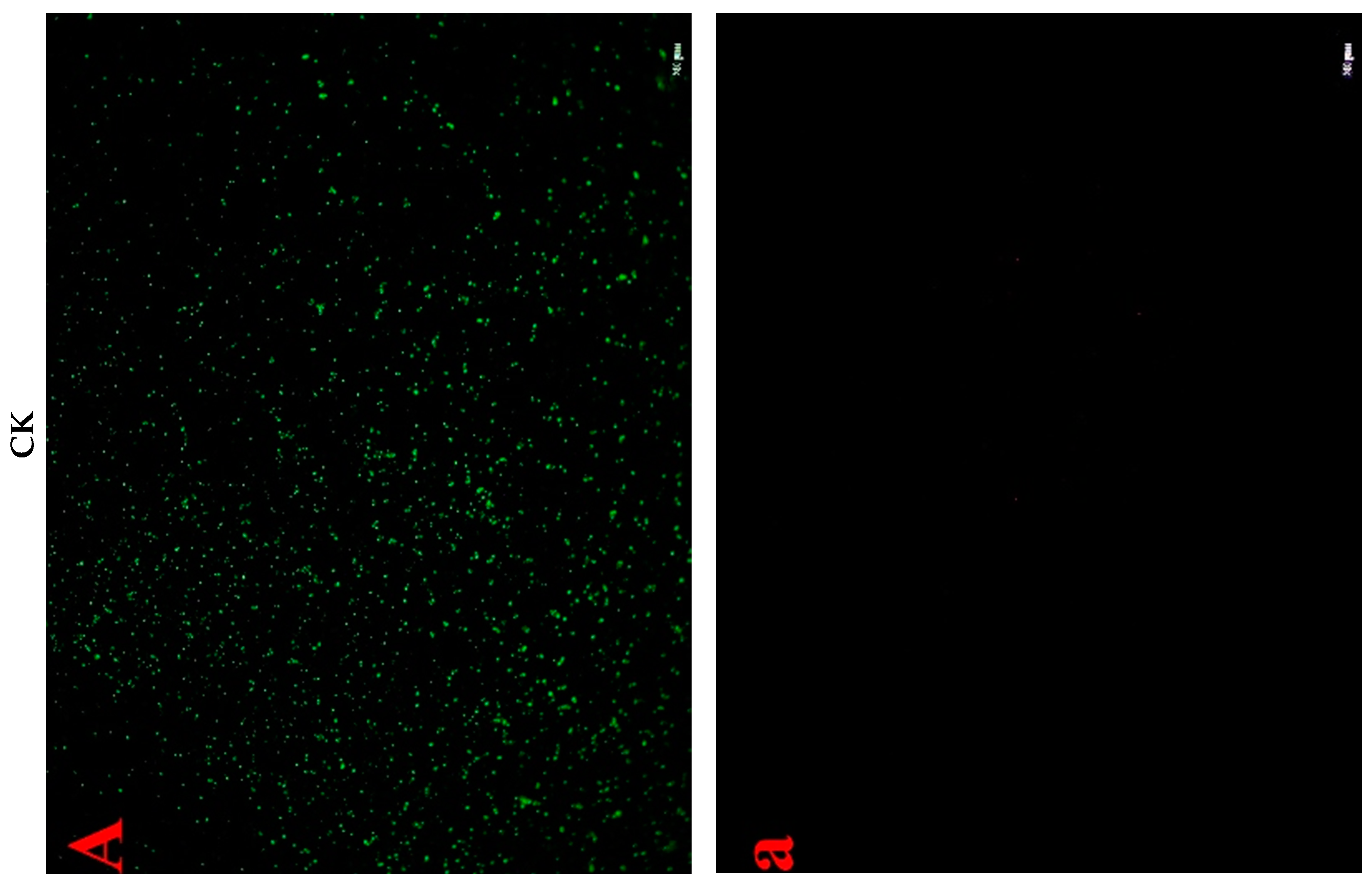
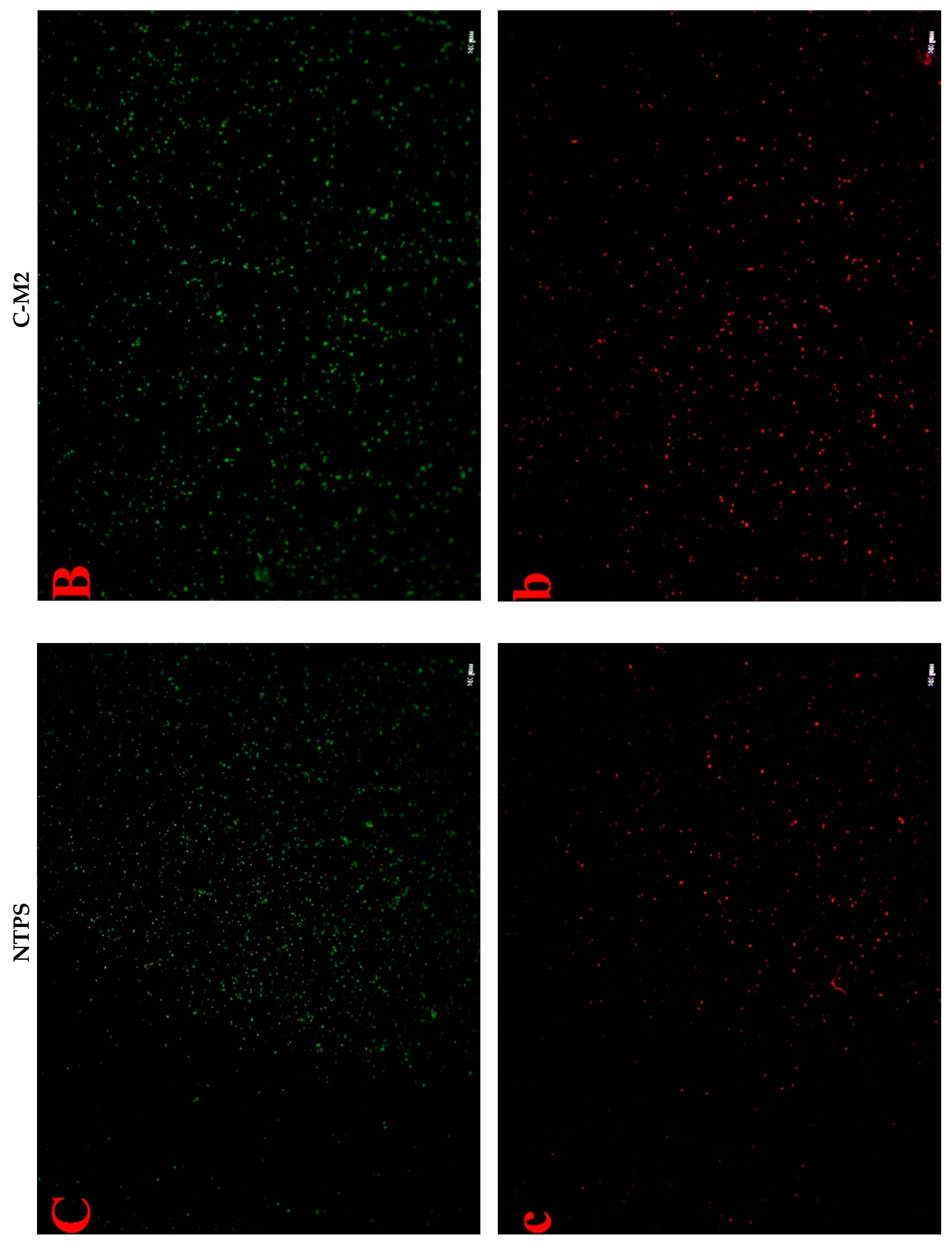
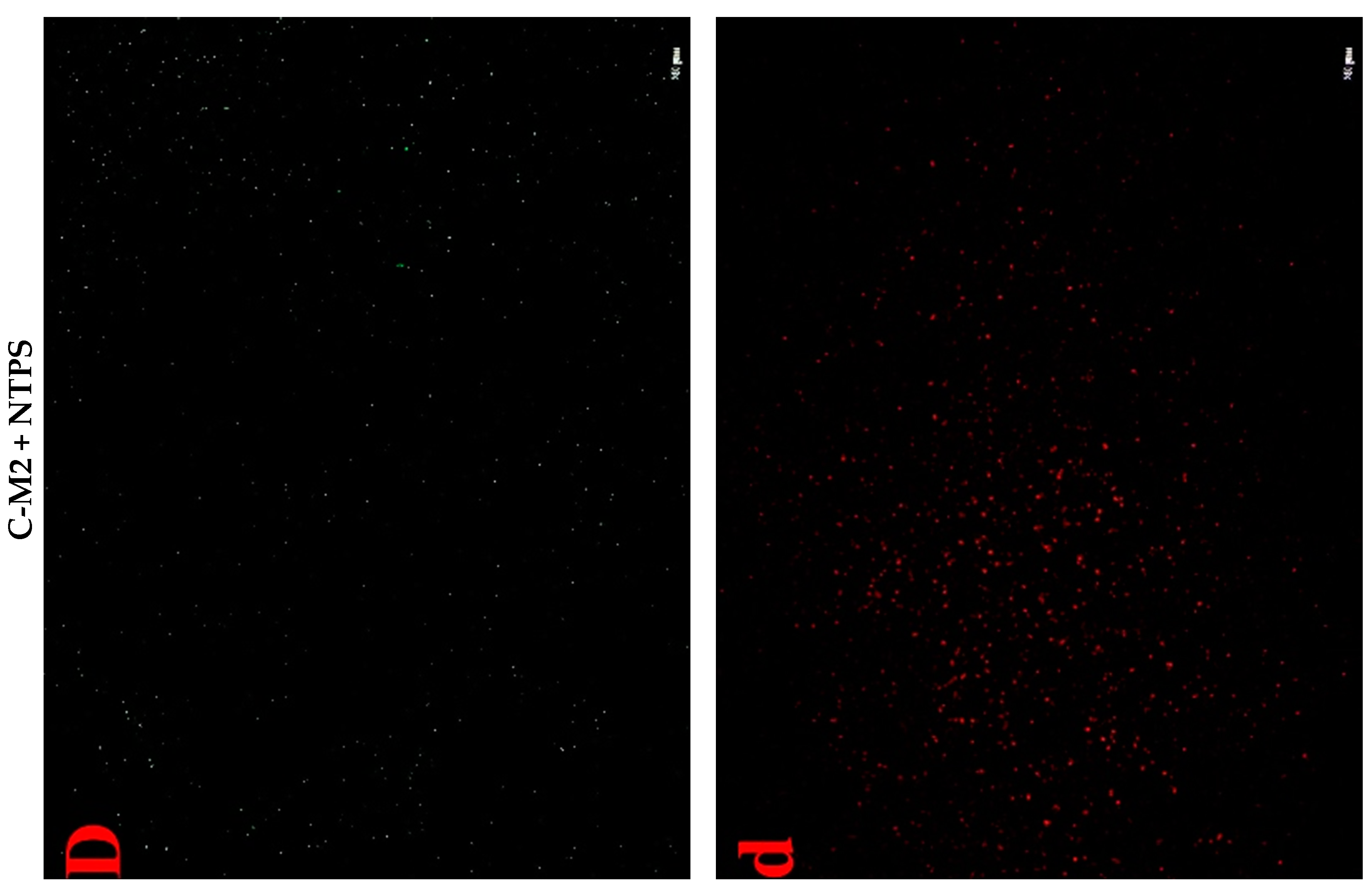
2.2. Effects of Lactocin C-M2 and NTPS on the Morphology
2.3. Effects of C-M2 and NTPS on the Cell Membrane Permeability
2.4. Effects of Lactocin C-M2 and NTPS on the Cell Membrane Integrity
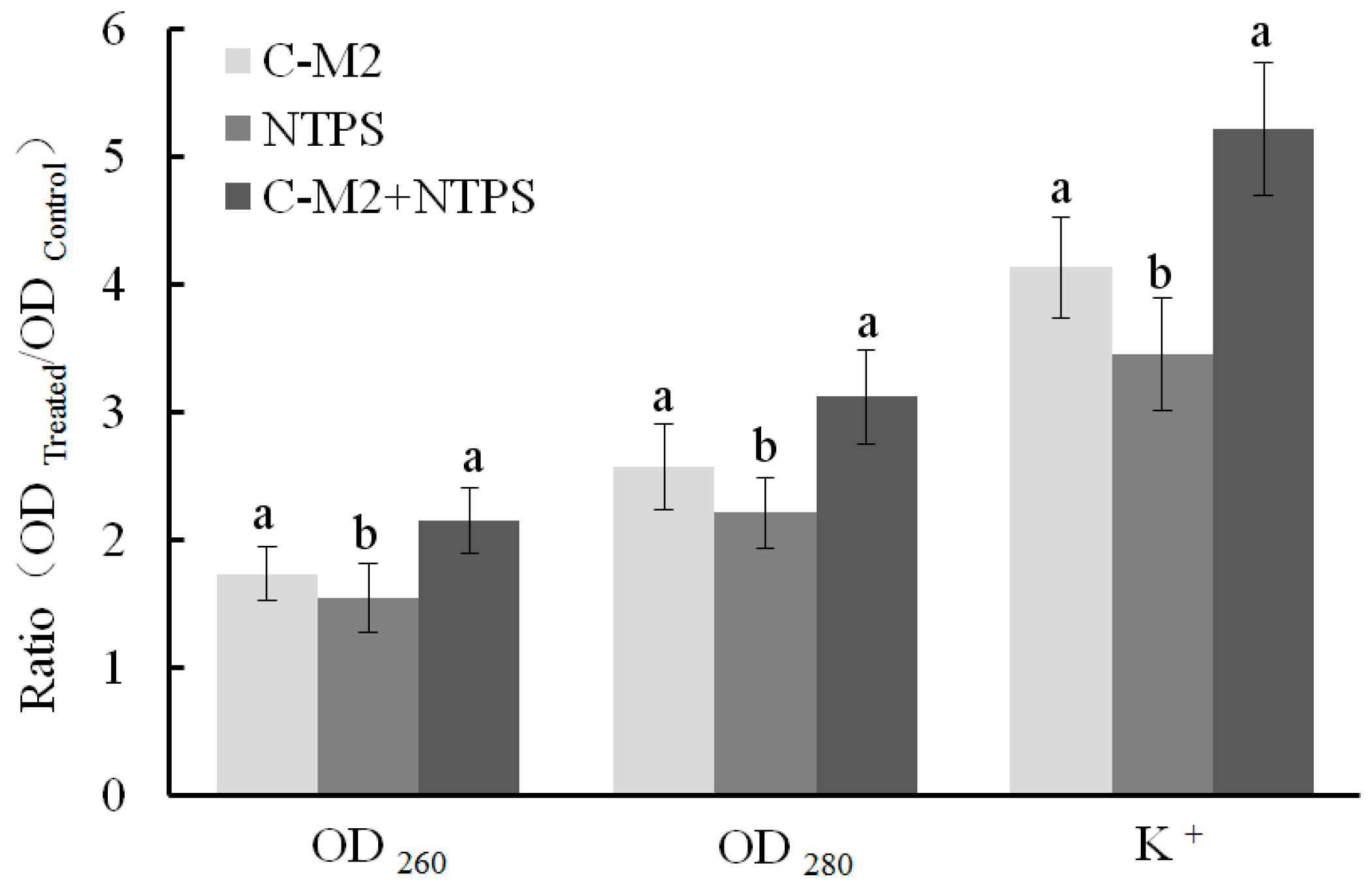
2.4.1. Released Cell Nucleic Acid, Protein, and K+
2.4.2. Fatty Acid Composition in Cell Membrane
2.4.3. Cell Fluorescent Staining
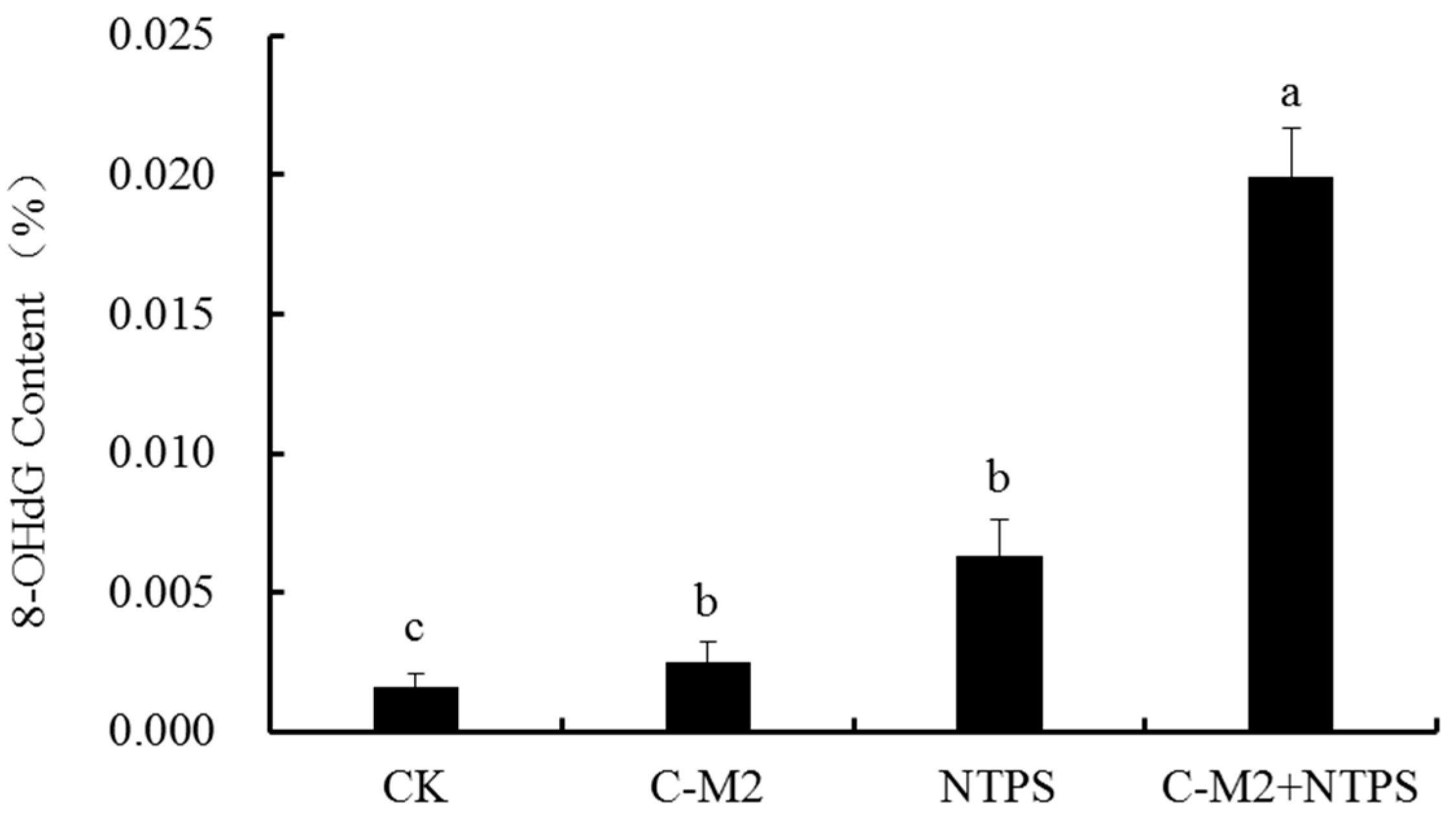
2.5. Effects of Lactocin C-M2 and NTPS on the DNA Damage of Morganella sp. wf-1
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Bacterial Strains, Culture Condition, and Preparation
4.3. Different Treatments by Lactocin C-M2 and NTPS
4.4. Viable Bacterial Counting
4.5. Transmission Electron Microscopy (TEM)
4.6. Cell Membrane Permeability
4.7. Release of Cell Nucleic Acid, Protein, and K+
4.8. Fatty Acid Composition in Cell Membranes
4.9. Confocal Laser Scanning Microscopy (CLSM)
4.10. DNA Damage in Cells
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Issa-Zacharia, A.; Kamitani, Y.; Muhimbula, H.; Iwasaki, K. Antimicrobial effect of slightly acidic electrolyzed water for inactivation of Salmonella spp. and Escherichia coli on fresh strawberries (Fragaria L.). Afr. J. Microbiol. Res. 2010, 4. [Google Scholar] [CrossRef]
- Shakila, R.J.; Vasundhara, T.S.; Vijaya Rao, D. Inhibitory effect of spices on in vitro histamine production and histidine decarboxylase activity ofMorganella morganii and on the biogenic amine formation in mackerel stored at 30 °C. Z. für Lebensm.-Unters. Forsch. 1996, 203, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.T.; Jun, J.W.; Giri, S.S.; Yun, S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Kang, J.W.; Han, S.J.; Kwon, J.; et al. Morganella psychrotolerans as a possible opportunistic pathogen in rainbow trout (Oncorhynchus mykiss) fisheries. Aquaculture 2020, 520, 735021. [Google Scholar] [CrossRef]
- Liu, G.; Song, Z.; Yang, X.; Gao, Y.; Wang, C.; Sun, B. Antibacterial mechanism of bifidocin A, a novel broad-spectrum bacteriocin produced by Bifidobacterium animalis BB04. Food Control 2016, 62, 309–316. [Google Scholar] [CrossRef]
- Singla, N.; Kaistha, N.; Gulati, N.; Chander, J. Morganella morganii could be an important Intensive Care Unit pathogen. Indian J. Crit. Care Med. 2010, 14, 154–155. [Google Scholar] [CrossRef] [Green Version]
- Niemira, B.A. Cold plasma decontamination of foods. Annu. Rev. Food Sci. Tech. 2012, 3, 125–142. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Zinoviadou, K.G.; Galanakis, C.M.; Brnčić, M.; Grimi, N.; Boussetta, N.; Mota, M.J.; Saraiva, J.A.; Patras, A.; Tiwari, B.; Barba, F.J. Fruit juice sonication: Implications on food safety and physicochemical and nutritional properties. Food Res. Int. 2015, 77, 743–752. [Google Scholar] [CrossRef]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Zhou, K.; Zhou, W.; Li, P.; Liu, G.; Zhang, J.; Dai, Y. Mode of action of pentocin 31-1: An antilisteria bacteriocin produced by Lactobacillus pentosus from Chinese traditional ham. Food Control 2008, 19, 817–822. [Google Scholar] [CrossRef]
- Acuña, L.; Picariello, G.; Sesma, F.; Morero, R.D.; Bellomio, A. A new hybrid bacteriocin, Ent35–MccV, displays antimicrobial activity against pathogenic Gram-positive and Gram-negative bacteria. FEBS Open Bio 2012, 2, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Li, D.; Sheng, Y.; Liu, X. Mode of action of sakacin C2 against Escherichia coli. Food Control 2011, 22, 657–661. [Google Scholar] [CrossRef]
- Shan, C.J.; Hu, Y.X.; Xia, X.D.; Liu, X.L.; Li, Y.; Wang, Y.; Zhang, J.H.; Zhou, J.Z. Purification and Characterization of the Bacteriocin Produced by Lactobacillus panis C-M2. Food Sci. 2017, 38, 20–26. [Google Scholar]
- Ferrari, G.; Maresca, P.; Ciccarone, R. The application of high hydrostatic pressure for the stabilization of functional foods: Pomegranate juice. J. Food Eng. 2010, 100, 245–253. [Google Scholar] [CrossRef]
- Schlüter, O.; Ehlbeck, J.; Hertel, C.; Habermeyer, M.; Roth, A.; Engel, K.H.; Holzhauser, T.; Knorr, D.; Eisenbrand, G. Opinion on the use of plasma processes for treatment of foods. Mol. Nutr. Food Res. 2013, 57, 920–927. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, G.; Shi, X.; Xu, G.; Yang, Y. Chemical Mechanisms of Bacterial Inactivation Using Dielectric Barrier Discharge Plasma in Atmospheric Air. IEEE Trans. Plasma Sci. 2008, 36, 1615–1620. [Google Scholar] [CrossRef]
- Herceg, Z.; Kovačević, D.B.; Kljusurić, J.G.; Jambrak, A.R.; Zorić, Z.; Dragović-Uzelac, V. Gas phase plasma impact on phenolic compounds in pomegranate juice. Food Chem. 2016, 190, 665–672. [Google Scholar] [CrossRef]
- Hertrich, S.M.; Boyd, G.; Sites, J.; Niemira, B.A. Cold Plasma Inactivation of Salmonella in Prepackaged, Mixed Salads Is Influenced by Cross-Contamination Sequence. J. Food Prot. 2017, 80, 2132–2136. [Google Scholar] [CrossRef]
- Fernández, A.; Noriega, E.; Thompson, A. Inactivation of Salmonella enterica serovar Typhimurium on fresh produce by cold atmospheric gas plasma technology. Food Microbiol. 2013, 33, 24–29. [Google Scholar] [CrossRef]
- Surowsky, B.; Fröhling, A.; Gottschalk, N.; Schlüter, O.; Knorr, D. Impact of cold plasma on Citrobacter freundii in apple juice: Inactivation kinetics and mechanisms. Int. J. Food Microbiol. 2014, 174, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lei, C.; Chen, J.; Yang, K.; Zhu, L.; Lin, D. Effect of natural and synthetic surface coatings on the toxicity of multiwalled carbon nanotubes toward green algae. Carbon 2015, 83, 198–207. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamamoto, T.; Kawai, Y.; Inoue, N.; Yamazaki, K. Mode of action of piscicocin CS526 produced by Carnobacterium piscicola CS526. J. Appl. Microbiol. 2005, 98, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek, E.; Sałek, K.; Guzik, U.; Dudzińska-Bajorek, B. Cell surface properties and fatty acids composition of Stenotrophomonas maltophilia under the influence of hydrophobic compounds and surfactants. New Biotech. 2013, 30, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.; Perrier-Cornet, J.-M.; Gervais, P. Damage in Escherichia coli Cells Treated with a Combination of High Hydrostatic Pressure and Subzero Temperature. Appl. Environ. Microbiol. 2007, 73, 6508–6518. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Li, C.; Wen, Y.; Liu, W. Antioxidant defense system responses and DNA damage of earthworms exposed to Perfluorooctane sulfonate (PFOS). Environ. Pollut. 2013, 174, 121–127. [Google Scholar] [CrossRef]
- Xie, Z.; Okinaga, T.; Niu, G.; Qi, F.; Merritt, J. Identification of a Novel Bacteriocin Regulatory System in Streptococcus mutans. Mol. Microbiol. 2010, 78, 1431–1447. [Google Scholar] [CrossRef] [Green Version]
- Engelke, G.; Gutowski-Eckel, Z.; Hammelmann, M.; Entian, K.D. Biosynthesis of the lantibiotic nisin: Genomic organization and membrane localization of the NisB protein. Appl. Environ. Microbiol. 1992, 58, 3730–3743. [Google Scholar] [CrossRef] [Green Version]
- Yoneyama, F.; Fukao, M.; Zendo, T.; Nakayama, J.; Sonomoto, K. Biosynthetic characterization and biochemical features of the third natural nisin variant, nisin Q, produced by Lactococcus lactis 61-14. J. Appl. Microbiol. 2008, 105, 1982–1990. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Wang, D.; Wang, X. A novel cold plasma jet generated by atmospheric dielectric barrier capillary discharge. Thin Solid Films 2006, 506–507, 404–408. [Google Scholar] [CrossRef]
- Cebrián, R.; Martínez-Bueno, M.; Valdivia, E.; Albert, A.; Maqueda, M.; Sánchez-Barrena, M.J. The bacteriocin AS-48 requires dimer dissociation followed by hydrophobic interactions with the membrane for antibacterial activity. J. Struct. Biol. 2015, 190, 162–172. [Google Scholar] [CrossRef]
- Yasuda, H.; Miura, T.; Kurita, H.; Takashima, K.; Mizuno, A. Biological Evaluation of DNA Damage in Bacteriophages Inactivated by Atmospheric Pressure Cold Plasma. Plasma Process. Polym. 2010, 7, 301–308. [Google Scholar] [CrossRef]
- Diao, W.-R.; Hu, Q.-P.; Zhang, H.; Xu, J.-G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Stoffels, E.; Gonzalvo, Y.A.; Whitmore, T.D.; Seymour, D.L.; Rees, J.A. Mass spectrometric detection of short-living radicals produced by a plasma needle. Plasma Source Sci. Technol. 2007, 16, 549–556. [Google Scholar] [CrossRef]
- Mecocci, P.; Polidori, M.C.; Mattioli, P.; Cecchetti, R.; Ingegni, T.; Cherubini, A.; Catani, M.; Senin, U. Plasma antioxidants and oxidative DNA damage in lymphocytes from normal aged people and Alzheimer disease patients. Neurobiol. Aging 2000, 21, 363–369. [Google Scholar] [CrossRef]
- Huang, M.; Wang, J.; Zhuang, H.; Yan, W.; Zhao, J.; Zhang, J. Effect of in-package high voltage dielectric barrier discharge on microbiological, color and oxidation properties of pork in modified atmosphere packaging during storage. Meat Sci. 2019, 149, 107–113. [Google Scholar] [CrossRef]
- Huang, M.; Zhuang, H.; Zhao, J.; Wang, J.; Yan, W.; Zhang, J. Differences in cellular damage induced by dielectric barrier discharge plasma between Salmonella Typhimurium and Staphylococcus aureus. Bioelectrochemistry 2020, 132, 107445. [Google Scholar] [CrossRef]
- Wu, H.; Rui, X.; Li, W.; Xiao, Y.; Zhou, J.; Dong, M. Whole-grain oats (Avena sativa L.) as a carrier of lactic acid bacteria and a supplement rich in angiotensin I-converting enzyme inhibitory peptides through solid-state fermentation. Food Funct. 2018, 9, 2270–2281. [Google Scholar] [CrossRef]
- Sharma, A.; Srivastava, S. Anti-Candida activity of two-peptide bacteriocins, plantaricins (Pln E/F and J/K) and their mode of action. Fungal Biol. 2014, 118, 264–275. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, S.; Yang, K.; Zhu, L.; Lin, D. Toxicity of perfluorooctane sulfonate and perfluorooctanoic acid to Escherichia coli: Membrane disruption, oxidative stress, and DNA damage induced cell inactivation and/or death. Environ. Pollut. 2016, 214, 806–815. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, F.; Liao, X.; Hu, X.; Chen, Y.; Deng, L. Analysis of Escherichia coli cell damage induced by HPCD using microscopies and fluorescent staining. Int. J. Food Microbiol. 2010, 144, 169–176. [Google Scholar] [CrossRef] [PubMed]


| Saturated Fatty Acids | CK | C-M2 | NTPS | C-M2 + NTPS | Unsaturated Fatty Acids | CK | C-M2 | NTPS | C-M2 + NTPS |
|---|---|---|---|---|---|---|---|---|---|
| C4:0 | 5.21 | 1.64 | 6.56 | 5.09 | C14:1 | 6.23 | 41.72 | 43.40 | 46.93 |
| C6:0 | 0.98 | 0.39 | 0.40 | 0.00 | C15:1 | 0.24 | 1.64 | 0.07 | 1.18 |
| C8:0 | 0.46 | 0.00 | 0.00 | 0.00 | C16:1 | 0.27 | 8.63 | 0.31 | 26.18 |
| C10:0 | 0.75 | 0.39 | 0.00 | 0.00 | C17:1 | 0.21 | 0.61 | 0.79 | 0.43 |
| C11:0 | 0.48 | 0.00 | 0.00 | 0.00 | C18:1n9t | 4.61 | 2.94 | 2.64 | 2.95 |
| C12:0 | 0.27 | 0.00 | 0.14 | 0.00 | C18:1n9c | 45.61 | 25.98 | 28.75 | 8.93 |
| C13:0 | 0.28 | 0.74 | 0.21 | 0.00 | C18:2n6t | 0.04 | 0.88 | 2.09 | 0.00 |
| C14:0 | 0.42 | 0.99 | 0.21 | 0.00 | C18:3n6 | 0.02 | 0.00 | 0.02 | 0.00 |
| C15:0 | 0.11 | 0.27 | 0.18 | 0.30 | C20:1 | 1.32 | 0.46 | 1.09 | 0.38 |
| C16:0 | 9.86 | 3.29 | 0.20 | 1.60 | C18:3n3 /C21:0 | 0.52 | 0.00 | 0.27 | 0.00 |
| C17:0 | 1.29 | 0.55 | 0.36 | 0.00 | C20:2 | 0.39 | 0.00 | 0.23 | 0.00 |
| C18:0 | 7.03 | 4.25 | 4.73 | 4.15 | C20:3n6 | 0.05 | 0.00 | 0.04 | 0.00 |
| C20:0 | 3.52 | 3.68 | 4.39 | 1.88 | C22:1n9 | 9.11 | 0.97 | 2.43 | 0.00 |
| C22:0 | 0.12 | 0.00 | 0.04 | 0.00 | C20:3n3 /C20:4n6 | 0.06 | 0.00 | 0.07 | 0.00 |
| C23:0 | 0.22 | 0.00 | 0.00 | 0.00 | C22:2 | 0.14 | 0.00 | 0.00 | 0.00 |
| C24:0 | 0.11 | 0.00 | 0.00 | 0.00 | C20:5n3 | 0.04 | 0.00 | 0.07 | 0.00 |
| C24:1 | 0.03 | 0.00 | 0.32 | 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, C.; Wu, H.; Zhou, J.; Yan, W.; Zhang, J.; Liu, X. Synergistic Effects of Bacteriocin from Lactobacillus panis C-M2 Combined with Dielectric Barrier Discharged Non-Thermal Plasma (DBD-NTP) on Morganella sp. in Aquatic Foods. Antibiotics 2020, 9, 593. https://doi.org/10.3390/antibiotics9090593
Shan C, Wu H, Zhou J, Yan W, Zhang J, Liu X. Synergistic Effects of Bacteriocin from Lactobacillus panis C-M2 Combined with Dielectric Barrier Discharged Non-Thermal Plasma (DBD-NTP) on Morganella sp. in Aquatic Foods. Antibiotics. 2020; 9(9):593. https://doi.org/10.3390/antibiotics9090593
Chicago/Turabian StyleShan, Chengjun, Han Wu, Jianzhong Zhou, Wenjing Yan, Jianhao Zhang, and Xiaoli Liu. 2020. "Synergistic Effects of Bacteriocin from Lactobacillus panis C-M2 Combined with Dielectric Barrier Discharged Non-Thermal Plasma (DBD-NTP) on Morganella sp. in Aquatic Foods" Antibiotics 9, no. 9: 593. https://doi.org/10.3390/antibiotics9090593





