Augmented Renal Clearance and How to Augment Antibiotic Dosing
Abstract
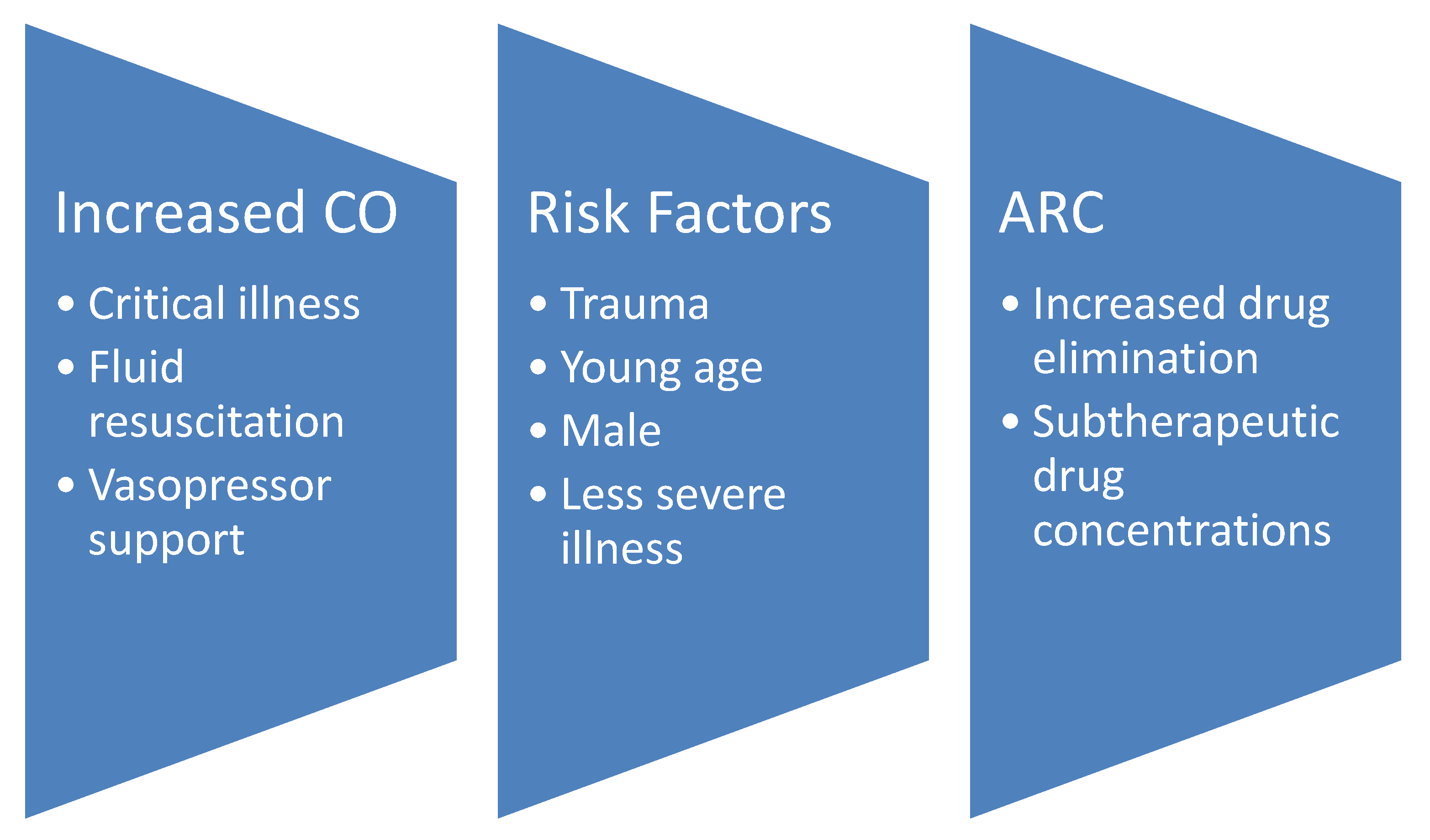
1. Introduction
2. Creatinine Clearance
3. Identifying Augmented Renal Clearance
4. Effects on Antibiotic Therapy
4.1. Beta-Lactams
4.2. Vancomycin
4.3. Additional Agents
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bilbao-Meseguer, I.; Rodriguez-Gascon, A.; Barrasa, H.; Isla, A.; Solinis, M.A. Augmented renal clearance in critically Ill patients: A systematic review. Clin. Pharmacokinet. 2018, 57, 1107–1121. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Manzi, J.; Levey, A.S.; Chen, J.; Deysher, A.E.; Greene, T.; Poggio, E.D.; Schmid, C.H.; Steffes, M.W.; Zhang, Y.L.; et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am. J. Kidney Dis. 2007, 50, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Bookstaver, P.B.; Johnson, J.W.; McCoy, T.P.; Stewart, D.; Williamson, J.C. Modification of Diet in Renal Disease and modified Cockcroft-Gault formulas in predicting aminoglycoside elimination. Ann. Pharm. 2008, 42, 1758–1765. [Google Scholar] [CrossRef]
- Chin, P.K.; Florkowski, C.M.; Begg, E.J. The performances of the Cockcroft-Gault, modification of diet in renal disease study and chronic kidney disease epidemiology collaboration equations in predicting gentamicin clearance. Ann. Clin. Biochem. 2013, 50, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Saez Fernandez, E.M.; Perez-Blanco, J.S.; Lanao, J.M.; Calvo, M.V.; Martin-Suarez, A. Evaluation of renal function equations to predict amikacin clearance. Expert. Rev. Clin. Pharmacol. 2019, 12, 805–813. [Google Scholar] [CrossRef]
- Glatard, A.; Bourguignon, L.; Jelliffe, R.W.; Maire, P.; Neely, M.N.; Goutelle, S. Influence of renal function estimation on pharmacokinetic modeling of vancomycin in elderly patients. Antimicrob. Agents Chemother. 2015, 59, 2986–2994. [Google Scholar] [CrossRef]
- Schwandt, A.; Denkinger, M.; Fasching, P.; Pfeifer, M.; Wagner, C.; Weiland, J.; Zeyfang, A.; Holl, R.W. Comparison of MDRD, CKD-EPI, and Cockcroft-Gault equation in relation to measured glomerular filtration rate among a large cohort with diabetes. J. Diabetes Complicat. 2017, 31, 1376–1383. [Google Scholar] [CrossRef]
- Cherry, R.A.; Eachempati, S.R.; Hydo, L.; Barie, P.S. Accuracy of short-duration creatinine clearance determinations in predicting 24-hour creatinine clearance in critically ill and injured patients. J. Trauma. 2002, 53, 267–271. [Google Scholar] [CrossRef]
- Baumann, T.J.; Staddon, J.E.; Horst, H.M.; Bivins, B.A. Minimum urine collection periods for accurate determination of creatinine clearance in critically ill patients. Clin. Pharm. 1987, 6, 393–398. [Google Scholar] [PubMed]
- O’Connell, M.B.; Wong, M.O.; Bannick-Mohrland, S.D.; Dwinell, A.M. Accuracy of 2- and 8-hour urine collections for measuring creatinine clearance in the hospitalized elderly. Pharmacotherapy 1993, 13, 135–142. [Google Scholar] [PubMed]
- Udy, A.A.; Roberts, J.A.; Shorr, A.F.; Boots, R.J.; Lipman, J. Augmented renal clearance in septic and traumatized patients with normal plasma creatinine concentrations: Identifying at-risk patients. Crit. Care (Lond. Engl.) 2013, 17, R35. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Roberts, J.A.; Boots, R.J.; Paterson, D.L.; Lipman, J. Augmented renal clearance: Implications for antibacterial dosing in the critically ill. Clin. Pharmacokinet. 2010, 49, 1–16. [Google Scholar] [CrossRef]
- Baptista, J.P.; Martins, P.J.; Marques, M.; Pimentel, J.M. Prevalence and risk factors for augmented renal clearance in a population of critically Ill patients. J. Intensive Care Med. 2018, 885066618809688. [Google Scholar] [CrossRef]
- Mulder, M.B.; Eidelson, S.A.; Sussman, M.S.; Schulman, C.I.; Lineen, E.B.; Iyenger, R.S.; Namias, N.; Proctor, K.G. Risk factors and clinical outcomes associated with augmented renal clearance in Trauma Patients. J. Surg. Res. 2019, 244, 477–483. [Google Scholar] [CrossRef]
- Declercq, P.; Nijs, S.; D’Hoore, A.; Van Wijngaerden, E.; Wolthuis, A.; de Buck van Overstraeten, A.; Wauters, J.; Spriet, I. Augmented renal clearance in non-critically ill abdominal and trauma surgery patients is an underestimated phenomenon: A point prevalence study. J. Trauma Acute Care Surg. 2016, 81, 468–477. [Google Scholar] [CrossRef]
- Barletta, J.F.; Mangram, A.J.; Byrne, M.; Sucher, J.F.; Hollingworth, A.K.; Ali-Osman, F.R.; Shirah, G.R.; Haley, M.; Dzandu, J.K. Identifying augmented renal clearance in trauma patients: Validation of the Augmented Renal Clearance in Trauma Intensive Care scoring system. J. Trauma Acute Care Surg. 2017, 82, 665–671. [Google Scholar] [CrossRef]
- Tsai, D.; Udy, A.A.; Stewart, P.C.; Gourley, S.; Morick, N.M.; Lipman, J.; Roberts, J.A. Prevalence of augmented renal clearance and performance of glomerular filtration estimates in Indigenous Australian patients requiring intensive care admission. Anaesth. Intensive Care 2018, 46, 42–50. [Google Scholar] [CrossRef]
- De Waele, J.J.; Dumoulin, A.; Janssen, A.; Hoste, E.A. Epidemiology of augmented renal clearance in mixed ICU patients. Minerva Anestesiol 2015, 81, 1079–1085. [Google Scholar]
- Van Der Heggen, T.; Dhont, E.; Peperstraete, H.; Delanghe, J.R.; Vande Walle, J.; De Paepe, P.; De Cock, P.A. Augmented renal clearance: A common condition in critically ill children. Pediatr. Nephrol. 2019, 34, 1099–1106. [Google Scholar] [CrossRef]
- Dhont, E.; Van Der Heggen, T.; De Jaeger, A.; Vande Walle, J.; De Paepe, P.; De Cock, P.A. Augmented renal clearance in pediatric intensive care: Are we undertreating our sickest patients? Pediatr. Nephrol. 2020, 35, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Akers, K.S.; Niece, K.L.; Chung, K.K.; Cannon, J.W.; Cota, J.M.; Murray, C.K. Modified Augmented Renal Clearance score predicts rapid piperacillin and tazobactam clearance in critically ill surgery and trauma patients. J. Trauma Acute Care Surg. 2014, 77, S163–S170. [Google Scholar] [CrossRef] [PubMed]
- Asin-Prieto, E.; Rodriguez-Gascon, A.; Isla, A. Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. J. Infect. Chemother. 2015, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Claus, B.O.; Hoste, E.A.; Colpaert, K.; Robays, H.; Decruyenaere, J.; De Waele, J.J. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J. Crit. Care 2013, 28, 695–700. [Google Scholar] [CrossRef]
- Roberts, J.A.; Kruger, P.; Paterson, D.L.; Lipman, J. Antibiotic resistance—What’s dosing got to do with it? Crit. Care Med. 2008, 36, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L. Antimicrobial pharmacodynamics: Critical interactions of ‘bug and drug’. Nat. Rev. Microbiol. 2004, 2, 289–300. [Google Scholar] [CrossRef]
- Udy, A.A.; Varghese, J.M.; Altukroni, M.; Briscoe, S.; McWhinney, B.C.; Ungerer, J.P.; Lipman, J.; Roberts, J.A. Subtherapeutic initial beta-lactam concentrations in select critically ill patients: Association between augmented renal clearance and low trough drug concentrations. Chest 2012, 142, 30–39. [Google Scholar] [CrossRef]
- McKinnon, P.S.; Paladino, J.A.; Schentag, J.J. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J. Antimicrob. Agents 2008, 31, 345–351. [Google Scholar] [CrossRef]
- Huttner, A.; Von Dach, E.; Renzoni, A.; Huttner, B.D.; Affaticati, M.; Pagani, L.; Daali, Y.; Pugin, J.; Karmime, A.; Fathi, M.; et al. Augmented renal clearance, low beta-lactam concentrations and clinical outcomes in the critically ill: An observational prospective cohort study. Int. J. Antimicrob. Agents 2015, 45, 385–392. [Google Scholar] [CrossRef]
- Wu, C.C.; Tai, C.H.; Liao, W.Y.; Wang, C.C.; Kuo, C.H.; Lin, S.W.; Ku, S.C. Augmented renal clearance is associated with inadequate antibiotic pharmacokinetic/pharmacodynamic target in Asian ICU population: A prospective observational study. Infect. Drug Resist. 2019, 12, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Troger, U.; Drust, A.; Martens-Lobenhoffer, J.; Tanev, I.; Braun-Dullaeus, R.C.; Bode-Boger, S.M. Decreased meropenem levels in Intensive Care Unit patients with augmented renal clearance: Benefit of therapeutic drug monitoring. Int. J. Antimicrob. Agents 2012, 40, 370–372. [Google Scholar] [CrossRef]
- Dulhunty, J.M.; Webb, S.A.; Paterson, D.L.; Bellomo, R.; Myburgh, J.; Roberts, J.A.; Lipman, J. A survey of antibiotic prescribing practices in Australian and New Zealand intensive care units. Crit. Care Resusc. 2010, 12, 162–170. [Google Scholar] [PubMed]
- Sinnollareddy, M.G.; Roberts, M.S.; Lipman, J.; Roberts, J.A. β-lactam pharmacokinetics and pharmacodynamics in critically ill patients and strategies for dose optimization: A structured review. Clin. Exp. Pharmacol. Physiol. 2012, 39, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Societe Francaise de Pharmacologie et Therapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Societe Francaise d’Anesthesie et Reanimation-SFAR). Crit. Care (Lond. Engl.) 2019, 23, 104. [Google Scholar] [CrossRef]
- Carrie, C.; Chadefaux, G.; Sauvage, N.; de Courson, H.; Petit, L.; Nouette-Gaulain, K.; Pereira, B.; Biais, M. Increased β-Lactams dosing regimens improve clinical outcome in critically ill patients with augmented renal clearance treated for a first episode of hospital or ventilator-acquired pneumonia: A before and after study. Crit. Care (Lond. Engl.) 2019, 23, 379. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Kuti, J.L.; Nicolau, D.P. Probability of pharmacodynamic target attainment with standard and prolonged-infusion antibiotic regimens for empiric therapy in adults with hospital-acquired pneumonia. Clin. Ther. 2009, 31, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Sutherland, C.A.; Kuti, J.L.; Nicolau, D.P. Optimal dosing of piperacillin-tazobactam for the treatment of Pseudomonas aeruginosa infections: Prolonged or continuous infusion? Pharmacotherapy 2007, 27, 1490–1497. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Sulaiman, H.; Mat-Nor, M.B.; Rai, V.; Wong, K.K.; Hasan, M.S.; Abd Rahman, A.N.; Jamal, J.A.; Wallis, S.C.; Lipman, J.; et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): A prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016, 42, 1535–1545. [Google Scholar] [CrossRef]
- Beranger, A.; Benaboud, S.; Urien, S.; Moulin, F.; Bille, E.; Lesage, F.; Zheng, Y.; Genuini, M.; Gana, I.; Renolleau, S.; et al. Piperacillin population pharmacokinetics and dosing regimen optimization in critically Ill children with normal and augmented renal clearance. Clin. Pharmacokinet. 2019, 58, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; et al. Association between augmented renal clearance and clinical outcomes in patients receiving beta-lactam antibiotic therapy by continuous or intermittent infusion: A nested cohort study of the BLING-II randomised, placebo-controlled, clinical trial. Int. J. Antimicrob. Agents 2017, 49, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Lomaestro, B.M.; Rotschafer, J.C.; Moellering, R.C.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Vancomycin therapeutic guidelines: A summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009, 49, 325–327. [Google Scholar] [CrossRef]
- Chu, Y.; Luo, Y.; Qu, L.; Zhao, C.; Jiang, M. Application of vancomycin in patients with varying renal function, especially those with augmented renal clearance. Pharm. Biol. 2016, 54, 2802–2806. [Google Scholar] [CrossRef]
- Molina, K.C.; Hall, S.T.; Barletta, J.F.; Mangram, A.J.; Dzandu, J.K.; Huang, V. Utilization of augmented renal clearance in Trauma Intensive Care Scoring System to improve Vancomycin dosing in Trauma patients at risk for augmented renal clearance. Surg. Infect. (Larchmt) 2020, 21, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, R.D.; Talledo, O.; Neely, S.; White, B.; Celii, A.; Cross, A.; Kennedy, R. Vancomycin dosing in critically ill trauma patients: The VANCTIC Study. J. Trauma Acute Care Surg. 2019, 87, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Ishii, H.; Shimoshikiryo, T.; Shimomura, T.; Tsuji, D.; Inoue, K.; Kadoiri, T.; Itoh, K. Augmented Renal Clearance in Patients with Febrile Neutropenia is Associated with Increased Risk for Subtherapeutic Concentrations of Vancomycin. Ther. Drug Monit. 2016, 38, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Morbitzer, K.A.; Rhoney, D.H.; Dehne, K.A.; Jordan, J.D. Enhanced renal clearance and impact on vancomycin pharmacokinetic parameters in patients with hemorrhagic stroke. J. Intensive Care 2019, 7, 51. [Google Scholar] [CrossRef]
- Avedissian, S.N.; Bradley, E.; Zhang, D.; Bradley, J.S.; Nazer, L.H.; Tran, T.M.; Nguyen, A.; Le, J. Augmented Renal Clearance Using Population-Based Pharmacokinetic Modeling in Critically Ill Pediatric Patients. Pediatr. Crit. Care Med. 2017, 18, e388–e394. [Google Scholar] [CrossRef]
- Lodise, T.P.; Drusano, G.L.; Zasowski, E.; Dihmess, A.; Lazariu, V.; Cosler, L.; McNutt, L.A. Vancomycin exposure in patients with methicillin-resistant Staphylococcus aureus bloodstream infections: How much is enough? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 59, 666–675. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. AJHP Off. J. Am. Soc. Health Syst. Pharm. 2020. [Google Scholar] [CrossRef]
- Elder, K.; Hill, D.M.; Hickerson, W.L. Characterization of variables for potential impact on vancomycin pharmacokinetics in thermal or inhalation injury. Burn. J. Int. Soc. for Burn Inj. 2018, 44, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Eyler, R.F.; Shvets, K. Clinical pharmacology of antibiotics. Clin. J. Am. Soc. Nephrol. 2019, 14, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.C.; Wisniewski, C.S.; Wolf, B.; Bosso, J.A. Comparison of Linezolid and Vancomycin for Methicillin-Resistant Staphylococcus aureus Pneumonia: Institutional Implications. Pharmacotherapy 2016, 36, 731–739. [Google Scholar] [CrossRef]
- Liu, P.; Capitano, B.; Stein, A.; El-Solh, A.A. Clinical outcomes of linezolid and vancomycin in patients with nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus stratified by baseline renal function: A retrospective, cohort analysis. BMC Nephrol. 2017, 18, 168. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.; Xie, J.; Wang, T.; Zheng, X.; He, H.; Dong, W.; Xing, J.; Dong, Y. Linezolid versus vancomycin for the treatment of suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia: A systematic review employing meta-analysis. Eur. J. Clin. Pharmacol. 2015, 71, 107–115. [Google Scholar] [CrossRef]
- French, G. Safety and tolerability of linezolid. J. Antimicrob. Chemother. 2003, 51, ii45–ii53. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, E.; Cho, Y.J.; Lee, Y.J.; Rhie, S.J. Linezolid-induced thrombocytopenia increases mortality risk in intensive care unit patients, a 10 year retrospective study. J. Clin. Pharm. Ther. 2019, 44, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Hanai, Y.; Matsuo, K.; Ogawa, M.; Higashi, A.; Kimura, I.; Hirayama, S.; Kosugi, T.; Nishizawa, K.; Yoshio, T. A retrospective study of the risk factors for linezolid-induced thrombocytopenia and anemia. J. Infect. Chemother. 2016, 22, 536–542. [Google Scholar] [CrossRef]
- Giunio-Zorkin, N.; Brown, G. Real-life frequency of new-onset Thrombocytopenia during Linezolid treatment. Can. J. Hosp. Pharm. 2019, 72, 133–138. [Google Scholar] [CrossRef]
- Barrasa, H.; Soraluce, A.; Uson, E.; Sainz, J.; Martin, A.; Sanchez-Izquierdo, J.A.; Maynar, J.; Rodriguez-Gascon, A.; Isla, A. Impact of augmented renal clearance on the pharmacokinetics of linezolid: Advantages of continuous infusion from a pharmacokinetic/pharmacodynamic perspective. Int. J. Infect. Dis. 2020, 93, 329–338. [Google Scholar] [CrossRef]
- Kashuba, A.D.; Nafziger, A.N.; Drusano, G.L.; Bertino, J.S., Jr. Optimizing aminoglycoside therapy for nosocomial pneumonia caused by gram-negative bacteria. Antimicrob. Agents Chemother. 1999, 43, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Carrie, C.; Delzor, F.; Roure, S.; Dubuisson, V.; Petit, L.; Molimard, M.; Breilh, D.; Biais, M. Population pharmacokinetic study of the suitability of standard dosing regimens of Amikacin in critically Ill patients with open-abdomen and negative-pressure wound therapy. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Arechiga-Alvarado, N.A.; Medellin-Garibay, S.E.; Milan-Segovia, R.D.C.; Ortiz-Alvarez, A.; Magana-Aquino, M.; Romano-Moreno, S. Population pharmacokinetics of amikacin administered once-daily in patients with different renal function. Antimicrob. Agents Chemother. 2020. [Google Scholar] [CrossRef]
- Avedissian, S.N.; Rhodes, N.J.; Kim, Y.; Bradley, J.; Valdez, J.L.; Le, J. Augmented renal clearance of aminoglycosides using population-based pharmacokinetic modelling with Bayesian estimation in the paediatric ICU. J. Antimicrob. Chemother. 2020, 75, 162–169. [Google Scholar] [CrossRef]
- Aitullina, A.; Krumina, A.; Svirskis, S.; Purvina, S. Colistin use in patients with extreme renal function: From dialysis to augmented clearance. Medicina 2019, 55, 33. [Google Scholar] [CrossRef] [PubMed]
- Pea, F.; Lewis, R.E. Overview of antifungal dosing in invasive candidiasis. J. Antimicrob. Chemother. 2018, 73, i33–i43. [Google Scholar] [CrossRef]
- Calandra, T.; Roberts, J.A.; Antonelli, M.; Bassetti, M.; Vincent, J.L. Diagnosis and management of invasive candidiasis in the ICU: An updated approach to an old enemy. Crit. Care (Lond. Engl.) 2016, 20, 125. [Google Scholar] [CrossRef]
- Cota, J.M.; FakhriRavari, A.; Rowan, M.P.; Chung, K.K.; Murray, C.K.; Akers, K.S. Intravenous antibiotic and antifungal agent pharmacokinetic-pharmacodynamic dosing in adults with severe burn injury. Clin. Ther. 2016, 38, 2016–2031. [Google Scholar] [CrossRef]
- FETROJA(R) (Cefiderocol) Prescribing Information; Shionogi & Co., Ltd.: Osaka, Japan, 2019.
- Caro, L.; Larson, K.B.; Nicolau, D.; De Waele, J.; Kuti, J.; Yu, B.; Gadzicki, E.; Adedoyin, A.; Zeng, Z.; Rhee, E.G. PK/PD and safety of 3 g Ceftolozane/Tazobactam in critically-Ill augmented renal clearance patients. (Abstract No. 0661). In Proceedings of the Society of Critical Care Medicine (SCCM) 46th Critical Care Congress, Honolulu, HI, USA, 21–25 January 2017. [Google Scholar]
- Caro, L.; Nicolau, D.P.; De Waele, J.J.; Kuti, J.L.; Larson, K.B.; Gadzicki, E.; Yu, B.; Zeng, Z.; Adedoyin, A.; Rhee, E.G. Lung penetration, bronchopulmonary pharmacokinetic/pharmacodynamic profile and safety of 3 g of ceftolozane/tazobactam administered to ventilated, critically ill patients with pneumonia. J. Antimicrob. Chemother. 2020. [Google Scholar] [CrossRef]
- Bhagunde, P.; Patel, P.; Lala, M.; Watson, K.; Copalu, W.; Xu, M.; Kulkarni, P.; Young, K.; Rizk, M.L. Population Pharmacokinetic analysis for Imipenem-Relebactam in healthy volunteers and patients with bacterial infections. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 748–758. [Google Scholar] [CrossRef]
- Li, J.; Lovern, M.; Green, M.L.; Chiu, J.; Zhou, D.; Comisar, C.; Xiong, Y.; Hing, J.; MacPherson, M.; Wright, J.G.; et al. Ceftazidime-Avibactam population Pharmacokinetic modeling and Pharmacodynamic target attainment across adult indications and patient subgroups. Clin. Transl. Sci. 2019, 12, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Soraluce, A.; Asin-Prieto, E.; Rodriguez-Gascon, A.; Barrasa, H.; Maynar, J.; Carcelero, E.; Soy, D.; Isla, A. Population pharmacokinetics of daptomycin in critically ill patients. Int. J. Antimicrob. Agents 2018, 52, 158–165. [Google Scholar] [CrossRef] [PubMed]
| 1. Cockcroft–Gault (CG) [2]: × 0.85 (if female) 2. Modification of Diet in Renal Disease (MDRD) [3]: eGFR = 175 × SCr−1.154 × age−0.203 × 0.742 (if female) × 1.212 (if black) 3. Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [4]: eGFR = 141 × min (SCr/κ, 1)α × max (SCr/κ, 1)−1.209 × 0.993age × 1.018 (if female) × 1.159 (if black) |
| 1. Use maximum approved dosing regimen |
| 2. Administer doses in a prolonged or continuous infusion |
| 3. Therapeutic drug monitoring |
| 4. Switch to an alternative agent that is not largely renally eliminated |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, I.H.; Nicolau, D.P. Augmented Renal Clearance and How to Augment Antibiotic Dosing. Antibiotics 2020, 9, 393. https://doi.org/10.3390/antibiotics9070393
Chen IH, Nicolau DP. Augmented Renal Clearance and How to Augment Antibiotic Dosing. Antibiotics. 2020; 9(7):393. https://doi.org/10.3390/antibiotics9070393
Chicago/Turabian StyleChen, Iris H., and David P. Nicolau. 2020. "Augmented Renal Clearance and How to Augment Antibiotic Dosing" Antibiotics 9, no. 7: 393. https://doi.org/10.3390/antibiotics9070393
APA StyleChen, I. H., & Nicolau, D. P. (2020). Augmented Renal Clearance and How to Augment Antibiotic Dosing. Antibiotics, 9(7), 393. https://doi.org/10.3390/antibiotics9070393




