Antimicrobial Photodynamic Therapy in the Control of COVID-19
Abstract
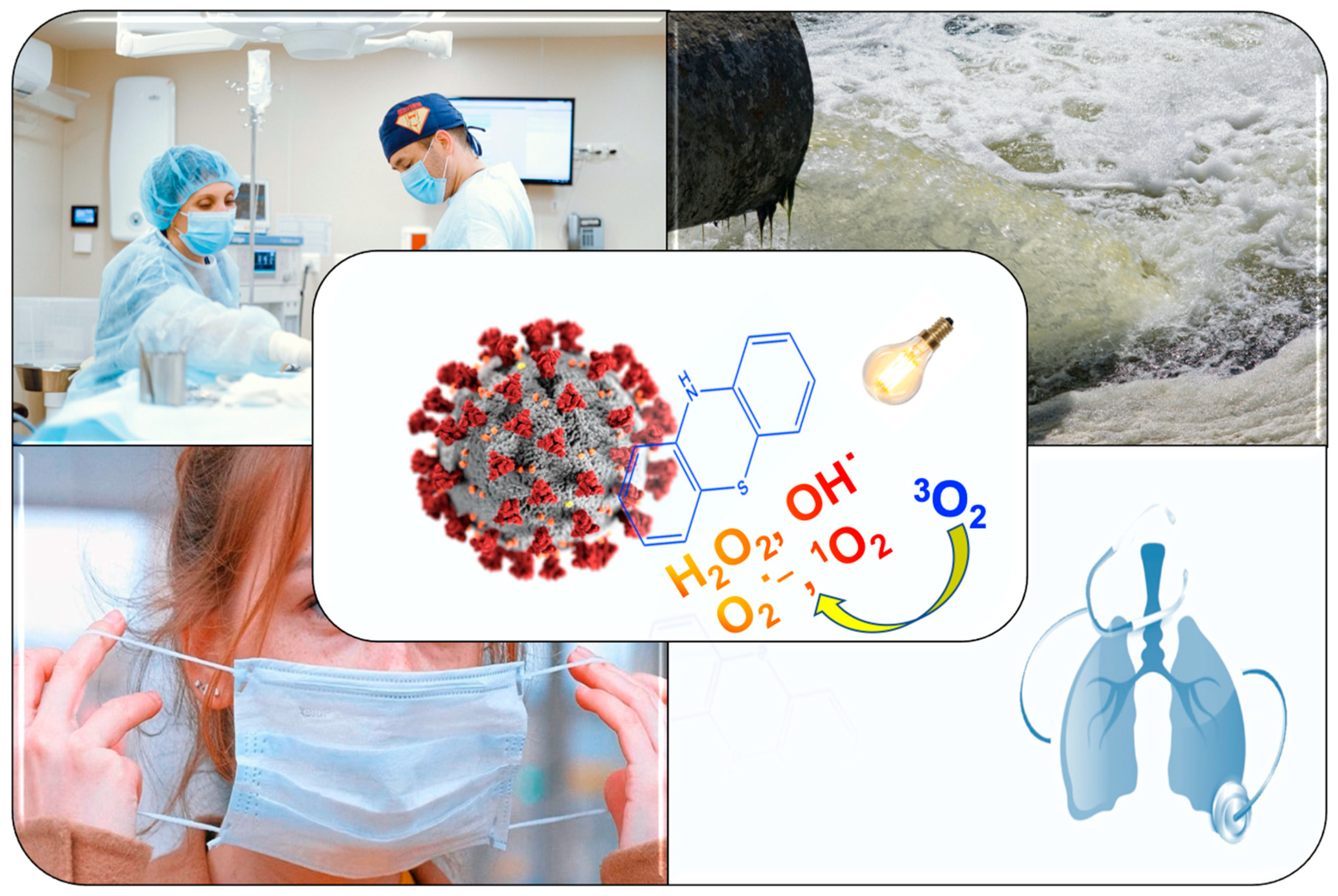
Main text
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, Z.-D.; Wang, Z.-Y.; Zhang, S.-F.; Li, X.; Li, L.; Li, C.; Cui, Y.; Fu, R.-B.; Dong, Y.-Z.; Chi, X.-Y.; et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital Wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020, 26. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.W.X.; Tan, Y.K.; Chia, P.Y.; Lee, T.H.; Ng, O.-T.; Wong, M.S.Y.; Marimuthu, K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA 2020, 323, 1610. [Google Scholar] [CrossRef]
- CDC. Infection Control: Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Finfection-control%2Fcontrol-recommendations.html (accessed on 5 May 2020).
- Publications & Data. European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/publications-data/infectionprevention-and-control-covid-19-healthcare-settings (accessed on 5 May 2020).
- Jaffe, S. Regulators split on antimalarials for COVID-19. Lancet 2020, 395, 1179. [Google Scholar] [CrossRef]
- Rana, D.R.; Dulal, S. No therapeutic application of chloroquine and hydroxychloroquine in clinical trials for COVID-19: A systematic review. MedRxiv 2020. [Google Scholar] [CrossRef]
- Borba, M.; Val, F.D.A.; Sampaio, V.S.; Alexandre, M.A.; Melo, G.C.; Brito, M.; Mourão, M.P.G.; Brito-Sousa, J.D.; Baia-Da-Silva, D.C.; Guerra, M.V.F.; et al. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: Preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study). JAMA 2020. [Google Scholar] [CrossRef]
- Lane, J.C.; Weaver, J.; Kostka, K.; Salles, T.D.; Abrahão, M.F.; Alghoul, H.; Alser, O.; Alshammari, T.M.; Biedermann, P.; Burn, E.; et al. Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: A multinational, network cohort and self-controlled case series study. MedRXIV 2020. [Google Scholar] [CrossRef]
- Colson, P.; Rolain, J.-M.; Lagier, J.-C.; Brouqui, P.; Raoult, D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents 2020, 55, 105932. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Working to Supply Remdesivir for COVID-19. Available online: https://www.gilead.com/purpose/advancing-global-health/covid-19/working-to-supply-remdesivir-for-covid-19 (accessed on 5 May 2020).
- FDA. Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment (accessed on 5 May 2020).
- Almeida, A.; Cunha, A.; Faustino, M.A.F.; Tomé, A.C.; Neves, M.G.P.M.S. Porphyrins as antimibrobial photosensitizing agents. In Photodynamic Inactivation of Microbial Pathogens: Medical and Environmental Applications; Hamblin, M.R., Jori, G., Eds.; Royal Society of Chemistry: Cambridge, UK, 2011; pp. 83–160. [Google Scholar]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2017, 17, 49–55. [Google Scholar] [CrossRef]
- Vieira, C.; Gomes, A.T.; Mesquita, M.Q.; Moura, N.M.M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. An Insight into the potentiation effect of potassium iodide on aPDT efficacy. Front. Microbiol. 2018, 9, 2665. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, L.M.; Rocha, D.M.G.C.; Ramos, C.I.V.; Gomes, M.C.; Almeida, A.; Faustino, M.A.F.; Paz, F.A.A.; Neves, M.G.P.M.S.; Cunha, A.; Tomé, J.P.C. Photoinactivation of planktonic and biofilm forms of Escherichia coli through the action of cationic zinc(II) phthalocyanines. ChemPhotoChem 2019, 3, 251–260. [Google Scholar] [CrossRef]
- Vieira, C.; Santos, A.; Mesquita, M.Q.; Gomes, A.T.P.C.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Advances in aPDT based on the combination of a porphyrinic formulation with potassium iodide: Effectiveness on bacteria and fungi planktonic/biofilm forms and viruses. J. Porphyr. Phthalocya. 2019, 23, 534–545. [Google Scholar] [CrossRef]
- Silva, E.M.P.; Giuntini, F.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Silva, A.M.S.; Santana-Marques, M.G.; Ferrer-Correia, A.J.; Cavaleiro, J.A.S.; et al. Synthesis of cationic β-vinyl substituted meso-tetraphenylporphyrins and their in vitro activity against herpes simplex virus type 1. Bioorg. Med. Chem. Lett. 2005, 15, 3333–3337. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Tomé, J.P.C.; Neves, M.D.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Faustino, M.A.F.; Almeida, A. Susceptibility of non-enveloped DNA- and RNA-type viruses to photodynamic inactivation. Photochem. Photobiol. Sci. 2012, 11, 1520. [Google Scholar] [CrossRef]
- Wiehe, A.; O’Brien, J.; Senge, M.O. Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef]
- Diogo, P.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Palma, P.; Baptista, I.P.; Gonçalves, T.; Santos, J.M. An insight into advanced approaches for photosensitizer optimization in endodontics—A critical review. J. Funct. Biomater. 2019, 10, 44. [Google Scholar] [CrossRef]
- Diogo, P.; Mota, M.S.; Fernandes, C.; Sequeira, D.B.; Palma, P.; Caramelo, F.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Gonçalves, T.; Santos, J.M. Is the chlorophyll derivative Zn(II)e 6 Me a good photosensitizer to be used in root canal disinfection? Photodiagnosis Photodyn. Ther. 2018, 22, 205–211. [Google Scholar] [CrossRef]
- Diogo, P.; Fernandes, C.; Caramelo, F.; Mota, M.S.; Miranda, I.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Uliana, M.P.; De Oliveira, K.T.; Santos, J.M.; et al. Antimicrobial photodynamic therapy against endodontic enterococcus faecalis and candida albicans mono and mixed biofilms in the presence of photosensitizers: A comparative study with classical endodontic irrigants. Front. Microbiol. 2017, 8, 626. [Google Scholar] [CrossRef]
- Almeida, A.; Cunha, A.; Gomes, N.C.; Alves, E.; Costa, L.; Faustino, M.A.F. Phage therapy and photodynamic therapy: Low environmental impact approaches to inactivate microorganisms in fish farming plants. Mar. Drugs 2009, 7, 268–313. [Google Scholar] [CrossRef]
- Oliveira, A.; Almeida, A.; Carvalho, C.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A. Porphyrin derivatives as photosensitizers for the inactivation of Bacillus cereus endospores. J. Appl. Microbiol. 2009, 106, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.; Carvalho, C.M.B.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tome, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Gomes, N.C.; Alves, E.; et al. Antimicrobial photodynamic therapy: Study of bacterial recovery viability and potential development of resistance after treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef]
- Carvalho, C.M.B.; Alves, E.; Costa, L.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Almeida, A.; Cunha, A.; et al. Functional cationic nanomagnet−porphyrin hybrids for the photoinactivation of microorganisms. ACS Nano 2010, 4, 7133–7140. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Faustino, M.A.F.; Cunha, A.; Gomes, N.C.; Almeida, A. Evaluation of resistance development and viability recovery by a non-enveloped virus after repeated cycles of aPDT. Antivir. Res. 2011, 91, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeu, M.; Reis, S.; Fontes, M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Photodynamic action against wastewater microorganisms and chemical pollutants: An effective approach with low environmental impact. Water 2017, 9, 630. [Google Scholar] [CrossRef]
- Castro, K.A.; Moura, N.M.M.; Fernandes, A.; Faustino, M.A.F.; Simões, M.M.; Cavaleiro, J.A.S.; Nakagaki, S.; Almeida, A.; Cunha, A.; Silvestre, A.J.; et al. Control of Listeria innocua biofilms by biocompatible photodynamic antifouling chitosan based materials. Dye. Pigment. 2017, 137, 265–276. [Google Scholar] [CrossRef]
- Marciel, L.S.C.; Mesquita, M.Q.; Ferreira, R.; Moreira, B.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. An efficient formulation based on cationic porphyrins to photoinactivate Staphylococcus aureus and Escherichia coli. Futur. Med. Chem. 2018, 10, 1821–1833. [Google Scholar] [CrossRef]
- Costa, L.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, A.; Almeida, A. Photodynamic inactivation of mammalian viruses and bacteriophages. Viruses 2012, 4, 1034–1074. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, A.; Tomé, J.P.C.; Almeida, A. An insight on bacterial cellular targets of photodynamic inactivation. Futur. Med. Chem. 2014, 6, 141–164. [Google Scholar] [CrossRef]
- Almeida, A.; Faustino, M.A.F.; Tomé, J.P.C. Photodynamic inactivation of bacteria: Finding the effective targets. Futur. Med. Chem. 2015, 7, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Esteves, A.C.; Correia, A.; Moreirinha, C.; Delgadillo, I.; Cunha, A.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. SDS-PAGE and IR spectroscopy to evaluate modifications in the viral protein profile induced by a cationic porphyrinic photosensitizer. J. Virol. Methods 2014, 209, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Gomes, N.C.; Almeida, A. Nucleic acid changes during photodynamic inactivation of bacteria by cationic porphyrins. Bioorganic Med. Chem. 2013, 21, 4311–4318. [Google Scholar] [CrossRef]
- Alves, E.; Santos, N.; Melo, T.P.; Maciel, E.; Dória, M.L.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Cunha, A.; et al. Photodynamic oxidation of Escherichia coli membrane phospholipids: New insights based on lipidomics. Rapid Commun. Mass Spectrom. 2013, 27, 2717–2728. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeu, M.; Coimbra, S.; Cunha, A.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Faustino, M.A.F.; Almeida, A. Indirect and direct damage to genomic DNA induced by 5,10,15-tris(1-methylpyridinium-4-yl)-20-(pentafluorophenyl)porphyrin upon photodynamic action. J. Porphyr. Phthalocya. 2016, 20, 331–336. [Google Scholar] [CrossRef]
- Woźniak, A.; Grinholc, M. Combined antimicrobial activity of photodynamic inactivation and antimicrobials–state of the art. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Tomb, R.M.; White, T.A.; Coia, J.E.; Anderson, J.G.; MacGregor, S.J.; MacLean, M. Review of the comparative susceptibility of microbial species to photoinactivation using 380–480 nm violet-blue light. Photochem. Photobiol. 2018, 94, 445–458. [Google Scholar] [CrossRef]
- Wainwright, M.; Crossley, K.B. Photosensitising agents—Circumventing resistance and breaking down biofilms: A review. Int. Biodeterior. Biodegrad. 2004, 53, 119–126. [Google Scholar] [CrossRef]
- Kim, M.-J.; Yuk, H.-G. Antibacterial mechanism of 405-nanometer light-emitting diode against salmonella at refrigeration temperature. Appl. Environ. Microbiol. 2016, 83. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Alves, E.; E Melo, T.P.; Simões, C.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Cunha, A.; Gomes, N.C.; Domingues, P.; et al. Photodynamic oxidation of Staphylococcus warneri membrane phospholipids: New insights based on lipidomics. Rapid Commun. Mass Spectrom. 2013, 27, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, Y.; Salmon-Divon, M.; Shporen, E.; Malik, Z. ALA induced photodynamic effects on Gram positive and negative bacteria. Photochem. Photobiol. Sci. 2004, 3, 430. [Google Scholar] [CrossRef]
- Schäfer, M. High sensitivity of Deinococcus radiodurans to photodynamically-produced singlet oxygen. Int. J. Radiat. Biol. 1998, 74, 249–253. [Google Scholar] [CrossRef]
- Heinemann, F.; Karges, J.; Gasser, G. Critical overview of the use of Ru(II) polypyridyl complexes as photosensitizers in one-photon and two-photon photodynamic therapy. Acc. Chem. Res. 2017, 50, 2727–2736. [Google Scholar] [CrossRef] [PubMed]
- North, J.; Coombs, R.; Levy, J. Photodynamic inactivation of free and cell-associated HIV-1 using the photosensitizer, benzoporphyrin derivative. J. Acquir. Immune Defic. Syndr. 1994, 7, 891–898. [Google Scholar] [PubMed]
- Wilson, M.; Yianni, C. Killing of methicillin-resistant Staphylococcus aureus by low-power laser light. J. Med. Microbiol. 1995, 42, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef]
- Grinholc, M.; Szramka, B.; Olender, K.; Graczyk, A. Bactericidal effect of photodynamic therapy against methicillin-resistant Staphylococcus aureus strain with the use of various porphyrin photosensitizers. Acta Biochim. Pol. 2007, 54, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Jori, G.; Coppellotti, O. Inactivation of pathogenic microorganisms by photodynamic techniques: Mechanistic aspects and perspective applications. Anti Infect. Agents Med. Chem. 2007, 6, 119–131. [Google Scholar] [CrossRef]
- Almeida, J.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Costa, L.; Faustino, M.A.F.; Almeida, A. Photodynamic inactivation of multidrug-resistant bacteria in hospital wastewaters: Influence of residual antibiotics. Photochem. Photobiol. Sci. 2014, 13, 626–633. [Google Scholar] [CrossRef]
- Le Gall, T.; Lemercier, G.; Chevreux, S.; Tucking, K.-S.; Ravel, J.; Thetiot, F.; Jonas, U.; Schönherr, H.; Montier, T. Ruthenium(II) polypyridyl complexes as photosensitizers for antibacterial photodynamic therapy: A structure-activity study on clinical bacterial strains. ChemMedChem 2018, 13, 2229–2239. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, Y.-Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Kömerik, N.; Wilson, M.; Poole, S. The effect of photodynamic action on two virulence factors of gram-negative bacteria. Photochem. Photobiol. 2000, 72, 676. [Google Scholar] [CrossRef]
- Tubby, S.; Wilson, M.; Nair, S.P. Inactivation of staphylococcal virulence factors using a light-activated antimicrobial agent. BMC Microbiol. 2009, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeu, M.; Rocha, S.; Cunha, A.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Effect of photodynamic therapy on the virulence factors of Staphylococcus aureus. Front. Microbiol. 2016, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Cheng, Q.; Yang, H.; Li, H.; Gong, N.; Liu, D.; Wu, J.; Lei, X. Effects of ALA-PDT on biofilm structure, virulence factor secretion, and QS in Pseudomonas aeruginosa. Photodiagnosis Photodyn. Ther. 2018, 24, 88–94. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Dias, C.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting current photoactive materials for antimicrobial photodynamic therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef] [PubMed]
- Bertoloni, G. Photosensitizing activity of hematoporphyrin on Staphylococcus aureus cells. Biochim. Biophys. Acta (BBA) Gen. Subj. 2000, 1475, 169–174. [Google Scholar] [CrossRef]
- El-Adly, A.A. Photoactive anionic porphyrin derivative against Gram-positive and Gram-negative bacteria. J. Appl. Sci. Res. 2008, 4, 1817–1821. [Google Scholar]
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends 2020, 14, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Touret, F.; De Lamballerie, X. Of chloroquine and COVID-19. Antivir. Res. 2020, 177, 104762. [Google Scholar] [CrossRef] [PubMed]
- Lentini, G.; Cavalluzzi, M.M.; Habtemariam, S. COVID-19, chloroquine repurposing, and cardiac safety concern: Chirality might help. Molecules 2020, 25, 1834. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Summa, M.; Patrick, L.; Schwartz, L. A cohort of cancer patients with no reported cases of SARS-CoV-2 infection: The possible preventive role of Methylene Blue. Substantia 2020, 4, 888. [Google Scholar]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef]
- Eickmann, M.; Gravemann, U.; Handke, W.; Tolksdorf, F.; Reichenberg, S.; Muller, T.; Seltsam, A. Inactivation of Ebola virus and Middle East respiratory syndrome coronavirus in platelet concentrates and plasma by ultraviolet C light and methylene blue plus visible light, respectively. Transfusion 2018, 58, 2202–2207. [Google Scholar] [CrossRef]
- Wainwright, M.; McLean, A. Rational design of phenothiazinium derivatives and photoantimicrobial drug discovery. Dye. Pigment. 2017, 136, 590–600. [Google Scholar] [CrossRef]
- Yao, T.-T.; Wang, J.; Xue, Y.-F.; Yu, W.-J.; Gao, Q.; Ferreira, L.; Ren, K.-F.; Ji, J. A photodynamic antibacterial spray-coating based on the host-guest immobilization of the photosensitizer methylene blue. J. Mater. Chem. B 2019, 7, 5089–5095. [Google Scholar] [CrossRef]
- Friedman, L.I.; Skripchenko, A.; Wagner, S.J. Photodynamic Inactivation of Pathogens in Blood by Phenothiazines and Oxygen. Patent WO/2001/049328, 28 December 2000. [Google Scholar]
- Brown, S.B.; O’Grady, C.C.; Griffiths, J.; Mellish, K.J.; Tunstall, R.G.; Roberts, D.J.H.; Vernon, D.I. Biologically Active Methylene Blue Derivatives. Patent WO2002GB02278, 30 May 2002. [Google Scholar]
- Shafirstein, G.; Battoo, A.; Harris, K.E.; Baumann, H.; Gollnick, S.O.; Lindenmann, J.; Nwogu, C.E. Photodynamic therapy of non–small cell lung cancer. Narrative review and future directions. Ann. Am. Thorac. Soc. 2015, 13, 265–275. [Google Scholar] [CrossRef]
- Song, W.; Kuang, J.; Li, C.-X.; Zhang, M.; Zheng, D.; Zeng, X.; Liu, C.; Zhang, X.-Z. Enhanced immunotherapy based on photodynamic therapy for both primary and lung metastasis tumor eradication. ACS Nano 2018, 12, 1978–1989. [Google Scholar] [CrossRef]
- Mokwena, M.G.; Kruger, C.; Ivan, M.-T.; Heidi, A.; Gift, M.M.; Ann, K.C. A review of nanoparticle photosensitizer drug delivery uptake systems for photodynamic treatment of lung cancer. Photodiagnosis Photodyn. Ther. 2018, 22, 147–154. [Google Scholar] [CrossRef]
- Fekrazad, R. Photobiomodulation and antiviral photodynamic therapy as a possible novel approach in COVID-19 management. Photobiomodulation Photomed. Laser Surg. 2020, 38, 255–257. [Google Scholar] [CrossRef]
- New Technology Reduces Healthcare-Associated Infections and Surgical Site Infections. Available online: https://ondinebio.com/wp-content/uploads/2019/03/SW9001-Rev-A-Steriwave-brochure.pdf (accessed on 8 May 2020).
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, A.; Nadais, H.; Almeida, A. Potential applications of porphyrins in photodynamic inactivation beyond the medical scope. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 34–57. [Google Scholar] [CrossRef]
- RIVM. Novel Coronavirus Found in Wastewater. Available online: https://www.rivm.nl/en/news/novel-coronavirus-found-in-wastewater (accessed on 5 May 2020).
- Medema, G.; Heijnen, L.; Elsinga, G.; Italiaander, R.; Brouwer, A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020. [Google Scholar] [CrossRef]
- Lodder, W.; Husman, A.M.D.R. SARS-CoV-2 in wastewater: Potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020, 5, 533–534. [Google Scholar] [CrossRef]
- Núñez-Delgado, A. What do we know about the SARS-CoV-2 coronavirus in the environment? Sci. Total. Environ. 2020, 727, 138647. [Google Scholar] [CrossRef] [PubMed]
- Frigon, D.; Biswal, B.K.; Mazza, A.; Masson, L.; Gehr, R. Biological and physicochemical wastewater treatment processes reduce the prevalence of virulent Escherichia coli. Appl. Environ. Microbiol. 2012, 79, 835–844. [Google Scholar] [CrossRef]
- Vaz-Moreira, I.; Nunes, O.C.; Manaia, C.M. Bacterial diversity and antibiotic resistance in water habitats: Searching the links with the human microbiome. FEMS Microbiol. Rev. 2014, 38, 761–778. [Google Scholar] [CrossRef]
- Prüss, A.; Giroult, E.; Rushbrook, P. Safe Management of Wastes from Health-Care Activities; World Health Organization: Hong Kong, China, 2017. [Google Scholar]
- Macauley, J.J.; Qiang, Z.; Adams, C.D.; Surampalli, R.; Mormile, M.R. Disinfection of swine wastewater using chlorine, ultraviolet light and ozone. Water Res. 2006, 40, 2017–2026. [Google Scholar] [CrossRef]
- Mansor, N.A.; Tay, K.S. Potential toxic effects of chlorination and UV/chlorination in the treatment of hydrochlorothiazide in the water. Sci. Total. Environ. 2020, 714, 136745. [Google Scholar] [CrossRef]
- Falås, P.; Andersen, H.R.; Ledin, A.; Jansen, J.; La, C. Occurrence and reduction of pharmaceuticals in the water phase at Swedish wastewater treatment plants. Water Sci. Technol. 2012, 66, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Prata, C.; Ribeiro, A.; Cunha, A.; Gomes, N.C.; Almeida, A. Ultracentrifugation as a direct method to concentrate viruses in environmental waters: Virus-like particle enumeration as a new approach to determine the efficiency of recovery. J. Environ. Monit. 2012, 14, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Castro, K.A.; Moura, N.M.; Simões, M.M.; Cavaleiro, J.A.; Faustino, M.D.A.F.; Cunha, Â.; Paz, F.A.A.; Mendes, R.F.; Almeida, A.; Freire, C.S.; et al. Synthesis and characterization of photoactive porphyrin and poly(2-hydroxyethyl methacrylate) based materials with bactericidal properties. Appl. Mater. Today 2019, 16, 332–341. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Moura, N.M.M.; Figueira, F.; Ferreira, R.I.; Simões, M.M.; Cavaleiro, J.A.S.; Faustino, M.A.F.; Silvestre, A.J.; Freire, C.S.; Tomé, J.P.C.; et al. New materials based on cationic porphyrins conjugated to chitosan or titanium dioxide: Synthesis, characterization and antimicrobial efficacy. Int. J. Mol. Sci. 2019, 20, 2522. [Google Scholar] [CrossRef]
- Alves, E.; Rodrigues, J.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Lin, Z.; Cunha, A.; Nadais, H.; Tomé, J.P.C.; Almeida, A. A new insight on nanomagnet–porphyrin hybrids for photodynamic inactivation of microorganisms. Dye. Pigment. 2014, 110, 80–88. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Menezes, J.C.J.M.D.S.; Pires, S.; Neves, M.G.P.M.S.; Simões, M.M.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Daniel-Da-Silva, A.L.; Almeida, A.; et al. Pyrrolidine-fused chlorin photosensitizer immobilized on solid supports for the photoinactivation of Gram negative bacteria. Dye. Pigment. 2014, 110, 123–133. [Google Scholar] [CrossRef]
- Carvalho, C.M.; Gomes, A.T.; Fernandes, S.C.; Prata, A.C.; Almeida, A.; Cunha, A.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; et al. Photoinactivation of bacteria in wastewater by porphyrins: Bacterial β-galactosidase activity and leucine-uptake as methods to monitor the process. J. Photochem. Photobiol. B Biol. 2007, 88, 112–118. [Google Scholar] [CrossRef]
- Alves, E.; Carvalho, C.M.B.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Mendo, S.; Almeida, A. Photodynamic inactivation of recombinant bioluminescent Escherichia coli by cationic porphyrins under artificial and solar irradiation. J. Ind. Microbiol. Biotechnol. 2008, 35, 1447–1454. [Google Scholar] [CrossRef]
- Almeida, M.A.; Cavaleiro, J.A.S.; Rocha, J.; Carvalho, C.M.B.; Costa, L.A.S.; Alves, E.S.C.F.; Cunha, M.A.S.D.A.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; et al. Nanomagnet-Porphyrin Hybrid Materials: Synthesis and Water Disinfection Application. Portuguese Patent No. PT 103828, 21 September 2009. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, A.; Faustino, M.A.F.; Neves, M.G.P.M.S. Antimicrobial Photodynamic Therapy in the Control of COVID-19. Antibiotics 2020, 9, 320. https://doi.org/10.3390/antibiotics9060320
Almeida A, Faustino MAF, Neves MGPMS. Antimicrobial Photodynamic Therapy in the Control of COVID-19. Antibiotics. 2020; 9(6):320. https://doi.org/10.3390/antibiotics9060320
Chicago/Turabian StyleAlmeida, Adelaide, M. Amparo F. Faustino, and Maria G. P. M. S. Neves. 2020. "Antimicrobial Photodynamic Therapy in the Control of COVID-19" Antibiotics 9, no. 6: 320. https://doi.org/10.3390/antibiotics9060320
APA StyleAlmeida, A., Faustino, M. A. F., & Neves, M. G. P. M. S. (2020). Antimicrobial Photodynamic Therapy in the Control of COVID-19. Antibiotics, 9(6), 320. https://doi.org/10.3390/antibiotics9060320






