Antimicrobial Lipids from Plants and Marine Organisms: An Overview of the Current State-of-the-Art and Future Prospects
Abstract
1. Introduction
1.1. Synergistic Effects between Natural Products and Antibiotics
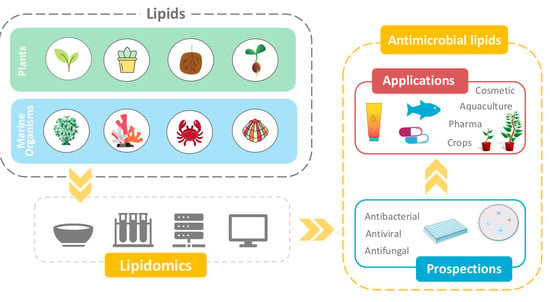
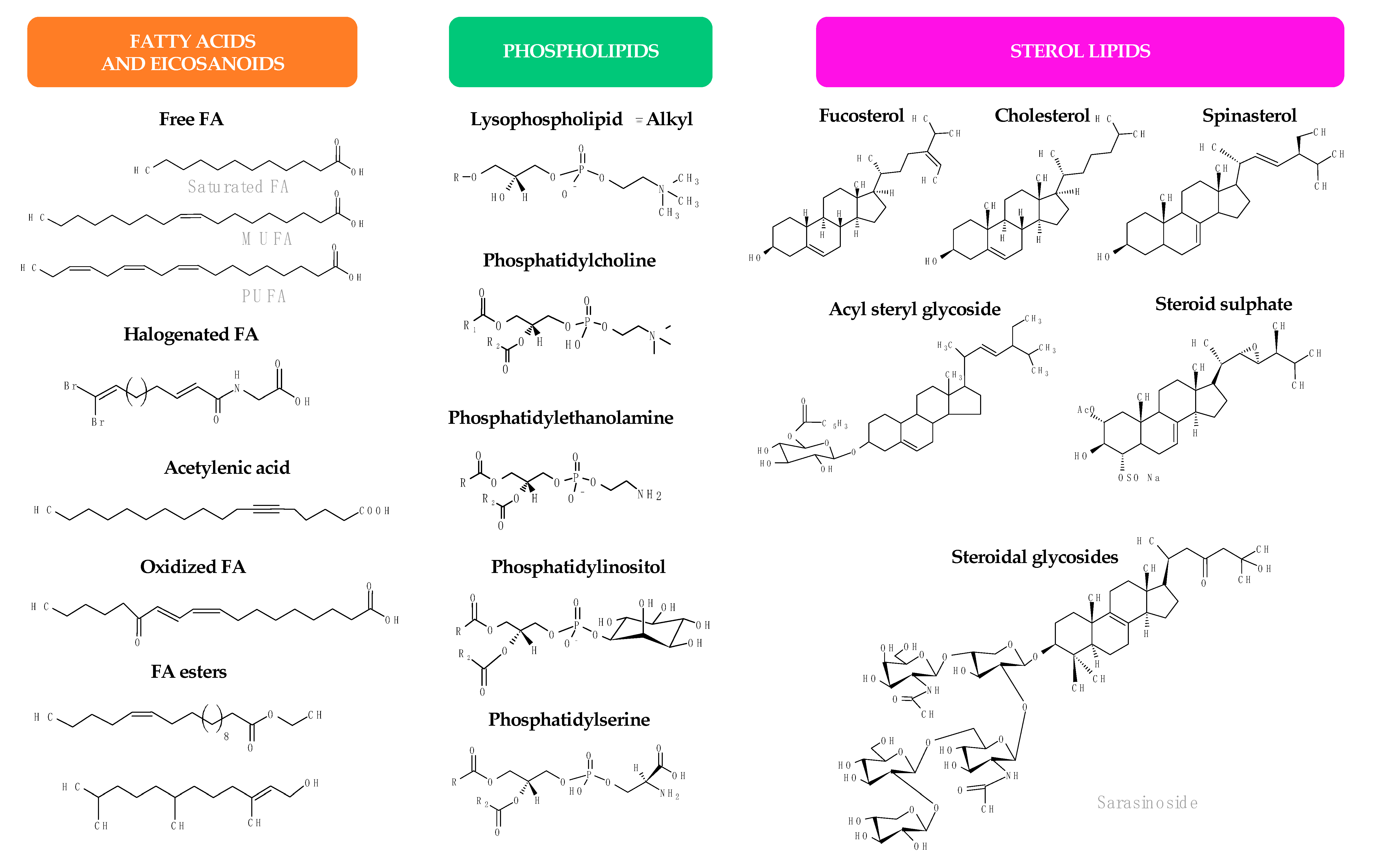
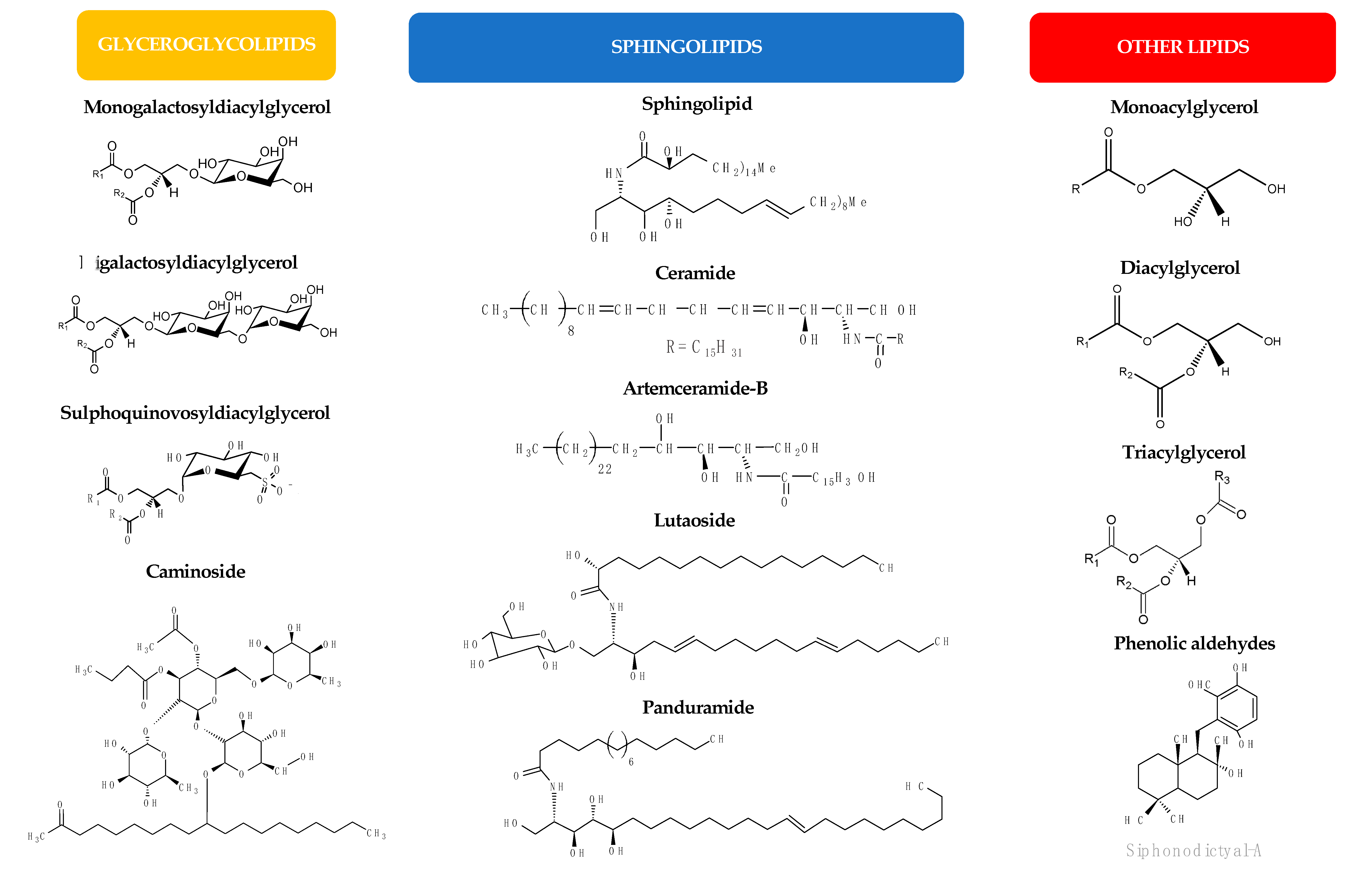
1.2. Antimicrobial Lipids
1.3. Aim of the Study
2. Antimicrobial Lipids from Plants
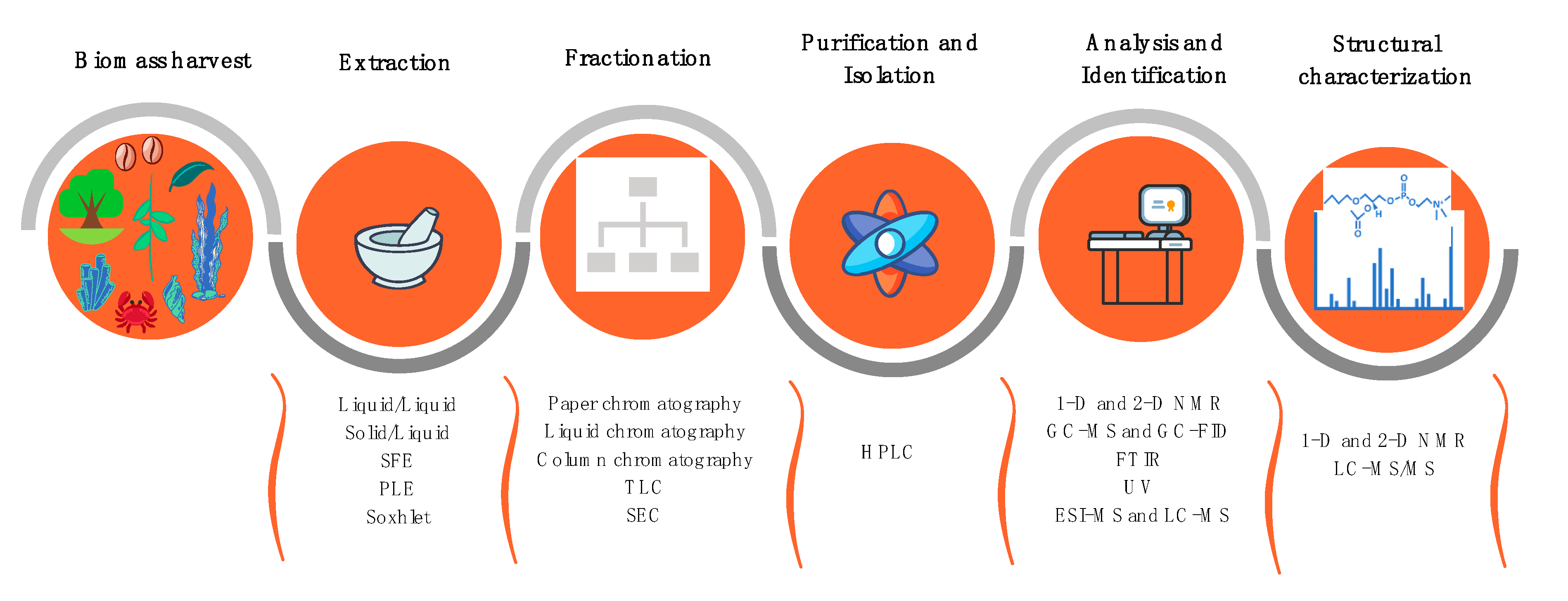
2.1. Extraction and Isolation of Plant Lipids
2.2. Susceptibility Testing, Inhibitory, and Microbicidal Activities of Plant Lipids
3. Antimicrobial Lipids from Marine Organisms
3.1. Marine Algae
3.1.1. Fatty Acids
3.1.2. Glycolipids
3.1.3. Other Lipids
3.1.4. Photosynthetic Pigments
3.2. Marine Invertebrates
3.2.1. Fatty Acids
3.2.2. Sterols
3.2.3. Polar Lipids
3.2.4. Other Lipids
3.2.5. Pigments
4. Cytotoxicity of Natural Antimicrobial Lipids against Mammalian Cells
5. Lipidomics for the Analysis of Bioactive Lipids in Plants and in Marine Organisms
6. Prospection and Applications of Antimicrobial Lipids
6.1. Cosmetic and Drug Formulations
6.2. Food Additives
6.3. Herbicides and Pesticides
6.4. Aquaculture
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMP | Antimicrobial peptide |
| AMR | Antimicrobial resistance |
| BuOH | Butanol |
| CC | Cytotoxic concentration |
| CH2Cl2 | Dichloromethane |
| CHCl3 | Chloroform |
| CLSI | Clinical and Laboratory Standards Institute (formerly NCCLS) |
| COSY | Correlation spectroscopy |
| CRE | Carbapenemase-resistant Enterobacteriaceae |
| DAG | Diacylglycerol |
| DGDG | Digalactosyldiacylglycerol |
| DOX | Dioxygenase |
| DPA | Docosapentaenoic |
| EC | Effective concentration |
| EPA | Eicosapentaenoic acid |
| ESBL | Extended-spectrum beta-lactamase |
| ESI-MS | Electrospray ionization-mass spectrometry |
| ESI-MS/MS | Electrospray ionization-tandem mass spectrometry |
| ETA | Eicosatetraenoic acid |
| Et2O | Diethyl ether |
| EtOAc | Ethyl acetate |
| EtOH | Ethanol |
| FA | Fatty acid |
| FAB-MS | Fast atom bombardment-mass spectrometry |
| FAME | Fatty acid methyl ester |
| FIA-MS | Flow-injection analysis-mass spectrometry |
| FT-NMR | Fourier transformed-nuclear magnetic resonance |
| G(-) | Gram-negative |
| G(+) | Gram-positive |
| GC | Gas-phase chromatography |
| GC-FID | Gas-phase chromatography-flame ionization detector |
| GC-MS | Gas-phase chromatography-mass spectrometry |
| GlCer | Glucosylceramide |
| GRAS | Generally recognized as safe |
| HMBC | Heteronuclear multiple bond correlation |
| HPLC | High-performance liquid chromatography |
| HPLC-DAD | High-performance liquid chromatography-diode-array detector |
| HPLC-ELSD | High-performance liquid chromatography-evaporative light scattering detector |
| HPLC-ESI-MS | High-performance liquid chromatography-electrospray ionization-mass spectrometry |
| HPLC-UV-HRMS | High-performance liquid chromatography-ultraviolet-high-resolution mass spectrometry |
| HPTLC | High-performance thin-layer chromatography |
| HR-APCI-MS | High-resolution atmospheric pressure chemical ionization-mass spectrometry |
| HR-EI-MS | High-resolution-electron ionization-mass spectrometry |
| HR-FAB-MS | High-resolution fast atom bombardment-mass spectrometry |
| HRMS | High resolution mass spectrometry |
| HSQC | Heteronuclear single quantum coherence |
| HSV | Herpes simplex virus |
| HSV-1 | Herpes simplex virus type 1 |
| HSV-2 | Herpes simplex virus type 2 |
| IC50 | Half maximal inhibitory concentration |
| IMTA | Integrated multi-trophic aquaculture |
| IR | Infrared |
| IZ | Inhibition zone |
| LC | Liquid chromatography |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| LC50 | Lethal concentration (ppm) at 50% |
| LOX | Lipoxygenase |
| LPC | Lysophosphatidylcholine |
| MAG | Monoacylglycerol |
| MBC | Minimum bactericidal concentration |
| MDR | Multi-drug resistant |
| MeOH | Methanol |
| MFC | Minimum fungicidal concentration |
| MGDG | Monogalactosyldiacylglycerol |
| MIC | Minimum inhibitory concentration |
| MIQ | Minimum inhibition quantity |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSSA | Methicillin-sensitive Staphylococcus aureus |
| MUFA | Monounsaturated fatty acid |
| NCCLS | National Committee for Clinical Laboratory Standards |
| NMR | Nuclear magnetic resonance |
| NOESY | Nuclear Overhauser effect spectroscopy |
| PACT | Photodynamic antimicrobial chemotherapy |
| PAF | Platelet-activating factor |
| PC-O | Phosphatidylcholine ether |
| PE | Phosphatidylethanolamine |
| PFA | Paraformaldehyde |
| PI | Phosphatidylinositol |
| ppm | Parts per million |
| PrOH | Propanol |
| PS | Phosphatidylserine |
| PUFA | Polyunsaturated fatty acid |
| QSI | Quorum sensing inhibitor |
| RP | Reversed-phase |
| SEC | Size exclusion chromatography |
| SFE | Supercritical fluid extraction |
| SI | Selectivity index |
| SiO2 | Silica gel |
| SM | Sphingomyelin |
| SPE | Solid-phase extraction |
| SQDG | Sulfoquinovosyldiacylglycerol |
| TI | Therapeutic index |
| TLC | Thin-layer chromatography |
| UFA | Unsaturated fatty acid |
| VII | Viral inhibition index |
| WHO | World Health Organization |
References
- Mikulic, M. Total Consumption of Antibiotics in WHO Countries in 2016 (In Metric Tons). Available online: https://www.statista.com/statistics/949926/consumed-amount-of-antibiotics-in-who-countries/ (accessed on 20 February 2020).
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 3 March 2020).
- World Health Organization. New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis. Available online: https://www.who.int/news-room/detail/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 4 March 2020).
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (accessed on 3 March 2020).
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Hogberg, L.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.; Colomb-Cotinat, M.; Kretzschmar, M.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- McCarthy, N. Antibiotic Resistance—Superbugs Kill 33,000 Europeans Every Year. Available online: https://www.statista.com/chart/16012/median-number-of-deaths-due-to-antibiotic-resistance-bacteria/ (accessed on 3 March 2020).
- World Health Organization. No Time to Wait: Securing the Future from Drug-Resistant Infections. Report to the Secretary-General of the United Nations. Available online: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/final-report/en/ (accessed on 4 March 2020).
- World Health Organization. Lack of New Antibiotics Threatens Global Efforts to Contain Drug-Resistant Infections. Available online: https://www.who.int/news-room/detail/17-01-2020-lack-of-new-antibiotics-threatens-global-efforts-to-contain-drug-resistant-infections (accessed on 3 March 2020).
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, A.; Tome, J.; Almeida, A. An insight on bacterial cellular targets of photodynamic inactivation. Future Med. Chem. 2014, 6, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, A.; Nadais, H.; Almeida, A. Potential applications of porphyrins in photodynamic inactivation beyond the medical scope. J. Photochem. Photobiol. C 2015, 22, 34–57. [Google Scholar] [CrossRef]
- Almeida, A.; Cunha, A.; Gomes, N.C.M.; Alves, E.; Costa, L.; Faustino, M.A.F. Phage therapy and photodynamic therapy: Low environmental impact approaches to inactivate microorganisms in fish farming plants. Mar. Drugs 2009, 7, 268–313. [Google Scholar] [CrossRef]
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to conventional antibiotics in the era of antimicrobial resistance. Trends Microbiol. 2019, 27, 323–338. [Google Scholar] [CrossRef]
- Hughes, C.C.; Fenical, W. Antibacterials from the sea. Chemistry 2010, 16, 12512–12525. [Google Scholar] [CrossRef]
- Gray, D.A.; Wenzel, M. Multitarget approaches against multiresistant superbugs. ACS Infect. Dis. 2020, 6, 1346–1365. [Google Scholar] [CrossRef]
- Smith, V.J.; Desbois, A.P.; Dyrynda, E.A. Conventional and unconventional antimicrobials from fish, marine invertebrates and micro-algae. Mar. Drugs 2010, 8, 1213–1262. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef]
- Hayashi, M.A.; Bizerra, F.C.; Da Silva, P.I. Antimicrobial compounds from natural sources. Front. Microbiol. 2013, 4, 195. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial free fatty acids and monoglycerides: Biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [PubMed]
- McGaw, L.J.; Jäger, A.K.; Van Staden, J. Antibacterial effects of fatty acids and related compounds from plants. S. Afr. J. Bot. 2002, 68, 417–423. [Google Scholar] [CrossRef]
- Bergsson, G.; Hilmarsson, H.; Thormar, H. Chapter 3—Antibacterial, antiviral and antifungal activities of lipids. In Lipids and Essential Oils as Antimicrobial Agents; Thormar, H., Ed.; Wiley: Chichester, UK, 2010; pp. 47–80. [Google Scholar]
- Pasdaran, A.; Hamedi, A. Chapter 14—Natural products as source of new antimicrobial compounds for skin infections. In The Microbiology of Skin, Soft Tissue, Bone and Joint Infections; Kon, K., Rai, M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 223–253. [Google Scholar]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Stefanović, O.D. Synergistic activity of antibiotics and bioactive plant extracts: A study against Gram-positive and Gram-negative bacteria. In Bacterial Pathogenesis and Antibacterial Control; Kırmusaoğlu, S., Ed.; IntechOpen: London, UK, 2018; pp. 23–48. [Google Scholar]
- De Menezes, C.B.A.; Afonso, R.S.; de Souza, W.R.; Parma, M.M.; de Melo, I.S.; Fugita, F.L.S.; Moraes, L.A.B.; Zucchi, T.D.; Fantinatti-Garboggini, F. Williamsia aurantiacus sp. nov. a novel actinobacterium producer of antimicrobial compounds isolated from the marine sponge. Arch. Microbiol. 2019, 201, 691–698. [Google Scholar] [CrossRef]
- Ismail, A.; Ktari, L.; Ahmed, M.; Bolhuis, H.; Boudabbous, A.; Stal, L.J.; Cretoiu, M.S.; El Bour, M. Antimicrobial activities of bacteria associated with the brown alga Padina pavonica. Front. Microbiol. 2016, 7, 1072. [Google Scholar] [CrossRef]
- Haq, A.; Siddiqi, M.; Batool, S.; Islam, A.; Khan, A.; Khan, D.; Khan, S.; Khan, H.; Shah, A.; Hasan, F.; et al. Comprehensive investigation on the synergistic antibacterial activities of Jatropha curcas pressed cake and seed oil in combination with antibiotics. AMB Express 2019, 9, 67. [Google Scholar] [CrossRef]
- Desbois, A.P.; Lawlor, K.C. Antibacterial activity of long-chain polyunsaturated fatty acids against Propionibacterium acnes and Staphylococcus aureus. Mar. Drugs 2013, 11, 4544–4557. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeong, M.R.; Choi, S.M.; Na, S.S.; Cha, J.D. Synergistic effect of fucoidan with antibiotics against oral pathogenic bacteria. Arch. Oral Biol. 2013, 58, 482–492. [Google Scholar] [CrossRef]
- Kawabata, S.; Nagayama, R.; Hirata, M.; Shigenaga, T.; Agarwala, K.L.; Saito, T.; Cho, J.; Nakajima, H.; Takagi, T.; Iwanaga, S. Tachycitin, a small granular component in horseshoe crab hemocytes, is an antimicrobial protein with chitin-binding activity. J. Biochem. 1996, 120, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Eom, S.; Lee, E.; Jung, Y.; Kim, H.; Jo, M.; Son, K.; Lee, H.; Kim, J.; Lee, M.; et al. In vitro antibacterial and synergistic effect of phlorotannins isolated from edible brown seaweed Eisenia bicyclis against acne-related bacteria. Algae 2014, 29, 47–55. [Google Scholar] [CrossRef]
- He, X.; Hwang, H.M.; Aker, W.G.; Wang, P.; Lin, Y.; Jiang, X. Synergistic combination of marine oligosaccharides and azithromycin against Pseudomonas aeruginosa. Microbiol. Res. 2014, 169, 759–767. [Google Scholar] [CrossRef]
- Ebada, S.S.; Lin, W.; Proksch, P. Bioactive sesterterpenes and triterpenes from marine sponges: Occurrence and pharmacological significance. Mar. Drugs 2010, 8, 313–346. [Google Scholar] [CrossRef]
- Pohl, C.H.; Kock, J.L.; Thibane, V.S. Antifungal free fatty acids: A review. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendéz-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; pp. 61–71. [Google Scholar]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.; Epand, R. Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochim. Biophys. Acta 2009, 1788, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Jacob, M.R.; ElSohly, H.N.; Nagle, D.G.; Smillie, T.J.; Walker, L.A.; Clark, A.M. Acetylenic acids inhibiting azole-resistant Candida albicans from Pentagonia gigantifolia. J. Nat. Prod. 2003, 66, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Ells, R.; Kock, J.L.; Van Wyk, P.W.; Botes, P.J.; Pohl, C.H. Arachidonic acid increases antifungal susceptibility of Candida albicans and Candida dubliniensis. J. Antimicrob. Chemother. 2009, 63, 124–128. [Google Scholar] [CrossRef]
- Sjögren, J.; Magnusson, J.; Broberg, A.; Schnürer, J.; Kenne, L. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl. Environ. Microbiol. 2003, 69, 7554–7557. [Google Scholar] [CrossRef]
- Dilika, F.; Bremner, P.; Meyer, J. Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia 2000, 71, 450–452. [Google Scholar] [CrossRef]
- Sun, C.; O’Connor, C.; Roberton, A. Antibacterial actions of fatty acids and monoglycerides against Helicobacter pylori. FEMS Immunol. Med. Microbiol. 2003, 36, 9–17. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Tiku, A.R. Antimicrobial compounds (phytoanticipins and phytoalexins) and their role in plant defense. In Co-Evolution of Secondary Metabolites. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 845–868. [Google Scholar]
- Prost, I.; Dhondt, S.; Rothe, G.; Vicente, J.; Rodriguez, M.J.; Kift, N.; Carbonne, F.; Griffiths, G.; Esquerré-Tugayé, M.T.; Rosahl, S.; et al. Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiol. 2005, 139, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Blee, E. Impact of phyto-oxylipins in plant defense. Trends Plant. Sci. 2002, 7, 315–321. [Google Scholar] [CrossRef]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant. Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Deboever, E.; Deleu, M.; Mongrand, S.; Lins, L.; Fauconnier, M.L. Plant-pathogen interactions: Underestimated roles of phyto-oxylipins. Trends Plant. Sci. 2020, 25, 22–34. [Google Scholar] [CrossRef]
- D’Oca, C.; Coelho, T.; Marinho, T.; Hack, C.; Duarte, R.; da Silva, P.; D’Oca, M. Synthesis and antituberculosis activity of new fatty acid amides. Bioorg. Med. Chem. Lett. 2010, 20, 5255–5257. [Google Scholar] [CrossRef]
- Dembitsky, V.; Shkrob, I.; Rozentsvet, O. Fatty acid amides from freshwater green alga Rhizoclonium hieroglyphicum. Phytochemistry 2000, 54, 965–967. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Srinivas, V.; Alekhya, G.; Prakash, B.; Kudapa, H.; Varshney, R. Evaluation of broad-spectrum Streptomyces sp. for plant growth promotion traits in chickpea (Cicer arietinum L.). Philipp. Agric. Sci. 2015, 98, 270–278. [Google Scholar]
- Tanvir, R.; Javeed, A.; Rehman, Y. Fatty acids and their amide derivatives from endophytes: New therapeutic possibilities from a hidden source. FEMS Microbiol. Lett. 2018, 365. [Google Scholar] [CrossRef]
- Gunstone, F.D.; Harwood, J.L. Occurrence and characterisation of oils and fats. In The Lipid Handbook with CD-ROM, 3rd ed.; Gunstone, F.D., Harwood, J.L., Dijkstra, A.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 51–156. [Google Scholar]
- Fafal, T.; Yilmaz, F.F.; Birincioğlu, S.S.; Hoşgör-Limoncu, M.; Kivçak, B. Fatty acid composition and antimicrobial activity of Asphodelus aestivus seeds. Hum. Vet. Med. 2016, 8, 103–107. [Google Scholar]
- Shukla, S.; Hegde, S.; Kumar, A.; Chaudhary, G.; Tewari, K.; Upreti, D.; Pal, M. Fatty acid composition and antibacterial potential of Cassia tora (leaves and stem) collected from different geographic areas of India. J. Food Drug Anal. 2018, 26, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Sati, A.; Sati, S.; Sati, N.; Sati, O. Chemical composition and antimicrobial activity of fatty acid methyl ester of Quercus leucotrichophora fruits. Nat. Prod. Res. 2017, 31, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Abdelillah, A.; Houcine, B.; Halima, D.; Meriel, C.S.; Imane, Z.; Eddine, S.D.; Abdallah, M.; Daoudi, C.S. Evaluation of antifungal activity of free fatty acids methyl esters fraction isolated from Algerian Linum usitatissimum L. seeds against toxigenic Aspergillus. Asian Pac. J. Trop. Biomed. 2013, 3, 443–448. [Google Scholar] [CrossRef]
- Nascimento, G.; Souza, D.; Santos, A.; Batista, J.; Rathinasabapathi, B.; Gagliardi, P.; Goncalves, J. Lipidomic profiles from seed oil of Carapa guianensis Aubl. and Carapa vasquezii Kenfack and implications for the control of phytopathogenic fungi. Ind. Crops Prod. 2019, 129, 67–73. [Google Scholar] [CrossRef]
- Sasmakov, S.; Gazizov, F.; Putieva, Z.; Wende, K.; Alresly, Z.; Lindequist, U. Neutral lipids, phospholipids, and biological activity of extracts from Zygophyllum oxianum. Chem. Nat. Compd. 2012, 48, 11–15. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Senthilkumar, A.; Venkatesalu, V. Antibacterial and antifungal efficacy of fatty acid methyl esters from the leaves of Sesuvium portulacastrum L. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 775–780. [Google Scholar]
- Chandrasekaran, M.; Kannathasan, K.; Venkatesalu, V. Antimicrobial activity of fatty acid methyl esters of some members of Chenopodiaceae. Z. Für Naturforsch. C J. Biosci. 2008, 63, 331–336. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Chandrasekaran, M.; Venkatesalu, V.; Hsu, M.J. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz. J. Microbiol. 2007, 38, 739–742. [Google Scholar] [CrossRef]
- Basa’ar, O.; Fatema, S.; Alrabie, A.; Mohsin, M.; Farooqui, M. Supercritical carbon dioxide extraction of Triognella foenum graecum Linn seeds: Determination of bioactive compounds and pharmacological analysis. Asian Pac. J. Trop. Biomed. 2017, 7, 1085–1091. [Google Scholar] [CrossRef]
- Kannathasan, K.; Senthilkumar, A.; Venkatesalu, V.; Chandrasekaran, M. Larvicidal activity of fatty acid methyl esters of Vitex species against Culex quinquefasciatus. Parasitol. Res. 2008, 103, 999–1001. [Google Scholar] [CrossRef] [PubMed]
- Trapp, M.; Kai, M.; Mithofer, A.; Rodrigues, E. Antibiotic oxylipins from Alternanthera brasiliana and its endophytic bacteria. Phytochemistry 2015, 110, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Sundar, R.D.V.; Segaran, G.; Shankar, S.; Settu, S.; Ravi, L. Bioactivity of Phoenix dactylifera seed and its phytochemical analysis. Int. J. Green Pharm. 2017, 11, S292–S297. [Google Scholar] [CrossRef]
- Singh, G.; Kumar, P.; Jindal, A. Antibacterial potential of sterols of some medicinal plants. Int. J. Pharm. Pharm. Sci. 2012, 43, 159–3162. [Google Scholar]
- Wikaningtyas, P.; Sukandar, E.Y. The antibacterial activity of selected plants towards resistant bacteria isolated from clinical specimens. Asian Pac. J. Trop. Biomed. 2016, 6, 16–19. [Google Scholar] [CrossRef]
- Wong, K.; Ali, D.; Boey, P. Chemical constituents and antibacterial activity of Melastoma malabathricum L. Nat. Prod. Res. 2012, 26, 609–618. [Google Scholar] [CrossRef]
- Salvador, M.; Ferreira, E.; Pral, E.; Alfieri, S.; Albuquerque, S.; Ito, I.; Dias, D. Bioactivity of crude extracts and some constituents of Blutaparon portulacoides (Amaranthaceae). Phytomedicine 2002, 9, 566–571. [Google Scholar] [CrossRef]
- Bharitkar, Y.P.; Bathini, S.; Ojha, D.; Ghosh, S.; Mukherjee, H.; Kuotsu, K.; Chattopadhyay, D.; Mondal, N.B. Antibacterial and antiviral evaluation of sulfonoquinovosyldiacylglyceride: A glycolipid isolated from Azadirachta indica leaves. Lett. Appl. Microbiol. 2014, 58, 184–189. [Google Scholar] [CrossRef]
- Ash, A.; Bharitkar, Y.; Murmu, S.; Hazra, A.; Ravichandiran, V.; Kar, P.; Mondal, N. Ultrastructural changes in Raillietina (Platyhelminthes: Cestoda), exposed to sulfonoquinovosyldiacylglyceride (SQDG), isolated from Neem (Azadirachta indica). Nat. Prod. Res. 2017, 31, 2445–2449. [Google Scholar] [CrossRef]
- Rashid, M.U.; Alamzeb, M.; Ali, S.; Khan, A.; Igoli, J.; Ferro, V.; Gray, A.; Khan, M. A new ceramide along with eight known compounds from the roots of Artemisia incisa Pamp. Rec. Nat. Prod. 2015, 9, 297–304. [Google Scholar]
- Tang, J.; Meng, X.; Liu, H.; Zhao, J.; Zhou, L.; Qiu, M.; Zhang, X.; Yu, Z.; Yang, F. Antimicrobial activity of sphingolipids isolated from the stems of cucumber (Cucumis sativus L.). Molecules 2010, 15, 9288–9297. [Google Scholar] [CrossRef] [PubMed]
- Khedr, A.; Ibrahim, S.; Mohamed, G.; Ross, S.; Yamada, K. Panduramides A-D, new ceramides from Ficus pandurata fruits. Phytochem. Lett. 2018, 23, 100–105. [Google Scholar] [CrossRef]
- Poumale, H.; Djoumessi, A.; Ngameni, B.; Sandjo, L.; Ngadjui, B.; Shiono, Y. A new ceramide isolated from Ficus lutea Vahl (Moraceae). Acta Chim. Slov. 2011, 58, 81–86. [Google Scholar] [PubMed]
- Wyatt, R.; Hodges, L.; Kalafatis, N.; Wright, P.; Wynne, P.; Macrides, T. Phytochemical analysis and biological screening of leaf and twig extracts from Kunzea ericoides. Phytother. Res. 2005, 19, 963–970. [Google Scholar] [CrossRef]
- Allaoua, Z.; Benkhaled, M.; Dibi, A.; Long, C.; Aberkane, M.C.; Bouzidi, S.; Kassah-Laouar, A.; Haba, H. Chemical composition, antioxidant and antibacterial properties of Pteranthus dichotomus from Algerian Sahara. Nat. Prod. Res. 2016, 30, 700–704. [Google Scholar] [CrossRef]
- Abubakar, M.N.; Majinda, R.R.T. GC-MS Analysis and preliminary antimicrobial activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis. Medicines 2016, 3, 3. [Google Scholar] [CrossRef]
- Keroletswe, N.; Majinda, R.R.T.; Masesane, I.B. A new 3-prenyl-2-flavene and other extractives from Baphia massaiensis and their antimicrobial activities. Nat. Prod. Commun. 2018, 13, 435–438. [Google Scholar] [CrossRef]
- Semwal, P.; Painuli, S.; Badoni, H.; Bacheti, R.K. Screening of phytoconstituents and antibacterial activity of leaves and bark of Quercus leucotrichophora A. Camus from Uttarakhand Himalaya. Clin. Phytosci. 2018, 4, 30. [Google Scholar] [CrossRef]
- Sanusi, S.B.; Bakar, M.A.; Mohamed, M.; Sabran, S.F.; Azizul, I. Antibacterial Activity and Phytochemical Analysis of Kembang semangkok (Scaphium macropodum) Stem Bark. In IOP Conference Series: Earth and Environmental Science, Proceedings of International Conference on Biodiversity; Takzim, J.D., Ed.; IOP Publishing: Bristol, UK, 2018; p. 12043. [Google Scholar] [CrossRef]
- Nguyen, H.; Ho, D.; Vo, H.; Le, A.; Nguyen, H.; Kodama, T.; Ito, T.; Morita, H.; Raal, A. Antibacterial activities of chemical constituents from the aerial parts of Hedyotis pilulifera. Pharm. Biol. 2017, 55, 787–791. [Google Scholar] [CrossRef]
- Chatterjee, R.; Singh, O.; Pachuau, L.; Malik, S.P.; Paul, M.; Bhadra, K.; Paul, S.; Kumar, G.S.; Mondal, N.B.; Banerjee, S. Identification of a sulfonoquinovosyldiacylglyceride from Azadirachta indica and studies on its cytotoxic activity and DNA binding properties. Bioorg. Med. Chem. Lett. 2010, 20, 6699–6702. [Google Scholar] [CrossRef]
- Yogesh, P.; Bhattacharya, S.; Das, T.; Roy, M.; Besra, S.; Gomes, A.; Mondal, N.; Banerjee, S. Anti-leukemic activity of sulfonoquinovosyldiacylglyceride (SQDG): A constituent of Azadirachta indica leaves. Med. Chem. Res. 2013, 22, 22–27. [Google Scholar] [CrossRef]
- Bachere, E.; Gueguen, Y.; Gonzalez, M.; de Lorgeril, J.; Garnier, J.; Romestand, B. Insights into the anti-microbial defense of marine invertebrates: The penaeid shrimps and the oyster Crassostrea gigas. Immunol. Rev. 2004, 198, 149–168. [Google Scholar] [CrossRef]
- Bhadury, P.; Wright, P. Exploitation of marine algae: Biogenic compounds for potential antifouling applications. Planta 2004, 219, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Rodriguez, A.; Taglialatela-Scafati, O.; Fusetani, N. Marine Pharmacology in 2012–2013: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2017, 15, 273. [Google Scholar] [CrossRef]
- El Baz, F.; El Baroty, G.; Abd El Baky, H.; Abd El-Salam, O.; Ibrahim, E. Structural characterization and biological activity of sulfolipids from selected marine algae. Grasas Aceites 2013, 64, 561–571. [Google Scholar] [CrossRef]
- Freile-Pelegrin, Y.; Morales, J. Antibacterial activity in marine algae from the coast of Yucatan, Mexico. Bot. Mar. 2004, 47, 140–146. [Google Scholar] [CrossRef]
- Gerasimenko, N.; Chaykina, E.; Busarova, N.; Anisimov, M. Antimicrobic and hemolytic activity of low-molecular metabolits of brown seaweed Laminaria cichorioides (Miyabe). Appl. Biochem. Microbiol. 2010, 46, 426–430. [Google Scholar] [CrossRef]
- Val, A.; Platas, G.; Basilio, A.; Cabello, A.; Gorrochategui, J.; Suay, I.; Vicente, F.; Portillo, E.; Río, M.; Reina, G.; et al. Screening of antimicrobial activities in red, green and brown macroalgae from Gran Canaria (Canary Islands, Spain). Int. Microbiol. 2001, 4, 35–40. [Google Scholar] [CrossRef]
- Shanab, S.M. Antioxidant and antibiotic activities of some seaweeds (Egyptian isolates). Int. J. Agric. Biol. 2007, 9, 220–225. [Google Scholar]
- Capillo, G.; Savoca, S.; Costa, R.; Sanfilippo, M.; Rizzo, C.; Lo Giudice, A.; Albergamo, A.; Rando, R.; Bartolomeo, G.; Spanò, N.; et al. New insights into the culture method and antibacterial potential of Gracilaria gracilis. Mar. Drugs 2018, 16, 492. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.L.; Shen, W.Z.; Rui, W.; Ma, X.J.; Cen, Y.Z. Antiviral activity of a sulfoquinovosyldiacylglycerol (SQDG) compound isolated from the green alga Caulerpa racemosa. Bot. Mar. 2007, 50, 185–190. [Google Scholar] [CrossRef]
- Yap, W.F.; Tay, V.; Tan, S.H.; Yow, Y.Y.; Chew, J. Decoding antioxidant and antibacterial potentials of Malaysian green seaweeds: Caulerpa racemosa and Caulerpa lentillifera. Antibiotics 2019, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Mattos, B.; Romanos, M.; de Souza, L.; Sassaki, G.; Barreto-Bergter, E. Glycolipids from macroalgae: Potential biomolecules for marine biotechnology? Rev. Bras. Farmacogn. 2011, 21, 244–247. [Google Scholar] [CrossRef]
- Paul, V.; Fenical, W. Chemical defense in tropical green algae, order Caulerpales. Mar. Ecol. Prog. Ser. 1986, 34, 157–169. [Google Scholar] [CrossRef]
- Stabili, L.; Acquaviva, M.; Angile, F.; Cavallo, R.; Cecere, E.; Del Coco, L.; Fanizzi, F.; Gerardi, C.; Narracci, M.; Petrocelli, A. Screening of Chaetomorpha linum lipidic extract as a new potential source of bioactive compounds. Mar. Drugs 2019, 17, 313. [Google Scholar] [CrossRef]
- Marín-Álvarez, A.; Murillo-Álvarez, J.I.; Muñoz-Ochoa, M.; Molina-Salinas, G.M. Chemical constituents and bioactivity of Codium amplivesiculatum Setchell & N. L. Gardener (Chlorophyta; Bryopsidales). CICIMAR Oceán. 2013, 28, 1–6. [Google Scholar]
- Garg, H.; Sharma, M.; Bhakuni, D.; Pramanik, B.; Bose, A. An antiviral sphingosine derivative from the green alga Ulva fasciata. Tetrahedron Lett. 1992, 33, 1641–1644. [Google Scholar] [CrossRef]
- Sharma, M.; Garg, H.; Chandra, K. Erythro-sphinga-4,8-dienine-N-palmitate—An antiviral agent from the green alga Ulva fasciata. Bot. Mar. 1996, 39, 213–215. [Google Scholar] [CrossRef]
- El Baroty, G.S.; El-Baz, F.K.; Abd-Elmoein, I.; Abd El Baky Hanaa, H.; Ali, M.M.; Ibrahim, E.A. Evaluation of glycolipids of some egyptian marine algae as a source of bioactive substances. Int. Res. J. Pharm. 2011, 2, 165–174. [Google Scholar]
- Al-Fadhli, A.; Wahidulla, S.; D’Souza, L. Glycolipids from the red alga Chondria armata (Kutz.) Okamura. Glycobiology 2006, 16, 902–915. [Google Scholar] [CrossRef]
- Mendes, M.; Pereira, R.; Pinto, I.S.; Carvalho, A.P.; Gomes, A.M. Antimicrobial activity and lipid profile of seaweed extracts from the North Portuguese Coast. Int. Food Res. J. 2013, 20, 3337–3345. [Google Scholar]
- Manilal, A.; Sujith, S.; Selvin, J.; Kiran, G.S.; Shakir, C.; Lipton, A.P. Antimicrobial potential of marine organisms collected from the southwest coast of India against multiresistant human and shrimp pathogens. Sci. Mar. 2010, 74, 287–296. [Google Scholar] [CrossRef]
- Ohta, K.; Mizushina, Y.; Hirata, N.; Takemura, M.; Sugawara, F.; Matsukage, A.; Yoshida, S.; Sakaguchi, K. Sulfoquinovosyldiacylglycerol, KM043, a new potent inhibitor of eukaryotic DNA polymerases and HIV-reverse transcriptase type 1 from a marine red alga, Gigartina tenella. Chem. Pharm. Bull. 1998, 46, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Acquaviva, M.I.; Biandolino, F.; Cavallo, R.A.; De Pascali, S.A.; Fanizzi, F.P.; Narracci, M.; Petrocelli, A.; Cecere, E. The lipidic extract of the seaweed Gracilariopsis longissima (Rhodophyta, Gracilariales): A potential resource for biotechnological purposes? New Biotechnol. 2012, 29, 443–450. [Google Scholar] [CrossRef]
- Feng, M.; Yu, X.; Yang, P.; Yang, H.; Lin, K.; Mao, S. Two new antifungal polyunsaturated fatty acid ethyl esters from the red alga Laurencia okamurai. Chem. Nat. Compd. 2015, 51, 418–422. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Suzuki, M.; Ishii, T.; Okino, T.; Abe, T.; Masuda, M. Antibacterial activity of halogenated sesquiterpenes from Malaysian Laurencia spp. Phytochemistry 2008, 69, 2490–2494. [Google Scholar] [CrossRef]
- De Souza, L.; Sassaki, G.; Romanos, M.; Barreto-Bergter, E. Structural characterization and anti-HSV-1 and HSV-2 activity of glycolipids from the marine algae Osmundaria obtusiloba isolated from Southeastern Brazilian coast. Mar. Drugs 2012, 10, 918–931. [Google Scholar] [CrossRef]
- García-Bueno, N.; Dumay, J.; Guerin, T.; Turpin, V.; Paillard, C.; Stiger-Pouvreau, V.; Pouchus, Y.-F.; Marín-Atucha, A.A.; Decottignes, P.; Fleurence, J. Seasonal variation in the antivibrio activity of two organic extracts from two red seaweed: Palmaria palmata and the introduced Grateloupia turuturu against the abalone pathogen Vibrio harveyi. Aquat. Living Resour. 2015, 28, 81–87. [Google Scholar] [CrossRef]
- Latorre, N.; Castaneda, F.; Meynard, A.; Rivas, J.; Contreras-Porcia, L. First approach of characterization of bioactive compound in Pyropia orbicularis during the daily tidal cycle. Lat. Am. J. Aquat. Res. 2019, 47, 826–840. [Google Scholar] [CrossRef]
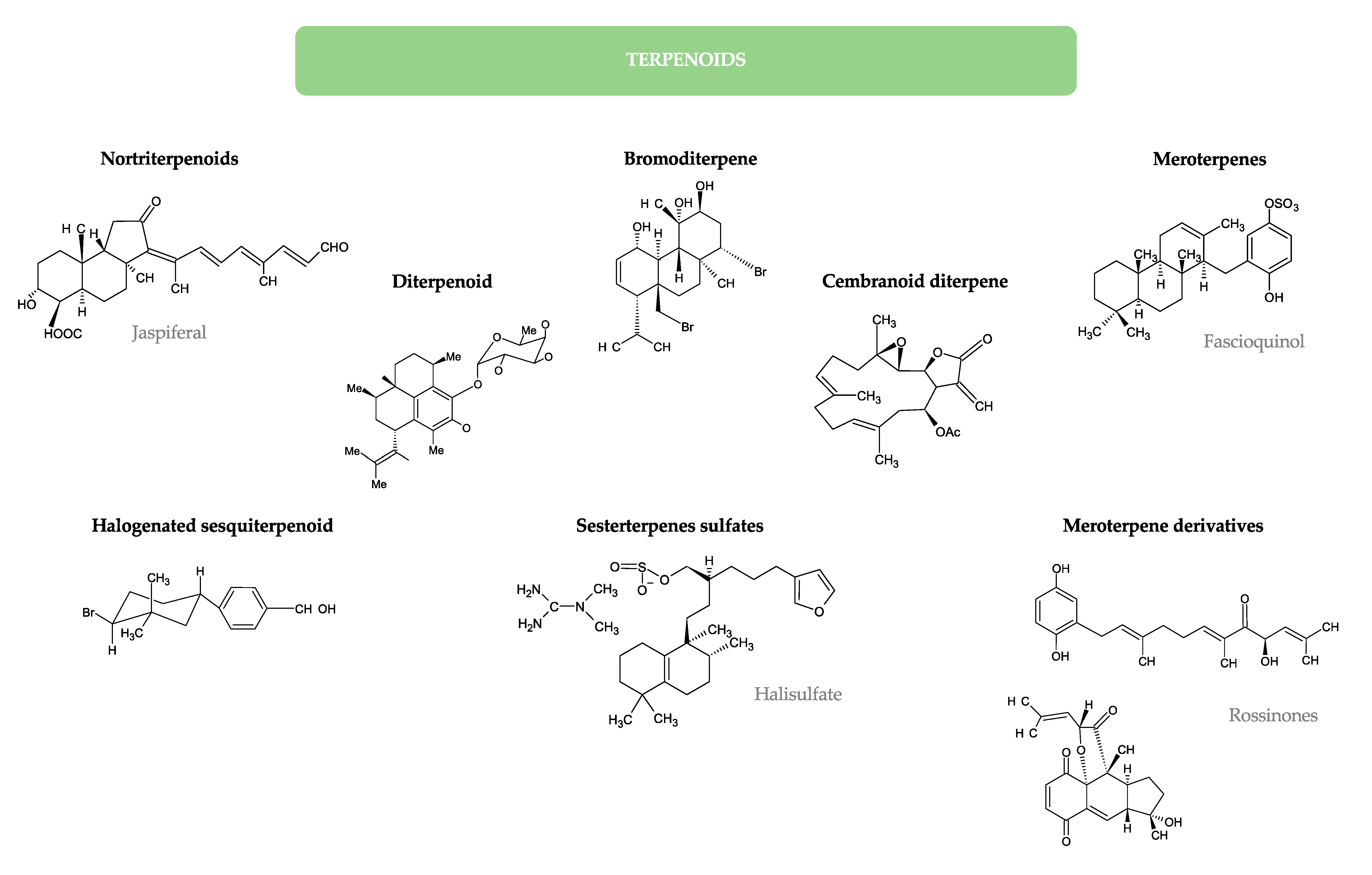
- Etahiri, S.; Bultel-Ponce, V.; Caux, C.; Guyot, M. New bromoditerpenes from the red alga Sphaerococcus coronopifolius. J. Nat. Prod. 2001, 64, 1024–1027. [Google Scholar] [CrossRef]
- Amiguet, V.; Jewell, L.; Mao, H.; Sharma, M.; Hudson, J.; Durst, T.; Allard, M.; Rochefort, G.; Arnason, J. Antibacterial properties of a glycolipid-rich extract and active principle from Nunavik collections of the macroalgae Fucus evanescens C. Agardh (Fucaceae). Can. J. Microbiol. 2011, 57, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Santoyo, S.; Jaime, L.; Reina, G.; Herrero, M.; Senoráns, F.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef] [PubMed]
- El Shafay, S.M.; Ali, S.S.; El-Sheekh, M.M. Antimicrobial activity of some seaweeds species from Red sea, against multidrug resistant bacteria. Egypt. J. Aquat. Res. 2016, 42, 65–74. [Google Scholar] [CrossRef]
- Gerasimenko, N.I.; Martyias, E.A.; Logvinov, S.V.; Busarova, N.G. Biological activity of lipids and photosynthetic pigments of Sargassum pallidum C. Agardh. Prikl. Biokhim. Mikrobiol. 2014, 50, 85–94. [Google Scholar] [CrossRef]
- Plouguerné, E.; de Souza, L.M.; Sassaki, G.L.; Cavalcanti, J.F.; Villela Romanos, M.T.; da Gama, B.A.; Pereira, R.C.; Barreto-Bergter, E. Antiviral sulfoquinovosyldiacylglycerols (SQDGs) from the Brazilian brown seaweed Sargassum vulgare. Mar. Drugs 2013, 11, 4628–4640. [Google Scholar] [CrossRef]
- Arunkumar, K.; Selvapalam, N.; Rengasamy, R. The antibacterial compound sulphoglycerolipid 1-0 palmitoyl-3-0(6’-sulpho-alpha-quinovopyranosyl)-glycerol from Sargassum wightii Greville (Phaeophyceae). Bot. Mar. 2005, 48, 441–445. [Google Scholar] [CrossRef]
- Mendiola, J.; Torres, C.; Tore, A.; Martín-Álvarez, P.; Santoyo, S.; Arredondo, B.; Senoráns, F.; Cifuentes, A.; Ibáñez, E. Use of supercritical CO2 to obtain extracts with antimicrobial activity from Chaetoceros muelleri microalga. A correlation with their lipidic content. Eur. Food Res. Technol. 2007, 224, 505–510. [Google Scholar] [CrossRef]
- Ohta, S.; Chang, T.; Kawashima, A.; Nagate, T.; Murase, M.; Nakanishi, H.; Miyata, H.; Kondo, M. Anti methicillin-resistant Staphylococcus aureus (MRSA) activity by linolenic acid isolated from the marine microalga Chlorococcum HS-101. Bull. Environ. Contam. Toxicol. 1994, 52, 673–680. [Google Scholar] [CrossRef]
- Herrero, M.; Ibáñez, E.; Cifuentes, A.; Reglero, G.; Santoyo, S. Dunaliella salina microalga pressurized liquid extracts as potential antimicrobials. J. Food Prot. 2006, 69, 2471–2477. [Google Scholar] [CrossRef]
- Findlay, J.A.; Patil, A.D. Antibacterial constituents of the diatom Navicula delognei. J. Nat. Prod. 1984, 47, 815–818. [Google Scholar] [CrossRef]
- Desbois, A.P.; Lebl, T.; Yan, L.; Smith, V.J. Isolation and structural characterisation of two antibacterial free fatty acids from the marine diatom, Phaeodactylum tricornutum. Appl. Microbiol. Biotechnol. 2008, 81, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.; Mearns-Spragg, A.; Smith, V. A fatty acid from the diatom Phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA). Mar. Biotechnol. 2009, 11, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Shiomi, Y.; Kawashima, A.; Aozasa, O.; Nakao, T.; Nagate, T.; Kitamura, K.; Miyata, H. Antibiotic effect of linoleic acid from Chlorococcum strain HS-101 and Dunaliella primolecta on methicillin-resistant Staphylococcus aureus. J. Appl. Phycol. 1995, 7, 121–127. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Thelen, J.; Fedosejevs, E.; Harwood, J. The lipid biochemistry of eukaryotic algae. Prog. Lipid Res. 2019, 74, 31–68. [Google Scholar] [CrossRef] [PubMed]
- Hölzl, G.; Dörmann, P. Structure and function, of glycoglycerolipids in plants and bacteria. Prog. Lipid Res. 2007, 46, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Morimoto, T.; Imamura, H.; Ueda, T.; Nagai, S.; Sakakibara, J.; Yamada, N. Studies on glycolipids. III. Glyceroglycolipids from an axenically cultured cyanobacterium, Phormidium tenue. Chem. Pharm. Bull. 1991, 39, 2277–2281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, C.; Yu, G.; Guan, H. Total synthesis and structure-activity relationship of glycoglycerolipids from marine organisms. Mar. Drugs 2014, 12, 3634–3659. [Google Scholar] [CrossRef]
- Banskota, A.; Stefanova, R.; Sperker, S.; Lall, S.; Craigie, J.; Hafting, J. Lipids isolated from the cultivated red alga Chondrus crispus inhibit nitric oxide production. J. Appl. Phycol. 2014, 26, 1565–1571. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.X.; Guan, H.S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef]
- Cerón, M.; García-Malea, M.; Rivas, J.; Acien, F.; Fernández, J.; Del Río, E.; Guerrero, M.; Molina, E. Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Appl. Microbiol. Biotechnol. 2007, 74, 1112–1119. [Google Scholar] [CrossRef]
- Hsu, C.; Chao, P.; Hu, S.; Yang, C. The antioxidant and free radical scavenging activities of chlorophylls and pheophytins. Food Nutr. Sci. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Bruce, D.L.; Duff, D.C.B.; Antia, N.J. The identification of two antibacterial products of the marine planktonic alga Isochrysis galbana. J. Gen. Microbiol. 1967, 48, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Carballeira, N.; Reyes, E.; Sostre, A.; Rodríguez, A.; Rodríguez, J.; González, F. Identification of the novel antimicrobial fatty acid (5Z,9Z)-14-methyl-5,9-pentadecadienoic acid in Eunicea succinea. J. Nat. Prod. 1997, 60, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Uchio, Y.; Yasumoto, K.; Kusumi, T.; Ooi, T. Brominated unsaturated fatty acids from marine sponge collected in Papua New Guinea. Chem. Pharm. Bull. 2008, 56, 378–382. [Google Scholar] [CrossRef]
- Dmitrenok, A.; Radhika, P.; Anjaneyulu, V.; Subrahmanyam, S.; Rao, P.; Dmitrenok, P.; Boguslavsky, V. New lipids from the soft corals of the Andaman Islands. Russ. Chem. Bull. 2003, 52, 1868–1872. [Google Scholar] [CrossRef]
- Angulo-Preckler, C.; Spurkland, T.; Avila, C.; Iken, K. Antimicrobial activity of selected benthic Arctic invertebrates. Polar Biol. 2015, 38, 1941–1948. [Google Scholar] [CrossRef]
- Lauritano, C.; Martínez, K.A.; Battaglia, P.; Granata, A.; de la Cruz, M.; Cautain, B.; Martín, J.; Reyes, F.; Ianora, A.; Guglielmo, L. First evidence of anticancer and antimicrobial activity in Mediterranean mesopelagic species. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Barnathan, G. Non-methylene-interrupted fatty acids from marine invertebrates: Occurrence, characterization and biological properties. Biochimie 2009, 91, 671–678. [Google Scholar] [CrossRef]
- Ely, R.; Supriya, T.; Naik, C.G. Antimicrobial activity of marine organisms collected off the coast of South East India. J. Exp. Mar. Bio. Ecol. 2004, 309, 121–127. [Google Scholar] [CrossRef]
- Chan-Higuera, J.E.; Carbonell-Barrachina, A.A.; Cárdenas-Lopez, J.L.; Kačániová, M.; Burgos-Hernández, A.; Ezquerra-Brauer, J.M. Jumbo squid (Dosidicus gigas) skin pigments: Chemical analysis and evaluation of antimicrobial and antimutagenic potential. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 349–353. [Google Scholar] [CrossRef]
- Seleghim, M.H.R.; Lira, S.P.; Kossuga, M.H.; Batista, T.; Berlinck, R.G.S.; Hajdu, E.; Muricy, G.; da Rocha, R.M.; do Nascimento, G.G.F.; Silva, M.; et al. Antibiotic, cytotoxic and enzyme inhibitory activity of crude extracts from Brazilian marine invertebrates. Rev. Bras. Farmacogn. 2007, 17, 287–318. [Google Scholar] [CrossRef]
- Lin, K.; Yang, P.; Yang, H.; Liu, A.-H.; Yao, L.-G.; Guo, Y.-W.; Mao, S.-C. Lysophospholipids from the guangxi sponge Spirastrella purpurea. Lipids 2015, 50, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Linington, R.G.; Robertson, M.; Gauthier, A.; Finlay, B.B.; van Soes, R.; Andersen, R.J. Caminoside A, an antimicrobial glycolipid isolated from the marine sponge Caminus sphaeroconia. Org. Lett. 2002, 4, 4089–4092. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Wahidulla, S.; D’Souza, L.; Rameshkumar, G. Antimicrobial lipids from the hemolymph of brachyuran crabs. Appl. Biochem. Biotechnol. 2010, 162, 1039–1051. [Google Scholar] [CrossRef]
- Haug, T.; Kjuul, A.K.; Stensvåg, K.; Sandsdalen, E.; Styrvold, O.B. Antibacterial activity in four marine crustacean decapods. Fish. Shellfish Immunol. 2002, 12, 371–385. [Google Scholar] [CrossRef]
- Schmitt, P.; Wilmes, M.; Pugnière, M.; Aumelas, A.; Bachère, E.; Sahl, H.-G.; Schneider, T.; Destoumieux-Garzón, D. Insight into invertebrate defensin mechanism of action oyster defensins inhibit peptidoglycan biosynthesis by binding to lipid II. J. Biol. Chem. 2010, 285, 29208–29216. [Google Scholar] [CrossRef]
- Mohanraju, R.; Marri, D.B.; Karthick, P.; Narayana, S.; Murthy, K.N.; Ramesh, C. Antibacterial activity of certain cephalopods from Andamans, India. Int J. Pharm Biol Sci. 2013, 32, 450–455. [Google Scholar]
- Cheng, L.; Jin, X.-K.; Li, W.-W.; Li, S.; Guo, X.-N.; Wang, J.; Gong, Y.-N.; He, L.; Wang, Q. Fatty acid binding proteins FABP9 and FABP10 participate in antibacterial responses in Chinese mitten crab, Eriocheir sinensis. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Kelly, J.R.; Scheibling, R.E. Fatty acids as dietary tracers in benthic food webs. Mar. Ecol. Prog. Ser. 2012, 446, 1–22. [Google Scholar] [CrossRef]
- Brinkmann, C.M.; Marker, A.; Kurtboke, D.I. An overview on marine sponge-symbiotic bacteria as unexhausted sources for natural product discovery. Diversity 2017, 9, 40. [Google Scholar] [CrossRef]
- Laport, M.S.; Santos, O.C.S.; Muricy, G. Marine sponges: Potential sources of new antimicrobial drugs. Curr. Pharm. Biotechnol. 2009, 10, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Blumbach, B.; Müller, I.M. Evolution of the innate and adaptive immune systems: Relationships between potential immune molecules in the lowest metazoan phylum (Porifera) and those in vertebrates. Transplantation 1999, 68, 1215–1227. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Klemt, M.; Thakur, N.L.; Schröder, H.C.; Aiello, A.; D’esposito, M.; Menna, M.; Fattorusso, E. Molecular/chemical ecology in sponges: Evidence for an adaptive antibacterial response in Suberites domuncula. Mar. Biol. 2004, 144, 19–29. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Feldlaufer, M.F.; Knox, D.A.; Lusby, W.R.; Shimanuki, H. Antimicrobial activity of fatty acids against Bacillus larvae, the causative agent of American foulbrood disease. Apidologie 1993, 24, 95–99. [Google Scholar] [CrossRef]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef]
- Matsunaga, S.; Okada, Y.; Fusetani, N.; van Soest, R. An antimicrobial C14 acetylenic acid from a marine sponge Oceanapia species. J. Nat. Prod. 2000, 63, 690–691. [Google Scholar] [CrossRef]
- Tasdemir, D.; Topaloglu, B.; Perozzo, R.; Brun, R.; O’Neill, R.; Carballeira, N.M.; Zhang, X.; Tonge, P.J.; Linden, A.; Ruedi, P. Marine natural products from the Turkish sponge Agelas oroides that inhibit the enoyl reductases from Plasmodium falciparum, Mycobacterium tuberculosis and Escherichia coli. Bioorg. Med. Chem. 2007, 15, 6834–6845. [Google Scholar] [CrossRef]
- Tianero, M.D.B.; Hanif, N.; de Voogd, N.J.; van Soest, R.W.M.; Tanaka, J. A new antimicrobial fatty acid from the calcareous sponge Paragrantia cf. waguensis. Chem. Biodivers. 2009, 6, 1374–1377. [Google Scholar] [CrossRef]
- Keffer, J.L.; Plaza, A.; Bewley, C.A. Motualevic acids A-F, antimicrobial acids from the sponge Siliquariaspongia sp. Org. Lett. 2009, 11, 1087–1090. [Google Scholar] [CrossRef]
- Bifulco, G.; Bruno, I.; Minale, L.; Riccio, R. Novel HIV-inhibitory halistanol sulfates FH from a marine sponge, Pseudoaxinissa digitata. J. Nat. Prod. 1994, 57, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.H.; Gross, S.S.; Gunasekera, M.; Koehn, F.E. Weinbersterol disulfate A and B, antiviral steroid sulfates from the sponge Petrosia weinbergi. Tetrahedron 1991, 47, 1185–1190. [Google Scholar] [CrossRef]
- Boonlarppradab, C.; Faulkner, D.J. Eurysterols A and B, cytotoxic and antifungal steroidal sulfates from a marine sponge of the genus Euryspongia. J. Nat. Prod. 2007, 70, 846–848. [Google Scholar] [CrossRef] [PubMed]
- DiGirolamo, J.A.; Li, X.-C.; Jacob, M.R.; Clark, A.M.; Ferreira, D. Reversal of fluconazole resistance by sulfated sterols from the marine sponge Topsentia sp. J. Nat. Prod. 2009, 72, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Matsunaga, S.; Fusetani, N.; van Soest, R.W.M. Acanthosterol sulfates A-J: Ten new antifungal steroidal sulfates from a marine sponge Acanthodendrilla sp. J. Nat. Prod. 1998, 61, 1374–1378. [Google Scholar] [CrossRef]
- Viegelmann, C.; Parker, J.; Ooi, T.; Clements, C.; Abbott, G.; Young, L.; Kennedy, J.; Dobson, A.D.W.; Edrada-Ebel, R. Isolation and identification of antitrypanosomal and antimycobacterial active steroids from the sponge Haliclona simulans. Mar. Drugs 2014, 12, 2937–2952. [Google Scholar] [CrossRef]
- Karthikeyan, S.C.; Velmurugan, S.; Donio, M.B.S.; Michaelbabu, M.; Citarasu, T. Studies on the antimicrobial potential and structural characterization of fatty acids extracted from Sydney rock oyster Saccostrea glomerata. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 332. [Google Scholar] [CrossRef]
- Carmely, S.; Roll, M.; Loya, Y.; Kashman, Y. The structure of eryloside A, a new antitumor and antifungal 4-methylated steroidal glycoside from the sponge Erylus lendenfeldi. J. Nat. Prod. 1989, 52, 167–170. [Google Scholar] [CrossRef]
- Dai, H.-F.; Edrada, R.A.; Ebel, R.; Nimtz, M.; Wray, V.; Proksch, P. Norlanostane triterpenoidal saponins from the marine sponge Melophlus sarassinorum. J. Nat. Prod. 2005, 68, 1231–1237. [Google Scholar] [CrossRef]
- Okada, Y.; Matsunaga, S.; van Soest, R.W.M.; Fusetani, N. Sokodosides, steroid glycosides with an isopropyl side chain, from the marine sponge Erylus placenta. J. Org. Chem. 2006, 71, 4884–4888. [Google Scholar] [CrossRef]
- Ryu, G.; Choi, B.W.; Lee, B.H.; Hwang, K.-H.; Lee, U.C.; Jeong, D.S.; Lee, N.H. Wondosterols AC, three steroidal glycosides from a Korean marine two-sponge association. Tetrahedron 1999, 55, 13171–13178. [Google Scholar] [CrossRef]
- Linington, R.G.; Robertson, M.; Gauthier, A.; Finlay, B.B.; MacMillan, J.B.; Molinski, T.F.; van Soest, R.; Andersen, R.J. Caminosides B-D, antimicrobial glycolipids isolated from the marine sponge Caminus sphaeroconia. J. Nat. Prod. 2006, 69, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Vanisree, M.; Subbaraju, G. Alcyonacean metabolites VIII-antibacterial metabolites from Labophytum crassum of the Indian Ocean. Asian J. Chem. 2002, 14, 957–960. [Google Scholar]
- Butler, M.S.; Capon, R.J. The luffarins (AZ), novel terpenes from an Australian marine sponge, Luffariella geometrica. Aust. J. Chem. 1992, 45, 1705–1743. [Google Scholar] [CrossRef]
- De Silva, E.D.; Scheuer, P.J. Three new sesterterpenoid antibiotics from the marine sponge Luffariella variabilis (Polejaff). Tetrahedron Lett. 1981, 22, 3147–3150. [Google Scholar] [CrossRef]
- De Silva, E.D.; Scheuer, P.J. Manoalide, an antibiotic sesterterpenoid from the marine sponge Luffariella variabilis (Polejaeff). Tetrahedron Lett. 1980, 21, 1611–1614. [Google Scholar] [CrossRef]
- Kobayashi, J.; Yuasa, K.; Kobayashi, T.; Sasaki, T.; Tsuda, M. Jaspiferals A ~ G, new cytotoxic isomalabaricane-type nortriterpenoids from Okinawan marine sponge Jaspis stellifera. Tetrahedron 1996, 52, 5745–5750. [Google Scholar] [CrossRef]
- Sullivan, B.; Djura, P.; Mcintyre, D.E.; Faulkner, D.J. Antimicrobial constituents of the sponge Siphonodictyon coralliphagum. Tetrahedron 1981, 37, 979–982. [Google Scholar] [CrossRef]
- Lee, D.; Shin, J.; Yoon, K.-M.; Kim, T.-I.; Lee, S.-H.; Lee, H.-S.; Oh, K.-B. Inhibition of Candida albicans isocitrate lyase activity by sesterterpene sulfates from the tropical sponge Dysidea sp. Bioorg. Med. Chem. Lett. 2008, 18, 5377–5380. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, X.M. A new sesquiterpenoid hydroquinone from the marine sponge Dysidea arenaria. Molecules 2008, 13, 1275–1281. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kubota, T.; Ito, J.; Mikami, Y.; Fromont, J.; Kobayashi, J. Nakijiquinones G-I, new sesquiterpenoid quinones from marine sponge. Bioorg. Med. Chem. 2008, 16, 7561–7564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Khalil, Z.G.; Capon, R.J. Fascioquinols A–F: Bioactive meroterpenes from a deep-water southern Australian marine sponge, Fasciospongia sp. Tetrahedron 2011, 67, 2591–2595. [Google Scholar] [CrossRef]
- Ishiyama, H.; Hashimoto, A.; Fromont, J.; Hoshino, Y.; Mikami, Y.; Kobayashi, J. Halichonadins A–D, new sesquiterpenoids from a sponge Halichondria sp. Tetrahedron 2005, 61, 1101–1105. [Google Scholar] [CrossRef]
- Ishiyama, H.; Kozawa, S.; Aoyama, K.; Mikami, Y.; Fromont, J.; Kobayashi, J. Halichonadin F and the Cu(I) complex of halichonadin C from the sponge Halichondria sp. J. Nat. Prod. 2008, 71, 1301–1303. [Google Scholar] [CrossRef]
- Appleton, D.R.; Chuen, C.S.; Berridge, M.V.; Webb, V.L.; Copp, B.R. Rossinones A and B, biologically active meroterpenoids from the Antarctic Ascidian, Aplidium species. J. Org. Chem. 2009, 74, 9195–9198. [Google Scholar] [CrossRef] [PubMed]
- Elkhayat, E.; Edrada, R.; Ebel, R.; Wray, V.; van Soest, R.; Wiryowidagdo, S.; Mohamed, M.; Müller, W.E.G.; Proksch, P. New luffariellolide derivatives from the Indonesian sponge Acanthodendrilla sp. J. Nat. Prod. 2004, 67, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; Weete, J.D.; Schinazi, R.F.; Wirtz, S.S.; Tharnish, P.; Scheuer, P.J.; Hamann, M.T. Mololipids, a new series of anti-HIV bromotyramine-derived compounds from a sponge of the order Verongida. J. Nat. Prod. 2000, 63, 501–503. [Google Scholar] [CrossRef]
- Ata, A.; Win, H.; Holt, D.; Holloway, P.; Segstro, E.; Jayatilake, G. New antibacterial diterpenes from Pseudopterogorgia elisabethae. Helv. Chim. Acta 2004, 87, 1090–1098. [Google Scholar] [CrossRef]
- Ibrahim, H.A.H. Antibacterial carotenoids of three Holothuria species in Hurghada, Egypt. Egypt. J. Aquat. Res. 2012, 38, 185–194. [Google Scholar] [CrossRef]
- Mendes, G.; Soares, A.; Martins, F.; de Albuquerque, M.; Costa, S.; Yoneshigue-Valentin, Y.; Gestinari, L.; Santos, N.; Romanos, M. Antiviral activity of the green marine alga Ulva fasciata on the replication of human metapneumovirus. Rev. Inst. Med. Trop. Sao Paulo 2010, 52, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Chen, Y.; Yao, F.; Chen, W.; Zhong, S.; Zheng, F.; Shi, G. Antioxidant, antibacterial and antischistosomal activities of extracts from Grateloupia livida (Harv). Yamada. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Alves, E.; Simoes, A.; Domingues, M. Fruit seeds and their oils as promising sources of value-added lipids from agro-industrial byproducts: Oil content, lipid composition, lipid analysis, biological activity and potential biotechnological applications. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Maciel, E.; Leal, M.C.; Lillebø, A.I.; Domingues, P.; Domingues, M.R.; Calado, R. Bioprospecting of marine macrophytes using MS-based lipidomics as a new approach. Mar. Drugs 2016, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Maciel, E.; Alves, E.; Domingues, P.; Domingues, R. Module 3–Lipidomics. In Advanced Analytical Chemistry for Life Sciences: AACLifeSci Course Companion Manual; Domingues, P., García, A., Skrzydlewska, E., Eds.; Medical University of Bialystok: Bialystok, Poland, 2018; pp. 120–134. [Google Scholar]
- Malviya, N.; Malviya, S. Bioassay guided fractionation-an emerging technique influence the isolation, identification and characterization of lead phytomolecules. Hosp. Pharm. 2017, 2, 5. [Google Scholar] [CrossRef][Green Version]
- Cunha, L.C.S.; de Morais, S.A.L.; de Aquino, F.J.T.; Chang, R.; de Oliveira, A.; Martins, M.M.; Martins, C.H.G.; Sousa, L.C.F.; Barros, T.T.; da Silva, C.V.; et al. Bioassay-guided fractionation and antimicrobial and cytotoxic activities of Cassia bakeriana extracts. Rev. Bras. Farmacogn. 2017, 27, 91–98. [Google Scholar] [CrossRef]
- Erenler, R.; Meral, B.; Sen, O.; Elmastas, M.; Aydin, A.; Eminagaoglu, O.; Topcu, G. Bioassay-guided isolation, identification of compounds from Origanum rotundifolium and investigation of their antiproliferative and antioxidant activities. Pharm. Biol. 2017, 55, 1646–1653. [Google Scholar] [CrossRef]
- Thorgeirsdóttir, T.O.; Kristmundsdóttir, T.; Thormar, H.; Axelsdóttir, Í.; Holbrook, W.P. Antimicrobial activity of monocaprin: A monoglyceride with potential use as a denture disinfectant. Acta Odontol. Scand. 2006, 64, 21–26. [Google Scholar] [CrossRef]
- Knightly, W.H. The physical properties of emulsifiers used in baking. In Physical Properties of Fats, Oils, and Emulsifiers; Widlak, N.W., Ed.; AOCS Press: Champaign, IL, USA, 1999; pp. 164–185. [Google Scholar]
- Le, P.N.T.; Desbois, A.P. Antibacterial effect of eicosapentaenoic acid against Bacillus cereus and Staphylococcus aureus: Killing kinetics, selection for resistance, and potential cellular target. Mar. Drugs 2017, 15, 334. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Guerra, C.A.; Cano-Díaz, C.; Egidi, E.; Wang, J.-T.; Eisenhauer, N.; Singh, B.K.; Maestre, F.T. The proportion of soil-borne pathogens increases with warming at the global scale. Nat. Clim. Chang. 2020, 1–5. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Benkendorff, K.; Davis, A.R.; Rogers, C.N.; Bremner, J.B. Free fatty acids and sterols in the benthic spawn of aquatic molluscs, and their associated antimicrobial properties. J. Exp. Mar. Biol. Ecol. 2005, 316, 29–44. [Google Scholar] [CrossRef]
| Botanical Name | Family | Common Name | Country of Collection | Plant Part | Extracting Solvent/Method | Isolated Lipids or Lipid Mixtures | Ref. |
|---|---|---|---|---|---|---|---|
| Sesuvium portulacastrum L. | Aizoaceae | Sea purslane | India | Leaves | MeOH/benzene/sulfuric acid (200:100:10, v/v) | FAME | [60] |
| Blutaparon portulacoides (A. St.-Hil.) Mears | Amaranthaceae | Capotiraguá | Brazil | Roots | EtOH | Acyl steryl glycosides (sitosteryl 3-β-O-glucoside 6’-O-palmitate and stigmasteryl 3-β-O-glucoside 6’-O-palmitate) | [70] |
| Arthrocnemum indicum (Willd.) Moq., Salicornia brachiata Roxb., Suaeda maritima (L.) Dumort. and Suaeda monoica Forsk. | Glasswort for Salicornia genus, herbaceous seepweed for S. maritima, and South-Indian seepweed for S. monoica | India | Shoots of A. indicum and S. brachiata, and leaves of S. maritima and S. monoica | Dry MeOH/benzene/sulfuric acid (200:100:10, v/v) | FAME | [61] | |
| Alternanthera brasiliana | Brazilian joyweed | Brazil | Root, stem and leaves | EtOH and EtOAc | Linoleate oxylipins | [65] | |
| Phoenix dactylifera L. | Arecaceae | Date palm | India | Seeds | CHCl3 and acetone | Sterol and triterpenes | [66] |
| Asphodelus aestivus Brot. | Asphodelaceae (formerly Liliaceae) | Summer asphodel | Turkey | Seeds | Petroleum ether with Soxhlet extractor | FA (C4:0, 6:0, 8:0, 10:0, 16:0, 18:0, 21:0, 24:0, 14:1, 15:1, 18:1n9t, 20:1, 24:1, 18:2, 18:2n6t, 18:2n6c, 20:2n6, 20:3n3, 22:6n3, and others unidentified) | [54] |
| Artemisia incisa Pamp. | Asteraceae | Pakistan | Roots | MeOH and recovered after elution on a SiO2 column with CH2Cl2/MeOH (9:1, v/v) following previous elution with n-hexane/EtOAc (5:4, v/v) | Artemceramide-B | [73] | |
| Pteranthus dichotomus Forssk. (also known as P. echinatus Desf.) | Caryophyllaceae | Algerian Sahara | Aerial parts | MeOH/H2O (80:20, v/v). Aqueous phase extracted successively with petroleum ether, EtOAc and n-BuOH. EtOAc fraction contained the sterols and steryl glycoside. BuOH fraction contained the glyceroglycolipids and the cerebroside. | BuOH fraction contained the compounds: 1-O-palmitoyl-3-O-(6-sulfo-α-D-quinovopyranosyl)-glycerol, 1,2-di-O-palmitoyl-3-O-(6-sulfo-α-D-quinovopyranosyl)-glycerol and soya cerebroside I. EtOAc fraction contained the compounds: stigmat-7-en-3-ol, spinasterol, β-sitosterol and β-sitosterol-3-O-glycoside | [78] | |
| Cucumis sativus L. | Cucurbitaceae | Cucumber | China | Stems | CHCl3 fraction of the crude methanolic extract | Sphingolipids [(2S,3S,4R,10E)-2-[(2’R)-2-hydroxytetracosanoylamino]-1,3,4-octadecanetriol-10-ene, 1-O-β-D-glucopyranosyl-(2S,3S,4R,10E)-2-[(2’R)-2-hydroxytetracosanoylamino]-1,3,4-octadecanetriol-10-ene and soya-cerebroside I] | [74] |
| Excoecaria agallocha | Euphorbiaceae | Blind-your-eye mangrove | India | Leaves | Dry MeOH, benzene and sulfuric acid (200:100:10, v/v) | FAME | [62] |
| Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC) | Fabaceae | Flat crown Albizia and African teak, respectively | Nigeria and Botswana, respectively | Heartwood of A. adianthifolia and stem bark of P. angolensis | n-hexane, CHCl3, MeOH, and 10% MeOH (aq) | n-hexadecanoic acid (palmitic acid); oleic acid; chondrillasterol; stigmasterol, 24S 5α-stigmast-7-en-3-ol; 9,12-octadecadienoic acid (Z,Z)-, methyl ester; trans-13-octadecanoic acid, methyl ester; tetradecanoic acid; hexadecanoic acid, methyl ester; octadecanoic acid | [79] |
| Baphia massaiensis | Jasmine pea | Botswana | Seeds | n-hexane/1-propanol (3:1, v/v) with Soxhlet extractor | Seed oil (total FA) | [80] | |
| Cassia tora L. (or Senna tora L. Roxb.) | Sickle Senna | India | Leaves and stem | Petroleum ether with Soxhlet extractor | FA (the major were palmitic acid, linoleic acid, linolenic acid, margaric acid, melissic acid, and behenic acid) | [55] | |
| Trigonella foenum-graecum L. | Fenugreek | India | Seeds | Supercritical fluid extraction (40–60 °C and 10–25 Mpa) | Conjugated linoleic acid methyl ester, saturated FAME, steroids | [63] | |
| Quercus leucotrichophora A. Camus | Fagaceae | Banjh oak | India | Fruits | 85% aqueous EtOH. Ethanolic extract fractionated with hexane and EtOAc using Soxhlet extractor. Hexane extract was analyzed. | FAME | [56] |
| Quercus leucotrichophora A. Camus | Banjh oak | Garhwal region of Himalaya | Leaves and bark | MeOH | FA; linoleic acid in stem bark and leaves extracts and cis-vaccenic acid in stem bark | [81] | |
| Vitex altissima L., V. negundo L. and V. trifolia L. | Lamiaceae | Peacock chaste tree, Chinese chaste tree, and simpleleaf chastetree, respectively | India | Leaves | Dry MeOH/benzene/sulfuric acid (200:100:10, v/v) | FAME | [64] |
| Linum usitatissimum L. | Linaceae | Common flax or linseed | Algeria | Seeds | Petroleum ether with Soxhlet extractor | FAME | [57] |
| Scaphium macropodum (Miq.) Beumee ex. Heyne | Malvaceae | Malva nut or Kembang semangkok | Malaysia | Stem bark | MeOH | Methyl hexadecanoate, hexadecanoic acid <n-> | [82] |
| Melastoma malabathricum L. | Melastomataceae | Planter’s rhododendron or Sendudok | Malaysia | Leaves | MeOH/H2O (4:1, v/v), defatted with petroleum ether and extracted with CHCl3. Lipids recovered after elution of the CHCl3 extract by SiO2 column with CHCl3/acetone/MeOH (10:9:1, v/v). | Steryl glycoside: β-sitosterol 3-O-β-D-glucopyranoside | [69] |
| Azadirachta indica A. Juss | Meliaceae | Neem | India | Leaves | MeOH. Recovered after elution on a SiO2 column with CHCl3/MeOH (9:1, v/v) | SQDG | [71] |
| Azadirachta indica A. Juss | Neem | India | Leaves | Petroleum ether (60–80 °C) for 24 h and extracted thrice with MeOH for 48 h each time at room temperature | SQDG | [72] | |
| Carapa guianensis Aubl. and Carapa vasquezii Kenfack | Andiroba | Brazil | Seed oil | n-hexane with Soxhlet extractor | FA, FAME, squalene, β-sitosterol | [58] | |
| Ficus lutea Vahl | Moraceae | Giant-leaved fig or Lagos rubbertree | Cameroon | Woods | CH2Cl2/MeOH (1:1, v/v) and elution with EtOAc/10% MeOH | Glycosphingolipid [1-O-β-D-glucopyranosyl-(2S,3R,5E,12E)-2N-[(2′R)-hydroxyhexadecanoyl]-octadecasphinga-5,12-dienine] named lutaoside | [76] |
| Ficus pandurata Hance | Fiddle leaf fig | Egypt | Fruits | 70% MeOH. MeOH extract fractionated on a SiO2 column and purified by semi-preparative HPLC to afford pure ceramides | Ceramides [panduramides A-D, and newbouldiamide] | [75] | |
| Kunzea ericoides (A. Rich) J. Thompson | Myrtaceae | Kanuka (Maori), white manuka (Maori) or the white tea tree (English) | Australia | Leaves and twigs | CH2Cl2:MeOH (1:1, v/v), CH2Cl2:MeOH (2:1, v/v) and CH2Cl2 (neat) | Steryl esters, triacylglycerols, free FA, sterols, and phospholipids | [77] |
| Pentagonia gigantifolia Ducke | Rubiaceae | Peru | Roots | 95% EtOH. EtOH extract was fractionated on a SiO2 column using CHCl3/MeOH from 0% to 100% MeOH. Fraction eluted with 2% MeOH/CHCl3 was separated on C18 SiO2 using 85% to 90% MeOH. | Acetylenic acids: 6-octadecynoic acid and 6-nonadecynoic acid | [38] | |
| Hedyotis pilulifera (Pit.) T.N. Ninh | Vietnam | Aerial parts | MeOH at 60 °C, suspended in water and successively partitioned with CHCl3 and EtOAc. EtOAc extract fractionated on a SiO2 column. | Triterpenoids, steroids, FA, glycolipids, and a ceramide | [83] | ||
| Withania somnifera (L.) Dunal, Euphorbia hirta L., Terminalia chebula Retz. | Solanaceae, Euphorbiaceae, Combretaceae | Ashwaganda, asthma-plant, black myrobalan | India | Fruits, leaf, stem, and root from W. somnifera and E. hirta and fruits, leaf, stem, and stem bark from T. chebula | EtOAc | Sterols fraction | [67] |
| Kaempferia pandurata Roxb. (synonym of Boesenbergia rotunda (L.) Mansf.) and Senna alata (L.) Roxb. | Zingiberaceae and Fabaceae, respectively | Fingerroot and candle bush, respectively | Indonesia | Leaf of S. alata and rhizome of K. pandurata | EtOH (96%) | Sterols and triterpenoid | [68] |
| Zygophyllum oxianum Boriss. | Zygophyllaceae | Beancaper | Uzbekistan | Leaves, stems, and fruit | Acetone and CHCl3:MeOH (2:1, v/v) for total lipid extraction. Total lipids from each plant part separated in SiO2 columns. Neutral lipids eluted with CHCl3; glycolipids with acetone; phospholipids with MeOH. | Total lipid extract from leaves, stems and aerial organs (hydrocarbons, triterpenol and steryl esters, triacylglycerols, free FA, sterols, phospholipids) | [59] |
| Botanical Name | Tested (Micro)Organisms | Antimicrobial Testing Method/Evaluation | Reference Antimicrobial (Positive Control) | MIC, MBC, Diameter of Inhibition Zone (in mm) or Other | Isolated Lipids or Lipid Mixtures | Ref. |
|---|---|---|---|---|---|---|
| Sesuvium portulacastrum L. | G(+) bacteria: Bacillus subtilis NCIM 2063, B. pumilus NCIM 2327, Micrococcus luteus NCIM 2376 and S. aureus NCIM 2901; G(-) bacteria: P. aeruginosa NCIM 5031, K. pneumoniae NCIM 2957 and E. coli NCIM 2256. Ten isolates of MRSA and of MRSA NCTC 6571. Human pathogenic yeast type fungi: Candida albicans, C. krusei, C. tropicalis and C. parapsilosis and mould fungi: Aspergillus niger, A. flavus, and A. fumigatus | Inhibition zone (IZ) by disk diffusion test and minimum inhibitory concentration (MIC) by broth macrodilution method | Ciprofloxacin for bacteria, methicillin, oxacillin and vancomycin for MRSA and amphotericin-B for fungi | MIC: 0.25 mg/mL for B. subtilis, 0.5 mg/mL for S. aureus, MRSA, P. aeruginosa, K. pneumoniae and C. albicans, and 1.0 mg/mL for E. coli; MBC: 0.5 mg/mL for B. subtilis, 1.0 mg/mL for S. aureus, MRSA and K. pneumoniae, and 2.0 mg/mL for P. aeruginosa and E. coli; MFC: 1 mg/mL for C. albicans | FAME | [60] |
| Blutaparon portulacoides (A. St.-Hil.) Mears | Trypanosoma cruzi, Leishmania amazonensis, S. aureus ATCC 25923 and 7+ penicillinase producer, Streptococcus epidermidis (6ep), E. coli ATCC 10538, Streptococcus mutans (9.1), Streptococcus sobrinus (180.3) | Crude extracts and isolated compounds added to the trypomastigote-containing blood samples and incubated 24 h at 4 °C. Trypanocidal activity evaluated by counting the remaining trypomastigotes. L. amazonensis amastigote viability assessed colorimetrically by the reduction of a tetrazolium salt (MTT). Antimicrobial activity measured by the well-diffusionmethod in double layer | Gentian violet for trypanocidal activity and gentamicin for antibacterial assays | MIC: 100–500 μg/mL in T. cruzi trypomastigotes and 14–500 μg/mL in L. amazonensis amastigotes; 50 μg/mL in E. coli, S. aureus ATCC 25923, S. aureus (7+) and 500 μg/mL in S. epidermidis, S. mutans, and S. sobrinus | Acyl steryl glycosides (sitosteryl 3-β-O-glucoside 6’-O-palmitate and stigmasteryl 3-β-O-glucoside 6’-O-palmitate) | [70] |
| Arthrocnemum indicum (Willd.) Moq., Salicornia brachiata Roxb., Suaeda maritima (L.) Dumort. and Suaeda monoica Forsk. | G(+) bacteria: B. subtilis NCIM 2063, B. pumilus NCIM 2327, M. luteus NCIM 2376, and S. aureus NCIM 2901; G(-) bacteria: P. aeruginosa NCIM 5031, K. pneumoniae NCIM 2957, and E. coli NCIM 2256; ten isolates of MRSA and of MRSA NCTC 6571; yeasts (C. albicans, C. krusei, C. tropicalis, and C. parapsilosis) and molds (A. niger, A. flavus, and A. fumigatus) | Disk diffusion method and broth macrodilution method | Ciprofloxacin for bacteria, methicillin, oxacillin and vancomycin for MRSA and amphotericin-B for fungi | MIC of 0.06 mg/mL of S. brachiata extracts against B. subtilis, S. aureus, and MRSA, and 0.5 mg/mL against P. aeruginosa; MIC of 0.5 mg/mL of all FAME extracts against E. coli and K. pneumoniae; MBC of 0.1 mg/mL of S. brachiata extracts against P. aeruginosa and of 1.0 mg/mL of all FAME extracts against E. coli and K. pneumoniae | FAME | [61] |
| Alternanthera brasiliana | E. coli ATCC 25922, B. subtilis ATCC 6623, P. aeruginosa ATCC 15442, M. luteus ATCC 9341, and S. aureus ATCC 25923 | Microdilution broth method according to NCCLS standardization | Tetracycline and norfloxacin | MIC: 50 μg/mL against B. subtilis, M. luteus, and S. aureus | Linoleate oxylipins | [65] |
| Phoenix dactylifera L. | Bacillus cereus and E. coli | Disk diffusion method | Streptomycin | 20 mm against E. coli and 17 mm against B. cereus at 1 mg/mL of the acetone extract | Sterol and triterpenes | [66] |
| Asphodelus aestivus Brot. | G(+) bacteria: S. aureus ATCC 6538-p, E. faecalis ATCC 29212; G(-) bacteria: E. coli ATCC 29998, K. pneumoniae ATCC 13883, P. aeruginosa ATCC 27853); yeasts: C. albicans ATCC 10239 and C. krusei ATCC 6258 | Disk diffusion method and broth microdilution tests according to the recommendations of Clinical and Laboratory Standards Institute (CLSI) | Ampicillin, ciprofloxacin and fluconazole | MIC: 512 μg/mL against S. aureus, E. faecalis, K. pneumoniae, and C. albicans | FA (4:0, 6:0, 8:0, 10:0, 16:0, 18:0, 21:0, 24:0, 14:1, 15:1, 18:1n9t, 20:1, 24:1, 18:2, 18:2n6t, 18:2n6c, 20:2n6, 20:3n3, 22:6n3, and others unidentified) | [54] |
| Artemisia incisa Pamp. | S. epidermidis and S. aureus | Agar well diffusion method and MIC determined by a referenced method | Streptomycin and tetracycline | S. epidermidis (0.0157 mg/mL) and S. aureus (0.0313 mg/mL) | Artemceramide-B | [73] |
| Pteranthus dichotomus Forssk. (also known as P. echinatus Desf.) | S. aureus ATCC 25923, E. coli ATCC 25922, K. pneumoniae ESBL, and Enterobacter sp. ESBL | Disk diffusion method | Gentamicin and ampicillin | P. dichotomus BuOH extracts at 0.25 g/mL (8 mm against E. coli, K. pneumoniae ESBL); P. dichotomus EtOAc extract at 0.5 g/mL (7 mm against E. coli), at 65 mg/mL (8.33 mm against S. aureus), and at 0.25 g/mL (7 mm against Enterobacter sp. ESBL) | BuOH fraction contained 1-O-palmitoyl-3-O-(6-sulfo-α-D-quinovopyranosyl)-glycerol, 1,2-di-O-palmitoyl-3-O-(6-sulfo-α-D-quinovopyranosyl)-glycerol and soya cerebroside I. EtOAc fraction contained stigmat-7-en-3-ol, spinasterol, β-sitosterol and β-sitosterol-3-O-glucoside | [78] |
| Cucumis sativus L. | Phytopathogenic fungi (Pythium aphanidermatum, Botryosphaeria dothidea, Fusarium oxysporum f.sp. cucumerinum, and Botrytis cinerea); phytopathogenic bacteria [G(-): Xanthomonas vesicatoria ATCC 11633, Pseudomonas lachrymans ATCC 11921, and G(+) B. subtilis ATCC 11562] | Mycelial radial growth inhibition assay and antifungal activity (pour plating method in potato dextrose agar medium) for fungi and agar-well diffusion assay for bacteria | Carbendazim for fungi and streptomycin sulfate for bacteria | 5.5–100 inhibitory rate of mycelia growth inhibitory activity; IC50 of B. subtilis (50.2–110.9 μg/mL), X. vesicatoria (25.6–64.5 μg/mL), P. lachrymans (15.3–37.3 μg/mL) for sphingolipids | Sphingolipids [(2S,3S,4R,10E)-2-[(2’R)-2-hydroxytetracosanoylamino]-1,3,4-octadecanetriol-10-ene, 1-O-β-D-glucopyranosyl-(2S,3S,4R,10E)-2-[(2’R)-2-hydroxytetracosanoylamino]-1,3,4-octadecanetriol-10-ene and soya-cerebroside I] | [74] |
| Excoecaria agallocha | G(+) bacteria: B. subtilis NCIM 2063, B. pumilus NCIM 2327, M. luteus NCIM 2376, S. aureus NCIM 2901; G(-) bacteria: P. aeruginosa NCIM 5031, K. pneumoniae NICM 2957, and E. coli NCIM 2256; yeasts: C. albicans, C. krusei, C. tropicalis, and C. parapsilosis | Disk diffusion method for antibacterial and antifungal susceptibility tests; MIC tested in Mueller-Hinton broth for bacteria and yeast nitrogen base for yeasts by two-fold serial dilution method | Ciprofloxacin and amphotericin B | MIC: 0.125 mg for B. subtilis and S. aureus, 0.5 mg for P. aeruginosa and K. pneumoniae, and 1.0 mg for E. coli; MBC: 0.25 mg for B. subtilis and S. aureus, 1.0 mg for P. aeruginosa and K. pneumoniae, and 2.0 mg for E. coli; MFC: 1 mg for C. albicans, C. krusei and C. parapsilosis | FAME | [62] |
| Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC) | Bacteria (E. coli, P. aeruginosa, B. subtilis, S. aureus) and yeast (C. albicans) | Modified agar overlay method | Chloramphenicol for bacteria and miconazole for fungi | MIQ: 1 μg of n-hexane and CHCl3 extracts of A. adianthifolia against E. coli; 50 μg of n-hexane and CHCl3 extracts of A. adianthifolia against P. aeruginosa; 50 μg of CHCl3 extract of P. angolensis against B. subtilis and 100 μg of n-hexane extract of P. angolensis against B. subtilis and C. albicans | n-hexadecanoic acid (palmitic acid); oleic acid; chondrillasterol; stigmasterol, 24S 5α-stigmast-7-en-3-ol; 9,12-octadecadienoic acid (Z,Z)-, methyl ester; trans-13-octadecanoic acid, methyl ester; tetradecanoic acid; hexadecanoic acid, methyl ester; octadecanoic acid | [79] |
| Baphia massaiensis | E. coli, B. subtilis, P. aeruginosa, S. aureus, and C. albicans | Agar well diffusion method | Not mentioned | 10 mm of inhibition zone against E. coli and S. aureus, and 16 mm against B. subtilis | Seed oil (total FA) | [80] |
| Cassia tora L. (or Senna tora L. Roxb.) | MRSA, MSSA, B. subtilis, and P. aeruginosa | Broth microdilution method | Ampicillin | MIC for all bacteria between 125–1000 μg/mL | FA (the major were palmitic acid, linoleic acid, linolenic acid, margaric acid, melissic acid, and behenic acid) | [55] |
| Trigonella foenum-graecum L. | G(-) bacteria: E. coli and P. aeruginosa; G(+) bacteria: S. aureus and Streptococcus pyogenes; acid-fast bacteria: M. tuberculosis; fungi: C. albicans, A. niger and A. clavatus; parasite Plasmodium falciparum (etiological agent of malaria) | Antimicrobial activity assessed by broth dilution method, anti-tuberculosis activity assessed by the slope method, in vitro anti-malarial assay according to a microassay protocol | Gentamycin, chloramphenicol, ciprofloxacin and norfloxacin for bacteria; isoniazid and rifampicin for mycobacteria; nystatin and greseofulvin for fungi; chloroquine and quinine as anti-malarials | MIC values of 100, 250, 125 μg/mL towards E. coli, S. aureus, and S. pyogenes and P. aeruginosa, respectively. MFC value of 250 μg/mL of C. albicans. MIC value of 100 μg/mL toward M. tuberculosis and of 0.29 μg/mL toward P. falciparum | Conjugated linoleic acid methyl ester, saturated FAME, steroids | [63] |
| Quercus leucotrichophora, A. Camus | G(+) bacteria: B. subtilis and S. aureus; G(-) bacteria: P. aeruginosa and E. coli | Disk diffusion method for antibacterial susceptibility tests; MIC was tested in Mueller-Hinton broth for bacteria by two-fold serial dilution method | Ciprofloxacin | MIC: 0.125 mg/mL for B. subtilis and S. aureus; 0.5 mg/mL for P. aeruginosa and 1.0 mg/mL for E. coli | FAME | [56] |
| Quercus leucotrichophora A. Camus | G(-) bacteria: E. coli MTCC-582 and P. aeruginosa MTCC-2295; G(+) bacteria: S. aureus MTCC-3160, B. subtilis MTCC-441 and S. pyogenes MTCC-1924 | Disk diffusion method | Ampicillin | IZ of both extracts against all microorganisms: 8.53 ± 0.50 to 19.07 ± 0.31 mm | FA; linoleic acid in stem bark and leaves extracts and cis-vaccenic acid in stem bark | [81] |
| Vitex altissima L., V. negundo L. and V. trifolia L. | Culex quinquefasciatus (early fourth-instar larvae) | Larvicidal activity analyzed according to standard procedures (WHO-VBC 81.807, 1981) | Not mentioned | V. trifolia (LC50 = 9.26 ppm and LC90 = 21.28 ppm) | FAME | [64] |
| Linum usitatissimum L. | A. flavus MTTC 2799 and A. ochraceus CECT 2092 | Determination of percent mycelial inhibition by growth radial technique on solid medium and by biomass technique on liquid medium | Not mentioned | Antifungal index of FAME in solid medium: 54.19 ± 0.85 at 10 μL in A. flavus and 40.48 ± 0.12 at 90 μL for A. ochraceus at 90 μL | FAME | [57] |
| Scaphium macropodum (Miq.) Beumee ex. Heyne | Mycobacterium smegmatis, E. coli, S. typhimurium, B. subtilis, and S. aureus | Inhibitory activity of the extract by disk diffusion method; broth microdilution assay (MTT assay) was used to determine the MIC; MBC was determined via streak plate method | Ampicillin and rifampicin | IZ of 10.67 ± 0.58 mm in S. aureus and of 9 mm in P. aeruginosa at 0.25 mg/mL. S. aureus showed the lowest MIC (0.78 mg/mL) and MBC (3.13 mg/mL). For M. smegmatis, MIC value was 3.13 mg/mL and MBC was 25 mg/mL | Methyl hexadecanoate, hexadecanoic acid <n-> | [82] |
| Melastoma malabathricum L. | S. aureus ATCC 25923, B. cereus ATCC 10876, P. aeruginosa ATCC 17853, S. typhi laboratory strain | Disk diffusion method | Rifampicin | P. aeruginosa (9 mm at 0.25 mg/mL), S. aureus (7 mm, 1 mg/mL), S. typhi (9 mm at 1 mg/mL), B. cereus (10.5 mm at 2 mg/mL) | Steryl glycoside: β-sitosterol 3-O-β-D-glucopyranoside | [69] |
| Azadirachta indica A. Juss | Multidrug-resistant clinical isolates of S. aureus, Salmonella enterica serovar typhi, S. dysenteriae, E. coli, Vibrio cholerae, K. pneumoniae, and P. aeruginosa | MIC determined by microbroth dilution method and antibacterial sensitivity of SQDG determined by disk diffusion method (CLSI protocol) | Not mentioned | MIC of 32 μg/mL for S. typhi and two isolates of S. dysenteriae; MIC of 64 μg/mL for three isolates of S. typhi, E. coli and V. cholerae and 256 μg/mL for K. pneumoniae | SQDG | [71] |
| Azadirachta indica A. Juss | Raillietina spp. (helminth parasite) | Ultrastructural changes by scanning electron microscopy | Praziquantel | Anthelmintic activity of SQDG with 0.5 and 1 mg/mL, respectively: paralysis time of 1 h and 0.7 h; death time of 1.6 h and 0.9 h | SQDG | [72] |
| Carapa guianensis and Carapa vasquezii | Phytopathogenic fungi: A. flavus, A. niger, and F. oxysporum | Fungal mycelial growth inhibition trials developed in 96-well microtiter plates adding 10 μL of conidia suspensions (2 × 105 conidia mL−1) and 90 μL yeast peptone dextrose. Inhibition of germination observed under light microscopy | 20 mM hydrogen peroxide | MIC (μg/mL): 125–250 of C. guianensis and 15.6–125 of C. vasquezii against the three phytopathogenic fungi | FA, FAME, squalene, β-sitosterol | [58] |
| Ficus lutea Vahl | Mucor miehei and B. subtilis | Disk diffusion method | Nystatin | IZ of 17 mm for M. miehei; of 16 mm for B. subtilis; and of 12 mm for C. albicans exposed to 40 μg of compound | Glycosphingolipid [1-O-β-D-glucopyranosyl-(2S,3R,5E,12E)-2N-[(2′R)-hydroxyhexadecanoyl]-octadecasphinga-5,12-dienine] named lutaoside | [76] |
| Ficus pandurata Hance | Yeast: C. albicans ATCC 90028, C. glabrata ATCC 90030, C. krusei ATCC 6258, A. fumigatus ATCC 90906, MRSA ATCC 33591, Cryptococcus neoformans ATCC 90113, S. aureus ATCC 2921, E. coli ATCC 35218, K. pneumoniae ATCC 13883, P. aeruginosa ATCC 27853, and Mycobacterium intracellulare ATCC 23068); chloroquine sensitive (D6, Sierra Leone) and resistant (W2, Indochina) strains of Plasmodium falciparum; parasite: Leishmania donovani promastigotes | Modified versions of the NCCLS methods | Antibacterial agent and antifungal agents not mentioned; antimalarial agents: chloroquine and artemisinin; anti-leishmanial agents: pentamidine and amphotericin B | No activity was observed for any compound | Ceramides [panduramides A-D, and newbouldiamide] | [75] |
| Kunzea ericoides (A. Rich) J. Thompson | E. coli ATCC 25922 and S. aureus ATCC 25923 | Broth microdilution method utilizing the redox dye resazurin | Not mentioned | 0.625–10 mg/mL for S. aureus and more than 10 mg/mL in E. coli | Steryl esters, triacylglycerols, free FA, sterols and phospholipids | [77] |
| Pentagonia gigantifolia Ducke | C. albicans ATCC 90028 and fluconazole-resistant C. albicans strains | MIC and MFC determined by using a modified version of the microdilution NCCLS methods; sphingolipid reversal assay | Amphotericin B, fluconazole and flucytosine | C. albicans ATCC 90028 (0.52 to 1.04 μg/mL) | Acetylenic acids: 6-octadecynoic acid and 6-nonadecynoic acid | [38] |
| Hedyotis pilulifera (Pit.) T.N. Ninh | S. aureus NBRC 100910, B. subtilis NBRC 13719, M. smegmatis NBRC 13167 | Microdilution method | Ampicillin | Oleanolic acid (MIC value of 2.5 μg/mL against M. smegmatis), rotungenic acid (MIC value of 2.5, 2.5, and 1.25 μg/mL against S. aureus, B. subtilis, and M. smegmatis, respectively), rotundic acid (MIC value of 5 μg/mL against B. subtilis) | Triterpenoids, steroids, FA, glycolipids, and a ceramide | [83] |
| Withania somnifera (L.) Dunal, Euphorbia hirta L., Terminalia chebula Retz. | E. coli MTCC 46, P. aeruginosa MTCC 1934), Proteus mirabilis MTCC 3310, Raoultella planticola MTCC 2271, Enterobacter aerogenes (now Klebsiella aerogenes) MTCC 2822, B. subtilis MTCC 121, S. aureus MTCC 3160 | Disk diffusion method for antibiotic susceptibility testing. Broth microdilution method for determination of MIC values | Streptomycin | MIC: W. somnifera leaf (0.039 mg/mL on P. aeruginosa); T. chebula fruits and stems (0.039 mg/mL on E. coli) and T. chebula stems and fruits (0.039 mg/mL on S. aureus): MBC of 0.039 mg/mL of T. chebula bark on S. aureus | Sterols fraction | [67] |
| Kaempferia pandurata Roxb. (synonym of Boesenbergia rotunda (L.) Mansf.) and Senna alata (L.) Roxb. | MRSA, extended spectrum beta-lactamase (ESBL), and carbapenemase-resistant Enterobacteriaceae (CRE) | Broth microdilution method | Tetracycline and vancomycin for MRSA, cefotaxime and meropenem for ESBL-producing bacteria and for CRE | MIC: K. pandurata extract (256 μg/mL) and S. alata extract (512 μg/mL) against MRSA | Sterols and triterpenoid | [68] |
| Zygophyllum oxianum Boriss. | S. aureus ATCC 29213 and B. subtilis ATCC 6059 | Modified disk diffusion method | Ampicillin, gentamicin sulfate, and nystatin | MIC: 2-mg leaves and stems extract (8 mm in S. aureus and 6 mm in B. subtilis, weak antibacterial activity); 2 mg-BuOH extract of whole air-dried aerial organs extract (5 mm in B. subtilis, 4 mm in E. coli and 20 mm in C. maltosa, good antifungal activity) | Total lipid extract from leaves, stems and aerial organs (hydrocarbons, triterpenol and steryl esters, triacylglycerols, free FA, sterols, phospholipids) | [59] |
| Scientific Name | Collection Site | Extracting Solvent(s)/Method | Isolated Lipids or Lipid Classes | Methods for Compounds Identification | Ref. |
|---|---|---|---|---|---|
| Macroalgae–Chlorophyta | |||||
| Caulerpa racemosa | Qionghai, Hainan, China | EtOH (95%). Extract partitioned with EtOAc and n-BuOH | SQDG [2S-1,2-di-O-palmitoyl-3-O-(6’sulfo-α-D-quinovopyranosyl) glycerol] | 1H and 13C NMR, ESI-MS | [95] |
| Caulerpa racemosa, Caulerpa lentillifera | Port Dickson, Malaysia | CHCl3, MeOH | PUFA, MUFA, Terpenoids | LC-MS | [96] |
| Caulerpa racemosa, Ulva fasciata | Buzios, Rio de Janeiro, Brazil | Acetone insoluble material extracted with CHCl3/MeOH (2:1 and 1:2, v/v). Lipid extract partitioned on SiO2 column, eluted with CHCl3, acetone or MeOH | Glycolipid-rich extracts (Sulfoglycolipids, Glycosyldiacylglycerols) | HPTLC | [97] |
| Caulerpa spp., Chlorodesmis fastigiata, Halimeda spp., Penicillus capitatus, Penicillus dumentosus, Penicillus pyriformis, Rhipocephalus phoenix, Udotea argentea, Udotea cyathiformis, Udotea flabellum, Udotea petiolata | Bahamas, Florida Keys, Puerto Rico, Belize, Guan, Hawaii, Australia, Mediterranean Sea | CH2Cl2. Chlorophylls removed with MgO3Si. Fractionation with SiO2 column and purification by HPLC | Sequiterpenoids, Diterpenoids | TLC, NMR, HPLC | [98] |
| Chaetomorpha linum | IMTA, Mar Piccolo of Taranto, Italy | CHCl3/MeOH (2:1, v/v), Soxhlet extractor, EtOH (95%) | Lipid extracts | 1H and 13C NMR, 1D and 2D NMR, GC-FID, TLC | [99] |
| Codium amplivesiculatum | Bahía Magdalena, Mexico | CH2Cl2/EtOH (97:3, v/v). Liquid/liquid extraction CH2Cl2/H2O. Fractionation with CH2Cl2. Crystallization in hot MeOH | Fraction with clerosterol as main constituent. Isolated clerosterol did not show activity | 1H NMR, IR | [100] |
| Ulva fasciata | Malvan, India | EtOH, fractionated by neutral alumina column with EtOAc/MeOH | Sphingosine (major component: N-palmitoyl-2-amino-1,3,4,5-tetrahydroxyoctadecane) | 1H and 13C NMR, FAB-MS, IR | [101] |
| Malvan, India | EtOH (90%). Extract fractionated. n-hexane fraction chromatographed on SiO2 and flash SiO2 | Ceramide (Erythro-sphinga-4,8-dienine-N-palmitate) | 1H and 13C NMR, ESI-MS, GC-MS, IR | [102] | |
| Mediterranean Sea, Egypt | CHCl3/MeOH (2:1, v/v). Glycolipid separation using acetone on SiO2 column | Glycolipid-rich extracts (DGDG) | GC-FID, LC-MS/MS | [103] | |
| Mediterranean Sea, Egypt | MeOH/CHCl3 (2:1, v/v). Sulfolipid isolation: diethylaminoethyl-cellulose column eluted with CHCl3/MeOH (6:4, v/v) and NH3 | Sulfolipids (SQDG) | GC-MS, GC-FID, LC-MS/MS, IR | [89] | |
| Ulva rigida | Cap Zebib and Ghar El Melh, Tunisia | CH2Cl2 and CH2Cl2/MeOH (1:1, v/v). Extracts fractionated on SiO2 column and TLC with n-hexane/EtOAc/CH2Cl2/MeOH | FA | 1H and 13C NMR, GC | [27] |
| Macroalgae–Rhodophyta | |||||
| Chondria armata | Goa, West coast of India; Mumbai, India | MeOH and CHCl3, Polar fractions: petroleum ether/EtOAc (1:1, v/v), MeOH/CHCl3 (2:98, v/v), MeOH/CHCl3 (5:95, v/v) | Neutral glycolipids [main compound MGDG(20:5/16:0)] | 1H and 13C NMR, ESI-MS/MS | [104] |
| Chondrus crispus, Gracilaria vermiculophylla, Porphyra dioica | IMTA and Portuguese coast, Portugal | EtOAc in Soxhlet extractor | FA | GC-FID | [105] |
| Falkenbergia (heteromorphic sporophyte of Asparagopsis taxiformis) | Kollam coast, India | MeOH. Fractionation on SiO2 column (petroleum ether/EtOAc and EtOAc/MeOH). Purification with TLC and RP HPLC | FA | GC-MS | [106] |
| Galaxaura cylindrica, Laurencia papillosa | Red Sea, Egypt | CHCl3/MeOH (2:1, v/v). Glycolipid separation using acetone on SiO2 columns | Glycolipid-rich extracts (DGDG) | LC-MS/MS | [103] |
| Red Sea, Egypt | MeOH/CHCl3 (2:1, v/v). Sulfolipid isolation: diethylaminoethyl-cellulose column (CHCl3/MeOH (6:4, v/v) and NH3) | Sulfolipids (SQDG) | GC-MS, GC-FID, LC-MS/MS, IR | [89] | |
| Gigartina tenella | Sagami Bay, Kanagawa, Japan | Acetone. Extract partitioned with EtOAc/H2O (3:1, v/v). Organic layer dissolved in EtOAc/MeOH/H2O (100:20:5, v/v) and chromatographed on SiO2 column | Glycolipid (Sulfolipids) | 1H and 13C NMR, HR-FAB-MS | [107] |
| Gracilaria gracilis | Ganzirri lagoon and Margi channel, Eastern Sicily, Italy | CHCl3, Et2O in Soxhlet extractor | FA | GC-FID | [94] |
| Gracilariopsis longissima | Mar Piccolo of Taranto, Italy | CHCl3/MeOH/H2O (2:1:1, v/v) | FA | 1H and 13C NMR, 1D and 2D NMR, GC-FID | [108] |
| Hypnea musciformis, Osmundaria obtusiloba, Porphyra acanthophora, Pterocladiella capillacea | Buzios, Rio de Janeiro, Brazil | Acetone insoluble material extracted with CHCl3/MeOH (2:1 and 1:2, v/v). Extract partitioned on SiO2 column (CHCl3, acetone or MeOH) | Glycolipid-rich extracts (Sulfolipids, Glycosyldiacylglycerols) | HPTLC | [97] |
| Jania corniculata, Laurencia papillosa | Suez Canal, Egypt | EtOH (70%), CH2Cl2 | FA | GC-MS | [93] |
| Laurencia okamurai | Nanji Island in the East China Sea, Zhejiang Province, China | EtOH (95%) extract partitioned with Et2O and fractionated by SiO2 (gradient system: petroleum ether –CH2Cl2 (10:0 → 1:9)), Sephadex column, and purification by semi-preparative C18 HPLC | FA ethyl esters [(9Z,12Z,15Z,18Z,21Z)-ethyl tetracosa-9,12,15,18,21-Pentaenoate, (10Z,13Z)-ethyl nonadeca-10,13-dienoate, (9Z,12Z)-ethyl nonadeca-9,12-dienoate, (Z)-ethyl octadec-13-enoate (4), and (Z)-ethyl hexadec-11-enoate] | 1H and 13C NMR, 1D and 2D NMR, IR, HR-EI-MS | [109] |
| Laurencia spp. | Pulau Tioman, Pahang, Pulau Karah, Terengganu, Pulau Nyireh, Terengganu, Malaysia | MeOH. Extract partitioned with Et2O and H2O and fractionated by SiO2 column (hexane/EtOAc) | Sesquiterpenes (Halogenated sesquiterpenes) | 1H and 13C NMR, LREIMS, HREIMS | [110] |
| Osmundaria obtusiloba | Buzios, Rio de Janeiro, Brazil | Acetone insoluble material extracted with CHCl3/MeOH (2:1 and 1:2, v/v). Extract partitioned (CHCl3/MeOH/0.75% KCl (8:4:3, v/v)). Fractionation on SiO2 column (CHCl3, acetone and MeOH). MeOH fraction purified on SiO2 column (CHCl3/MeOH, 90:10, v/v) | Glycolipids (Sulfoglycolipids) | 1H and 13C NMR, ESI-MS/MS | [111] |
| Palmaria palmata, Grateloupia turuturu | Batz-sur-Mer, France | CH2Cl2/MeOH (2:1, v/v), MeOH/H2O (1:1, v/v) | Polar lipids | 1H and 13C NMR | [112] |
| Pyropia orbicularis | Maitencillo, Chile | MeOH, acetone, CH2Cl2, n-hexane. Soxhlet extractor. n-hexane extract fractionated on SiO2 column (2, 10, 20, 30 and 100% acetone) | Phospholipids (main compounds PC-O, PE, PS, PI, SM, GlCer), Glycolipids (MGDG), Triacylglycerol, DAG | LC-ESI-MS/MS | [113] |
| Sphaerococcus coronopifolius | Atlantic coast of Morocco | MeOH/CH2Cl2. Extract separated on SiO2 column (hexane, gradients of hexane/CH2Cl2 and CH2Cl2/acetone, and MeOH) | Bromoditerpenes (Sphaerolabdadiene-3,14-diol (1), Sphaerococcenol) | 1H and 13C NMR, HRMS, EIMS, CIMS, FTIR, UV | [114] |
| Macroalgae–Ochrophyta | |||||
| Dictyota cervicornis, Dictyota menstrualis | Buzios, Rio de Janeiro, Brazil | Acetone insoluble material extracted with CHCl3/MeOH (2:1 and 1:2, v/v). Extract partitioned on SiO2 column (CHCl3, acetone or MeOH) | Glycolipid-rich extracts (Sulfoglycolipids, Glycosyldiacylglycerols) | HPTLC | [97] |
| Dictyota fasciola, Taonia atomaria | Mediterranean Sea, Egypt | CHCl3/MeOH (2:1, v/v). Glycolipid separation using acetone on SiO2 column | Glycolipid-rich extracts (DGDG) | GC-FID, LC-MS/MS | [103] |
| Mediterranean Sea, Egypt | MeOH/CHCl3 (2:1, v/v). Sulfolipid isolation: diethylaminoethyl-cellulose column (CHCl3/MeOH (6:4, v/v) and NH3) | Glycolipids (Sulfolipids) | GC-MS, GC-FID, LC-MS/MS, IR | [89] | |
| Fucus evanescens | West coast of Ungava Bay, Canada | EtOAc (99%), CH2Cl2. EtOAc algal extract acetylated and organic layer purified by flash chromatography (0% → 50% EtOAc in hexane and flushed with 100% EtOAc and 5% MeOH in 95% EtOAc) | Glycolipid-rich extracts | 1H and 13C NMR | [115] |
| Himanthalia elongata | Las Palmas, Spain | Pressurized liquid extraction Hexane, EtOH, H2O | Sterol (Fucosterol), FA | GC-MS, HPLC-DAD | [116] |
| Laminaria cichorioides | Khasan region of the Primorskii Territory, in the Troitsa Gulf (the Sea of Japan), Russia | EtOH (96%). Lipophilic fraction extracted with CHCl3. Fractionation of lipid classes on SiO2 column | Glycolipids (MGDG, DGDG, SQDG), Free FA, PUFA | [91] | |
| Sargassum dentifolium | Suez Canal, Egypt | EtOH (70%), CH2Cl2 | FA | GC-MS | [93] |
| Sargassum fusiforme, Sargassum vulgare | Red Sea, Egypt | Et2O, MeOH, EtOH, CHCl3 | Terpenoids, FA | GC-MS | [117] |
| Sargassum pallidum | Trinity Bay in the Peter the Great Gulf, Russia | EtOH; EtOH/acetone (1:1, v/v), EtOH/CHCl3 (1:1, v/v). Fractionation of lipid classes on SiO2 column (hexane, Et2O/hexane with increasing ether concentration (95:5 → 50:50, v/v) and CHCl3) | Glycolipids (MGDG, SQDG; DGDG), Free FA/Esters, Triacylglycerols, DAG | [118] | |
| Sargassum vulgare | Sepetiba Bay, Brazil | CHCl3/MeOH (2:1 and 1:2, v/v). Fractionated on SiO2 column (CHCl3, acetone and MeOH) | Glycolipid-rich extracts (Sulfoglycolipids) | 1H and 13C NMR, ESI-MS-MS | [119] |
| Sargassum wightii | Gulf of Mannar, India | MeOH. Isolation on SiO2 column (hexane/EtOAc, EtOAc/MeOH). Purification on flash SiO2 column (CHCl3/MeOH gradients) | Glycolipid (Sulfoglycerolipid, 1-O-palmitoyl-3-O(6′-sulfo-α-quinovopyranosyl)-glycerol) | 1H and 13C NMR, IR | [120] |
| Microalgae | |||||
| Chaetoceros muelleri | Supercritical fluid extraction, EtOH (99.5%) | Triacylglycerol, DAG, MAG, sterols (cholesterol), FA | HPLC-ELSD, GC-FID | [121] | |
| Chlorococcum HS-101 | Japan | MeOH extract, partitioned with hexane. SiO2 column (MeOH/CHCl3 gradient). Active fraction recovered with MeOH/CHCl3 (5:95, v/v) | FA | 1H and 13C NMR, GC-MS | [122] |
| Dunaliella salina | Jerusalem, Israel | Pressurized liquid extracts: hexane, petroleum ether, EtOH | FA Sesquiterpenoids (Neophytadiene), Diterpenoid (Phytol) | GC-MS | [123] |
| Navicula delognei | Lepreau Ledges, New Brunswick, Canada | MeOH, CHCl3, extract chromatographed on SiO2 column (CHCl3 and CHCl3/MeOH) | FA [(6Z,9Z,12Z,15Z)-hexadecatetraenoic acid, (6Z,9Z,12Z,15Z)-octadecatetraenoic acid), Ester ((E)-phytol (5Z,8Z,11Z,14Z,17Z)-eicosapentaenoate] | 1H and 13C NMR, GC-MS | [124] |
| Phaeodactylum tricornutum | Experimental Phycology and Culture Collection of Algae at the University of Göttingen (Germany) | EtOAc and MeOH extracts applied to SiO2 Sep Pak cartridges. EtOAc extract eluted with 10% step increases of hexane/EtOAc until 100% EtOAc. MeOH extract eluted with 10% step increases of EtOAc/MeOH until 100% MeOH | Free FA (palmitoleic acid, HTA) | 1H and 13C NMR, ESI-MS | [125] |
| MeOH/H2O (5:1, v/v). Extract redissolved in MeOH (70%) and fractioned on Sep Pak cartridge (MeOH (70%) followed by constant volumes of MeOH (5% steps → 100%)). Fractionated by RP HPLC | FA (EPA) | 1H NMR, ESI-MS | [126] | ||
| Synechocystis sp. | Las Palmas, Spain | Pressurized liquid extraction: hexane, EtOH, H2O | FA, Sesquiterpenoids (Neophytadiene) | GC-MS, HPLC-DAD | [116] |
| Scientific Name | Antimicrobial Activity | Tested Microorganisms | Antimicrobial Testing Method/Evaluation | Reference Antimicrobial (Positive Control) | MIC, MBC, Diameter of Inhibition Zone (IZ, in mm) or Other | Ref. |
|---|---|---|---|---|---|---|
| Macroalgae–Chlorophyta | ||||||
| Caulerpa racemosa | Antiviral | Viruses: Cox B3, HSV | Cytopathic effect (CPE) reduction assay, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method | Acyclovir (HSV), Ribavirin (Cox B3) | IC50/CC50 (µg/mL)/SI Cox B3: 31.3/500/16 HSV: 7.9/250 | [95] |
| Caulerpa racemosa, Caulerpa lentillifera | Antibacterial | G(+):MRSA (MTCC 381123) G(-): E. coli K1 (MTCC 710859) | Disk diffusion method, crude extracts (CHCl3 and MeOH) | Penicillin-streptomycin | PI: 97.7% (C. racemosa) | [96] |
| Caulerpa racemosa, Ulva fasciata | Antiviral | Viruses: HSV-1-ACVs, HSV-1-ACVr | Titer reduction | [97] | ||
| Caulerpa spp., Chlorodesmis fastigiata, Halimeda spp., Penicillus capitatus, Penicillus dumentosus, Penicillus pyriformis, Rhipocephalus phoenix, Udotea argentea, Udotea cyathiformis, Udotea flabellum, Udotea petiolata | Antibacterial, Antifungal | G(-):Serratia marinoruba, Vibrio splendida, V. harveyi, V. leiognathi, Vibrio sp. Undescribed bacteria: VJP Cal8101, VJP Cal8102, VJP Cal8103 Fungi: Leptosphaeria sp. Lulworthia sp., Alternaria sp., Dreschleria haloides, Lindra thallasiae, Undescribed fungi: VJP Cal8104, VJP Cal8105 | Plate assay-disk method | IZ (mm) > 2 | [98] | |
| Chaetomorpha linum | Antibacterial | G(+): Pseudomonas sp., Staphylococcus sp., Streptococcus agalactiae, Enterococcus sp. G(-): Vibrio alginolyticus, V. harveyi, V. mediterranei, V. ordalii, V. parahaemolyticus, V. salmonicida, V. vulnificus Yeast: C. albicans, Candida famata, C. glabrata | Disk diffusion method | IZ (mm) V. ordalii: 8–12 V. vulnificus: 8–12 | [99] | |
| Codium amplivesiculatum | Antibacterial | G(+): S. aureus (ATCC BAA-42, resistant to methicillin, penicillin, ampicillin/sulbactam, oxacillin, cefalotine) G(-): V. parahaemolyticus (17802) | Disk diffusion method | MIC (µg/mL)/IZ (mm) S. aureus: 125/15 V. parahaemolyticus: >250/8 | [100] | |
| Ulva fasciata | Antiviral | Virus: Semeliki Forest Virus (SFV) | 20 mg/mouse/7 days by giving 50% protection | [101] | ||
| Antiviral | Viruses: Japanese encephalitis virus (JEV), encephalomyocarditis (EMC) virus | 96-well microtiter plates | CC50 (µg/mL)/EC50 (µg/mL)/TI JEV: 7.8/3.9/2.0 | [102] | ||
| Antifungal, Antiviral | Yeast: C. albicans; Fungus: A. niger Virus: HSV-1 | Disk diffusion method; plaque reduction assay (antiviral) | MIC (µg/mL)/IZ (mm) C. albicans: 60/8, A. niger: 80/13 HSV-1: 9.37–15.62% | [103] | ||
| Antibacterial, Antiviral | G(+):B. subtilis RRL B-94 G(-): E. coli NRRL B-3703 Virus: HSV-1 | Disk diffusion method | Chloramphenicol (bacteria), Acyclovir (virus) | MIC (µg/mL)/IZ (mm) PI(%) E. coli: 60/13 B. subtilis: 40/16 HSV-1: 18.75–46.87% | [89] | |
| Ulva rigida | Antibacterial | G(+): S. agalactiae, S. aureus (ATCC 25923), S. aureus (ATCC 6538), E. faecalis (ATCC 29212), Micrococcus sp. G(-): Vibrio tapetis (CECT4600), V. anguillarum (ATCC 12964T), V. alginolyticus (ATCC 17749T), E. coli O126-B16 (ATTC 14948), E. coli (ATCC 25922), E. coli (ATCC 8739), Pseudomonas cepacia, P. fluorescens (AH2), P. aeruginosa (ATCC 27853), Aeromonas salmonicida (LMG3780), A. hydrophila B3, S. typhimurium (C52) Yeast: C. albicans (ATCC10231) | Disk diffusion method and broth microdilution technique | IZ (mm) 6.3–16.3 | [27] | |
| Macroalgae–Rhodophyta | ||||||
| Chondria armata | Antibacterial, Antifungal | G(+): S. aureus G(-): E. coli, P. aeruginosa, S. typhi, Salmonella flexneri, Klebsiella sp., V. cholerae Yeast: C. albicans, C. neoformans, Rhodotorula sp. Fungi: Aspergillus fumigatus, A. niger | Disk diffusion method | Streptomycin, Nystatin | 1 < IZ ≤ 4 | [104] |
| Chondrus crispus, Gracilaria vermiculophylla, Porphyra dioica | Antibacterial, Antifungal | G(+): Listeria innocua (NCTC 11286), B. cereus (ATCC 11778), E. faecalis (LMG S 19456 5002), Lactobacillus brevis (LMG 6906), S. aureus (ATCC 6538), MRSA G(-): E. coli (ATCC 8739), Salmonella enteritidis (ATCC 3076), P. aeruginosa (ATTC 10145) Yeast: Candida spp. (CCUG 49242) | Disk diffusion method | Ampicillin (L. innocua), Cycloheximide (Candida spp.), Chloramphenicol (other microorganisms) | IZ (mm) C. crispus: 5 < IZ ≤ 20 G. vermiculophylla: 10 < IZ ≤ 15 P. dioica: 5 < IZ ≤ 12 | [105] |
| Falkenbergia (heteromorphic sporophyte of Asparagopsis taxiformis) | Antibacterial | G(+): S. epidermidis, S. aureus, B. subtilis G(-): V. vulnificus (MTCC 1145), V. parahaemolyticus (MTCC 451), V. harveyi (MTCC 3438), V. alginolyticus (MTCC 4439), V. alcaligenes (MTCC 4442), P. aeruginosa, K. pneumoniae | Broth dilution method | Chloramphenicol, Nalidixic acid | IZ (mm)/MIC (µg)/MBC (µg) S. epidermidis: 21/1250/270 S. aureus: 21/750/170 B. subtilis: 23/750/180 V. vulnificus: 31/750/90 V. parahaemolyticus: 28/750/110 V. harveyi: 26/750/60 V. alginolyticus: 32/500/80 V. alcaligenes: 33/500/50 P. aeruginosa: 19/1250/420 K. pneumoniae: 15/1250/380 | [106] |
| Galaxaura cylindrica, Laurencia papillosa | Antiviral | Virus: HSV-1 | Plaque reduction assay | Acyclovir | Inhibition (%) L. papillosa: 9.37–31.25 G. cylindrica: 15.62–28.12 | [103] |
| Antibacterial, Antiviral | G(+): B. subtilis NRRL B-94 G(-): E. coli NRRL B-3703 Virus: (HSV-1) | Disk diffusion method | Chloramphenicol (bacteria), Acyclovir (virus) | MIC (µg/mL)/IZ (mm) PI(%) L. papillosa E. coli: -/8 B. subtilis: -/11 HSV-1: 40.62–59.37% G. cylindrica E. coli: 80/11 B. subtilis: 80/11 HSV-1: 45.87–59.37% | [89] | |
| Gigartina tenella | Antiviral | HIV-reverse transcriptase type 1 | IC50 11.2 µM | [107] | ||
| Gracilaria gracilis | Antibacterial | G(+): B. subtilis G(-): V. fischeri, V. cholerae, P. aeruginosa, Salmonella sp., A. hydrophila | Disk diffusion method | Chloramphenicol | MIC: 5 µg/disk IZ (mm) B. subtilis 10.3–17.6 (CHCl3)/10–15.6 (Et2O) | [94] |
| Gracilariopsis longissima | Antibacterial, Antifungal | G(+): S. agalactiae, Enterococcus sp. G(-): P. aeruginosa, V. salmonicida, V. fluvialis, V. vulnificus, V. cholerae non-O1, V. alginolyticus Yeast: C. albicans, C. famata, C. glabrata | Disk diffusion method | IZ (mm) V. alginolyticus: 25 V. fluvialis: 8 V. vulnificus: 15 V. cholerae non-O-1:10 | [108] | |
| Hypnea musciformis, Osmundaria obtusiloba, Porphyra acanthophora, Pterocladiella capillacea | Antiviral | Virus: HSV-1-ACVs, HSV-1-ACVr | Titer reduction | PI(%)/VII O. obtusiloba ACVs-HSV-1: 82.2–99.5/0.75–2.35 HSV-1-ACVr: 99.7–99.9/2.5–4.5 | [97] | |
| Jania corniculata, Laurencia papillosa | Antibacterial, Antifungal | G(+): B. subtilis, Staphylococcus albus, E. faecalis G(-): E. coli Yeast: C. albicans Fungus: A. flavus | Disk diffusion method | 11 < IZ ≤ 15 No IZ (A. flavus) | [93] | |
| Laurencia okamurai | Antifungal | Yeast: C. neoformans (32609), C. glabrata (537) Fungi: Trichophyton rubrum (Cmccftla), A. fumigatus (07544) | Broth dilution method | Amphotericin B, fluconazole, voriconazole, ketoconazole | MIC80 (µg/mL) C. neoformans: 8–64 C. glabrata: 4–64 A. fumigatus: >64 T. rubrum: 64 | [109] |
| Laurencia spp. | Antibacterial | G(-): Chromobacterium violaceum, P. mirabilis, P. vulgaris, Erwinia sp., V. parahaemolyticus, V. alginolyticus | Disk diffusion method | MIC (µg/disk) C. violaceum: 10–40 P. mirabilis: 20–40 P. vulgaris: 20–40 Erwinia sp.: 10–30 V. parahaemolyticus: 20–40 V. alginolyticus: 20–30 | [110] | |
| Osmundaria obtusiloba | Antiviral | Virus: HSV-1, HSV-2 | Titer reduction | Acyclovir | EC50 (µg/mL)/SI/PI(%) HSV-1: 42/1.7/75 HSV-2: 12/6/96 | [111] |
| Palmaria palmata, Grateloupia turuturu | Antibacterial | G(-): V. harveyi ORM4 | Broth microdilution method | 0.2 < PI ≤ 7.9% | [112] | |
| Pyropia orbicularis | Antibacterial | G( + ): S. aureus, B. cereus G(-): E. coli | Disk diffusion method | Kanamycin | 16 < IZ < 26 | [113] |
| Sphaerococcus coronopifolius | Antibacterial, Antiplasmodial | G(+): S. aureus (ATCC # 6538) Parasitic protozoa: P. falsciparum (FCB1) | Antibacterial: Disk-diffusion, Antimalarial: inhibition of [3H]-hypoxanthine uptake by P. falsciparum cultured in human blood | S. aureus-MIC: 0.104–0–146 µM P. falciparum-IC50: 1 µM | [114] | |
| Macroalgae–Ochrophyta | ||||||
| Dictyota cervicornis, Dictyota menstrualis | Antiviral | Virus: HSV-1-ACVs, HSV-1-ACVr | Titer reduction | [97] | ||
| Dictyota fasciola, Taonia atomaria | Antibacterial, Antifungal, Antiviral | G(+): B. subtilis G(-): E. coli Fungi: C. albicans, A. niger Virus: HSV-1 | Disk diffusion method (antibacterial); Plaque reduction assay (antiviral) | D. fasciola: Inhibition (%) HSV-1: 50.00–81.25% T. atomaria: MIC (µm/mL)/IZ(mm) B. subtilis: 80/9, E. coli: 80/7, C. albicans: 80/10, A. niger: 60/12 Inhibition (%) HSV-1: 31.25–34.37% | [103] | |
| Antibacterial, Antiviral | G(+):B. subtilis NRRL B-94 G(-): E. coli NRRL B-3703 Virus: HSV-1 | Disk diffusion method (antibacterial); Plaque reduction assay (antiviral) | Chloramphenicol (bacteria), Acyclovir (virus) | MIC (µg/mL)/IZ (mm) PI(%) D. fasciola E. coli: -/8 B. subtilis: -/10 HSV-1: 46.87–70.12% T. atomaria: E. coli: 60/15 B. subtilis: 40/13 HSV-1: 43.75–56.25% | [89] | |
| Fucus evanescens | Antibacterial | G(+): B. cereus, Clostridium difficile, MRSA, Propionibacterium acnes (ATCC and clinical isolate), S. pyogenes G(-): Acinetobacter baumannii, E. coli, Haemophilus influenzae, K. pneumoniae, Legionella pneumophila, P. aeruginosa | Disk diffusion method | MIC100: 50 µg/mL | [115] | |
| Himanthalia elongata | Antibacterial | G(+): S. aureus ATCC 25923, G(-): E. coli ATCC 11775 Yeast: C. albicans ATCC 60193 Fungi: A. niger ATCC 16404 | Broth microdilution method | MBC (mg/mL) S. aureus: 6.25 E. coli: 6.00 MFC (mg/mL) C. albicans: 8 A. niger: 12 | [116] | |
| Laminaria cichorioides | Antibacterial, Antifungal | G(+): S. aureus ATCC 21027 G(-): E. coli ATCC 15034 Yeast: Safale S04, C. albicans KMM 455 Fungi: A. niger KMM 4634, F. oxysporum KMM 4639 | Disk diffusion method | Fucoxanthin, Nitrofungin | IZ (mm) S. aureus: 2–5 E. coli:1–6 C. albicans: 1–6 A. niger: 1–3 F. oxysporum: 1–4 | [91] |
| Sargassum dentifolium | Antibacterial, Antifungal | G(+): B. subtilis, S. albus, E. faecalis G(-): E. coli Yeast: C. albicans Fungus: A. flavus | Disk diffusion method | 11 < IZ (mm) ≤ 12 No IZ (A. flavus) | [93] | |
| Sargassum fusiforme, Sargassum vulgare | Antibacterial | Multidrug resistant: S. aureus, P. aeruginosa, Shigella flexneri, E. coli, Corynebacterium sp. | Agar well diffusion | 9.33 < IZ (mm) ≤ 23.33 MIC: 50–100 mg/mL | [117] | |
| Sargassum pallidum | Antibacterial, Antifungal | G(+): S. aureus ATCC 21027 G(-): E. coli ATCC 15034 Yeast: C. albicans KMM 455 Fungi: A. niger KMM 4634, F. oxysporum KMM 4639, Septoria glycines | Agar well diffusion | IZ (mm) S. aureus: 0.7–14.5 E. coli: 0.5–6.7 C. albicans: 1.0–4.5 A. niger: 2.0–5.7 F. oxysporum: 1.0–5.2 S. glycines: 2.0–5.7 | [118] | |
| Sargassum vulgare | Antiviral | Virus: HSV-1, HSV-2 | Titer reduction | Acyclovir | PI (%) HSV-1: 96.0–99.9 HSV-2: 99.9 | [119] |
| Sargassum wightii | Antibacterial | Xanthomonas oryzae pv. oryzae CAS ar01 | Disk diffusion method | IZ (mm): 3.0–13.5 | [120] | |
| Microalgae | ||||||
| Chaetoceros muelleri | Antibacterial, Antifungal | G(+): Staphyloccocus aureus (ATCC 25923) G(-):E. coli (ATCC 11775) Yeast: C. albicans (ATCC 60193) | Broth microdilution method | Chloramphenicol, Amphotericin B | MBC (mg/mL) E. coli: 12–15 S. aureus: 12–17 C. albicans: 7–9 | [121] |
| Chlorococcum HS-101 | Antibacterial | G(+): MRSA, S. aureus ATCC 25923 | Disk diffusion method | Gentamicin, Amikacin, Cephalosporin, Habekacin, Ampicillin, Vancomycin, Oxytetracyclin, Erythromycin, Cefmetazole, Fosfomycin, Imipenem, Minomycin | IZ (mm): 18.7–28.3 | [122] |
| Dunaliella salina | Antibacterial, Antifungal | G(+): S. aureus ATCC 25923 G(-): E. coli ATCC 11775 Yeast: C. albicans ATCC 60193 Fungi: A. niger ATCC 16404 | Disk diffusion method | Chloramphenicol (bacteria), Amphotericin B (yeast and fungi) | MBC (mg/mL) E. coli: 6–30 S. aureus: 8–30 MFC (mg/mL) C. albicans: 12–30 A. niger: 32–>35 | [123] |
| Navicula delognei f. elliptica | Antibacterial | G(+): Staphyloccus aureus (ATCC 25923), S. epidermidis (ATCC 12228) G(-): S. typhimurium (ATCC 14028), P. vulgaris (ATCC 13315), Enterobacter cloacae (ATCC 23355), E. coli (ATCC 25922), K. pneumoniae (ATCC 13883), Serratia marcescens (ATCC 8100) | Disk diffusion method | Ampicillin, Tetracycline, Chloramphenicol | IZ (mm) S. aureus >4 S. typhimurium >4 S. epidermidis >2 P. vulgaris >2 E. coli IZ noticeable No IZ (E. cloacae, K. pneumoniae, S. marcescens) | [124] |
| Phaeodactylum tricornutum | Antibacterial, Antifungal | G(+): S. aureus (SH1000), Bacillus weihenstephanensis (10390), MRSA 252, MRSA 16a, S. epidermidis, M. luteus (NCIMB 9278), Planococcus citreus (NCIMB 1493), B. cereus (883-00) G(-): Alteromonas haloplanktis (NCIMB 19), A. hydrophila (NCIMB 1108), Photobacterium phosphoreum (NCIMB 64), Psychrobacter immobilis (NCIMB 308), Listonella anguillarum (MT1637), E. coli B, P. aeruginosa (NCIMB 10775) Yeast: C. glabrata, Candida neoformis, Candida sp., Saccharomyces cerevisiae BY4741a | Disk diffusion method | Ampicillin | IC50 (µM)/MBC (µM) S. aureus: 10–40/40–80 | [125] |
| Antibacterial, Antifungal | G(+):M. luteus (NCIMB 9278), Planococcus citreus (NCIMB 1493), B. cereus (883-00), S. aureus (SH1000), B. weihenstephanensis (10390), MRSA 252, MRSA16a, S. epidermidis G(-): Alteromonas haloplanktis (NCIMB 19), A. hydrophila (NCIMB 1108), Photobacterium phosphoreum (NCIMB 64), Psychrobacter immobilis (NCIMB308), Listonella anguillarum (MT1637), E. coli B, P. aeruginosa (NCIMB 10775) Yeast:C. glabrata, C. neoformis, Candida sp., S. cerevisiae BY4741a | Agar well diffusion | Growth inhibition (mm2) ≤50–>50 S. aureus: 25–190 mm2 | [126] | ||
| Synechocystis sp. | Antibacterial | G(+): S. aureus ATCC 25923 G(+): E. coli ATCC 11775, Yeast: C. albicans ATCC 60193 Fungi: A. niger ATCC 16404 | Broth microdilution method | MBC (mg/mL) S. aureus: 7 E. coli: 5.6 MFC (mg/mL) C. albicans: 12 A. niger: 14 | [116] | |
| Scientific Name | Phylum (Class) | Collection Site | Extracting Solvent(s)/Method | Isolated Lipids or Lipid Classes | Compound Identification Methods | Ref. |
|---|---|---|---|---|---|---|
| Acanthodendrilla sp. | Porifera (Demospongiae) | Gokasho Bay, Tokyo, Japan | MeOH. Aqueous residue extracted with Et2O and n-BuOH. Organic extract fractionated by SiO2 (MeOH/CHCl3), purified by ODS column and C18 RP HPLC | Steroid sulfates | 1H and 13C NMR | [169] |
| Porifera (Demospongiae) | Kundingarengkeke Island, Indonesia | EtOH, acetone and MeOH. Crude extract partitioned between aqueous MeOH and hexane, EtOAc, and BuOH. Hexane extract fractionated on normal-phase SiO2 column (n-hexane/EtOAc, 7:3, v/v). Purification by semi-preparative HPLC | Sesterterpenes (Luffariellolide derivatives, Acantholides) | 1H and 13C NMR, ESI-MS, HR-EI-MS | [190] | |
| Agelas oroides | Porifera (Demospongiae) | Gökçeada, Northern Aegean Sea, Turkey | MeOH, MeOH/CHCl3 (1:1, v/v) and CHCl3. Extract dissolved in MeOH/H2O (9:1, v/v) partitioned against n-hexane. n-hexane, CH3Cl and MeOH extracts fractionated on SiO2 (EtOAc (0 → 100%) in hexane). Sephadex LH20 and C18 flash column | FA | 1H and 13C NMR, 1D and 2D NMR, GC-MS, ESI-MS | [162] |
| Caminus sphaeroconia | Porifera (Demospongiae) | Dominica | MeOH extracts chromatographed on Sepahdex LH 20 (MeOH and EtOAc/MeOH/H2O 20:5:2). Purification by gradient on SiO2 (CH2Cl2 to CH2Cl2/MeOH 9:1, v/v) | Glycolipid (Caminoside) | 1H and 13C NMR, ESI-MS | [147] |
| Dominica | MeOH extract purified by Sephadex LH-20 (MeOH). Sephadex LH-20 (EtOAc/MeOH/H2O (20:5:2, v/v)) | Glycolipid (Caminoside) | 1H and 13C NMR, ESI-MS | [176] | ||
| Dysidea arenaria | Porifera (Demospongiae) | Hainan Island, South China Sea, China | CHCl3-soluble portion was repartitioned between petroleum ether and 90% MeOH. MeOH extract on flash SiO2 column (ether/EtOAc gradient) | Sesquiterpenoid (Sesquiterpenoid hydroquinone) | 1H and 13C-NMR, ESI-MS | [184] |
| Dysidea sp. | Porifera (Demospongiae) | Lakshadweep Islands, Kerala, India | EtOAc and MeOH. EtOAc extract chromatographed on Sephadex LH20 (MeOH/CHCl3, 1:1, v/v), SiO2 (2% EtOAc petroleum ether) | Sesterterpenes (Sesterterpene sulfates) | 1H and 13C NMR, HR-FAB-MS | [183] |
| Erylus lendenfeldi | Porifera (Demospongiae) | Gulf of Eilat, Red Sea | MeOH/CHCl3, RP on a C18 column (decreasing percentage of H2O in MeOH) | Steroidal glycoside (Eryloside) | 1H and 13C NMR, UV, IR | [172] |
| Erylus placenta | Porifera (Demospongiae) | Hachijo Island, Japan | n-PrOH/H2O (3:1, v/v). Extracts partitioned between H2O and CHCl3. H2O layer partitioned between n-BuOH and H2O. BuOH fraction separated by C18 flash (n-PrOH/H2O (1:9, 3:7, 5:5, and 8:2, v/v) and CHCl3/MeOH/H2O(6:4:1, v/v)) | Steroidal glycoside (Sokodosides) | 1H and 13C NMR, GC-FID, UV | [174] |
| Euryspongia sp. | Porifera (Demospongiae) | Light House Reef, Koror, Palau | MeOH. Extracts fractionated by HP20SS column (acetone/H2O) | Steroid sulfates (Eurysterols) | 1H and 13C NMR, ESI-MS, UV, IR | [167] |
| Fasciospongia sp. | Porifera (Demospongiae) | Cape Leeuwin, Western Australia | EtOH extract partitioned into n-BuOH and H2O soluble fractions. n-BuOH fraction subsequently defatted by sequential trituration in n-hexane and CH2Cl2 soluble fractions. CH2Cl2 fractions subjected to SPE or HLPC | Meroterpene (Meroterpene sulfate fascioquinol) | 1H and 13C NMR, HR-ESI-MS, UV | [186] |
| Halichondria sp. | Porifera (Demospongiae) | Unten Port, Okinawa, Japan | Methanolic extract partitioned between H2O and EtOAc. EtOAc soluble material subjected to SiO2 column (CHCl3/MeOH, (95:5, v/v) and petroleum ether/Et2O (9:1, v/v)) | Sesquiterpenoids (Halichonadins) | 1H and 13C NMR, EI-MS, IR | [187] |
| Porifera (Demospongiae) | MeOH extract partitioned between H2O and EtOAc. EtOAc-soluble material subjected to SiO2 column (n-hexane/EtOAc, 1:1 → MeOH). MeOH fraction subjected to SiO2 column (CHCl3/MeOH, 95:5 → 7:3). CHCl3/MeOH (7:3) fraction separated on SiO2 column (EtOAc/MeOH, 5:1 → MeOH) | Sesquiterpenoid (Halichonadins) | 1H and 13C NMR, ESI-MS, HR-ESI-MS, IR | [188] | ||
| Haliclona simulans | Porifera (Demospongiae) | Kilkieran Bay, Galway, Ireland | Acetone and MeOH extracts subjected to HP20 chromatography (100% H2O → 100% MeOH). Fractionated on flash forward system. SiO2 column (100% hexane → 100% EtOAc) | Steroids (24-vinyl-cholest-9-ene-3β,24-diol, 20-methyl-pregn-6-en-3β-ol,5α,8α-epidioxy, 24-methylenecholesterol) | 1H- and 13C NMR, GC-MS | [170] |
| Jaspis stellifera | Porifera (Demospongiae) | Ishigaki Island, Okinawa, Japan | MeOH. EtOAc soluble material subjected to SiO2 column (CHCI3/MeOH, 9:1 and hexane/EtOAc, 3:7, v/v). EtOAc-soluble material subjected to SiO2 columns and C18 HPLC | Nortriterpenoids (Jaspiferals) | 1H and 13C NMR, EI-MS, UV, IR | [181] |
| Luffariella geometrica | Porifera (Demospongiae) | Great Australian Bight, Australia | CH2Cl2. Sequential fractionation to obtain pure compounds | Sesterterpenes (Luffarins) | 1H and 13C NMR, EI-MS, UV, IR | [178] |
| Luffariella variabilis | Porifera (Demospongiae) | Western Carolines, Palau | CH₂Cl₂, purified by chromatography | Sesterterpenoids (Manoalide) | 1H and 13C NMR, UV, IR | [180] |
| Western Carolines, Palau | CH₂Cl₂, purified by chromatography | Sesterterpenoids (Manoalides) | 1H and 13C NMR, UV, IR | [179] | ||
| Melophlus sarasinorum | Porifera (Demospongiae) | Makassar, Sulawesi Island, Indonesia | Acetone and MeOH. Extract partitioned between EtOAc and H2O. Aqueous extract on HP20 (MeOH, H2O). MeOH eluate on C18 (MeOH and H2O, gradient elution) | Steroidal glycosides (Sarasinoside) | 1H and 13C NMR, HR-ESI-MS, LC-MS, UV | [173] |
| Oceanapia sp. | Porifera (Demospongiae) | Kamagi Bay, Sada Peninsula, Japan | MeOH extracts partitioned between ether and H2O. Organic phase partitioned between n-hexane and MeOH/H2O (9:1, v/v). Aqueous MeOH fraction subjected to C18. Purification on SiO2 column (CHCl3, CHCl3/MeOH (9:1), CHCl3/MeOH/H2O (6:4:1), and MeOH) | Acetylenic acid | 1H and 13C NMR, FAB-MS, UV, IR | [161] |
| Paragrantia cf. waguensis | Porifera (Calcarea) | Onna village, Okinawa, Japan | MeOH extract partitioned between H2O and EtOAc. EtOAc extract subjected to Sephadex LH20 (CH2Cl2/MeOH, 1:1, v/v). Fraction separation on RP HPLC | Acetylenic acid | 1H and 13C NMR, ESI-MS, UV, IR | [163] |
| Petrosia weinbergi | Porifera (Demospongiae) | Acklin Island, Bahamas | MeOH/CHCl3 (1:1, v/v). Aqueous suspension extracted with EtOAc, EtOAc/n-BuOH (1:1), and n-BuOH. Active extracts fractionated by C18 HPLC | Steroid sulfates (Weinbersterol disulfates) | 1H and 13C NMR, FAB-MS, IR | [166] |
| Poecillastra wondoensis, Rhabdastrella wondoensis (two-sponge association) | Porifera (Demospongiae) | Cheju Island, South Korea | MeOH (70%). Extract partitioned with Et2O and H2O. Aqueous phase extracted with n-BuOH, subjected to C18 flash chromatography and Sephadex LH-20. Purification on C18 HPLC | Steroidal glycosides (Wondosterols) | 1H and 13C NMR, FAB-MS, UV, IR | [175] |
| Pseudoceratina purpurea | Porifera (Demospongiae) | Kaunakakai Harbor, O’ahu island, Hawaii, USA | EtOH and methylene chloride. Combined extracts partitioned (hexane, methylene chloride and BuOH) Isolation: SiO2 flash column (hexane). Purification: Sephadex LH-20 | Bromotyramine homoserine-derived (Mololipids) | 1H and 13C NMR, HR-FAB-MS, UV, IR | [191] |
| Axinyssa digitata | Porifera (Demospongiae) | Tunisia | Acetone extract partitioned between H2O and Et2O. Aqueous residue re-extracted with n-BuOH and chromatographed on Sephadex LH-20 column (MeOH) and C18 HPLC | Steroid sulfates (Halistanol sulfates) | 1H and 13C NMR, FAB-MS | [165] |
| Siliquariaspongia sp. | Porifera (Demospongiae) | Motualevu reef, Fiji | H2O and MeOH/CH2Cl2 (1:1, v/v). n-BuOH-soluble material from the aqueous extract and CHCl3-soluble material from the organic extract chromatographed on Sephadex LH-20 (MeOH/H2O, 3:1, v/v). Purification by RP HPLC | Brominated long-chain acids (Motualevic acids) | 1H- and 13C-NMR, LC-MS, HR-ESI-MS, FT-IR | [164] |
| Siphonodictyon coralliphagum | Porifera (Demospongiae) | Lighthouse Reef and Glover Reef, Belize | EtOH. Aqueous suspension extracted with CH2Cl2, EtOAc and n-BuOH. EtOAc extract fractionated by chromatography and purified on SiO2 plates | Phenolic aldehydes (Siphonodictyal) | 1H and 13C NMR, IR, UV | [182] |
| Spheciospongia purpurea | Porifera (Demospongiae) | Weizhou Island, Guangxi Autonomous Region, China | Acetone. Extract resuspended in H2O and partitioned with Et2O. Et2O extract fractionated on SiO2 (petroleum ether/acetone, 1:0 → 0:1), Sephadex LH-20 (CH2Cl2/MeOH, 1:1). Purification by C18 HPLC | Lysophospholipids (PAF(16:0), PAF (16:1 n-5), PAF (18:0), PAF (18:1 n-7), PAF (18:1 n-11), PAF (18:1 n-13)) | 1H and 13C NMR, IR, ESI-MS, HR-ESI-MS, LC-MS/MS | [146] |
| Family Spongiidae | Porifera (Demospongiae) | Unten Port, Okinawa, Japan | MeOH extract partitioned between EtOAc and H2O. H2O-soluble portions extracted with n-BuOH. EtOAc and n-BuOH, soluble materials purified by SiO2 columns and C18 HPLC | Sesquiterpenoid (Sesquiterpenoid quinones, Nakijiquinones) | 1H and 13C NMR, FAB-MS, UV, IR | [185] |
| Suberites domuncula | Porifera (Demospongiae) | Rovinj, Croatia | MeOH and CHCl3. Combined extracts partitioned between H2O and BuOH. Organic layer fractionated by medium-pressure on C18 (linear gradient H2O → MeOH → CHCl3). Purification by RP HPLC | Lysophospholipids (PAF) | 1H NMR, FIA-MS, LC-MS/MS | [157] |
| Topsentia sp. | Porifera (Demospongiae) | Chuuk, Federated States of Micronesia | CH2Cl2/MeOH (1:1, v/v). Extract fractionated by SPE using C18 cartridges | Steroid sulfates (Eurysterols) | 1H and 13C NMR, HR-ESI-MS, IR, UV | [168] |
| Xestospongia sp. | Porifera (Demospongiae) | Rasch Pass of Madang, Papua New Guinea | Hexane, CH2Cl2 and EtOAc. Hexane extract subjected to flash column (gradient hexane/EtOAc (95:5, v/v) → EtOAc) | Brominated FA | 1H and 13C NMR, IR, UV, HR-EI-MS, HR-APCI-MS, HR-FAB-MS | [138] |
| Hyas araneus, Podopthalmus vigil, Lauridromia dehanni, Charybdis helleri, Portunus sanguinolentus, Portunus pelagicus | Arthropoda (Malacostraca) | Vellar Estuary, India | MeOH | Lysoglycerolipids/glycerides FA/esters | 1H and 13C NMR, ESI-MS/MS, FT-IR | [148] |
| Meganyctiphanes norvegica | Arthropoda (Malacostraca) | Straits of Messina, central Mediterranean Sea, Italy | H2O/acetone (1:1, v/v). Supernatant recovered with CH₂Cl₂. Extracts fractionated by semi-preparative RP HPLC-DAD on SB-C8 column | FA (EPA, DHA, ETA) | LC-MS, HPLC-UV-HR-MS | [141] |
| Aplidium sp. | Chordata (Ascidiacea) | Northland, New Zealand | MeOH-CH2Cl2 extract fractionated with RF C18 flash column (MeOH/H2O), Sephadex LH20 (MeOH), semi-preparative C18 HPLC | Meroterpene derivatives (Rossinones) | 1H and 13C NMR, HR-FAB-MS, UV, IR | [189] |
| Eunicea succinea | Cnidaria (Anthozoa) | Mona Island, Puerto Rico, | CHCl3/MeOH (1:1, v/v). Extract fractionated by SiO2 column chromatography | FA ((5Z,9Z)-14-methyl-5,9-pentadecadienoic acid) | 1H and 13C NMR, GC-MS, HR-MS, IR | [137] |
| Lobophytum crassum | Cnidaria (Anthozoa) | Rameswaram, India | Aqueous EtOH (95%), MeOH. Extract partitioned with H2O and EtOAc. EtOAc extract fractionated on SiO2 column (gradient hexane/EtOAc) | Cembranoid diterpene Ceramide | 1H and 13C NMR, FAB-MS, IR | [177] |
| Antillogorgia elisabethae | Cnidaria (Anthozoa) | Bahamas | MeOH. Extract redissolved in EtOH/H2O (2:8, v/v). EtOH/H2O extract reextracted with EtOAc, SiO2 column (hexane, 0 → 100% EtOAc/hexane, 0 → 100% MeOH/EtOAc). Fractions subjected to RP HPLC (0 → 100% H2O/Acetonitrile) | Diterpenes (Elisabethin) | 1H, 13C NMR, HR-EI-MS, UV, IR | [192] |
| Sinularia grandilobata, Sinularia sp. | Cnidaria (Anthozoa) | Andaman Islands, India | EtOH. Extract reextracted with EtOAc. Combined extracts fractionated on SiO2 column (gradient system hexane/EtOAc (100:0 → 0:100)) | Sphingolipids Glycolipids | 1H and 13C NMR, EI-MS | [139] |
| Holothuria scabra | Echinodermata (Echinozoa) | Red Sea, Egypt | EtOH (70%), MeOH, EtOAc and CHCl3/MeOH (2:1, v/v) | Pigments (Carotenoids) | HPLC-UV/VIS, GC-MS | [193] |
| Dosidicus gigas | Mollusca (Cephalopoda) | Hermosillo, Mexico | Acidified MeOH (MeOH/HCl, 99:1, v/v) | Pigments (Ommochrome) | 1H and 13C NMR, FT-IR, | [144] |
| Saccostrea glomerata | Mollusca (Bivalvia) | Kovalam, Tamilnadu, India | Hexane, EtOAc and MeOH. Purification on SiO2 column (hexane/EtOAc and EtOAc/MeOH) | Sterols [Cholesta-5,22-dien-3β-ol, Cholesterol, Ergosta-5,22-dien-3-ol, (3β,22E)-], FA (6-Octadecenoic acid, Octadecanoic acid) | GC-MS, FT-IR | [171] |
| Scientific Name | Activity | Tested (Micro)Organisms | Antimicrobial Testing Method/Evaluation | Reference Antimicrobial (Positive Control) | MIC, Diameter of Inhibition Zone (IZ) or Other | Ref. |
|---|---|---|---|---|---|---|
| Acanthodendrilla sp. | Antifungal | Yeast: S. cerevisiae (A364A, STX338-2C, 14028g, GT160-45C) | Disk diffusion method | IZ (mm): 7–11 | [169] | |
| Antibacterial Antifungal | G(+): S. aureus, B. subtilis G(-): E. coli, Yeast: C. albicans Fungi: Cladosporium herbarum | Disk diffusion method | IZ (mm) S. aureus: 7–11 B. subtilis: 7–12 E. coli: 7–12 C. albicans: 9–10 C. herbarum: 10–20 | [190] | ||
| Agelas oroides | Antibacterial, Antiplasmodial | Acid-fast bacterium: M. tuberculosis Parasitic protozoa: P. falciparum, Trypanosoma brucei rhodesiense, T. cruzi, L. donovani G(-): E. coli | [3H]-hypoxanthine incorporation assay, 96-well microtiter plates, inhibition of enzymatic activity | Artemisinin, Benznidazole, Melarsoprol, Miltefosine, Podophyllotoxin, Triclosan | IC50 (µg/mL) Antibacterial M. tuberculosis: 9.4–>50 E. coli: 0.07–>50 Antiprotozoal: 0.35–>30 | [162] |
| Caminus sphaeroconia | Antibacterial | G(+): MRSA, Enterococcus (VRE) G(-): E. coli | in vitro inhibition | MIC (µg/mL) MRSA: 12 VRE: 12 E. coli: > 100 | [147] | |
| Antibacterial | G(+): MRSA, Enterococcus (VRE) G(-): Xanthomonas maltophilia Plant pathogen: Pythium ultimum | Disk diffusion method | MIC (µg/disk) MRSA: 6.3–>100 VRE: 3.1–>100 X. maltophilia: 25–>100 P. ultimum: 25–>100 | [176] | ||
| Dysidea arenaria | Antiviral | Virus: HIV-1 | PFA | IC50 (µM) 16.4–239.7 | [184] | |
| Dysidea sp. | Antibacterial | G(+): S. aureus, B. subtilis, M. luteus G(-): P. vulgaris, S. typhimurium, E. coli | Broth macrodilution method | Linezolid | MIC (µg/mL) 0.117–>15 | [183] |
| Erylus lendenfeldi | Antifungal | Yeast: C. albicans | MIC (µg/mL) 15.6 | [172] | ||
| Erylus placenta | Antifungal | G(+): S. aureus G(-): E. coli Fungus: Mortierella ramanniana Yeasts: S. cerevisiae (cdc28, act1-1, erg6) | Disk diffusion method | IZ (mm) M. ramanniana: 11–12 S. cerevisiae: 8–18 | [174] | |
| Euryspongia sp. | Antifungal | Yeasts: C. albicans (ATCC 32354, wild-type) (ATCC 90873, amphotericin B-resistant) | Liquid antifungal assay | Amphotericin B | MIC (µg/mL): 15.6–62.5 | [167] |
| Fasciospongia sp. | Antibacterial | G(+): S. aureus (ATCC 25923, ATCC 9144), B. subtilis (ATCC 6051, ATCC 6633) G(-): E. coli (ATCC 11775), P. aeruginosa (ATCC 10145) Yeast: C. albicans (ATCC 90028) | 96-well microtiter plate | Penicillin, Fluconazole | IC50 (µM) S. aureus: 0.95–2.5 B. subtilis: 0.3–7.0 | [186] |
| Halichondria sp. | Antibacterial Antifungal | G(+): M. luteus, B. subtilis G(-): E. coli Yeasts: C. neoformans, C. albicans, Fungi: Paecilomyces variotii, A. niger, A. fumigatus | Broth microdilution method | MIC (µg/mL) M. luteus: 0.52 C. neoformans: 0.0625 C. albicans: 2.09 P. variotii: 1.04 A. niger: 1.04 A. fumigatus: 1.04 | [187] | |
| Antibacterial Antifungal | G(+): M. luteus Yeast: C. neoformans Fungi: T. mentagrophytes | MIC (µg/mL) M. luteus: 4 T. mentagrophytes: 8–16 C. neoformans: 16 | [188] | |||
| Haliclona simulans | Anti-mycobacterial Antitrypanosomal | Acid-fast bacterium: Mycobacterium marinum Parasitic trypanosomatida: T. brucei | Broth microdilution method | Gentamycin | MIC (µM): M. marinum: 156.9–288.8 T. b. brucei: 4.58–21.56 | [170] |
| Jaspis stellifera | Antibacterial Antifungal | G(+): Sarcina lutea Yeast: C. neoformans Fungi: T. mentagrophytes | MIC (µg/mL) S. lutea: 50 C. neoformans: 50 T. mentagrophytes: 12.5 | [181] | ||
| Luffariella geometrica | Antibacterial | G(+): S. aureus, Micrococcus sp. Yeast: S. cerevisiae | Disk diffusion method | EC (µg/disk) S. aureus: 100 Micrococcus sp.: 100 | [178] | |
| Luffariella variabilis | Antibacterial | G(+): Streptomyces pyogenes, S. aureus | Active against S. pyogenes, S. aureus | [180] | ||
| Antibacterial | G(+): S. aureus, B. subtilis G(-): E. coli, P. aeruginosa Yeast: C. albicans | Active against S. aureus, B. subtilis | [179] | |||
| Melophlus sarasinorum | Antibacterial Antifungal | G(+): B. subtilis (DSM2109) G(+): E. coli (DSM10290) Yeast: S. cerevisiae | Disk diffusion method | IZ (mm) B. subtilis: 9 S. cerevisiae: 10–13 | [173] | |
| Oceanapia sp. | Antibacterial Antifungal | G(+): B. subtilis, S. aureus G(-): E. coli, P. aeruginosa Yeast: S. cerevisiae, C. albicans (GT160-45C, cdc5, act1-1, YAT2296c) Fungi: Penicillium chrysogenum, Mortierella ramanniana | Disk diffusion method | IZ (mm) S. cerevisiae: 6.5–10 C. albicans: 8 E. coli: 8.5–12.0 P. aeruginosa: 8.5–13.0 B. subtilis: 11.0 S. aureus: 9.5–13.5 | [161] | |
| Paragrantia cf. waguensis | Antibacterial | G(+): S. aureus (IAM 12084) G(-): E. coli (ATCC 12600) | Broth microdilution method | Rifampicin, Nalidixic acid | MIC (µg/mL) S. aureus: 64 E. coli: 128 | [163] |
| Petrosia weinbergi | Antiviral | Viruses: Feline leukemia virus (FeLV), HIV | EC50 (µg/mL) FeLV: 4.0–5.2 HIV: 1.0 | [166] | ||
| Poecillastra wondoensis, Rhabdastrella wondoensis (two-sponge association) | Antibacterial | G(-): P. aeruginosa, E. coli | Disk diffusion method | Active concentration 10 µg/disk | [175] | |
| Pseudoceratina purpurea | Antiviral | Virus: HIV-1 | EC50 (µM): 52.2 | [191] | ||
| Axinyssa digitata | Antiviral | Viruses: HIV-1, HIV-2 | EC50 (µg/mL) HIV-1: 3–6 HIV-2: Not referred | [165] | ||
| Siliquariaspongia sp. | Antibacterial | G(+): S. aureus, MRSA | Disk diffusion method, Microbroth dilution | MIC50 (µg/mL) S. aureus: 1.2–10.9 S. aureus (MRSA): 3.9–400 | [164] | |
| Siphonodictyon coralliphagum | Antibacterial | G(+): S. aureus, B. subtilis | Active against S. aureus, B. subtilis | [182] | ||
| Spheciospongia purpurea | Antifungal | Yeasts: C. neoformans (32609), C. glabrata (537) Fungi: T. rubrum (Cmccftla), A. fumigatus (07544), | Broth dilution | Amphotericin B, Fluconazole, Voriconazole, Ketoconazole | MIC80 (µg/mL) C. neoformans: 4–32 C. glabrata: 8–64 A. fumigatus: >64 T. rubrum: >64 | [146] |
| Family Spongiidae | Antibacterial Antifungal | G(+): B. subtilis, M. luteus, S. aureus G(-): E. coli Yeasts: C. albicans, C. neoformans Fungi: A. niger | MIC (µm/mL) B. subtilis: 33.3 E. coli: >33.3 M. luteus: 16.7–33.3 S. aureus: 33.3 C. neoformans: 8.35 C. albicans: 8.35 A. niger: 16.7 | [185] | ||
| Suberites domuncula | Antibacterial | Bacterium SB1 (strain isolated from S. domuncula with >98.0% similarity to the alpha-Proteobacterium (MBIC3368) | Disk diffusion method | IZ (mm): 4.5–6.8 | [157] | |
| Topsentia sp. | Antifungal | G(+):MRSA, Acid-fast bacterium: M. intracellulare Parasitic protozoa: P. falciparum (D6 and W2 clones), L. donovani Yeasts: C. albicans, C. glabrata, Candida krusei, S. cerevisiae, C. neoformans Fungus: A. fumigatus | Broth microdilution method | Beauvericin | FIC (µM) C. albicans: 0.2–1.8 S. cerevisiae: 0.08–1.34 | [168] |
| Xestospongia sp. | Antibacterial | G(+): MRSA, S. mutans, S. sobrinus | Disk diffusion method | IZ (mm) MRSA: 12 S. mutans: 17 S. sobrinus: 14 | [138] | |
| Hyas araneus, Podopthalmus vigil, Lauridromia dehanni, Charybdis helleri, Portunus sanguinolentus, Portunus pelagicus | Antibacterial Antifungal | G(+): S. aureus, MRSA, S. pyogenes G(-): E. coli, P. aeruginosa, S. typhi, S. flexneri, Klebsiella sp., V. cholerae, Acinetobacter sp. Yeasts: Rhodotorula sp., C. albicans, C. neoformans Fungi: A. fumigatus, A. niger | Disk diffusion method | Penicillin, Ketoconazole | IZ (mm) S. pyogenes: 1 S. typhi: 1–3 S. flexneri: 1–6 V. cholerae: 1–4 Acinetobacter sp.: 1 | [148] |
| Meganyctiphanes norvegica | Antibacterial | G(+): MRSA, MB5393, MSSA, ATCC 29213 G(-): E. coli (ATCC 25922), K. pneumoniae (ATCC 700603) Acid-fast bacterium: M. tuberculosis (H37Ra ATCC 25177) | Well plate, REMA method | Vancomycin hydrochloride, Aztreonam, Gentamycin sulfate | MIC (µg/mL) MRSA: 80–320 MSSA: 320 M. tuberculosis: 320 | [141] |
| Aplidium sp. | Antibacterial Antiviral Antifungal | G(+): B. subtilis Fungi: T. mentagrophytes Virus: HSV-1 | Disk diffusion method | IZ (mm) B. subtilis/T. mentagrophytes: 3–6 Antiviral activity at 2 μg/disk | [189] | |
| Eunicea succinea | Antibacterial | G(+): S. aureus (ATCC 25923), E. faecalis (ATCC 29212) G(-): P. aeruginosa (ATCC 27853), E. coli (ATCC 25922) | Broth microdilution method | MIC (µmol/mL)/IC50 (µg/mL) S. aureus: 0.24/36 E. faecalis: 0.16/<10 | [137] | |
| Lobophytum crassum | Antibacterial | G(+): S. epidermidis, B. subtilis, S. aureus G(-): P. aeruginosa | Disk diffusion method | Ampicillin | IZ (mm) S. epidermidis: 9.5–16.5 B. subtilis: 8.5–18.0 S. aureus: 9.0–19.5 P. aeruginosa: 9.0–14.0 | [177] |
| Antillogorgia elisabethae | Antibacterial | G(+): S. pyogenes (ATCC 19615), S. aureus (ATCC 25923), E. faecalis (ATCC 19433) G(-): E. coli (ATCC 25933), P. aeruginosa (ATCC 27853) | Disk diffusion method | MIC (µg/mL)/IZ (mm) S. pyogenes: 0.8–1.0/12–17 S. aureus: 2.0–2.3/8–11 E. faecalis: 3.2–3.8/8–9 | [192] | |
| Sinularia grandilobata, Sinularia sp. | Antibacterial Antifungal | G(+): B. subtilis (MTCC 441), Bacillus pumilus (NCIM 2327) G(-): E. coli (MTCC 443), P. aeruginosa (MTCC 1688) Yeast: C. albicans (MTCC 183) Fungi: A. niger (MTCC 1344), Rhizopus oryzae (MTCC 1987) | Disk diffusion method | IZ (mm) B. subtilis: 11–18 B. pumilus: 11–16 E. coli: 11–17 P. aeruginosa: 11–17 C. albicans: 8–17 A. niger: 10–16 R. oryzae: 10–15 | [139] | |
| Holothuria scabra | Antibacterial | G(+): S. aureus (ATCC 6538), E. faecalis G(-): P. aeruginosa (ATCC 8739), V. damsela, E. coli | Well-cut diffusion technique | AU S. aureus: 1.2–2.8 E. faecalis: 1.7–3.2 P. aeruginosa: 1.4–1.8 V. damsela: 1.6 E. coli: 1.2 | [193] | |
| Dosidicus gigas | Antibacterial Antifungal | G(+): B. cereus (CCM 2010), Clostridium perfringens (CCM 4991), Listeria monocytogenes (CCM 4699), S. aureus subs. aureus (CCM 2461) G(-): Haemophilus influenza (CCM 4456), K. pneumoniae (CCM 2318), S. enterica subs. enterica (CCM 3807) Yeasts: C. albicans (CCM 8186), C. glabrata (CCM 8270), C. tropicalis (CCM 8223) Fungi: Aspergillus clavatus, A. flavus, Aspergillus versicolor, Penicillium chrisogenum, Penicillium griseofulvum, Penicillium expansum | Disk diffusion method | Inhibition (%) B. cereus: 39.4 C. perfringens: 45.5 L. monocytogenes: 60.7 S. aureus subs. aureus: 57.8 H. influenza: 54.5 K. pneumoniae: 39.4 S. enterica subs. enterica: 93.9 C. albicans: 66.7 C. glabrata: 42.4 C. tropicalis: 33.3 A. clavatus: 48.4 A. flavus: 42.4 A. versicolor: 42.4 P. chrisogenum: 39.4 P. griseofulvum: 42.4 P. expansum: 48.5 | [144] | |
| Saccostrea glomerata | Antibacterial Antifungal | G(+): S. aureus G(-): P. aeruginosa, V. harveyi, A. hydrophila, P. aeruginosa, V. harveyi, V. parahaemolyticus Yeast: C. albicans Fungi: A. niger, A. flavus, Fusarium sp. Virus: White spot syndrome virus (WSSV) | Disk diffusion method | IZ (mm) P. aeruginosa: 4.1–16.0 V. harveyi: 3.8–14.9 A. hydrophila: 5.1–14.5 A. niger: No activity–High activity C. albicans: No activity–High activity Fusarium sp: No activity–High activity Antiviral activity: PI < 91.85% | [171] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, E.; Dias, M.; Lopes, D.; Almeida, A.; Domingues, M.d.R.; Rey, F. Antimicrobial Lipids from Plants and Marine Organisms: An Overview of the Current State-of-the-Art and Future Prospects. Antibiotics 2020, 9, 441. https://doi.org/10.3390/antibiotics9080441
Alves E, Dias M, Lopes D, Almeida A, Domingues MdR, Rey F. Antimicrobial Lipids from Plants and Marine Organisms: An Overview of the Current State-of-the-Art and Future Prospects. Antibiotics. 2020; 9(8):441. https://doi.org/10.3390/antibiotics9080441
Chicago/Turabian StyleAlves, Eliana, Marina Dias, Diana Lopes, Adelaide Almeida, Maria do Rosário Domingues, and Felisa Rey. 2020. "Antimicrobial Lipids from Plants and Marine Organisms: An Overview of the Current State-of-the-Art and Future Prospects" Antibiotics 9, no. 8: 441. https://doi.org/10.3390/antibiotics9080441
APA StyleAlves, E., Dias, M., Lopes, D., Almeida, A., Domingues, M. d. R., & Rey, F. (2020). Antimicrobial Lipids from Plants and Marine Organisms: An Overview of the Current State-of-the-Art and Future Prospects. Antibiotics, 9(8), 441. https://doi.org/10.3390/antibiotics9080441