Encapsulation of the Antistaphylococcal Endolysin LysRODI in pH-Sensitive Liposomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Protein Purification and Encapsulation
2.3. Time-Kill Curves
2.4. Treatment of Actively Growing Bacterial Cultures and Biofilms
2.5. Statistical Analysis
3. Results and Discussion
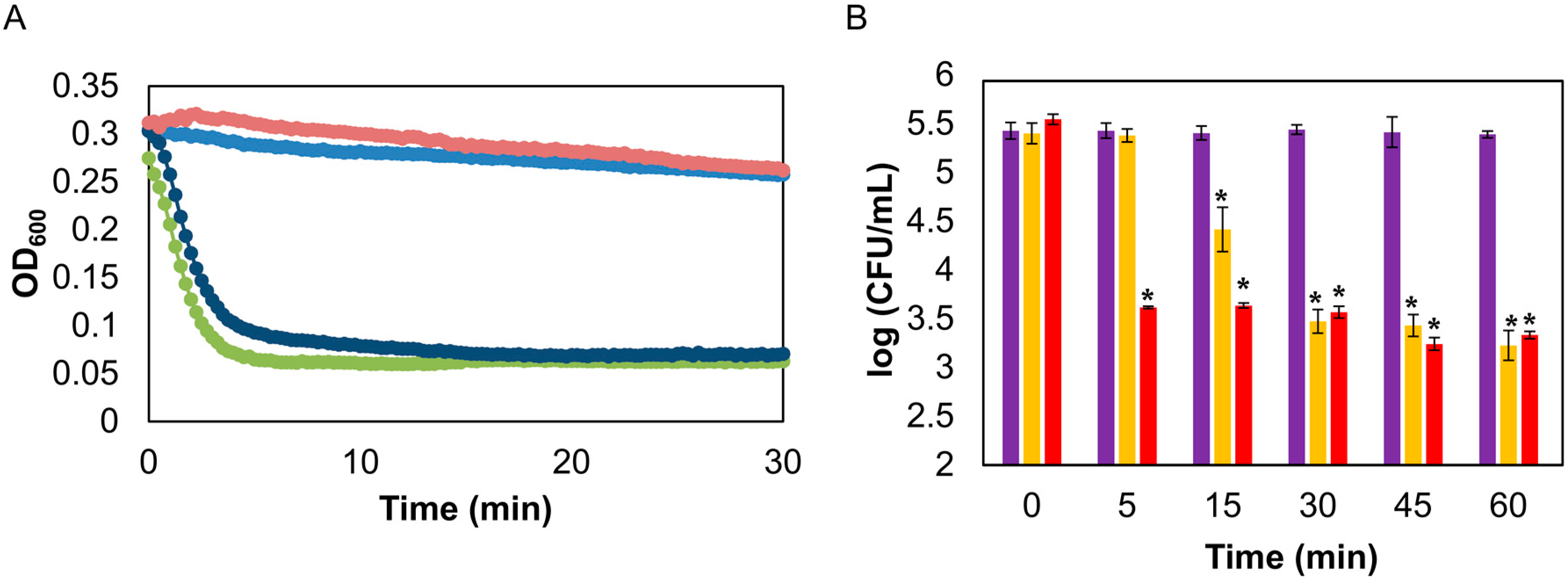
3.1. Protein Encapsulation and Efficacy in Time-Kill Curve Assay
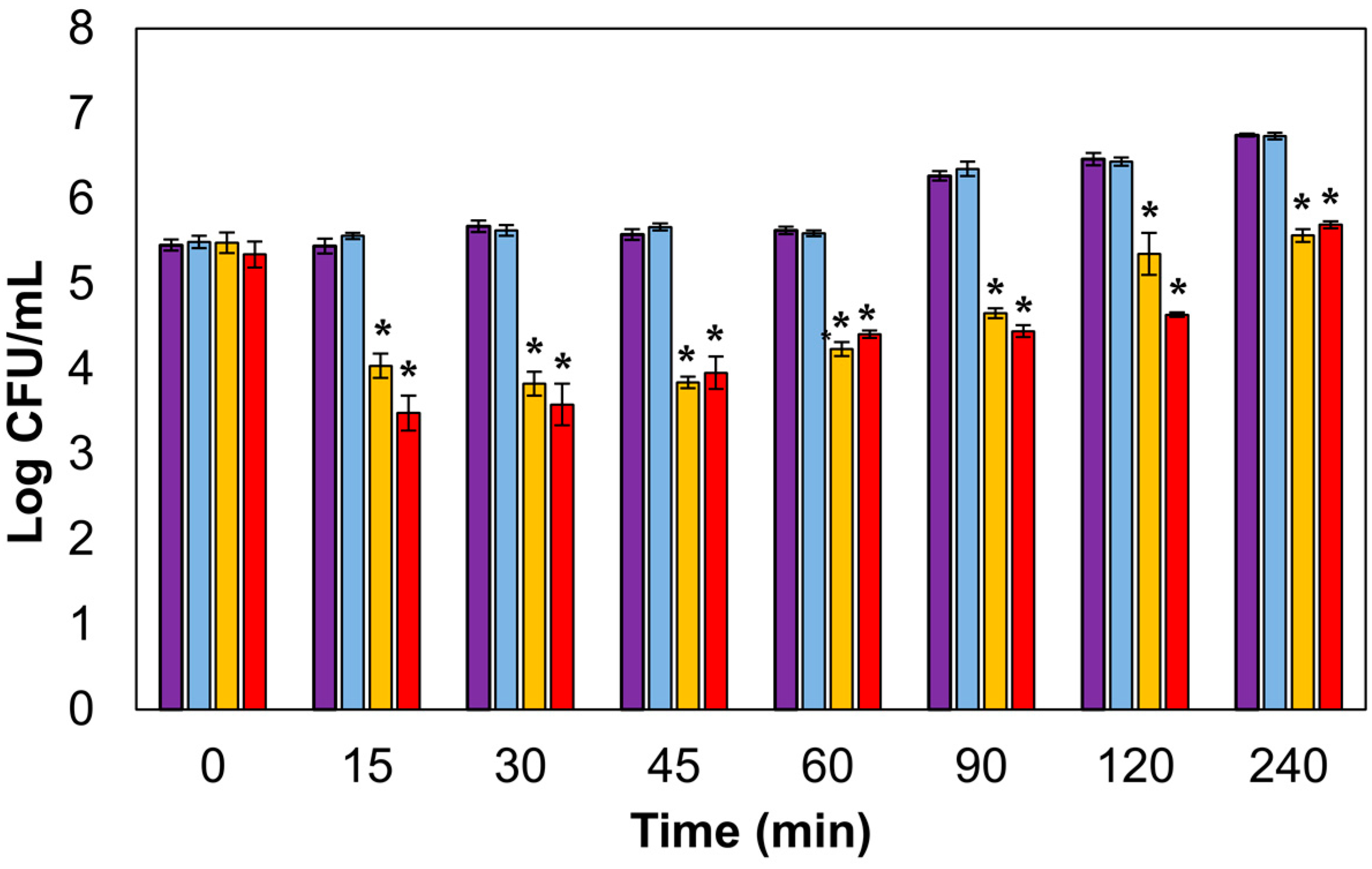
3.2. Lytic Activity in Actively Growing Cultures
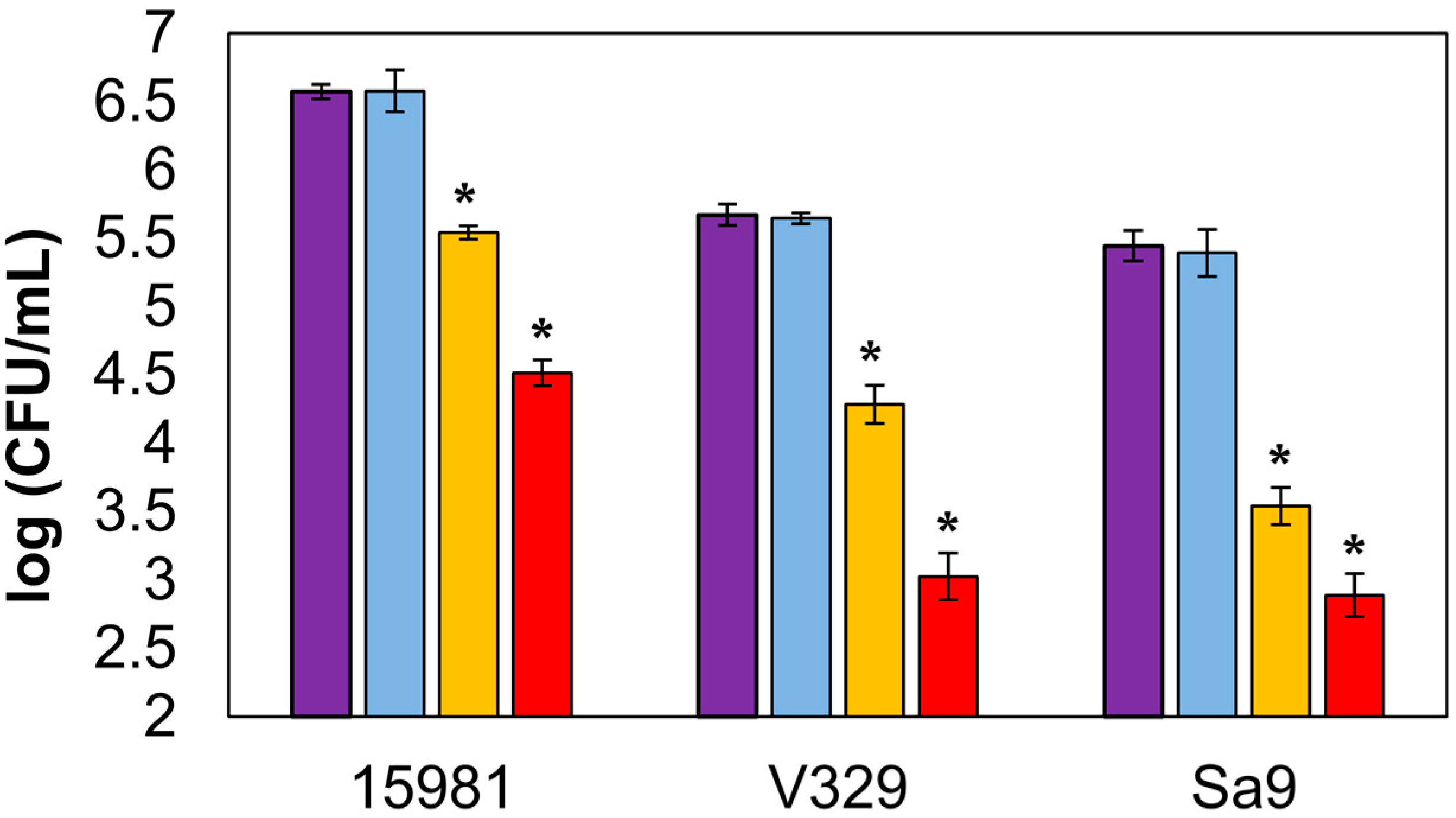
3.3. Antibiofilm Activity of Encapsulated LysRODI
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Esposito, S.; Noviello, S.; Leone, S. Epidemiology and microbiology of skin and soft tissue infections. Curr. Opin. Infect. Dis. 2016, 29, 109–115. [Google Scholar] [CrossRef]
- Nelson, D.C.; Schmelcher, M.; Rodriguez-Rubio, L.; Klumpp, J.; Pritchard, D.G.; Dong, S.; Donovan, D.M. Endolysins as antimicrobials. Adv. Virus Res. 2012, 83, 299–365. [Google Scholar]
- Fischetti, V.A. Bacteriophage lysins as effective antibacterials. Curr. Opin. Microbiol. 2008, 11, 393–400. [Google Scholar] [CrossRef]
- Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; Donovan, D.M.; García, P. Enhanced staphylolytic activity of the Staphylococcus aureus bacteriophage vB_SauS-phiIPLA88 HydH5 virion-associated peptidoglycan hydrolase: Fusions, deletions, and synergy with LysH5. Appl. Environ. Microbiol. 2012, 78, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Fernández, L.; Rodríguez, A.; García, P. Are phage lytic proteins the secret weapon to kill Staphylococcus aureus? MBio 2018, 9, e01923-17. [Google Scholar] [CrossRef] [PubMed]
- Totte, J.; de Wit, J.; Pardo, L.; Schuren, F.; van Doorn, M.; Pasmans, S. Targeted anti-staphylococcal therapy with endolysins in atopic dermatitis and the effect on steroid use, disease severity and the microbiome: Study protocol for a randomized controlled trial (MAAS trial). Trials 2017, 18, 404. [Google Scholar] [CrossRef] [PubMed]
- Totté, J.E.E.; van Doorn, M.B.; Pasmans, S. Successful treatment of chronic Staphylococcus aureus-related dermatoses with the topical endolysin Staphefekt SA.100: A report of 3 cases. Case Rep. Dermatol. 2017, 9, 19–25. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Kell, A.J.; Stewart, G.; Ryan, S.; Peytavi, R.; Boissinot, M.; Huletsky, A.; Bergeron, M.G.; Simard, B. Vancomycin-modified nanoparticles for efficient targeting and preconcentration of Gram-positive and Gram-negative bacteria. ACS Nano 2008, 2, 1777–1788. [Google Scholar] [CrossRef]
- Hathaway, H.; Ajuebor, J.; Stephens, L.; Coffey, A.; Potter, U.; Sutton, J.M.; Jenkins, A.T. Thermally triggered release of the bacteriophage endolysin CHAPK and the bacteriocin lysostaphin for the control of methicillin resistant Staphylococcus aureus (MRSA). J. Control. Release 2017, 245, 108–115. [Google Scholar] [CrossRef]
- Bai, J.; Yang, E.; Chang, P.S.; Ryu, S. Preparation and characterization of endolysin-containing liposomes and evaluation of their antimicrobial activities against gram-negative bacteria. Enzyme Microb. Technol. 2019, 128, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Vandenheuvel, D.; Martínez, B.; Rodríguez, A.; Lavigne, R.; García, P. Two phages, phiIPLA-RODI and phiIPLA-C1C, lyse mono- and dual-species staphylococcal biofilms. Appl. Environ. Microbiol. 2015, 81, 3336–3348. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Garrido, V.; Fernández, L.; Portilla, S.; Rodríguez, A.; Grilló, M.J.; García, P. Phage lytic protein LysRODI prevents staphylococcal mastitis in mice. Front. Microbiol. 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Madera, C.; Martínez, B.; Rodríguez, A. Biocontrol of Staphylococcus aureus in curd manufacturing processes using bacteriophages. Int. Dairy J. 2007, 17, 7. [Google Scholar] [CrossRef]
- Cucarella, C.; Solano, C.; Valle, J.; Amorena, B.; Lasa, I.; Penadés, J.R. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 2001, 183, 2888–2896. [Google Scholar] [CrossRef]
- Valle, J.; Toledo-Arana, A.; Berasain, C.; Ghigo, J.M.; Amorena, B.; Penadés, J.R.; Lasa, I. SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 2003, 48, 1075–1087. [Google Scholar] [CrossRef]
- Obeso, J.M.; Martínez, B.; Rodríguez, A.; García, P. Lytic activity of the recombinant staphylococcal bacteriophage PhiH5 endolysin active against Staphylococcus aureus in milk. Int. J. Food Microbiol. 2008, 128, 212–218. [Google Scholar] [CrossRef]
- Sanderson, N.M.; Jones, M.N. Encapsulation of vancomycin and gentamicin within cationic liposomes for inhibition of growth of Staphylococcus epidermidis. J. Drug Target. 1996, 4, 181–189. [Google Scholar] [CrossRef]
- Rukavina, Z.; Vanić, Ž. Current trends in development of liposomes for targeting bacterial biofilms. Pharmaceutics 2016, 8, 18. [Google Scholar] [CrossRef]
- Vanić, Ž.; Holaeter, A.M.; Škalko-Basnet, N. (Phospho)lipid-based nanosystems for skin administration. Curr. Pharm. Des. 2015, 21, 4174–4192. [Google Scholar] [CrossRef]
- Lee, Y.; Thompson, D.H. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Foulston, L.; Elsholz, A.K.; DeFrancesco, A.S.; Losick, R. The extracellular matrix of Staphylococcus aureus biofilms comprises cytoplasmic proteins that associate with the cell surface in response to decreasing pH. MBio 2014, 5, e01667-14. [Google Scholar] [CrossRef] [PubMed]
- Hajiahmadi, F.; Alikhani, M.Y.; Shariatifar, H.; Arabestani, M.R.; Ahmadvand, D. The bactericidal effect of lysostaphin coupled with liposomal vancomycin as a dual combating system applied directly on methicillin-resistant Staphylococcus aureus infected skin wounds in mice. Int. J. Nanomed. 2019, 14, 5943–5955. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portilla, S.; Fernández, L.; Gutiérrez, D.; Rodríguez, A.; García, P. Encapsulation of the Antistaphylococcal Endolysin LysRODI in pH-Sensitive Liposomes. Antibiotics 2020, 9, 242. https://doi.org/10.3390/antibiotics9050242
Portilla S, Fernández L, Gutiérrez D, Rodríguez A, García P. Encapsulation of the Antistaphylococcal Endolysin LysRODI in pH-Sensitive Liposomes. Antibiotics. 2020; 9(5):242. https://doi.org/10.3390/antibiotics9050242
Chicago/Turabian StylePortilla, Silvia, Lucía Fernández, Diana Gutiérrez, Ana Rodríguez, and Pilar García. 2020. "Encapsulation of the Antistaphylococcal Endolysin LysRODI in pH-Sensitive Liposomes" Antibiotics 9, no. 5: 242. https://doi.org/10.3390/antibiotics9050242
APA StylePortilla, S., Fernández, L., Gutiérrez, D., Rodríguez, A., & García, P. (2020). Encapsulation of the Antistaphylococcal Endolysin LysRODI in pH-Sensitive Liposomes. Antibiotics, 9(5), 242. https://doi.org/10.3390/antibiotics9050242





