NSAIDs as a Drug Repurposing Strategy for Biofilm Control
Abstract
:1. Introduction
2. Results and Discussion
2.1. Minimum Inhibitory and Bactericidal Concentrations of Selected NSAIDs and Antibiotics
2.2. Effect of Different Doses of the Selected NSAIDs on Culturability of E. coli and S. aureus Planktonic Cells
2.3. Effect of Selected NSAIDs and Antibiotics on the Control of E. coli and S. aureus Biofilms
2.4. Effect of Dual Combinations of Selected NSAIDs with KAN and TET on the Control of E. coli and S. aureus Biofilms
3. Materials and Methods
3.1. Bacteria
3.2. Antibiotics and NSAIDs
3.3. Antibacterial Susceptibility Tests
3.3.1. Antibacterial Activity Assessment of Antibiotics by Disc Diffusion Method
3.3.2. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Selected Antibiotics and NSAIDs
3.4. Dose–Response Curves
3.5. Biofilm Control
3.5.1. Biofilm Formation
3.5.2. Exposure to NSAIDs
3.5.3. Biofilm Control Analysis
Mass Quantification by Crystal Violet Staining
Metabolic Activity Quantification by Alamar Blue Assay
Biofilm Culturable Cells Quantification by Plate Count Method
3.5.4. Biofilm Control Activity Classification
- Low efficacy: I or R < 25%;
- Moderate efficacy: 25% ≤ I or R < 60%;
- High efficacy: 60% ≤ I or R < 90%;
- Excellent efficacy: 90% ≤ I or R ≤ 100%.
3.5.5. Dual Combinations of NSAIDs with Antibiotics
- <0.5—synergistic (+++);
- 0.5 to 2—additive (++);
- 2 to 4—indifferent (+);
- >4—antagonistic (–).
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Santajit, S.; Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worthington, R.J.; Melander, C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013, 31, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, A.; Saavedra, M.J.; Simões, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef]
- Borges, A.; Abreu, A.; Malheiro, M.; Saavedra, M.J.; Simões, M. Biofilm prevention and control by dietary phytochemicals. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education, 2013 ed.; Microbiology Book Series; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; Volume 1, pp. 32–41. [Google Scholar]
- Meireles, A.; Borges, A.; Giaouris, E.; Simões, M. The current knowledge on the application of anti-biofilm enzymes in the food industry. Food Res. Int. 2016, 86, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Patel, R. Biofilms and antimicrobial resistance. Clin. Orthop. Relat. Res. 2005, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Abreu, A.C.; Serra, S.; Borges, A.; Saavedra, M.J.; Salgado, A.J.; Simões, M. Evaluation of the best method to assess antibiotic potentiation by phytochemicals against Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2014, 79, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Lima-E-Silva, A.; Silva, P.M. Non-antibiotic compounds: The activity of the NSAID diclofenac on bacteria—A review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 340–351. [Google Scholar] [CrossRef]
- Baptista, J.; Simões, M.; Borges, A. Effect of plant-based catecholic molecules on the prevention and eradication of Escherichia coli biofilms: A structure activity relationship study. Int. Biodeterior. Biodegrad. 2019, 141, 101–113. [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D.; Sutherland, A.D. New strategies for combating multidrug-resistant bacteria. Trends Mol. Med. 2007, 13, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Bin Zaman, S.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A review on antibiotic resistance: Alarm bells are ringing. Cureus 2017, 9, 1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aminov, R. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conly, J.; Johnston, B. Where are all the new antibiotics? The new antibiotic paradox. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 159–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dheman, N.; Mahoney, N.; Cox, E.M.; Farley, J.J.; Amini, T.; Lanthier, M.L. An analysis of antibacterial drug development trends in the US, 1980–2019. Clin. Infect. Dis. Oxford Acad. 2020. Available online: https://academic.oup.com/cid/article/doi/10.1093/cid/ciaa859/5862690 (accessed on 21 July 2020). [CrossRef]
- Takahashi, Y.; Tatsuma, T. Metal oxides and hydroxides as rechargeable materials for photocatalysts with oxidative energy storage abilities. Electrochemistry 2014, 82, 749–751. [Google Scholar] [CrossRef] [Green Version]
- Vanhaelen, Q.; Mamoshina, P.; Aliper, A.M.; Artemov, A.; Lezhnina, K.; Ozerov, I.; Labat, I.; Zhavoronkov, A. Design of efficient computational workflows for in silico drug repurposing. Drug Discov. Today 2017, 22, 210–222. [Google Scholar] [CrossRef]
- Park, K. A review of computational drug repurposing. Transl. Clin. Pharmacol. 2019, 27, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Baquero, F.; Coque, T.M.; De La Cruz, F. Ecology and evolution as targets: The need for novel eco-evo drugs and strategies to fight antibiotic resistance. Antimicrob. Agents Chemother. 2011, 55, 3649–3660. [Google Scholar] [CrossRef] [Green Version]
- Ventola, C.L. The antibiotic resistance crisis: Part 2: Management strategies and new agents. Pharm. Ther. 2015, 40, 344–352. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4422635/ (accessed on 6 July 2020).
- Yan, Q. Translational bioinformatics methods for drug discovery and development. Transl. Bioinforma. Syst. Biol. Methods Pers. Med. 2017, 97–110. [Google Scholar] [CrossRef]
- Corsello, S.M.; Bittker, J.A.; Liu, Z.; Gould, J.; McCarren, P.; Hirschman, J.E.; Johnston, S.E.; Vrcic, A.; Wong, B.; Khan, M.; et al. The drug repurposing hub: A next-generation drug library and information resource. Nat. Med. 2017, 23, 405–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.J.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2018, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Kaul, G.; Shukla, M.; Dasgupta, A.; Chopra, S. Update on drug-repurposing: Is it useful for tackling antimicrobial resistance? Futur. Microbiol. 2019, 14, 829–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, P.; Curtis, N. Antimicrobial effects of antipyretics. Antimicrob. Agents Chemother. 2017, 61, e02268-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, E.W.L.; Yee, Z.Y.; Raja, I.; Yap, J.K.Y. Synergistic effect of non-steroidal anti-inflammatory drugs (NSAIDs) on antibacterial activity of cefuroxime and chloramphenicol against methicillin-resistant Staphylococcus aureus. J. Glob. Antimicrob. Resist. 2017, 10, 70–74. [Google Scholar] [CrossRef]
- Abdul-Hussein, Z.R.; Zainab, R. Antibacterial Effect of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Antibacterial Effect of Non-Steroidal. 2014. Available online: https://www.iasj.net/iasj?func=fulltext&aId=98815 (accessed on 4 January 2019).
- Umaru, T.; Nwamba, C.O.; Kolo, I.; Nwodo, U.U. Antimicrobial activity of non-steroidal anti-inflammatory drugs with respect to immunological response: Diclofenac sodium as a case study. Afr. J. Biotechnol. 2009, 8, 7332–7339. [Google Scholar]
- Shirin, H.; Kancherla, S.; Kancherla, K.; Holt, P.R.; Sordillo, E.M.; Moss, S.F.; Weinstein, I.B. Non-steroidal anti-inflammatory drugs have bacteriostatic and bactericidal activity against Helicobacter pylori. J. Gastroenterol. Hepatol. 2006, 21, 1388–1393. [Google Scholar] [CrossRef]
- Oliveira, I.M.; Borges, A.; Borges, F.; Simões, M. Repurposing ibuprofen to control Staphylococcus aureus biofilms. Eur. J. Med. Chem. 2019, 166, 197–205. [Google Scholar] [CrossRef]
- Crofford, L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013, 15, S2. [Google Scholar] [CrossRef] [Green Version]
- Rao, P.; Knaus, E.E. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond. J. Pharm. Pharm. Sci. 2008, 11, 81s–110s. [Google Scholar] [CrossRef] [Green Version]
- Mazumdar, K.; Dastidar, S.G.; Park, J.H.; Dutta, N.K. The anti-inflammatory non-antibiotic helper compound diclofenac: An antibacterial drug target. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wang, Y.; Whittell, L.R.; Jergic, S.; Liu, M.; Harry, E.J.; Dixon, N.E.; Kelso, M.; Beck, J.L.; Oakley, A.J. DNA replication is the target for the antibacterial effects of nonsteroidal anti-inflammatory drugs. Chem. Biol. 2014, 21, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohsen, A.; Gomaa, A.; Mohamed, F.; Ragab, R.; Eid, M.; Ahmed, A.-H.; Khalaf, A.; Kamal, M.; Mokhtar, S.; Mohamed, H.; et al. Antibacterial, anti-biofilm activity of some non-steroidal anti-inflammatory drugs and n-acetyl cysteine against some biofilm producing uropathogens. Am. J. Epidemiol. Infect. Dis. 2015, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Davies, N.M.; Reynolds, J.K.; Undeberg, M.R.; Gates, B.J.; Ohgami, Y.; Vega-Villa, K.R. Minimizing risks of NSAIDs: Cardiovascular, gastrointestinal and renal. Expert Rev. Neurother. 2006, 6, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- eMC. FELDENE—Summary of Product Characteristics (SmPC). 2020. Available online: https://www.medicines.org.uk/emc/product/2901/smpc (accessed on 31 July 2020).
- Garg, S.K. Development Characterization and Invivo Study of Floating Tablet of Aceclofenac. INFLIBNET, 2014. Available online: https://shodhganga.inflibnet.ac.in/handle/10603/28939 (accessed on 9 June 2019).
- Medicines Agency. Information for the Package Leaflet Regarding Lactose Used as an Excipient in Medicinal Products for Human Use. 2018. Available online: www.ema.europa.eu/contact (accessed on 9 June 2019).
- Abbas, H.A.; Gad, A.I. Eradication of biofilms formed by bacteria isolated from diabetic foot infections by potential antibiofilm agents alone and in combination with ciprofloxacin. Afr. J. Microbiol. Res. 2014, 8, 3882–3892. [Google Scholar] [CrossRef]
- Laudy, A.E.; Mrówka, A.; Krajewska, J.; Tyski, S. The influence of efflux pump inhibitors on the activity of non-antibiotic NSAIDS against gram-negative rods. PLoS ONE 2016, 11, e0147131. [Google Scholar] [CrossRef]
- Patel, J.B.; Franklin, R.; Cockerill, I. M100-S25 Performance Standards for Antimicrobial Susceptibility Testing. Clin. Lab. Stan. Inst. 2015, 35, 44–49. [Google Scholar]
- Wootton, M. BSAC Methods for Antimicrobial Susceptibility Testing. 2013. Available online: http://bsac.org.uk/wp-content/uploads/2012/02/Version-12-Apr-2013_final.pdf (accessed on 12 June 2019).
- Fini, A.; Bassini, G.; Monastero, A.; Cavallari, C. Diclofenac salts, VIII. Effect of the counterions on the permeation through porcine membrane from aqueous saturated solutions. Pharmaceutics 2012, 4, 413–429. [Google Scholar] [CrossRef]
- Tocris Bioscience. Diclofenac Sodium Salt Supplier CAS 15307-79-6. Available online: https://www.tocris.com/products/diclofenac-sodium-salt_4454 (accessed on 18 August 2020).
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malheiro, J.F.; Maillard, J.Y.; Borges, F.; Simões, M. Biocide potentiation using cinnamic phytochemicals and derivatives. Molecules 2019, 24, 3918. [Google Scholar] [CrossRef] [Green Version]
- Abbas, H.A. Inhibition of virulence factors of Pseudomonas aeruginosa by diclofenac sodium. Roum. Arch. Microbiol. Immunol. 2016, 74, 79–85. [Google Scholar]
- El-Mowafy, S.A.; El Galil, K.H.; El-Messery, S.M.; Shaaban, M.I. Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microb. Pathog. 2014, 74, 25–32. [Google Scholar] [CrossRef]
- Cramton, S.E.; Gerke, C.; Schnell, N.F.; Nichols, W.W.; Goötz, F. The Intercellular Adhesion (ica) Locus Is Present in Staphylococcus aureus and Is Required for Biofilm Formation. Infect. Immun. 1999, 67, 5427–5433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Baky, R.M.A.; Sherein, G.E.G.; El-Gendy, S.G. Effect of non-steroidal anti-inflammatory drugs and dexamethazone on the biofilm formation and expression of some adhesion-related genes of Candida albicans and Staphylococcus aureus. Afr. J. Microbiol. Res. 2016, 10, 694–707. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.F.R.; El-baky, M.A.; Ahmed, A.B.F.; Waly, N.G.; Gad, G.F.M. Antibacterial activity of some non-steroidal anti-inflammatory drugs against bacteria causing urinary tract infection. Am. J. Infect. Dis. Microbiol. 2017, 5, 66–73. [Google Scholar] [CrossRef]
- Saavedra, M.J.; Borges, A.; Dias, C.; Aires, A.; Bennett, R.N.; Rosa, E.A.; Simões, M. Antimicrobial activity of phenolics and glucosinolate hydrolysis products and their synergy with streptomycin against pathogenic bacteria. Med. Chem. 2010, 6, 174–183. [Google Scholar] [CrossRef]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus. Evid. Based Complement. Altern. Med. 2015, 2015, 795435. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.; Simões, L.C.; Saavedra, M.J.; Simões, M. The action of selected isothiocyanates on bacterial biofilm prevention and control. Int. Biodeterior. Biodegrad. 2014, 86, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Borges, A.; Lopez-Romero, J.; Oliveira, D.; Giaouris, E.; Simões, M. Prevention, removal and inactivation of Escherichia coli and Staphylococcus aureus biofilms using selected monoterpenes of essential oils. J. Appl. Microbiol. 2017, 123, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Lemos, M.; Borges, A.; Teodósio, J.; Araújo, P.A.; Mergulhão, F.J.; Melo, L.; Simões, M. The effects of ferulic and salicylic acids on Bacillus cereus and Pseudomonas fluorescens single- and dual-species biofilms. Int. Biodeterior. Biodegrad. 2014, 86, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Salutas Pharma GmbH. Information Leaflet: Diclofenac Sodium; INFARMED, 2014. Available online: https://extranet.infarmed.pt/INFOMED-fo/pesquisa-avancada.xhtml (accessed on 31 July 2020).
- Bayer Bitterfeld GmbH. Information Leaflet: Acetylsalicylic Acid; INFARMED, 2008. Available online: https://extranet.infarmed.pt/INFOMED-fo/pesquisa-avancada.xhtml (accessed on 31 July 2020).
- Laboratorios CINFA. Information Leaflet: Piroxicam. INFARMED, 2008. Available online: http://app7.infarmed.pt/infomed/download_ficheiro.php?med_id=37208&tipo_doc=fi (accessed on 31 July 2020).
- McPherson, M.L.; Cimino, N.M. Topical NSAID formulations. Pain Med. 2013, 14, S35–S39. [Google Scholar] [CrossRef]
- Møiniche, S.; Dahl, J.; Kehlet, H. Short-term topical piroxicam has no anti-inflammatory or antinociceptive effects after burn injury. Curr. Ther. Res. 1993, 53, 466–472. [Google Scholar] [CrossRef]
- Campione, E.; Diluvio, L.; Paternò, E.J.; Chimenti, S. Topical treatment of actinic keratoses with piroxicam 1% gel. Am. J. Clin. Dermatol. 2010, 11, 45–50. [Google Scholar] [CrossRef]
- Van den Ouweland, F.A.; Eenhoorn, P.C.; Tan, Y.; Gribnau, F.W.J. Transcutaneous absorption of naproxen gel. Eur. J. Clin. Pharmacol. 1989, 36, 209–211. [Google Scholar] [CrossRef]
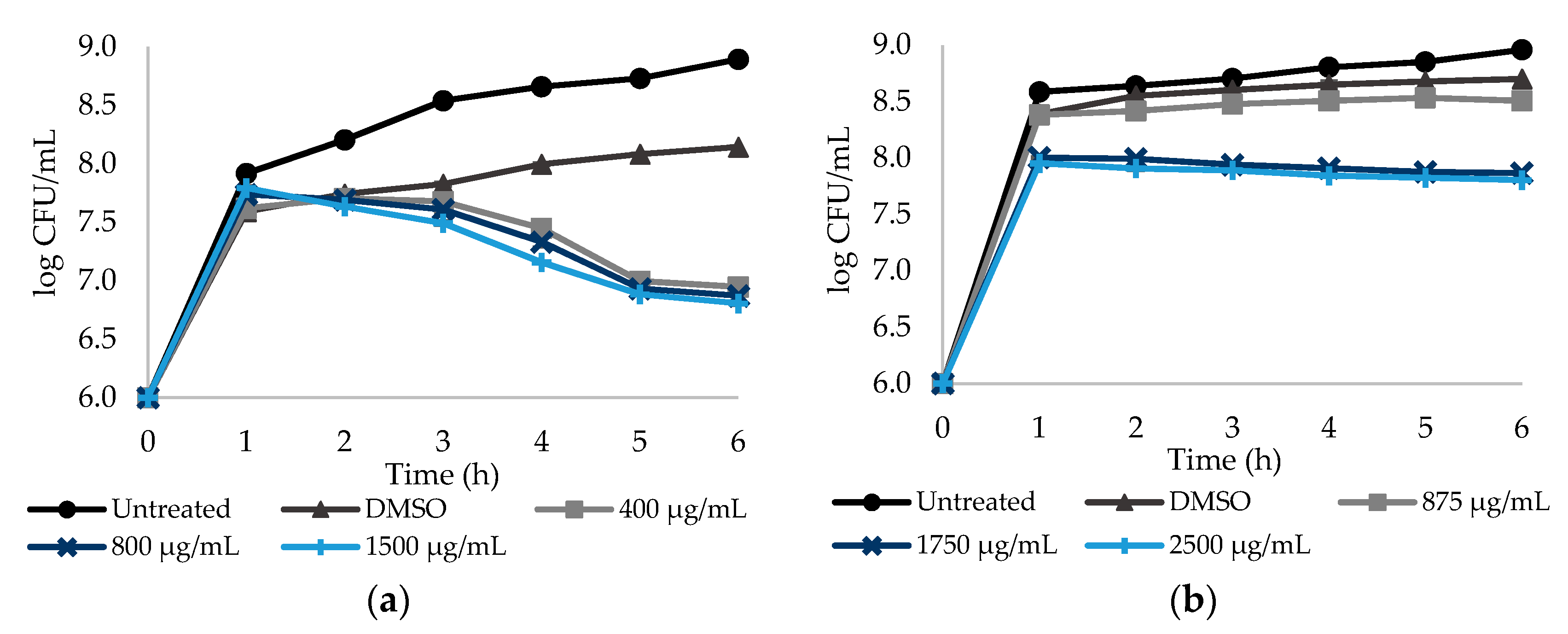
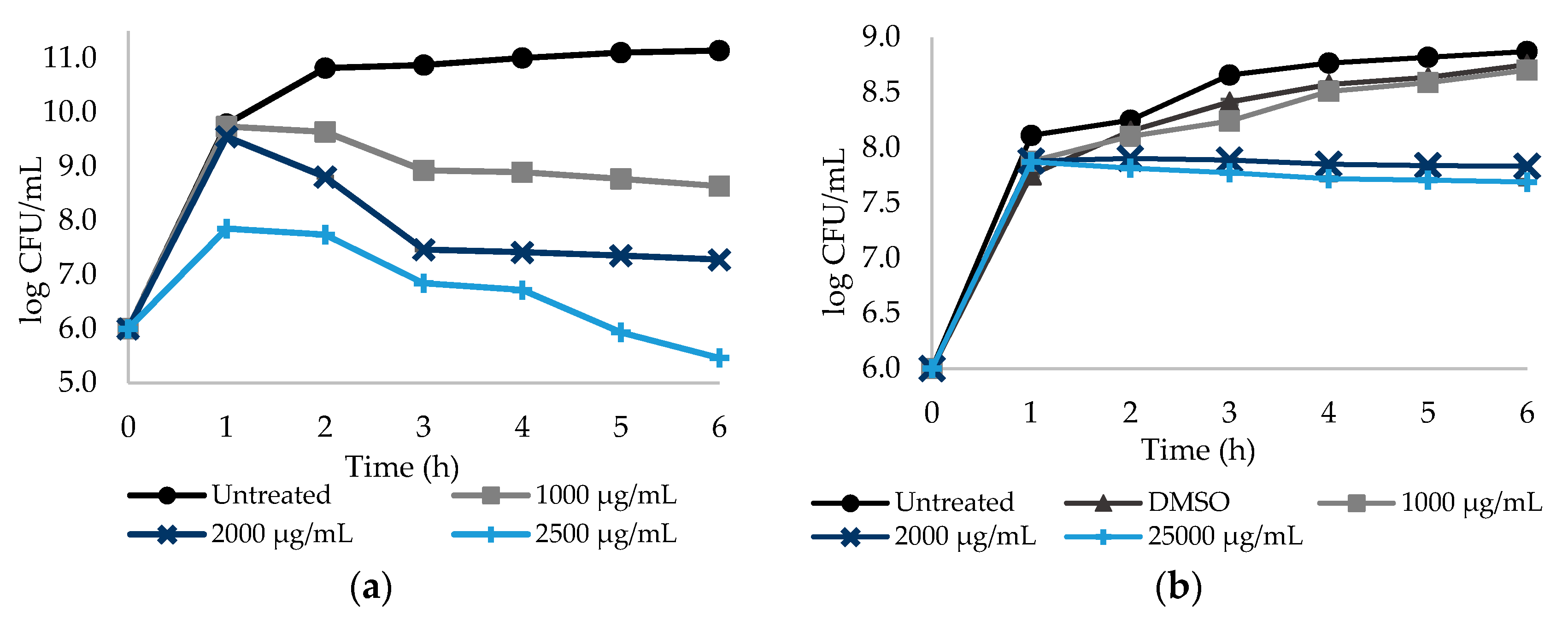
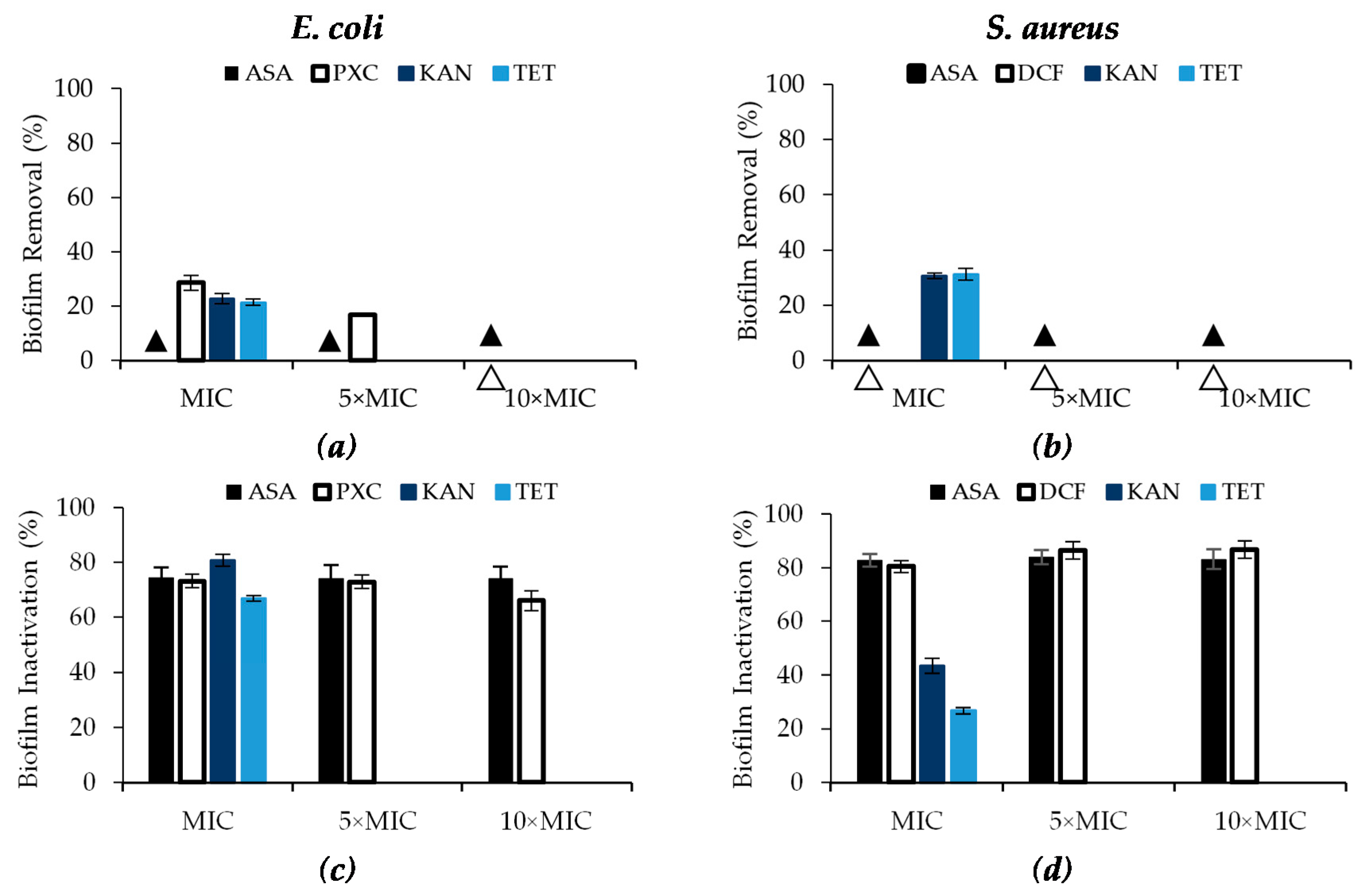

| NSAIDs | Bacteria | MIC (μg/mL) |
|---|---|---|
| PXC | E. coli | 800 ± 0 * |
| S. aureus | >2000 | |
| DCF | E. coli | >2000 |
| S. aureus | 2000 ± 0 | |
| ASA | E. coli | 1750 ± 0 |
| S. aureus | 2000 ± 0 | |
| NPX | E. coli | >2000 |
| S. aureus | >2000 |
| Bacterial Strain | Antibiotic | IZD (mm) |
|---|---|---|
| E. coli CECT 434 | CIP5μg/disc | 38.8 ± 3.1 |
| TET30μg/disc | 26.5 ± 1.2 | |
| STR10μg/disc | 39.7 ± 3.3 | |
| KAN30μg/disc | 29.8 ± 2.1 | |
| S. aureus CECT 976 | CIP5μg/disc | 43.2 ± 2.1 |
| TET30μg/disc | 37.3 ± 1.4 | |
| STR10μg/disc | 43.7 ± 0.0 | |
| KAN30μg/disc | 28.5 ± 0.2 |
| Antibiotic | MIC/MBC (μg/mL) | E. coli | S. aureus |
|---|---|---|---|
| KAN | MIC | 24 | 3 |
| MBC | 24 | 24 | |
| TET | MIC | 6 | 4 |
| MBC | 64 | 48 |
| Bacterial Strain | NSAID/Antibiotic | Concentration (μg/mL) | Biofilm Removal | Biofilm Inactivation | Biofilm Culturability |
|---|---|---|---|---|---|
| E. coli | PXC + KAN | MIC + MIC | + | + | ++ |
| 5MIC + MIC | + | + | ++ | ||
| 10MIC + MIC | ++ | + | ++ | ||
| PXC + TET | MIC + MIC | - | ++ | ++ | |
| 5MIC + MIC | + | ++ | ++ | ||
| 10MIC + MIC | ++ | ++ | ++ | ||
| ASA + KAN | MIC + MIC | ++ | ++ | + | |
| 5MIC + MIC | ++ | ++ | ++ | ||
| 10MIC + MIC | ++ | ++ | ++ | ||
| ASA + TET | MIC + MIC | ++ | ++ | ++ | |
| 5MIC + MIC | ++ | ++ | ++ | ||
| 10MIC + MIC | ++ | ++ | ++ | ||
| S. aureus | DCF + KAN | MIC + MIC | + | ++ | + |
| 5MIC + MIC | ++ | ++ | + | ||
| 10MIC + MIC | ++ | ++ | ++ | ||
| DCF + TET | MIC + MIC | ++ | ++ | ++ | |
| 5MIC + MIC | ++ | ++ | + | ||
| 10MIC + MIC | ++ | ++ | ++ | ||
| ASA + KAN | MIC + MIC | ++ | ++ | + | |
| 5MIC + MIC | ++ | ++ | ++ | ||
| 10MIC + MIC | ++ | ++ | ++ | ||
| ASA + TET | MIC + MIC | ++ | ++ | ++ | |
| 5MIC + MIC | ++ | ++ | ++ | ||
| 10MIC + MIC | ++ | ++ | ++ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leão, C.; Borges, A.; Simões, M. NSAIDs as a Drug Repurposing Strategy for Biofilm Control. Antibiotics 2020, 9, 591. https://doi.org/10.3390/antibiotics9090591
Leão C, Borges A, Simões M. NSAIDs as a Drug Repurposing Strategy for Biofilm Control. Antibiotics. 2020; 9(9):591. https://doi.org/10.3390/antibiotics9090591
Chicago/Turabian StyleLeão, Cláudia, Anabela Borges, and Manuel Simões. 2020. "NSAIDs as a Drug Repurposing Strategy for Biofilm Control" Antibiotics 9, no. 9: 591. https://doi.org/10.3390/antibiotics9090591
APA StyleLeão, C., Borges, A., & Simões, M. (2020). NSAIDs as a Drug Repurposing Strategy for Biofilm Control. Antibiotics, 9(9), 591. https://doi.org/10.3390/antibiotics9090591







