Abstract
Extensive documentation is available on plant essential oils as a potential source of antimicrobials, including natural drugs against Candida spp. Yeasts of the genus Candida are responsible for various clinical manifestations, from mucocutaneous overgrowth to bloodstream infections, whose incidence and mortality rates are increasing because of the expanding population of immunocompromised patients. In the last decade, although C. albicans is still regarded as the most common species, epidemiological data reveal that the global distribution of Candida spp. has changed, and non-albicans species of Candida are being increasingly isolated worldwide. The present study aimed to review the anti-Candida activity of essential oils collected from 100 species of the Lamiaceae family growing in the Mediterranean area and the Middle East. An overview is given on the most promising essential oils and constituents inhibiting Candida spp. growth, with a particular focus for those natural products able to reduce the expression of virulence factors, such as yeast-hyphal transition and biofilm formation. Based on current knowledge on members of the Lamiaceae family, future recommendations to strengthen the value of these essential oils as antimicrobial agents include pathogen selection, with an extension towards the new emerging Candida spp. and toxicological screening, as it cannot be taken for granted that plant-derived products are void of potential toxic and/or carcinogenic properties.
1. Introduction
Candida spp. are the most important cause of opportunistic mycoses worldwide. Yeasts of the genus Candida are associated with different clinical manifestations ranging from superficial infections, those involving the skin and mucosal surfaces, to systemic and potentially life-threatening diseases in otherwise healthy individuals. Overall, Candida spp. are one of the primary causes of catheter-associated bloodstream infections in intensive care units of U.S. and European hospitals, and the fourth most common cause of nosocomial bloodstream infection in the USA [1,2]. These severe infections are associated with high mortality rates that are difficult to ascertain, as many patients who acquire candidemia have an underlying medical condition. However, data from population-based surveillance studies report mortality rates ranging from 29% in the USA to 72% in Brazil [3].
Although more than 100 species of Candida have been described, the most challenging infections are caused by C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei. C. albicans is still regarded as the most common species, even though during the last 20 years a progressive shift in the etiology of candidiasis from C. albicans to other species has been observed. Currently, approximately half of the cases of candidiasis are caused by non-albicans species, such as C. glabrata, C. parapsilosis, C. tropicalis, C. krusei, C. guilliermondii, and C. dubliniensis. The overall species distribution of Candida spp. is dependent upon geographic location and patient population, however, the steadily increasing isolation rate of C. glabrata in the USA, Canada, northern European countries, and Australia, C. parapsilosis in Latin America, southern European countries, and Africa, and C. tropicalis in Asia is well documented [3,4].
Among Candida spp, antifungal resistance is observed towards the three main antifungal drug classes: the azoles, the echinocandins, and the polyens. While primary resistance to fluconazole is found naturally in C. krusei, secondary resistance mainly concerns C. albicans C. glabrata, C. parapsilosis, C. tropicalis, C. krusei to echinocandins and C. albicans, C. glabrata, and C. tropicalis to amphotericin B. In addition, coevolution of azole and echinocandin multidrug resistance in C. glabrata has substantially increased along the last decade [5,6].
Current epidemiology of candidiasis has been further modified by the recent identification of C. auris, first discovered in Japan in 2009. By 2018, cases of C. auris infections had become widespread across the globe. The infection is classified as ‘urgent threat’, as the yeast is multidrug resistant, causes high mortality (17–72%), spreads easily in hospital settings, and is difficult to identify [7,8].
Despite the latest developments of diagnostic tools and therapeutic options, candidiasis still remains difficult to treat regardless of its etiology, due to the characteristics of Candida spp., such as resistance to antifungal drugs, expression of virulence factors, and the ability to form biofilms, which is nowaday considered the dominant form of pathogens in natural infections [9,10]. Each Candida species exhibits differences in terms of biofilm formation; architectures, cellular morphologies (yeast cells, hyphae, and pseudohyphae), and extracellular matrix composition are not only species-specific but, in some cases, also strain-specific. Fighting these structures turns out to be even more difficult from a clinical point of view [10,11].
In order to cope with this scenario, the international research community is engaged in discovering new, potent, and promising compounds to be used alone and/or in combination with commercially available antifungal drugs. In addition, an alternative approach to combat Candida infections has recently gained attention, that is, to target functions crucial for the pathogen’s virulence [12].
Plants provide unlimited opportunities for isolation of new compounds because of the unmatched availability of chemical diversity and, therefore, many studies describe the potential antimicrobial properties of plant extracts, essential oils (EOs), and pure secondary metabolites. Faced with the ever-growing amount of scientific data on anti-Candida inhibitors, there is a need to critically review current knowledge on natural products.
This review aims to discuss the scientific documentation on the anti-Candida properties of EOs from plants of the Lamiaceae family growing in the Mediterranean area and the Middle East. Current knowledge regarding changes in the epidemiological features of Candida spp. and the emergence of pathogens with decreased antifungal susceptibilities are discussed in order to provide an up-to-date overview of antimicrobial research.
2. Essential Oils from the Lamiaceae Family
The Lamiaceae family, commonly known as the mint family, is a large family of flowering plants, with 236 genera and more than 7500 species with an almost worldwide distribution, but with the majority of species inhabiting Eurasia and Africa [13]. It is one of the plant families well known for its fragrant species, many of which are of highly valued in the cosmetic, perfumery, food, and pharmaceutical industries [14]. The fragrance of Lamiaceae plants is due to EOs, mixtures of specialized plant metabolites that are produced and accumulated within specialized structures, such as glandular hairs (trichomes), oil ducts, or secretory pockets, localized in different plant organs. Leaves and flowers represent the main storage organs for EOs, but other parts of the plant, including seeds, fruits, rhizomes, and even bark can also contain them. From these organs, EOs can be extracted by a variety of methods, including steam-distillation, hydro-distillation, dry-distillation, solvent and supercritical fluid extraction [15,16], even though only steam- and water-distillation can be used to obtain an EO as defined by the ISO in document ISO 9235.2.
From a chemical point of view, EOs are complex mixtures of organic volatile compounds belonging to different classes. The chemical complexity of these oils is remarkable, and up to 300 different compounds can be present in an EO [17]. Among them, terpenes represent the predominant compounds, but phenylpropanoids also occur. Within the class of terpenes, monoterpenes and sesquiterpenes are the most abundant constituents, although their derivatives, including alcohols, aldehydes, ketones, esters, and phenols, are also common in variable proportions. Synthesis of terpenes and phenylpropanoids occurs in the plant cell through different metabolic pathways; monoterpenes and sesquiterpenes are synthesized through the non-mevalonate and the mevalonate pathways, respectively, while phenylpropanoids arise from phenylalanine and tyrosine that, in turn, originate from the shikimate pathway.
Monoterpenes of EOs comprise both aromatic and acyclic structures, with the former representing the largest group of naturally occurring monoterpenes. Among them, common compounds include α-terpinene, β-terpinene, γ-terpinene, limonene, α-pinene, β-pinene, p-cymene, and its hydroxylated derivatives tymol and carvacrol, as well as the notable pulegone and piperitone. Some acyclic monoterpenes are also important constituents of EOs, including linalool, geraniol, and citronellol. As concerns sesquiterpenes, they show a wide structural diversity, with linear, branched, or cyclic structures [18,19]. Examples of compounds belonging to the latter group include the azulenes, that are responsible for the blue color of some EOs, α-bisabolene and its oxygenated derivatives, α- and β-bisabolol, and caryophyllene. The latter is present, as β-caryophyllene, in many EOs and, in some cases, represents the major component [20]. Phenylpropanoids occur less frequently in EOs and, when present, are usually less abundant than terpenes. Examples of important phenylpropanoids include cinnamaldehyde, myristicin, dillapiole, anethole, chavicol, eugenol and their methylated derivatives [21].
The plant species herein reviewed were sourced from Wos of Science and PubMed databases by using “Candida”, “essential oil”, “Lamiaceae” as key words, alone and as combinations. Only papers published from 2010 to March 2020 were analyzed. Plant species growing in the Mediterranean and Middle East was included as a specific criterion to filter reports; a total of 100 plants were identified and are, therefore, considered in this review. Italy, Iran, and Turkey were the most representative countries (17.0, 13.8, and 9.6%, respectively), followed by Portugal (8.5%), and Algeria (7.5%), while other countries of these areas had a relative distribution in the range 1.0 to 7.0% (Figure 1).
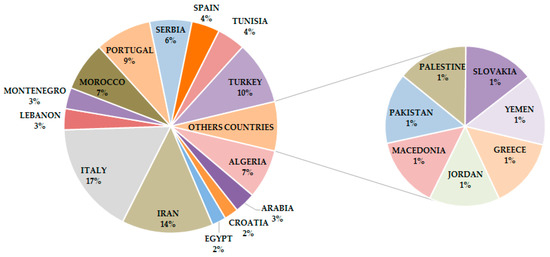
Figure 1.
Frequency distribution of reviewed plant species producing EOs with anti-Candida activity.
The 100 most frequently investigated plants belong to 24 genera, among which are Thymus, Mentha, Salvia, and Origanum, with percentage distributions of 22.6% 11.3%, 9.4%, and 8.5%, respectively. Calamintha, Stachys, and Lavandula are also well represented (5.7%). The compositions of the EOs are listed in Table S1; scientific plant names were checked using The Plant List database [22] and chemical components were unified according to PubChem [23].
3. Selection of Candida Spp.
The EOs from Lamiaceae plants that have been identified as active towards Candida spp. were assayed in vitro against reference strains and/or clinical isolates (Table 1). Reference strains were tested in 76.4% of the papers and were obtained from four organizations: American Type Culture Collection (ATCC), Agricultural Research Service Culture Collection (NRRL—Northern Regional Research Laboratory), CBS-KNAW Culture Collection (CBS), and Moroccan Coordinated Collections of Microorganisms (CCMM). Testing reference strains should be preferred in primary screening studies as these microorganisms are well-characterized and widely used, thus allowing for a direct comparison of literature data. Clinical strains from biological specimens were assayed as unique targeted pathogens in 21.3% of the papers and the majority of these isolates were not defined for their susceptibility to relevant antimicrobial agents. This information should be included in the research studies for a proper description of the tested samples and may represent an added value to determine the effectiveness of natural compounds towards pathogens circulating in the population.

Table 1.
General overview of the targeted Candida spp. and other relevant information of the reviewed research papers.
C. albicans constituted the primary target for the assessment of the anti-Candida activity of the EOs, both as reference strain and as clinical isolate. Testing a single Candida spp. is not a drawback, but a feature to be considered in relation to epidemiological changes occurring in C. albicans. In addition, the recent emergence of novel, multiresistant species, such as C. auris, amplifies the call for testing this pathogen. Thus, it is desirable that researchers improve their antimicrobial evaluations on natural products considering current clinical needs.
4. Anti-Candida Activity
Antimicrobial activity of natural extracts and pure compounds can be evaluated by measuring the growth response of microorganisms to samples that are placed in contact with them. Different methodologies for in vitro tests are available and roughly classified in two main groups: diffusion assays (agar disk, agar well, agar plug diffusion methods), and dilution assays (agar dilution and broth micro-, macro-dilution methods) [113]. As these methodologies incorporate viable cells whose growth can be influenced by unpredictable factors, a carefully standardization of the techniques is of utmost importance. Some methods were subjected to standardization by the CLSI (Clinical and Laboratory Standards Institute) and EUCAST (European Committee on Antimicrobial Susceptibility Testing), marking the major remarkable steps on the procedures.
The two most common methods used to investigate anti-Candida activity of EOs collected in the Mediterranean area and the Middle East were agar disk diffusion and Minimum Inhibitory Concentration (MIC) assays. As is desirable, the test of choice resulted in MIC measurements and 95.5% of EOs were assayed by broth dilution tests. Indeed, dilution methods in broth are the gold standard for antimicrobial susceptibility testing, and are mainly used to establish the in vitro activity of new antifungal agents, such as substances of biological, semi-synthetic, or synthetic origin that inhibit the growth of fungi or are lethal to them [114]. Agar-based assays are practical tools, due to their simplicity and capacity to analyze a large number of samples, however they are qualitative tests where diffusion plays an important role in determining the size of growth inhibition zones. Problems may arise especially when investigating EOs, as they are lipophilic and volatile; thus, they do not easily diffuse through agar and their evaporation may impact on the outcome of the assay. In addition, papers herein reviewed report data obtained in experiments where the EO samples were placed on different reservoirs in terms of types and dimensions, making comparisons between results on potency unreliable. As an example, the anti-Candida activity of Thymus capitatus (L.), Hoffmanns. and Link [78], was tested in an agar well (Ø 10 mm) bored into the culture medium, while Mentha cervina L., Ocimum basilicum L., and Origanum vulgare L. [59] were spotted on sterile paper discs Ø 9 mm), while standard guidelines indicate sterile paper discs of Ø 6 mm.
Results concerning the anti-Candida activities of the selected EOs are reported in Table S2 and are expressed as MIC values obtained by broth micro- and macro-dilution tests. This specific criterion was selected in order to compare a more homogeneous dataset.
Activities extend over a wide range of values (0.39–12,480 µg/mL; 0.125–40 µL/mL) regardless of the EO and the targeted Candida spp. Classification of the EO’s potency as strong, moderate, or weak, is a very difficult procedure and even authors of the selected publications point out significant discrepancies in anti-Candida properties. For example, Origanum ehrenbergii Boiss. and O. syriacum L. with MICs of 800 µg/mL are considered inactive by Al Hafi et al. [28], while Micromeria inodora (Desf.) Benth. with a MIC of 1000 µg/mL is regarded as a moderate inhibitor by Benomari and coworkers [38]. In many papers, the anti-Candida inhibitory potential is evaluated with reference to antifungal commercial drugs, e.g., fluconazole and amphotericin B; however, the activity of pure compounds cannot be compared with complex and structurally diverse natural mixtures, and their MIC values have to be considered as positive controls.
In an attempt to categorize EO potencies, the overall MIC values obtained for C. albicans reference strains are plotted in Figure 2. The frequency of distribution of MIC values indicate that more than half of the tested EOs is active in the 100–1000 µg/mL and 0.31–2 µL/mL range, indicating 1000 µg/mL and 2 µL/mL as thresholds to define an EO as an antifungal inhibitor. Table 2 reports MIC values of the EOs displaying inhibitory activity towards Candida spp. reference strains.
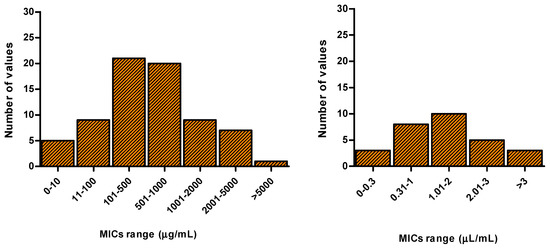
Figure 2.
Frequency of distribution of Minimum Inhibitory Concentration (MIC) values related to the selected EOs. Studies reporting MICs obtained from plant species analyzed in different seasons or collected in different regions were excluded from the analysis.

Table 2.
Minimum Inhibitory Concentrations (MICs) of the EOs with anti-Candida activity.
In terms of potency against C. albicans strains, some EOs showed a strong activity, with MIC levels <100 µg/mL or <0.3 µL/mL. Even though the concentration of 100 µg/mL has been adopted as a general endpoint criterion for plant-derived mixtures in all anti-infective bioassays [115], less than 10% of selected species meets that criterion, while most species produce an EO with a lower level of activity. This can be related to the intrinsic chemical nature of EOs and, in particular, to the low solubility of most of their components in aqueous media. Plants producing an EO with MIC values on C. albicans <1000 µg/mL or <2 µL/mL include several species of Thymus, Coridothymus, Origanum, Mentha, Calamintha, Satureja, Salvia, Lavandula, Plectranthus, and Stachys. Within this group, it is worth noting that some EOs were active at concentrations <100 µg/mL, such as Mentha mozaffarianii [102], Mentha suaveolens Ehrh [51], Salvia mirzayanii Rech.f. and Esfand [56], Stachys spruneri Boiss. [71], Thymus willdenowii Boiss [84], Plectranthus barbatus Andrews, and Plectranthus caninus Roth [55]. When considering MIC values expressed as µL/mL, the two most active species were Nepeta cataria L. [108] and Zataria multiflora Boiss [82], with MIC values <0.3 ml/L.
The most frequently represented chemical constituents of EOs endowed with anti-Candida activity belong to the group of monoterpenes, and include p-cymene (40 plants), linalool (35 plants), γ-terpinene (33 plants), carvacrol (31 plants), 1-8-cineole (30 plants), α-pinene (28 plants), and thymol (27 plants). The sesquiterpene β-caryophyllene is present as a constituent in 15 out of 100 plants.
Given the high chemical complexity of EOs and considering that the biological effect are often the result of a synergistic interaction occurring among the various components of the mixture, it is difficult to univocally identify the most active components. Based on the evaluation of the MIC values on C. albicans reference strains, it appears that some monoterpenes and derivatives were present as major constituents in EOs endowed with a powerful antifungal activity. Among these, terpinyl-acetate, α-terpineol, β-linalool, and γ-terpinene confer a strong anti-Candida activity to the EO when present as major components, as in the case of Salvia mirzayanii Rech.f. and Esfand [56], Plectranthus caninus Roth [55], and Thymus willdenowii Boiss. On the other hand, compounds making up a larger proportion of the EO are not necessarily responsible for the activity, and this makes it even more difficult to establish a correlation between chemical composition and biological efficacy.
The antifungal activity of single acyclic and cyclic monoterpenes has been deeply investigated [116,117,118]; α-terpineol, terpinen-4-ol, 1-8-cineol, and β-linalool were reported to show the most rapid killing activity, while γ-terpinene, α-terpinene, terpinolene, and p-cymene, showed a slower, although still significant, antifungal activity. This suggests that the alcohol moiety, more than the cyclic or acyclic structure itself, is important for a rapid inhibitory effect on fungal growth, and this has been related to the higher water solubility of alcohols in both aqueous media and microbial membranes [118,119]. Indeed, the antifungal effect of linalool has been demonstrated to be the result of the combined action on membrane integrity and cell cycle arrest [120,121]; moreover, an inhibitory action on biofilm formation, occurring through an impairment in filament production, has been documented [122]. The alteration of cell permeability caused by the insertion of small molecules of monoterpenes between the fatty acid chains of membrane phospholipids seems to be a common effect [123,124]. A high inhibitory activity was related to the presence of an aromatic ring in the monoterpene molecule [125], and thymol and carvacrol have been the subject of several investigations aimed at unravelling their mechanism of action. This involves the inhibition of ergosterol biosynthesis, with disruption of membrane integrity [126]. Recent papers reported that carvacrol exerts its potent antifungal activity by altering the integrity of endoplasmic reticulum, thus impairing the normal protein folding capacity of C. albicans cells.
5. Anti-Virulence Activity
The pathogenicity of Candida spp. is attributed to several virulence factors including yeast-hyphal transition, adherence to surfaces, production of hydrolytic enzymes (proteases, lipases, hemolysins), signal transduction pathway, and biofilm formation. In human infections, the formation of a germ tube and mycelium is an essential process for host tissue invasion, damage to mucosal epithelia, escape from host immune cells, and blood dissemination; additionally, filamentation is pivotal for robust biofilm development. Thus, natural products affecting both morphogenetic transitions between yeast and filamentous morphologies and biofilm formation represent alternative candidates for the treatment of candidiasis; currently, strategies targeting the expression of virulence factors are a promising approach for the treatment of infectious diseases, since these drugs can reduce the risk of resistance development in host infections [9,12].
Among the EOs investigated in the publications reviewed herein, the anti-Candida activity through inhibition of virulence factors was evaluated for 13 of them. In almost all papers, the targeted yeast was C. albicans reference strains and the EOs were tested in vitro for their effect on the hyphal development process and/or for their anti-biofilm activity. Several approaches are available to determine these properties [127]; the most common test to study yeast-to-hyphae transition is by culturing C. albicans in medium supplemented with serum at 10%, followed by light microscope observation, while biofilm production, inhibition, and eradication can be assessed by crystal violet staining of the biofilm mass obtained on tissue-culture plates. A representative sketch describing the effects of an anti-virulence agent on a C. albicans culture is depicted in Figure 3.
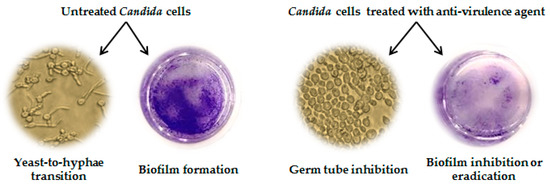
Figure 3.
C. albicans cultures grown under conditions inducing yeast-to-hyphae transition and in presence of a representative agent inhibiting the expression of virulence factors. Untreated cells undergo transition to filamentous forms and produce a strong biofilm, stained with crystal violet. Treated cells are blastospores and budding cells but not hyphae, and the biofilm is faintly stained.
Table 3 reports the data of the anti-virulence effects for 13 EOs; the yeast-to-hyphae transition was affected at sub-inhibitory concentrations for species of Lavandula, Mentha, Origanum, Thymus, and Ziziphora tenuior L., the latter species being also capable of decreasing biofilm biomass, together with some other species of Satureja, Thymbra, and Thymus. These findings add relevant information to the anti-Candida activities of these EOs, considering that biofilms display innate resistance to multiple drug classes and are capable of withstanding antifungal concentrations 1000-fold higher than those that inhibit planktonic cells [128].

Table 3.
Plant species producing EOs with anti-virulence activity against C. albicans reference strains.
Thymol, carvacrol, and 1,8-cineole were reported to be effective in inhibiting germ tube formation, an initial stage in the transition from yeast to hyphae, with an efficacy comparable to that of the “quorum sensing” molecule farnesol [129]. In another study carried out by Boni et al. [130], pulegone and carvone were found to be highly active molecules in blocking the progress of biofilm formation and in damaging mature biofilms at low concentrations. These findings can account for the anti-virulence activity of EOs shown in Table 3.
6. Synergistic Interaction with Commercial Antifungal Drugs
Drug combination approaches to tackle Candida infections are of great interest; indeed, much effort is put in the search for molecules leading to synergistic interactions with commercial drugs to achieve lower effective doses of drugs and to restore antifungal activities when drug resistance arises [131,132]. However, the majority of these combination studies were performed in vitro, thus lacking evidence for the in vivo and clinical effects, which limits the possible development of anti-candidiasis drugs with some general concerns about potency and potential toxicity.
In particular, studies examining the effects of EOs and conventional drugs are under-represented in antifungal drug discovery research and more investigations are required. Indeed, among the papers herein reviewed, synergistic interaction was described only for three EOs from Lamiaceae plants of the Mediterranean area and Middle East: Thymus vulgaris, Coridothymus capitatus, and Mentha x piperita. Synergy was demonstrated for T. vulgaris in combination with amphotericin B, even though a non standardized assay was applied [60]. The most common method aimed at evaluating the interaction between molecules is the checkerboard assay and the type of interaction is usually described by the fractional inhibitory concentration (FIC) index, a value that takes into account the potency of the combination of molecules in comparison to their individual activities [133]. Coridothymus capitatus differentially interacted with itraconazole, depending on the targeted Candida spp.; of clinical relevance is the synergistic effect of the EO with itraconazole against C. krusei, which is intrinsically resistant to fluconazole [74]. The combinatorial interaction of Mentha x piperita with commercial antifungal drugs (several azoles and amphotericin B) was investigated on different reference and clinical strains of Candida spp. [92,103]. Overall, results point to the synergy of the EO with amphotericin B, fluconazole, and miconazole towards reference strains, and with itraconazole towards clinical isolates of C. albicans, C. glabrata, and C. krusei with different azole-sensitivity. Remarkably, when investigations were carried out for menthol and menthone, the two main components of the EO, only an additive effect was recorded, confirming that the activity of an EO is due to the whole phytocomplex that, in fact, better expresses its effectiveness.
All synergistic combinations can be ascribed to the cell wall or cell membrane disruption mediated by the EO, which enhances penetration of antifungal drugs across the cell barriers [130,134].
7. Cytotoxicity
Assessment of the toxicological profile of natural compounds is a very important step in a drug discovery perspective. Cell viability and proliferation assays on mammalian cells should always be included in the investigations, since they allow to discriminate between a specific antimicrobial activity and a non-specific cytotoxicity. Many cell types and bioassays can be used for this purpose; natural compounds, at least at bioactive concentrations, should be evaluated on normal and/or malignant cells and a variety of methodologies are available, such as enzyme activity, cell membrane permeability, cell adherence, ATP production, co-enzyme production, and nucleotide uptake activity [135].
Unexpectedly, cytotoxicity was evaluated in only 20.2% of the investigated papers; cell viability was ascertained primarily by using the standardized MTT assay, an enzyme-based method relying on cellular dehydrogenase activity, and in more than one cell line. Artemia spp. (brine shrimps) have also been used as a biological model in three research papers [39,40,69], however, they are only suitable for ecotoxicity studies [136].
Table 4 reports the MIC values of 20 EOs tested against Candida spp. reference strains and data concerning their effects on mammalian cells, expressed either as percentage of cell viability compared to control cells, at a defined concentration, or as the IC50 value, the concentration at which viability was reduced by 50%.

Table 4.
Safety profile of 20 EOs from the Lamiaceae family.
Fungi and mammalian cells share similar cellular and biochemical pathways, thus some degree of toxicity for EOs is not surprising. Cytotoxicity against cancer cell lines could be considered an added value for EOs in the framework of anti-tumor drug development [33]; on the contrary, reduced cell viability on non malignant cells should be carefully considered when EOs are investigated as the potential source of antimicrobial agents. As reported in Table 4, only seven EOs were assayed on normal cells, thus indicating the need for a more careful choice of cells to be used in the experiments. The selectivity index (SI), the ratio between cytotoxicity and antimicrobial activity, is a widely accepted parameter used to express the in vitro efficacy of a test sample and to rule out a general effect on eukaryotic cells; to obtain the SI value, inhibitory activity towards a specific target has to be expressed as IC50 rather than MIC. Considering that the potency of EOs is reported as MIC, the SI cannot be measured and conclusive statements on the selective activity of EOs and on their safety is hazardous. Indeed, several EOs listed in Table 4 strongly interfere with cell proliferation of the tested cell lines with IC50 values <30 µg/mL, a threshold defining the cytotoxicity of natural products by the American National Cancer Institute and in scientific literature [51,99,137,138]. Thus, additional studies on EOs should be conducted in the perspective of their pharmaceutical use as antimicrobial agents. Multiple, independent assays may be necessary to confirm experimental outcomes, as results may differ depending on the cell type used. However, as an in vitro proof-of-concept on herbal safety, testing at least one type of mammalian cell model with a standardized methodology is strongly recommended in all studies.
8. Conclusions
The present review discusses data obtained from 89 research papers regarding the anti-Candida activity of EOs collected from 100 species of Lamiaceae plants. EOs, as well as other plant-derived natural products, represent a main source of new drug molecule today, thus investigations on their biological potential is of clinical relevance. For this reason, studies aimed at evaluating the antimicrobial activity of natural products should be conceived following as much as possible the standardized guidelines of the International Committees (CLSI and EUCAST). Some modifications of protocols may be requested because of the complexity of the tested material, however, these changes should not upset the basics of microbiology.
Most of the studies were screening publications with the intention to identify plants with a potential antimicrobial activity, indeed they tested a wide panel of microorganisms, including other fungi and bacteria, and about 10% of species produce an EO very potent against Candida species. The identification of plant species particularly active as anti-Candida can represent a starting point for a taxonomic approach aimed at screening closely related species that may potentially contain important chemicals in their EO, an approach that turned sometimes very successful for medicinal plants. On the other hand, the revision of papers has highlighted, in most cases, the insufficiency of adequate toxicological analyses that represent a mandatory requirement to define the safety profile of an EO.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6382/9/7/395/s1. Table S1: Main chemical components of EOs of selected Lamiaceae family plants; Table S2: Minimum Inhibitory Concentrations (MICs) of the EOs collected from 100 species of Lamiaceae family.
Author Contributions
F.B. and F.A. conceptualized the review and wrote the first manuscript draft with imputs from G.P and G.A.G.; all authors revised, and finalized the manuscript; G.P., F.B. and F.A. prepared tables and figures. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Stefania Biondi for the English proofreading of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial Bloodstream Infections in US Hospitals: Analysis of 24,179 Cases from a Prospective Nationwide Surveillance Study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Maraolo, A.E.; Simeon, V.; Magnè, F.; Pace, M.C.; Gentile, I.; Chiodini, P.; Viscoli, C.; Sanguinetti, M.; Mikulska, M.; et al. Changes in the relative prevalence of candidaemia due to non- albicans Candida species in adult in-patients: A systematic review, meta-analysis and meta-regression. Mycoses 2020, 63, 334–342. [Google Scholar] [CrossRef]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018, 73, i4–i13. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, S.; Rafei, R.; Osman, M.; El Safadi, D.; Mallat, H.; Papon, N.; Dabboussi, F.; Bouchara, J.-P.; Hamze, M. The epidemiology of Candida species in the Middle East and North Africa. J. Mycol. Med. 2019, 29, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.L.; de Almeida Júnior, J.N.; Guinea, J. Emerging multidrug-resistant Candida species. Curr. Opin. Infect. Dis. 2017, 30, 528–538. [Google Scholar] [CrossRef]
- Jha, A.; Kumar, A. Anticandidal agent for multiple targets: The next paradigm in the discovery of proficient therapeutics/overcoming drug resistance. Future Med. Chem. 2019, 11, 2955–2974. [Google Scholar] [CrossRef] [PubMed]
- Lone, S.A.; Ahmad, A. Candida auris—The growing menace to global health. Mycoses 2019, 62, 620–637. [Google Scholar] [CrossRef]
- Cortegiani, A.; Misseri, G.; Fasciana, T.; Giammanco, A.; Giarratano, A.; Chowdhary, A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J. Intensive Care 2018, 6, 69. [Google Scholar] [CrossRef]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Galocha, M.; Pais, P.; Cavalheiro, M.; Pereira, D.; Viana, R.; Teixeira, M.C. Divergent Approaches to Virulence in C. albicans and C. glabrata: Two Sides of the Same Coin. Int. J. Mol. Sci. 2019, 20, 2345. [Google Scholar] [CrossRef]
- Scorzoni, L.; de Paula e Silva, A.C.A.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.M.A.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Willis, K.J. State of the World’s Plants. Available online: https://stateoftheworldsplants.org/ (accessed on 15 May 2020).
- Barnes, J. Quality, efficacy and safety of complementary medicines: Fashions, facts and the future. Part I. Regulation and quality. Br. J. Clin. Pharmacol. 2003, 55, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Kubeczka, K.-H. History and sources of essential oil research. In Handbook of Essential Oils; CRC Press: Boca Raton, FL, USA, 2009; pp. 12–47. [Google Scholar]
- Lahlou, M. Methods to study the phytochemistry and bioactivity of essential oils. Phytother. Res. PTR 2004, 18, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Sell, C.S. The Chemistry of Fragrances: From Perfumer to Consumer; Royal Society of Chemistry: Cambridge, UK, 2006; Volume 38. [Google Scholar]
- Hüsnü, K.; Başer, C.; Demirci, F. Chemistry of Essential Oils. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Berger, R.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 43–86. ISBN 978-3-540-49339-6. [Google Scholar]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. PTR 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Sabulal, B.; Dan, M.; John J, A.; Kurup, R.; Pradeep, N.S.; Valsamma, R.K.; George, V. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity. Phytochemistry 2006, 67, 2469–2473. [Google Scholar] [CrossRef]
- Clifford, M.N. Miscellaneous phenols in foods and beverages – nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1126–1137. [Google Scholar] [CrossRef]
- Home—The Plant List. Available online: http://www.theplantlist.org/ (accessed on 3 June 2020).
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 3 June 2020).
- Abdelli, W.; Bahri, F.; Sysak, A.; Szumny, A.; Pawlak, A.; Obmińska-Mrukowicz, B. Chemical composition, antimicrobial and cytotoxic activity of essential oils of Algerian Thymus vulgaris L. Acta Pol. Pharm. Drug Res. 2019, 76, 1051–1059. [Google Scholar] [CrossRef]
- Abu-Darwish, M.S.; Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Paoli, M.; Tomi, F.; Efferth, T.; Salgueiro, L. Ziziphora tenuior L. essential oil from Dana Biosphere Reserve (Southern Jordan); Chemical characterization and assessment of biological activities. J. Ethnopharmacol. 2016, 194, 963–970. [Google Scholar] [CrossRef]
- Ahmadi, F.; Sadeghi, S.; Modarresi, M.; Abiri, R.; Mikaeli, A. Chemical composition, in vitro anti-microbial, antifungal and antioxidant activities of the essential oil and methanolic extract of Hymenocrater longiflorus Benth., of Iran. Food Chem. Toxicol. 2010, 48, 1137–1144. [Google Scholar] [CrossRef]
- Al Hafi, M.; El Beyrouthy, M.; Ouaini, N.; Stien, D.; Rutledge, D.; Chaillou, S. Chemical Composition and Antimicrobial Activity of Satureja, Thymus, and Thymbra Species Grown in Lebanon. Chem. Biodivers. 2017, 14, e1600236. [Google Scholar] [CrossRef]
- Al Hafi, M.; El Beyrouthy, M.; Ouaini, N.; Stien, D.; Rutledge, D.; Chaillou, S. Chemical Composition and Antimicrobial Activity of Origanum libanoticum, Origanum ehrenbergii, and Origanum syriacum Growing Wild in Lebanon. Chem. Biodivers. 2016, 13, 555–560. [Google Scholar] [CrossRef]
- Alves, M.; Gonçalves, M.J.; Zuzarte, M.; Alves-Silva, J.M.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Unveiling the Antifungal Potential of Two Iberian Thyme Essential Oils: Effect on C. albicans Germ Tube and Preformed Biofilms. Front. Pharmacol. 2019, 10, 446. [Google Scholar] [CrossRef]
- Asdadi, A.; Hamdouch, A.; Oukacha, A.; Moutaj, R.; Gharby, S.; Harhar, H.; El Hadek, M.; Chebli, B.; Idrissi Hassani, L.M. Study on chemical analysis, antioxidant and in vitro antifungal activities of essential oil from wild Vitex agnus-castus L. seeds growing in area of Argan Tree of Morocco against clinical strains of Candida responsible for nosocomial infections. J. Mycol. Médicale 2015, 25, e118–e127. [Google Scholar] [CrossRef]
- Ashraf, S.N.; Zubair, M.; Rizwan, K.; Tareen, R.B.; Rasool, N.; Zia-Ul-Haq, M.; Ercisli, S. Compositional studies and Biological activities of Perovskia abrotanoides Kar. oils. Biol. Res. 2014, 47, 12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ali, N.A.A.; Chhetri, B.K.; Dosoky, N.S.; Shari, K.; Al-Fahad, A.J.; Wessjohann, L.; Setzer, W.N. Antimicrobial, antioxidant, and cytotoxic activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) essential oils. Medicines 2017, 4, 17. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Bakchiche, B.; ALSalamat, H.A.; Rezzoug, M.; Gherib, A.; Flamini, G. Chemical composition, antioxidant, antimicrobial and Antiproliferative activities of essential oil of Mentha spicata L. (Lamiaceae) from Algerian Saharan atlas. BMC Complement. Altern. Med. 2018, 18, 201. [Google Scholar] [CrossRef] [PubMed]
- Bellete, B.; Rabérin, H.; Flori, P.; Akssi, S.E.; Sung, R.T.M.; Taourirte, M.; Hafid, J. Antifungal effect of the essential oil of Thymus broussonetii Boiss endogenous species of Morocco. Nat. Prod. Res. 2012, 26, 1692–1696. [Google Scholar] [CrossRef] [PubMed]
- Benabed, K.; Gourine, N.; Ouinten, M.; Bombarda, I.; Yousfi, M. Chemical Composition, Antioxidant and Antimicrobial Activities of the Essential Oils of Three Algerian Lamiaceae Species. Curr. Nutr. Food Sci. 2017, 13, 97–109. [Google Scholar] [CrossRef][Green Version]
- Bendif, H.; Boudjeniba, M.; Miara, M.D.; Biqiku, L.; Bramucci, M.; Lupidi, G.; Quassinti, L.; Vitali, L.A.; Maggi, F. Essential Oil of Thymus munbyanus subsp. coloratus from Algeria: Chemotypification and in vitro Biological Activities. Chem. Biodivers. 2017, 14, e1600299. [Google Scholar] [CrossRef]
- Benabdelkader, T.; Zitouni, A.; Guitton, Y.; Jullien, F.; Maitre, D.; Casabianca, H.; Legendre, L.; Kameli, A. Essential Oils from Wild Populations of Algerian Lavandula stoechas L.: Composition, Chemical Variability, and in vitro Biological Properties. Chem. Biodivers. 2011, 8, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Benomari, F.Z.; Djabou, N.; Medbouhi, A.; Khadir, A.; Bendahou, M.; Selles, C.; Desjobert, J.-M.; Costa, J.; Muselli, A. Chemical Variability and Biological Activities of Essential Oils of Micromeria inodora (Desf.) Benth. from Algeria. Chem. Biodivers. 2016, 13, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Bogavac, M.; Karaman, M.; Janjušević, L.; Sudji, J.; Radovanović, B.; Novaković, Z.; Simeunović, J.; Božin, B. Alternative treatment of vaginal infections - in vitro antimicrobial and toxic effects of Coriandrum sativum L. and Thymus vulgaris L. essential oils. J. Appl. Microbiol. 2015, 119, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Bogavac, M.A.; Karaman, M.A.; Suđi, J.J.; Radovanović, B.B.; Janjušević, L.N.; Ćetković, N.B.; Tešanović, K.D. Antimicrobial Potential of Rosmarinus officinalis Commercial Essential Oil in the Treatment of Vaginal Infections in Pregnant Women. Nat. Prod. Commun. 2017, 12, 1934578X1701200. [Google Scholar] [CrossRef]
- Božović, M.; Garzoli, S.; Sabatino, M.; Pepi, F.; Baldisserotto, A.; Andreotti, E.; Romagnoli, C.; Mai, A.; Manfredini, S.; Ragno, R. Essential Oil Extraction, Chemical Analysis and Anti-Candida Activity of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Ball—New Approaches. Molecules 2017, 22, 203. [Google Scholar] [CrossRef]
- Chenni, M.; El Abed, D.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Comparative Study of Essential Oils Extracted from Egyptian Basil Leaves (Ocimum basilicum L.) Using Hydro-Distillation and Solvent-Free Microwave Extraction. Molecules 2016, 21, 113. [Google Scholar] [CrossRef]
- Cutillas, A.-B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Composition and Antioxidant, Antienzymatic and Antimicrobial Activities of Volatile Molecules from Spanish Salvia lavandulifolia (Vahl) Essential Oils. Molecules 2017, 22, 1382. [Google Scholar] [CrossRef]
- Cutillas, A.-B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Salvia officinalis L. Essential Oils from Spain: Determination of Composition, Antioxidant Capacity, Antienzymatic, and Antimicrobial Bioactivities. Chem. Biodivers. 2017, 14, e1700102. [Google Scholar] [CrossRef]
- Cutillas, A.-B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Thyme essential oils from Spain: Aromatic profile ascertained by GC–MS, and their antioxidant, anti-lipoxygenase and antimicrobial activities. J. Food Drug Anal. 2018, 26, 529–544. [Google Scholar] [CrossRef]
- Cutillas, A.-B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Thymus mastichina L. essential oils from Murcia (Spain): Composition and antioxidant, antienzymatic and antimicrobial bioactivities. PLoS ONE 2018, 13, e0190790. [Google Scholar] [CrossRef]
- Delogu, G.; Juliano, C.C.A.; Usai, M. Thymus catharinae Camarda essential oil: β-cyclodextrin inclusion complexes, evaluation of antimicrobial activity. Nat. Prod. Res. 2016, 30, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Debbabi, H.; El Mokni, R.; Chaieb, I.; Nardoni, S.; Maggi, F.; Caprioli, G.; Hammami, S. Chemical Composition, Antifungal and Insecticidal Activities of the Essential Oils from Tunisian Clinopodium nepeta subsp. nepeta and Clinopodium nepeta subsp. glandulosum. Molecules 2020, 25, 2137. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.; Nardoni, S.; Bertelloni, F.; Pistelli, L.; Mancianti, F. Antimicrobial Activity of Five Essential Oils against Bacteria and Fungi Responsible for Urinary Tract Infections. Molecules 2018, 23, 1668. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.; Nardoni, S.; Bertelloni, F.; Giovanelli, S.; Ruffoni, B.; D’Ascenzi, C.; Pistelli, L.; Mancianti, F. Activity of Salvia dolomitica and Salvia somalensis Essential Oils against Bacteria, Molds and Yeasts. Molecules 2018, 23, 396. [Google Scholar] [CrossRef] [PubMed]
- El-Kashoury, E.-S.A.; El-Askary, H.I.; Kandil, Z.A.; Salem, M.A.; Sleem, A.A. Chemical composition and biological activities of the essential oil of Mentha suaveolens Ehrh. Z. Naturforschung C J. Biosci. 2012, 67, 571–579. [Google Scholar] [CrossRef]
- Fani, M.; Kohanteb, J. In Vitro Antimicrobial Activity of Thymus vulgaris Essential Oil Against Major Oral Pathogens. J. Evid. Based Complement. Altern. Med. 2017, 22, 660–666. [Google Scholar] [CrossRef]
- Fraternale, D.; Flamini, G.; Bisio, A.; Albertini, M.C.; Ricci, D. Chemical Composition and Antimicrobial Activity of Salvia x jamensis Essential Oil. Nat. Prod. Commun. 2012, 7, 1934578X1200700. [Google Scholar] [CrossRef]
- Garzoli, S.; Božović, M.; Baldisserotto, A.; Andreotti, E.; Pepi, F.; Tadić, V.; Manfredini, S.; Ragno, R. Sideritis romana L. subsp. purpurea (Tal. ex Benth.) Heywood, a new chemotype from Montenegro. Nat. Prod. Res. 2018, 32, 1056–1061. [Google Scholar] [CrossRef]
- Gelmini, F.; Squillace, P.; Testa, C.; Sparacino, A.C.; Angioletti, S.; Beretta, G. GC–MS characterisation and biological activity of essential oils from different vegetative organs of Plectranthus barbatus and Plectranthus caninus cultivated in north Italy. Nat. Prod. Res. 2015, 29, 993–998. [Google Scholar] [CrossRef]
- Ghasemi, E.; Sharafzadeh, S.; Amiri, B.; Alizadeh, A.; Bazrafshan, F. Variation in Essential Oil Constituents and Antimicrobial Activity of the Flowering Aerial Parts of Salvia mirzayanii Rech. & Esfand. Ecotypes as a Folkloric Herbal Remedy in Southwestern Iran. J. Essent. Oil Bear. Plants 2020, 23, 51–64. [Google Scholar] [CrossRef]
- Goldansaz, S.M.; Jeloudar, Z.; Safaeian, R.; Sonboli, A. Comparison of the chemical constitutions, antibacterial, anti-Candida, and antioxidant activity of Nepeta asterotricha Rech. F. essential oil. Am. J. Essent. Oils Nat. Prod. 2019, 7, 15–22. [Google Scholar]
- Goze, I.; Alim, A.; Cetinus, S.A.; Çetin, A.; Durmus, N.; Atas, A.T.; Vural, N. In Vitro Antimicrobial, Antioxidant, and Antispasmodic Activities and the Composition of the Essential Oil of Origanum acutidens (Hand.-Mazz.) Ietswaart. J. Med. Food 2010, 13, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Helal, I.M.; El-Bessoumy, A.; Al-Bataineh, E.; Joseph, M.R.P.; Rajagopalan, P.; Chandramoorthy, H.C.; Ben Hadj Ahmed, S. Antimicrobial Efficiency of Essential Oils from Traditional Medicinal Plants of Asir Region, Saudi Arabia, over Drug Resistant Isolates. BioMed Res. Int. 2019, 2019, 8928306. [Google Scholar] [CrossRef]
- Hussain, S.S.; Agoumi, A.; Amghar, S.; Boukachabine, K. Anticandida Activity of the Marketed Essential Oil of Thymus Vulgaris L and its Concomitant Action with Amphotericin B. Therapies 2011, 66, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Abdallah, H.M.; Mohamed, G.A.; Farag, M.A.; Alshali, K.Z.; Alsherif, E.A.; Ross, S.A. Volatile oil profile of some lamiaceous plants growing in Saudi Arabia and their biological activities. Z. Für Naturforschung C 2017, 72, 35–41. [Google Scholar] [CrossRef]
- Imani, Z.; Asgarpanah, J.; Hashemi, F.; Hashemi Hezaveh, J. Composition and antifungal activity of Zhumeria majdae essential oil. Curr. Med. Mycol. 2015, 1, 13–19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- İşcan, G.; Köse, Y.B.; Demirci, B.; Can Başer, K.H. Anticandidal Activity of the Essential Oil of Nepeta transcaucasicaGrossh. Chem. Biodivers. 2011, 8, 2144–2148. [Google Scholar] [CrossRef]
- İşcan, G.; Demirci, B.; Demirci, F.; Göger, F.; Kırımer, N.; Köse, Y.B.; Başer, K.H.C. Antimicrobial and Antioxidant Activities of Stachys lavandulifolia subsp. lavandulifolia Essential Oil and its Infusion. Nat. Prod. Commun. 2012, 7, 1934578X1200700. [Google Scholar] [CrossRef]
- DemiRci, B.; Köse, Y.B.; İŞcan, G. Antimicrobial essential oil of Origanum boissieri Ietswaart. J. Res. Pharm. 2020, 24, 233–239. [Google Scholar] [CrossRef]
- Jamali, C.A.; El Bouzidi, L.; Bekkouche, K.; Lahcen, H.; Markouk, M.; Wohlmuth, H.; Leach, D.; Abbad, A. Chemical Composition and Antioxidant and Anticandidal Activities of Essential Oils from Different Wild Moroccan Thymus Species. Chem. Biodivers. 2012, 9, 1188–1197. [Google Scholar] [CrossRef]
- Jaradat, N.; Adwan, L.; K’aibni, S.; Shraim, N.; Zaid, A.N. Chemical composition, anthelmintic, antibacterial and antioxidant effects of Thymus bovei essential oil. BMC Complement. Altern. Med. 2016, 16, 418. [Google Scholar] [CrossRef] [PubMed]
- Karadağ, A.E.; Demirci, B.; Kültür, Ş.; Demirci, F.; Başer, K.H.C. Antimicrobial, anticholinesterase evaluation and chemical characterization of essential oil Phlomis kurdica Rech. fil. Growing in Turkey. J. Essent. Oil Res. 2020, 1–5. [Google Scholar] [CrossRef]
- Karaman, M.; Bogavac, M.; Radovanović, B.; Sudji, J.; Tešanović, K.; Janjušević, L. Origanum vulgare essential oil affects pathogens causing vaginal infections. J. Appl. Microbiol. 2017, 122, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Stien, D.; Eparvier, V.; Ouaini, N.; El Beyrouthy, M. Report on the Medicinal Use of Eleven Lamiaceae Species in Lebanon and Rationalization of Their Antimicrobial Potential by Examination of the Chemical Composition and Antimicrobial Activity of Their Essential Oils. Evid. Based Complement. Altern. Med. 2016, 2016, 2547169. [Google Scholar] [CrossRef] [PubMed]
- Koutsaviti, A.; Milenković, M.; Tzakou, O. Antimicrobial Activity of the Essential Oil of Greek Endemic Stachys spruneri and its Main Component, Isoabienol. Nat. Prod. Commun. 2011, 6, 1934578X1100600. [Google Scholar] [CrossRef]
- Kremer, D.; Kosir, I.; Kosalec, I.; Koncic, M.; Potocnik, T.; Cerenak, A.; Bezic, N.; Srecec, S.; Dunkic, V. Investigation of Chemical Compounds, Antioxidant and Antimicrobial Properties of Teucrium arduini L. (Lamiaceae). Curr. Drug Targets 2013, 14, 1006–1014. [Google Scholar] [CrossRef]
- Lazarević, J.S.; Ðorđević, A.S.; Kitić, D.V.; Zlatković, B.K.; Stojanović, G.S. Chemical Composition and Antimicrobial Activity of the Essential Oil of Stachys officinalis (L.) Trevis. (Lamiaceae). Chem. Biodivers. 2013, 10, 1335–1349. [Google Scholar] [CrossRef]
- Marino, A.; Nostro, A.; Mandras, N.; Roana, J.; Ginestra, G.; Miceli, N.; Taviano, M.F.; Gelmini, F.; Beretta, G.; Tullio, V. Evaluation of antimicrobial activity of the hydrolate of Coridothymus capitatus (L.) Reichenb. fil. (Lamiaceae) alone and in combination with antimicrobial agents. BMC Complement. Med. Ther. 2020, 20, 89. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Porcedda, S.; Falconieri, D.; Maxia, A.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L. Chemical composition and biological assays of essential oils of Calamintha nepeta (L.) Savi subsp. nepeta (Lamiaceae). Nat. Prod. Res. 2010, 24, 1734–1742. [Google Scholar] [CrossRef]
- Milenković, M.; Stošović, J.; Slavkovska, V. Synergy between Essential Oils of Calamintha Species (Lamiaceae) and Antibiotics. Nat. Prod. Commun. 2018, 13, 1934578X1801300. [Google Scholar] [CrossRef]
- Minooeianhaghighi, M.H.; Sepehrian, L.; Shokri, H. Antifungal effects of Lavandula binaludensis and Cuminum cyminum essential oils against Candida albicans strains isolated from patients with recurrent vulvovaginal candidiasis. J. Mycol. Méd. 2017, 27, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Mkaddem, M.G.; Romdhane, M.; Ibrahim, H.; Ennajar, M.; Lebrihi, A.; Mathieu, F.; Bouajila, J. Essential Oil of Thymus capitatus Hoff. et Link. from Matmata, Tunisia: Gas Chromatography-Mass Spectrometry Analysis and Antimicrobial and Antioxidant Activities. J. Med. Food 2010, 13, 1500–1504. [Google Scholar] [CrossRef] [PubMed]
- El Mokni, R.; Majdoub, S.; Chaieb, I.; Jlassi, I.; Joshi, R.K.; Hammami, S. Chromatographic analysis, antimicrobial and insecticidal activities of the essential oil of Phlomis floccosa D. Don. Biomed. Chromatogr. 2019, 33. [Google Scholar] [CrossRef] [PubMed]
- Mothana, R.A.; Khaled, J.M.; Noman, O.M.; Kumar, A.; Alajmi, M.F.; Al-Rehaily, A.J.; Kurkcuoglu, M. Phytochemical analysis and evaluation of the cytotoxic, antimicrobial and antioxidant activities of essential oils from three Plectranthus species grown in Saudi Arabia. BMC Complement. Altern. Med. 2018, 18, 237. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, M.; Saharkhiz, M.J.; Pakshir, K.; Amini Akbarabadi, S.; Alikhani Khordshami, M.; Asadian, F.; Zareshahrabadi, Z.; Zomorodian, K. Chemical compositions and antifungal activities of Satureja macrosiphon against Candida and Aspergillus species. Curr. Med. Mycol. 2020, 5, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Niczad, A.; Sharafzadeh, S.; Alizadeh, A.; Amiri, B.; Bazrafshan, F. Variability in Essential Oil Constituent, Phenolic Content, Antioxidant and Antimicrobial Activities of Different Ecotypes of Zataria multiflora Boiss. from Iran. J. Essent. Oil Bear. Plants 2019, 22, 1435–1449. [Google Scholar] [CrossRef]
- Omayma, B.; Sabah, E.G.; Idrissi, M.E.; Bouymajane, A. Chemical composition, antimicrobial and antioxidant activities of the essential oil of Rosmarinus Officinalis L, cultivated in Fes- Meknes region. Green Appl. Chem. 2020, 8, 1–9. [Google Scholar]
- Ouknin, M.; Romane, A.; Costa, J.; Majidi, L. Comparative study of the chemical profiling, antioxidant and antimicrobial activities of essential oils of different parts of Thymus willdenowii Boiss & Reut. Nat. Prod. Res. 2019, 33, 2398–2401. [Google Scholar] [CrossRef]
- Outaleb, T.; Yekkour, A.; Hazzit, M.; Zitouni, A.; Sabaou, N. Phytochemical profiling, antioxidant and antimicrobial effectiveness of Rosmarinus tournefortii De Noe extracts issued from different regions of Algeria. J. Essent. Oil Res. 2020, 1–13. [Google Scholar] [CrossRef]
- Özek, G.; Demirci, F.; Özek, T.; Tabanca, N.; Wedge, D.E.; Khan, S.I.; Başer, K.H.C.; Duran, A.; Hamzaoglu, E. Gas chromatographic–mass spectrometric analysis of volatiles obtained by four different techniques from Salvia rosifolia Sm., and evaluation for biological activity. J. Chromatogr. A 2010, 1217, 741–748. [Google Scholar] [CrossRef]
- Palmeira-de-Oliveira, A.; Gaspar, C.; Palmeira-de-Oliveira, R.; Silva-Dias, A.; Salgueiro, L.; Cavaleiro, C.; Pina-Vaz, C.; Martinez-de-Oliveira, J.; Queiroz, J.A.; Rodrigues, A.G. The anti-Candida activity of Thymbra capitata essential oil: Effect upon pre-formed biofilm. J. Ethnopharmacol. 2012, 140, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Petretto, G.L.; Fancello, F.; Zara, S.; Foddai, M.; Mangia, N.P.; Sanna, M.L.; Omer, E.A.; Menghini, L.; Chessa, M.; Pintore, G. Antimicrobial Activity against Beneficial Microorganisms and Chemical Composition of Essential Oil of Mentha suaveolens ssp. insularis Grown in Sardinia: Mentha insularis activity against beneficial microflora…. J. Food Sci. 2014, 79, M369–M377. [Google Scholar] [CrossRef] [PubMed]
- Pietrella, D.; Angiolella, L.; Vavala, E.; Rachini, A.; Mondello, F.; Ragno, R.; Bistoni, F.; Vecchiarelli, A. Beneficial effect of Mentha suaveolens essential oil in the treatment of vaginal candidiasis assessed by real-time monitoring of infection. BMC Complement. Altern. Med. 2011, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Cocco, V.; Falconieri, D.; Porcedda, S.; Marongiu, B.; Maxia, A.; Frau, M.A.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L. Isolation of the Volatile Oil from Satureja thymbra by Supercritical Carbon Dioxide Extraction: Chemical Composition and Biological Activity. Nat. Prod. Commun. 2011, 6, 1934578X1100601. [Google Scholar] [CrossRef]
- Piras, A.; Porcedda, S.; Falconieri, D.; Maxia, A.; Gonçalves, M.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of essential oil from Mentha spicata L. and Mentha pulegium L. growing wild in Sardinia island (Italy). Nat. Prod. Res. 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Carocci, A.; Catalano, A.; Clodoveo, M.L.; Franchini, C.; Corbo, F.; Carbonara, G.G.; Carrieri, A.; Fracchiolla, G. Elucidation of the synergistic action of Mentha Piperita essential oil with common antimicrobials. PLoS ONE 2018, 13, e0200902. [Google Scholar] [CrossRef]
- Saad, A.; Fadli, M.; Bouaziz, M.; Benharref, A.; Mezrioui, N.-E.; Hassani, L. Anticandidal activity of the essential oils of Thymus maroccanus and Thymus broussonetii and their synergism with amphotericin B and fluconazol. Phytomedicine 2010, 17, 1057–1060. [Google Scholar] [CrossRef]
- Saei-Dehkordi, S.S.; Tajik, H.; Moradi, M.; Khalighi-Sigaroodi, F. Chemical composition of essential oils in Zataria multiflora Boiss. from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem. Toxicol. 2010, 48, 1562–1567. [Google Scholar] [CrossRef]
- Şerbetçi, T.; Demirci, B.; Güzel, Ç.B.; Kültür, Ş.; Ergüven, M.; Başer, K.H.C. Essential Oil Composition, Antimicrobial and Cytotoxic Activities of Two Endemic Stachys Cretica Subspecies (Lamiaceae) from Turkey. Nat. Prod. Commun. 2010, 5, 1934578X1000500. [Google Scholar] [CrossRef]
- Sevindik, E.; Aydin, S.; Kurtoglu, C.; Tin, B. Evaluation of essential oil composition of Origanum onites L.(Lamiaceae) plant and antifungal activity on some strong pathogen fungi. AFS-Adv. Food Sci. 2019, 41, 32–35. [Google Scholar]
- Sharifzadeh, A.; Khosravi, A.R.; Ahmadian, S. Chemical composition and antifungal activity of Satureja hortensis L. essentiall oil against planktonic and biofilm growth of Candida albicans isolates from buccal lesions of HIV+ individuals. Microb. Pathog. 2016, 96, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Souri, N.; Monsef-Esfehani, M.R.; Vazirian, M.; Samadi, N.; Sadati Lamardi, S.N. Analysis of Essential Oil Composition and Antimicrobial Effect of Stachys discolor subsp. mazandarana. Tradit. Integr. Med. 2020, 5, 1–6. [Google Scholar] [CrossRef]
- Spagnoletti, A.; Guerrini, A.; Tacchini, M.; Vinciguerra, V.; Leone, C.; Maresca, I.; Simonetti, G.; Sacchetti, G.; Angiolella, L. Chemical composition and bio-efficacy of essential oils from italian aromatic plants: Mentha suaveolens, Coridothymus capitatus, Origanum hirtum and Rosmarinus officinalis. Nat. Prod. Commun. 2016, 11, 1934578X1601101023. [Google Scholar] [CrossRef]
- Tadić, V.; Bojović, D.; Arsić, I.; Đorđević, S.; Aksentijevic, K.; Stamenić, M.; Janković, S. Chemical and Antimicrobial Evaluation of Supercritical and Conventional Sideritis scardica Griseb., Lamiaceae Extracts. Molecules 2012, 17, 2683–2703. [Google Scholar] [CrossRef] [PubMed]
- Tadić, V.; Oliva, A.; Božović, M.; Cipolla, A.; De Angelis, M.; Vullo, V.; Garzoli, S.; Ragno, R. Chemical and Antimicrobial Analyses of Sideritis romana L. subsp. purpurea (Tal. ex Benth.) Heywood, an Endemic of the Western Balkan. Molecules 2017, 22, 1395. [Google Scholar] [CrossRef]
- Teymouri, M.; Alizadeh, A. Chemical composition and antimicrobial activity of the essential oil of Mentha mozaffarianii Jamzad growing wild and cultivated in Iran. Nat. Prod. Res. 2018, 32, 1320–1323. [Google Scholar] [CrossRef]
- Tullio, V.; Roana, J.; Scalas, D.; Mandras, N. Evaluation of the Antifungal Activity of Mentha x piperita (Lamiaceae) of Pancalieri (Turin, Italy) Essential Oil and Its Synergistic Interaction with Azoles. Molecules 2019, 24, 3148. [Google Scholar] [CrossRef]
- Vale-Silva, L.; Gonçalves, M.; Cavaleiro, C.; Salgueiro, L.; Pinto, E. Antifungal Activity of the Essential Oil of Thymus x viciosoi against Candida, Cryptococcus, Aspergillus and Dermatophyte Species. Planta Med. 2010, 76, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Vale-Silva, L.; Silva, M.-J.; Oliveira, D.; Gonçalves, M.-J.; Cavaleiro, C.; Salgueiro, L.; Pinto, E. Correlation of the chemical composition of essential oils from Origanum vulgare subsp. virens with their in vitro activity against pathogenic yeasts and filamentous fungi. J. Med. Microbiol. 2012, 61, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Vitali, L.A.; Dall’Acqua, S.; Maggi, F.; Martonfi, P.; Papa, F.; Petrelli, D.; Sut, S.; Lupidi, G. Antimicrobial and antioxidant activity of the essential oil from the Carpathian Thymus alternans Klokov. Nat. Prod. Res. 2017, 31, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, G.; Demirci, B.; Aytaç, Z.; Demirci, F. Characterization of the Essential Oil and Anticandidal Evaluation of Thymus pallasicus Hayek & Velen. from Turkey. Nat. Volatiles Essent. Oils 2019, 6, 1–5. [Google Scholar]
- Zomorodian, K.; Saharkhiz, M.J.; Rahimi, M.J.; Shariatifard, S.; Pakshir, K.; Khashei, R. Chemical Composition and Antimicrobial Activities of Essential Oil of Nepeta Cataria L. Against Common Causes of Oral Infections. J. Dent. Tehran Iran 2013, 10, 329–337. [Google Scholar]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Canhoto, J.; Vale-Silva, L.; Silva, M.J.; Pinto, E.; Salgueiro, L. Chemical composition and antifungal activity of the essential oils of Lavandula viridis L’Hér. J. Med. Microbiol. 2011, 60, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Vale-Silva, L.; Gonçalves, M.J.; Cavaleiro, C.; Vaz, S.; Canhoto, J.; Pinto, E.; Salgueiro, L. Antifungal activity of phenolic-rich Lavandula multifida L. essential oil. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1359–1366. [Google Scholar] [CrossRef]
- Zuzarte, M.; Gonçalves, M.J.; Cruz, M.T.; Cavaleiro, C.; Canhoto, J.; Vaz, S.; Pinto, E.; Salgueiro, L. Lavandula luisieri essential oil as a source of antifungal drugs. Food Chem. 2012, 135, 1505–1510. [Google Scholar] [CrossRef]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Benzarti, A.; Marongiu, B.; Maxia, A.; Piras, A.; Salgueiro, L. Antifungal and anti-inflammatory potential of Lavandula stoechas and Thymus herba-barona essential oils. Ind. Crops Prod. 2013, 44, 97–103. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Guinea, J.; Cuenca-Estrella, M.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Howard, S.J. EUCAST DEFINITIVE DOCUMENT E.DEF 9.3. 2015, p. 23. Available online: https://www.aspergillus.org.uk/sites/default/files/pictures/Lab_protocols/EUCAST_E_Def_9_3_Mould_testing_definitive_0.pdf (accessed on 12 May 2020).
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J. Appl. Microbiol. 2003, 95, 853–860. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmad, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Ocimum sanctum essential oil and its active principles exert their antifungal activity by disrupting ergosterol biosynthesis and membrane integrity. Res. Microbiol. 2010, 161, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Zore, G.B.; Thakre, A.D.; Jadhav, S.; Karuppayil, S.M. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomed. Int. J. Phytother. Phytopharm. 2011, 18, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Lai, W.-L.; Chuang, K.-C.; Lee, M.-H.; Tsai, Y.-C. The inhibitory activity of linalool against the filamentous growth and biofilm formation in Candida albicans. Med. Mycol. 2013, 51, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef]
- Rivera-Yañez, C.R.; Terrazas, L.I.; Jimenez-Estrada, M.; Campos, J.E.; Flores-Ortiz, C.M.; Hernandez, L.B.; Cruz-Sanchez, T.; Garrido-Fariña, G.I.; Rodriguez-Monroy, M.A.; Canales-Martinez, M.M. Anti-Candida Activity of Bursera morelensis Ramirez Essential Oil and Two Compounds, α-Pinene and γ-Terpinene-An In Vitro Study. Molecules 2017, 22, 2095. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2011, 30, 41–50. [Google Scholar] [CrossRef]
- Marcos-Zambrano, L.J.; Escribano, P.; Bouza, E.; Guinea, J. Production of biofilm by Candida and non-Candida spp. isolates causing fungemia: Comparison of biomass production and metabolic activity and development of cut-off points. Int. J. Med. Microbiol. 2014, 304, 1192–1198. [Google Scholar] [CrossRef]
- Taff, H.T.; Mitchell, K.F.; Edward, J.A.; Andes, D.R. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013, 8, 1325–1337. [Google Scholar] [CrossRef]
- Raut, J.S.; Shinde, R.B.; Chauhan, N.M.; Mohan Karuppayil, S. Terpenoids of plant origin inhibit morphogenesis, adhesion, and biofilm formation by Candida albicans. Biofouling 2013, 29, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Giovana, C.B.; de Feiria Simone, N.B.; de Laet Santana, P.; Paula, C.A.; Marcelo, F.G.B.; Marcelle, M.B.-R.; Janaina, P.B.; de Oliveira Thais, R.; Jose, F.H. Antifungal and cytotoxic activity of purified biocomponents as carvone, menthone, menthofuran and pulegone from Mentha spp. Afr. J. Plant Sci. 2016, 10, 203–210. [Google Scholar] [CrossRef]
- Cui, J.; Ren, B.; Tong, Y.; Dai, H.; Zhang, L. Synergistic combinations of antifungals and anti-virulence agents to fight against Candida albicans. Virulence 2015, 6, 362–371. [Google Scholar] [CrossRef]
- De Cremer, K.; Staes, I.; Delattin, N.; Cammue, B.P.; Thevissen, K.; De Brucker, K. Combinatorial drug approaches to tackle Candida albicans biofilms. Expert Rev. Anti Infect. Ther. 2015, 13, 973–984. [Google Scholar] [CrossRef] [PubMed]
- van Vuuren, S.; Viljoen, A. Plant-Based Antimicrobial Studies—Methods and Approaches to Study the Interaction between Natural Products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef]
- Konuk, H.B.; Ergüden, B. Phenolic –OH group is crucial for the antifungal activity of terpenoids via disruption of cell membrane integrity. Folia Microbiol. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Sittampalam, G.S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C.P., Baell, J., Bejcek, B., Caaveiro, J.M.M., Chung, T.D.Y., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Libralato, G.; Prato, E.; Migliore, L.; Cicero, A.M.; Manfra, L. A review of toxicity testing protocols and endpoints with Artemia spp. Ecol. Indic. 2016, 69, 35–49. [Google Scholar] [CrossRef]
- Saeed, M.E.M.; Abdelgadir, H.; Sugimoto, Y.; Khalid, H.E.; Efferth, T. Cytotoxicity of 35 medicinal plants from Sudan towards sensitive and multidrug-resistant cancer cells. J. Ethnopharmacol. 2015, 174, 644–658. [Google Scholar] [CrossRef]
- Kuete, V.; Seo, E.-J.; Krusche, B.; Oswald, M.; Wiench, B.; Schröder, S.; Greten, H.J.; Lee, I.-S.; Efferth, T. Cytotoxicity and pharmacogenomics of medicinal plants from traditional korean medicine. Evid. Based Complement. Altern. Med. ECAM 2013, 2013, 341724. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).