Development of Antimicrobial Stewardship Programmes in Low and Middle-Income Countries: A Mixed-Methods Study in Nigerian Hospitals
Abstract
1. Introduction
2. Methods
2.1. Study Setting
2.2. Study Design
2.3. Data Collection
3. Results
3.1. Survey of Antibiotic Use in Hospitals in Bayelsa State, Nigeria
3.2. Interviews with Prescribers
4. Discussion
4.1. Feasibility and Priorities for Implementation
4.2. Set up and Operation of the AMS Plan
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Antimicrobial Stewardship Programmes in Health-Care Facilities in Low-and Middle-Income Countries: A WHO Practical Toolkit; WHO: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/handle/10665/329404 (accessed on 1 March 2020).
- Merrett GL, B.; Bloom, G.; Wilkinson, A.; MacGregor, H. Towards the just and sustainable use of antibiotics. J. Pharm. Policy Pract. 2016, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Tangcharoensathien, V.; Chanvatik, S.; Sommanustweechai, A. Complex determinants of inappropriate use of antibiotics. Bull. World Health Organ. 2018, 96, 141. [Google Scholar] [CrossRef] [PubMed]
- Franco, B.E.; Martínez, M.A.; Rodríguez MA, S.; Wertheimer, A.I. The determinants of the antibiotic resistance process. Infect. Drug Resist. 2009, 2, 1. [Google Scholar] [PubMed]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef]
- Holloway, K.A. Promoting the rational use of antibiotics. In Regional Health Forum; World Health Organization Regional Office for South-East Asia: New Delhi, India, 2011; Volume 15, pp. 122–130. [Google Scholar]
- Xiao, Y.; Zhang, J.; Zheng, B.; Zhao, L.; Li, S.; Li, L. Changes in Chinese policies to promote the rational use of antibiotics. PLoS Med. 2013, 10, e1001556. [Google Scholar] [CrossRef]
- Holloway, K.; van Dijk, L. The World Medicines Situation. Rational Use of Medicines; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Nwokike, J.; Clark, A.; Nguyen, P.P. Medicines quality assurance to fight antimicrobial resistance. Bull. World Health Organ. 2018, 96, 135. [Google Scholar] [CrossRef]
- Yip, W.; Powell-Jackson, T.; Chen, W.; Hu, M.; Fe, E.; Hu, M.; Jian, W.; Lu, M.; Han, W.; Hsiao, W.C. Capitation combined with pay-for-performance improves antibiotic prescribing practices in rural China. Health Aff. 2014, 33, 502–510. [Google Scholar] [CrossRef]
- Petti, C.A.; Polage, C.R.; Quinn, T.C.; Ronald, A.R.; Sande, M.A. Laboratory medicine in Africa: A barrier to effective health care. Clin. Infect. Dis. 2006, 42, 377–382. [Google Scholar] [CrossRef]
- Planta, M.B. The role of poverty in antimicrobial resistance. J. Am. Board Fam. Med. 2007, 20, 533–539. [Google Scholar] [CrossRef]
- Okeke, I.N.; Lamikanra, A.; Edelman, R. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg. Infect. Dis. 1999, 5, 18. [Google Scholar] [CrossRef]
- Liu, C.P.; Shih, S.C.; Wang, N.Y.; Wu, A.Y.; Sun, F.J.; Chow, S.F.; Chow, S.-F.; Chen, T.-L.; Yan, T.-R. Risk factors of mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteremia. J. Microbiol. Immunol. Infect. 2016, 49, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Andreatos, N.; Shehadeh, F.; Pliakos, E.E.; Mylonakis, E. The impact of antibiotic prescription rates on the incidence of MRSA bloodstream infections: A county-level, US-wide analysis. Int. J. Antimicrob. Agents 2018, 52, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Anong, D.N.; Akoachere, J.-F.K. Prescribing patterns and associated factors of antibiotic prescription in primary health care facilities of Kumbo East and Kumbo West Health Districts, North West Cameroon. PLoS ONE 2018, 13, e0193353. [Google Scholar]
- Feikin, D.R.; Olack, B.; Bigogo, G.M.; Audi, A.; Cosmas, L.; Aura, B.; Burke, H.; Njenga, M.K.; Williamson, J.; Breiman, R.F. The burden of common infectious disease syndromes at the clinic and household level from population-based surveillance in rural and urban Kenya. PLoS ONE 2011, 6, e16085. [Google Scholar] [CrossRef]
- Alzahrani, M.S.; Maneno, M.K.; Daftary, M.N.; Wingate, L.M.; Ettienne, E.B. Factors associated with prescribing broad-spectrum antibiotics for children with upper respiratory tract infections in ambulatory care settings. Clin. Med. Insights Pediatrics 2018, 12, 1179556518784300. [Google Scholar] [CrossRef]
- Tekleab, A.M.; Asfaw, Y.M.; Weldetsadik, A.Y.; Amaru, G.M. Antibiotic prescribing practice in the management of cough or diarrhea among children attending hospitals in Addis Ababa: A cross-sectional study. Pediatric Health Med. Ther. 2017, 8, 93. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.; El-Menyar, A.; Al-Thani, H.; Zarour, A.; Parchani, A.; Asim, M.; El-Enany, R.; Al-Tamimi, H.; Latifi, R. Adherence of surgeons to antimicrobial prophylaxis guidelines in a tertiary general hospital in a rapidly developing country. Adv. Pharmacol. Sci. 2013, 2013, 842593. [Google Scholar] [CrossRef]
- Elias, C.; Moja, L.; Mertz, D.; Loeb, M.; Forte, G.; Magrini, N. Guideline recommendations and antimicrobial resistance: The need for a change. BMJ Open 2017, 7, e016264. [Google Scholar] [CrossRef]
- Cox, J.A.; Vlieghe, E.; Mendelson, M.; Wertheim, H.; Ndegwa, L.; Villegas, M.V.; Gould, I.; Hara, G.L. Antibiotic stewardship in low-and middle-income countries: The same but different? Clin. Microbiol. Infect. 2017, 23, 812–818. [Google Scholar] [CrossRef]
- Van Dijck, C.; Vlieghe, E.; Cox, J.A. Antibiotic stewardship interventions in hospitals in low-and middle-income countries: A systematic review. Bull. World Health Organ. 2018, 96, 266. [Google Scholar] [CrossRef]
- Pearson, M.; Doble, A.; Glogowski, R.; Ibezim, S.; Lazenby, T.; Haile-Redai, A. Antibiotic Prescribing and Resistance: Views From Low-and Middle Income Prescribing and Dispensing Professionals; Report to the World Health Organization, researched and compiled by students and staff of the Antimicrobial Resistance Centre at the London School of Hygiene and Tropical Medicine (LSHTM); WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Tangcharoensathien, V.; Sattayawutthipong, W.; Kanjanapimai, S.; Kanpravidth, W.; Brown, R.; Sommanustweechai, A. Antimicrobial resistance: From global agenda to national strategic plan, Thailand. Bull. World Health Organ. 2017, 95, 599. [Google Scholar] [CrossRef] [PubMed]
- Fantom, N.; Serajuddin, U. The World Bank’s Classification of Countries by Income; The World Bank: Washington, DC, USA, 2016. [Google Scholar]
- Uzochukwu, B.S.C.; Ughasoro, M.D.; Etiaba, E.; Okwuosa, C.; Envuladu, E.; Onwujekwe, O.E. Health care financing in Nigeria: Implications for achieving universal health coverage. Niger. J. Clin. Pract. 2015, 18, 437–444. [Google Scholar] [CrossRef]
- Federal Ministry of Health; WHO. Nigerian Standard Treatment Guidelines; Ministry of Health Abuja: Abuja, Nigeria, 2016; p. 1089.
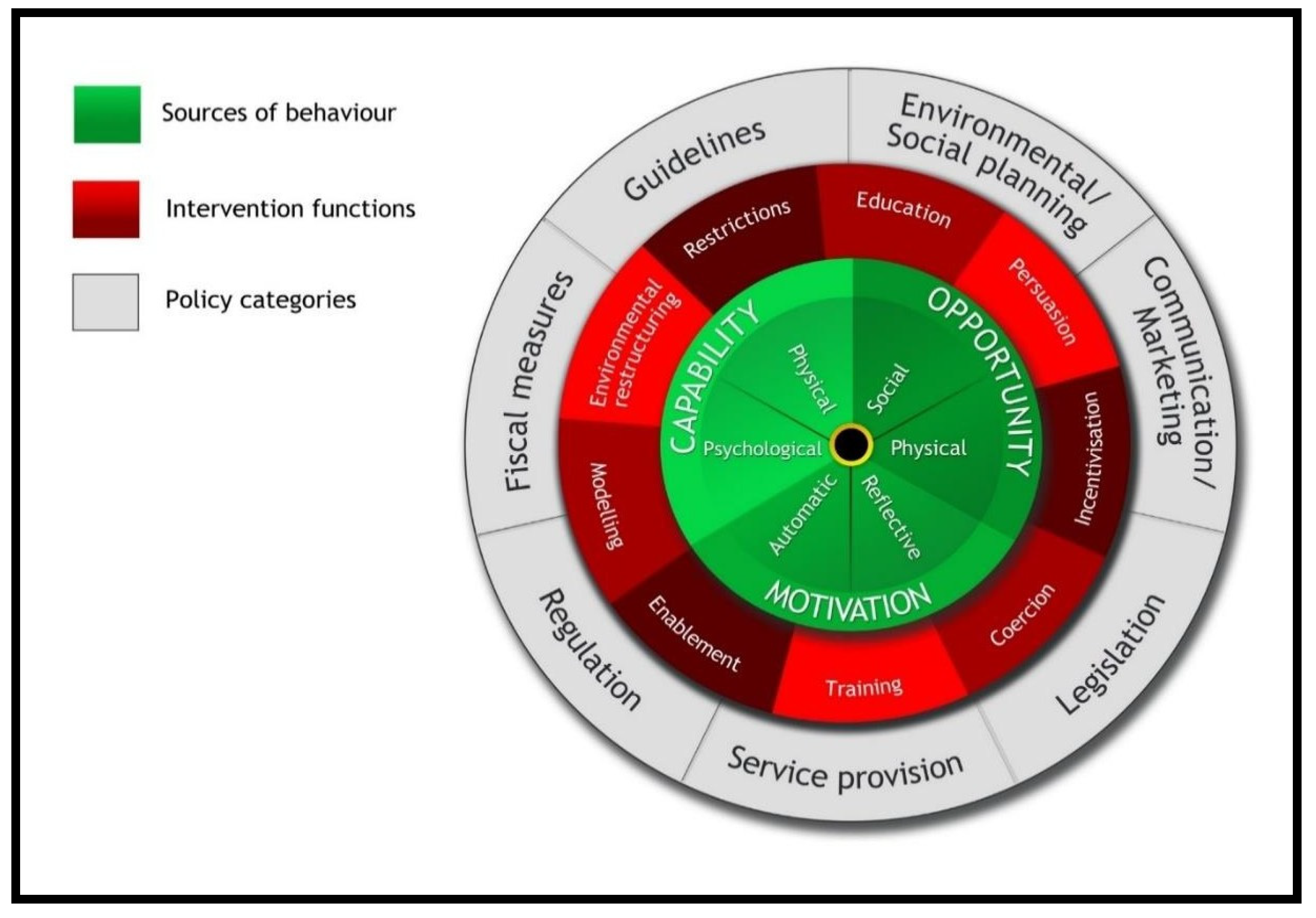
- Michie, S.; Atkins, L.; West, R. The Behaviour Change Wheel. A Guide to Designing Interventions, 1st ed.; Silverback Publishing: Great Britain, UK, 2014; pp. 1003–1010. [Google Scholar]
- Craig, P.; Dieppe, P.; Macintyre, S.; Michie, S.; Nazareth, I.; Petticrew, M. Developing and evaluating complex interventions: The new Medical Research Council guidance. BMJ 2008, 337, a1655. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Johnston, M.; Francis, J.; Hardeman, W.; Eccles, M. From theory to intervention: Mapping theoretically derived behavioural determinants to behaviour change techniques. Appl. Psychol. 2008, 57, 660–680. [Google Scholar] [CrossRef]
| Characteristics | Number of Participants n = 17 (%) | |
|---|---|---|
| Gender | Male | 12(70) |
| Female | 5 (30) | |
| Years of practice | ≤5 | 7 (40) |
| 6–10 | 4 (24) | |
| 11–15 | 4 (24) | |
| ≥16 | 2 (12) | |
| Rank | Medical officer | 7 (40) |
| Senior medical officer | 3(18) | |
| Principal medical officer | 4 (24) | |
| Consultant | 3 (18) | |
| Specialty | Internal Medicine | 6 (35) |
| Paediatrics | 5 (30) | |
| Surgery | 3 (18) | |
| Obstetrics & Gynaecology | 2 (12) | |
| Level of care | Tertiary | 11(65) |
| Secondary | 6 (35) | |
|
|
|
|
Steps to improve the availability of drugs:
|
|
|
| Policy Category | Intervention Functions | Suggestions and Recommendations from Stages 1 and 2 | Implementation Category |
|---|---|---|---|
| Communication/marketing | Education Persuasion Training | Increase awareness of problems and rational prescribing Use of fliers and posters as well as on-line and face-to-face methods. Education and training via short courses, workshops, use of local data to educate prescribers, highlight best practice and address problems | ST |
| Guidelines | Persuasion Restriction Modelling Enablement | Provision of up-to-date guidelines and treatment protocols Enablement, and models, of best practice Strict protocols to restrict access to reserved products, including restricted pharmacy dispensing | ST MT |
| Fiscal | Environmental restructuring Enablement | Improvements to lab facilities to provide enabling environment for rational prescribing. Wider coverage of health insurance, Stocking of low-cost generics to promote affordability for patients Invest in quality assurance units to safeguard product quality, so quality is not a barrier to optimal prescribing | LT |
| Regulation | Training, Restriction, Coercion Enablement | Implementation of prescribing guidelines Regular auditing of practice to regulate and inform improvements to practice Collection and use of local data to provide directly relevant feedback to practitioners and teams | ST S/MT |
| Legislation | Environmental restructuring Restriction, Enablement | Support at Government/health policy levels for prioritization of structural changes, and wider enforcement. | LT |
| Environmental/social planning | Environmental restructuring Enablement | At Government/health policy and institutional levels: interventions as above to ensure enabling environment | M/LT |
| Service provision | Education Training Restriction Environmental restructuring Enablement | Implementation of guidelines Availability and use of laboratory facilities Regular auditing of practice to identify and address problems; and ensure continued enabling environment | MT/LT S/M/LT |
| Increasing awareness and education about antibiotic resistance It was recommended that initiatives to increase awareness should target patients, other health professionals as well as prescribers. Stakeholders perceived that there was a general awareness, and one hospital reported to have already held a presentation. Thus, there were some opportunities to build on existing initiatives. Stakeholders saw important challenges in addressing attitudes and behaviours. Securing engagement and attendance and promoting a desire among practitioners to change was highlighted. Recommendations for AMS action plan and implementation:
|
| Provision of policies and guidelines Stakeholders reported that in the past prescribing guidelines had been developed, but these were commonly seen as not up-to-date or not readily accessible. A potential challenge in the development of guidelines was having sufficient expertise for their development. However, it was acknowledged that a wide range of stakeholders e.g. DTC, infectious disease clinicians and scientists, pharmacists would bring together their professional expertise. Ensuring engagement, ownership and acceptance by all stakeholders was seen as important for co-operation, compliance and enforcement. In one setting, restricted dispensing had already been accepted by the DTC, which included a procedure for review and approval of restricted products. Acceptance and implementation may be facilitated by a collaborative approach (scientists and health professionals) to their development.
|
| Monitoring and surveillance of antibiotic use On-going monitoring of the use of antibiotics and local research on infections and resistance could inform more rational use and was also of value in the development of policies and guidelines. A monitoring or surveillance programme was also seen as a way of engaging professional groups and bringing them together in a shared AMS programme. In one site discussions had already begun. The principal barrier identified was having sufficient personnel with expertise to lead for an on-going surveillance and research programme. Possible action points were:
|
| Improved laboratory and diagnostic services Improvement of laboratory services and training of scientists to reduce empirical prescribing was identified as a requirement to guide judicious antibiotic prescribing. The key challenge highlighted was the recruitment of scientists with sufficient expertise. It was suggested that pharmaceutical companies may be able to assist, e.g. with the provision of sensitivity discs for their products. Thus, as part of an action plan:
|
| Procurement and quality assurance Steps to ensure continuity in availability, affordability and trust in the quality of products (especially low-cost generics) was seen as essential for the successful operation of an AMS programme in short, medium, and longer term. Barriers to access to quality medicines was seen as encompassing manufacturing and regulation, affordability for patients and prescribing practices. In terms of quality assurance, facilities, personnel, and expertise was highlighted as a challenge. Possible collaboration between manufacturing and regulatory bodies was also mentioned. Despite the challenges, it was viewed that the AMS team should:
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kpokiri, E.E.; Taylor, D.G.; Smith, F.J. Development of Antimicrobial Stewardship Programmes in Low and Middle-Income Countries: A Mixed-Methods Study in Nigerian Hospitals. Antibiotics 2020, 9, 204. https://doi.org/10.3390/antibiotics9040204
Kpokiri EE, Taylor DG, Smith FJ. Development of Antimicrobial Stewardship Programmes in Low and Middle-Income Countries: A Mixed-Methods Study in Nigerian Hospitals. Antibiotics. 2020; 9(4):204. https://doi.org/10.3390/antibiotics9040204
Chicago/Turabian StyleKpokiri, Eneyi E., David G. Taylor, and Felicity J. Smith. 2020. "Development of Antimicrobial Stewardship Programmes in Low and Middle-Income Countries: A Mixed-Methods Study in Nigerian Hospitals" Antibiotics 9, no. 4: 204. https://doi.org/10.3390/antibiotics9040204
APA StyleKpokiri, E. E., Taylor, D. G., & Smith, F. J. (2020). Development of Antimicrobial Stewardship Programmes in Low and Middle-Income Countries: A Mixed-Methods Study in Nigerian Hospitals. Antibiotics, 9(4), 204. https://doi.org/10.3390/antibiotics9040204





