Anti-Fungal Efficacy and Mechanisms of Flavonoids
Abstract
1. Introduction
2. Fungal Diseases and Their Complications
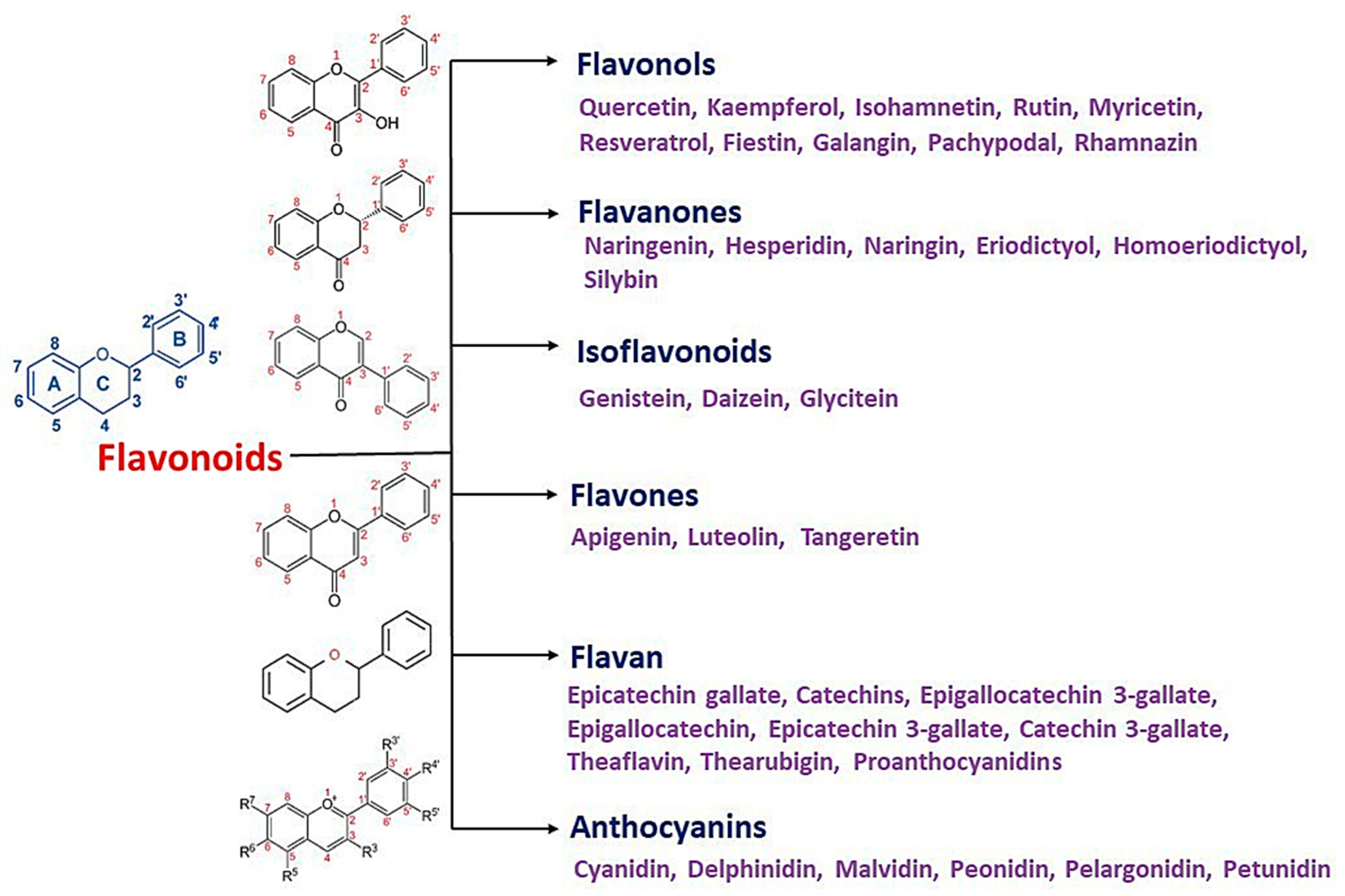
3. Flavonoids
4. Antifungal Activities of Flavonoids
5. Mechanism of Actions of Antifungal Flavonoids
5.1. Induced Plasma Membrane Disruption
5.2. Inhibition of Cell Wall Formation
5.3. Induced Mitochondrial Dysfunction
5.4. Inhibition of Cell Division
5.5. Inhibition of Efflux Pumps
5.6. Inhibition of RNA/DNA and Protein Synthesis
5.7. Synergistic Action between Flavonoids and Antifungals
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bongomin, F.; Gago, S.; Oladele, R.; Denning, D. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, T.; Wan, J.; Li, X.; Yuan, L.; Sun, S. Antifungal effects of phytocompounds on Candida species alone and in combination with fluconazole. Int. J. Antimicrobial. Agents 2017, 49, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, R.; Sandle, T.; Al-Aboody, M.S.; AlFonaisan, M.K.; Alturaiki, W.; Mickymaray, S.; Premanathan, M.; Alsagaby, S.A. Distribution of biocide resistant genes and biocides susceptibility in multidrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii—A first report from the Kingdom of Saudi Arabia. J. Infect. Public Health 2018, 11, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Chung, S.K.; Vanamala, J.; Xu, B. Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes. Int. J. Mol. Sci. 2018, 19, 3720. [Google Scholar] [CrossRef]
- Ganesan, K.; Guo, S.; Fayyaz, S.; Zhang, G.; Xu, B. Targeting Programmed Fusobacterium nucleatum Fap2 for Colorectal Cancer Therapy. Cancers 2019, 11, 1592. [Google Scholar] [CrossRef]
- Sanglard, D. Emerging Threats in Antifungal-Resistant Fungal Pathogens. Front. Med. 2016, 3. [Google Scholar] [CrossRef]
- de Almeida, R.F.M.; Santos, F.C.; Marycz, K.; Alicka, M.; Krasowska, A.; Suchodolski, J.; Panek, J.J.; Jezierska, A.; Starosta, R. New diphenylphosphane derivatives of ketoconazole are promising antifungal agents. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Vinodhini, R.M.K.; Al Aboody, M.S.; Suresh, M. Prevalence And Antifungal Susceptibility Pattern of Candida dubliniensis Isolated From Urine Samples. Int. J. Recent Sci. Res. 2016, 7, 13474–13480. [Google Scholar]
- Devi, A.C.D.D.; Suresh, M.; Thajuddin, N. Diagnostic value of real time PCR and associated bacterial and fungal infections in female genital tuberculosis. Biomed. Pharmacol. J. 2015, 3, 73–79. [Google Scholar]
- Mickymaray, S.; Al Aboody, M.S.; Rath, P.K.; Annamalai, P.; Nooruddin, T. Screening and antibacterial efficacy of selected Indian medicinal plants. Asian Pac. J. Trop. Biomed. 2016, 6, 185–191. [Google Scholar] [CrossRef]
- Ng, K.P.; Kuan, C.S.; Kaur, H.; Na, S.L.; Atiya, N.; Velayuthan, R.D. Candidaspecies epidemiology 2000-2013: a laboratory-based report. Trop. Med. Int. Health 2015, 20, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Looking intoCandida albicansinfection, host response, and antifungal strategies. Virulence 2015, 6, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Polke, M.; Hube, B.; Jacobsen, I.D. Candida Survival Strategies. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 139–235. [Google Scholar] [CrossRef]
- Hurtado, J.C.; Castillo, P.; Fernandes, F.; Navarro, M.; Lovane, L.; Casas, I.; Quintó, L.; Marco, F.; Jordao, D.; Ismail, M.R.; et al. Mortality due to Cryptococcus neoformans and Cryptococcus gattii in low-income settings: an autopsy study. Sci.Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Molina-Leyva, A.; Ruiz-Carrascosa, J.C.; Leyva-Garcia, A.; Husein-Elahmed, H. Cutaneous Cryptococcus laurentii infection in an immunocompetent child. Int. J. Infect. Dis. 2013, 17, e1232–e1233. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.; Sehring, M.; Chambers, J.; Patel, P. Perspectives on non-neoformanscryptococcal opportunistic infections. J. Community Hosp. Intern. Med. Perspect. 2017, 7, 214–217. [Google Scholar] [CrossRef]
- Calista, F.; Tomei, F.; Assalone, P.; Traficante, D.; Di Pilla, G.; Pepe, C.; Di Lullo, L. Cryptococcus laurentii Diarrhea in a Neoplastic Patient. Case Rep. Oncol. Med. 2015, 2015, 216458. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Kannaiyan, M.; Meseret Abebe, G.; Kanimozhi, C.; Thambidurai, P.; Ashokapuram Selvam, S.; Vinodhini, R.; Suresh, M. Prevalence of extended-spectrum beta-lactamase producing enterobacteriaceae members isolated from clinically suspected patients. Asian J. Pharma. Clin. Res. 2018, 11, 364. [Google Scholar] [CrossRef]
- Sinaga, M.; Ganesan, K.; Kumar Nair, S.K.P.; Gani, S.B. Preliminary Phytochemical Analysis and In Vitro Antibacterial Activity of Bark and Seeds of Ethiopian Neem (Azadirachta Indica A. Juss). World J. Pharmacy Pharma. Sci. 2016, 5, 1714–1723. [Google Scholar] [CrossRef]
- Kriengkauykiat, J.; Ito, J.I.; Dadwal, S.S. Epidemiology and treatment approaches in management of invasive fungal infections. Clin. Epidemiol. 2011, 3, 175. [Google Scholar] [CrossRef]
- Roemer, T.; Krysan, D.J. Antifungal Drug Development: Challenges, Unmet Clinical Needs, and New Approaches. Cold Spring Harbor Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef]
- Banu, G.S.; Kumar, G. In-vitro antibacterial activity of flower extracts of Woodfordia fruticosa Kurz. Int. J. Curr. Res. Chem. Pharma. Sci. 2014, 1, 127–130. [Google Scholar]
- Mickymaray, S.; Alturaiki, W. Antifungal Efficacy of Marine Macroalgae against Fungal Isolates from Bronchial Asthmatic Cases. Molecules 2018, 23, 3032. [Google Scholar] [CrossRef]
- Mickymaray, S.; Al Aboody, M.S. In vitro antioxidant and bactericidal efficacy of 15 common spices: novel therapeutics for urinary tract infections? Medicina 2019, 55, 289. [Google Scholar] [CrossRef]
- Mickymaray, S. One-step Synthesis of Silver Nanoparticles Using Saudi Arabian Desert Seasonal Plant Sisymbrium irio and Antibacterial Activity Against Multidrug-Resistant Bacterial Strains. Biomolecules 2019, 9, 662. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Banu, G.S.; Murugesan, A.G.; Pandian, M.R. Hypoglycaemic effect of Helicteres isora bark extract in rats. J. Ethnopharmacol. 2006, 107, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, K.; Punitha, T.; Vinodhini, R.; Mickymaray, S.; Shonga, A.; Tomass, Z.; Thajuddin, N. Efficacy of different solvent extracts of Aristolochia krisagathra and Thottea ponmudiana for potential antimicrobial activity. J. Pharmacy Res. 2015, 9, 35–40. [Google Scholar]
- Zhang, T.; Jayachandran, M.; Ganesan, K.; Xu, B. Black Truffle Aqueous Extract Attenuates Oxidative Stress and Inflammation in STZ-Induced Hyperglycemic Rats via Nrf2 and NF-κB Pathways. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Wu, Z.; Ganesan, K.; Khalid, S.; Chung, S.M.; Xu, B. Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2019, 303, 62–69. [Google Scholar] [CrossRef]
- Sukalingam, K.; Ganesan, K.; Xu, B. Protective Effect of Aqueous Extract from the Leaves of Justicia tranquebariesis against Thioacetamide-Induced Oxidative Stress and Hepatic Fibrosis in Rats. Antioxidants 2018, 7, 78. [Google Scholar] [CrossRef]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, M.A.; Andrade, J.C.; Alves, A.I.S.; dos Santos, F.d.A.G.; Leite-Andrade, M.C.; Sales, D.L.; Nunes, M.; Ribeiro, P.R.V.; Melo Coutinho, H.D.; Morais-Braga, M.F.B.; et al. Use of the natural products from the leaves of the fruitfull tree Persea americana against Candida sp. biofilms using acrylic resin discs. Sci. Total Environ. 2020, 703, 134779. [Google Scholar] [CrossRef] [PubMed]
- K Singla, R.; K Dubey, A. Molecules and Metabolites from Natural Products as Inhibitors of Biofilm in Candida spp. pathogens. Curr. Topics Med. Chem. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Seleem, D.; Pardi, V.; Murata, R.M. Review of flavonoids: A diverse group of natural compounds with anti-Candida albicans activity in vitro. Archiv. Oral Biol. 2017, 76, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Mishra, A.; Mishra, A.K. The chemistry and pharmacology of Cleome genus: A review. Biomed. Pharmacother. 2018, 101, 37–48. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Jawhari, F.Z.; Almehdi, A.M.; Elmsellem, H.; Fikri Benbrahim, K.; Bousta, D.; Bari, A. Antibacterial, antifungal and antioxidant activity of total polyphenols of Withania frutescens.L. Bioorg. Chem. 2019, 93, 103337. [Google Scholar] [CrossRef]
- de Andrade Monteiro, C.; Ribeiro Alves dos Santos, J. Phytochemicals and Their Antifungal Potential Against Pathogenic Yeasts. In Phytochemicals in Human Health; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Lagrouh, F.; Dakka, N.; Bakri, Y. The antifungal activity of Moroccan plants and the mechanism of action of secondary metabolites from plants. J. Mycol. Med. 2017, 27, 303–311. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Hube, B. Importance of the Candida albicans cell wall during commensalism and infection. Curr. Opin. Microbiol. 2012, 15, 406–412. [Google Scholar] [CrossRef]
- Veeraraghavan, B.; Jesudason, M.R.; Prakasah, J.A.J.; Anandan, S.; Sahni, R.D.; Pragasam, A.K.; Bakthavatchalam, Y.D.; Selvakumar, R.J.; Dhole, T.N.; Rodrigues, C.; et al. Antimicrobial susceptibility profiles of gram-negative bacteria causing infections collected across India during 2014–2016: Study for monitoring antimicrobial resistance trend report. Indian J. Med. Microbiol. 2018, 36, 32–36. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Translat. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef]
- Limper, A.H.; Adenis, A.; Le, T.; Harrison, T.S. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017, 17, e334–e343. [Google Scholar] [CrossRef]
- Suresh, M.; Rath, P.K.; Panneerselvam, A.; Dhanasekaran, D.; Thajuddin, N. Anti-mycobacterial effect of leaf extract of Centella asiatica. Res. J. Pharm. Technol. 2010, 3, 872–876. [Google Scholar]
- Suresh, M.; Rath, P.K.; Panneerselvam, A.; Dhanasekaran, D.; Thajuddin, N. Antifungal activity of selected Indian medicinal plant salts. J.Glob. Pharm.Technol. 2010, 2, 71–74. [Google Scholar]
- Revathi, P.; Senthinath, T.J.; Vigneswari, R. Antimicrobial resistance pattern of gram negative bacteria to 3rd and 4th generation cephalosporins. BMC Proceedings 2011, 5. [Google Scholar] [CrossRef]
- Kannaiyan, M.; Manuel, V.N.; Raja, V.; Thambidurai, P.; Mickymaray, S.; Nooruddin, T. Antimicrobial activity of the ethanolic and aqueous extracts of Salacia chinensis Linn. against human pathogens. Asian Pac. J. Trop. Dis. 2012, 2, S416–S420. [Google Scholar] [CrossRef]
- Kothavade, R.J.; Kura, M.M.; Valand, A.G.; Panthaki, M.H. Candida tropicalis: its prevalence, pathogenicity and increasing resistance to fluconazole. J. Med. Microbiol. 2010, 59, 873–880. [Google Scholar] [CrossRef]
- Tobudic, S.; Kratzer, C.; Presterl, E. Azole-resistant Candida spp.—Emerging pathogens? Mycoses 2012, 55, 24–32. [Google Scholar] [CrossRef]
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2015, 42, 905–927. [Google Scholar] [CrossRef]
- Sobel, J.D. Recurrent vulvovaginal candidiasis. Am. J. Obst. Gynecol. 2016, 214, 15–21. [Google Scholar] [CrossRef]
- Pana, Z.D.; Roilides, E.; Warris, A.; Groll, A.H.; Zaoutis, T. Epidemiology of Invasive Fungal Disease in Children. J. Pediatr. Infect. Dis. Soc. 2017, 6, S3–S11. [Google Scholar] [CrossRef]
- Lin, S.J.; Schranz, J.; Teutsch, S.M. Aspergillosis Case-Fatality Rate: Systematic Review of the Literature. Clin. Infect. Dis. 2001, 32, 358–366. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.M.; Morgan, J.; Wannemuehler, K.A.; Mirza, S.A.; Gould, S.M.; Mhlongo, N.; Moeng, P.; Maloba, B.R.; Crewe-Brown, H.H.; Brandt, M.E.; et al. Population-based surveillance for cryptococcosis in an antiretroviral-naive South African province with a high HIV seroprevalence. AIDS 2006, 20, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Gaitanis, G.; Magiatis, P.; Hantschke, M.; Bassukas, I.D.; Velegraki, A. The Malassezia Genus in Skin and Systemic Diseases. Clin. Microbiol. Rev. 2012, 25, 106–141. [Google Scholar] [CrossRef] [PubMed]
- Prohic, A.; Jovovic Sadikovic, T.; Krupalija-Fazlic, M.; Kuskunovic-Vlahovljak, S. Malasseziaspecies in healthy skin and in dermatological conditions. Int. J. Dermatol. 2015, 55, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Olicón-Hernández, D.R.; Camacho-Morales, R.L.; Pozo, C.; González-López, J.; Aranda, E. Evaluation of diclofenac biodegradation by the ascomycete fungus Penicillium oxalicum at flask and bench bioreactor scales. Sci. Total Environ. 2019, 662, 607–614. [Google Scholar] [CrossRef]
- Armstrong-James, D.; Meintjes, G.; Brown, G.D. A neglected epidemic: fungal infections in HIV/AIDS. Trends Microbiol. 2014, 22, 120–127. [Google Scholar] [CrossRef]
- Green, M.D.; Apel, A.J.G.; Naduvilath, T.; Stapleton, F.J. Clinical outcomes of keratitis. Clin. Exp. Ophthalmol. 2007, 35, 421–426. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Lionakis, M.S.; Lewis, R.E.; Chamilos, G.; Healy, M.; Perego, C.; Safdar, A.; Kantarjian, H.; Champlin, R.; Walsh, T.J.; et al. Zygomycosis in a Tertiary-Care Cancer Center in the Era ofAspergillus-Active Antifungal Therapy: A Case-Control Observational Study of 27 Recent Cases. J. Infect. Dis. 2005, 191, 1350–1360. [Google Scholar] [CrossRef]
- Geramizadeh, B.; Foroughi, R.; Keshtkar-Jahromi, M.; Malek-Hosseini, S.A.; Alborzi, A. Gastrointestinal basidiobolomycosis, an emerging infection in the immunocompetent host: a report of 14 patients. J. Med. Microbiol. 2012, 61, 1770–1774. [Google Scholar] [CrossRef]
- Nucci, M.; Anaissie, E. Fusarium Infections in Immunocompromised Patients. Clin. Microbiol. Rev. 2007, 20, 695–704. [Google Scholar] [CrossRef]
- Hay, R.J. Tinea Capitis: Current Status. Mycopathologia 2016, 182, 87–93. [Google Scholar] [CrossRef]
- Nweze, E.I.; Eke, I.E. Dermatophytes and dermatophytosis in the eastern and southern parts of Africa. Med. Mycol. 2017, 56, 13–28. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.-M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2016, 105, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Aspects Med. 2018, 61, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Felice, M.R.; Giuffrè, L.; El Aamri, L.; Hafidi, M.; Criseo, G.; Romeo, O.; Scordino, F. Looking for New Antifungal Drugs from Flavonoids: Impact of the Genetic Diversity of Candida albicans on the in-vitro Response. Curr. Med. Chem. 2019, 26, 5108–5123. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-Rich Dry Common Beans (Phaseolus vulgaris L.) and Their Health Benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Ganesan, K.; Xu, B. New Insight into Mycochemical Profiles and Antioxidant Potential of Edible and Medicinal Mushrooms: A Review. Int. J. Med. Mushrooms 2019, 21, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Polyphenol-Rich Lentils and Their Health Promoting Effects. Int. J. Mol. Sci. 2017, 18, 2390. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Anti-Obesity Effects of Medicinal and Edible Mushrooms. Molecules 2018, 23, 2880. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A critical review on phytochemical profile and health promoting effects of mung bean (Vigna radiata). Food Sci. Hum. Wellness 2018, 7, 11–33. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Hegarty, V.M.; May, H.M.; Khaw, K.-T. Tea drinking and bone mineral density in older women. Am. J. Clin. Nutr. 2000, 71, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Bojić, M.; Maleš, Ž.; Antolić, A.; Babić, I.; Tomičić, M. Antithrombotic activity of flavonoids and polyphenols rich plant species. Acta Pharma. 2019, 69, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Jayachandran, M.; Xu, B. A critical review on hepatoprotective effects of bioactive food components. Crit. Rev. Food Sci. Nutr. 2017, 58, 1165–1229. [Google Scholar] [CrossRef]
- Abdulwanis Mohamed, Z.; Mohamed Eliaser, E.; Mazzon, E.; Rollin, P.; Cheng Lian Ee, G.; Abdull Razis, A.F. Neuroprotective Potential of Secondary Metabolites from Melicope lunu-ankenda (Rutaceae). Molecules 2019, 24, 3109. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Banu, G.S.; Murugesan, A.G.A. Influence of Helicteres isora L. bark extracts on glycemic control and renoprotective activity in streptozotocin-induced diabetic rats. Int. J. Pharma Sci. Nanotechnol. 2008, 1, 275–280. [Google Scholar]
- Ganesan, K.; Xu, B. Molecular targets of vitexin and isovitexin in cancer therapy: A critical review. Ann. N.Y. Acad. Sci. 2017, 1401, 102–113. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Telomerase Inhibitors from Natural Products and Their Anticancer Potential. Int. J. Mol. Sci. 2017, 19. [Google Scholar] [CrossRef]
- Pandian, M.R.; Banu, G.S.; Kumar, G. A study of the antimicrobial activity of Alangium salviifolium. Indian J. Pharmacol. 2006, 38, 203. [Google Scholar] [CrossRef]
- Mickymaray, S. Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef]
- Banu, G.S.; Kumar, G. Preliminary Screening of Endophytic Fungi from Medicinal Plants in India for Antimicrobial and Antitumor Activity. Int. J. Pharma. Sci. Nanotechnol. 2009, 2, 566–571. [Google Scholar]
- Sukalingam, K.; Ganesan, K.; Xu, B. Trianthema portulacastrum L. (giant pigweed): Phytochemistry and pharmacological properties. Phytochem. Rev. 2017, 16, 461–478. [Google Scholar] [CrossRef]
- Abd El Maksoud, A.I.; Taher, R.F.; Gaara, A.H.; Abdelrazik, E.; Keshk, O.S.; Elawdan, K.A.; Morsy, S.E.; Salah, A.; Khalil, H. Selective Regulation of B-Raf Dependent K-Ras/Mitogen-Activated Protein by Natural Occurring Multi-kinase Inhibitors in Cancer Cells. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, J.; Zhang, C.; Li, Y. Baicalein restrains proliferation, migration, and invasion of human malignant melanoma cells by down-regulating colon cancer associated transcript-1. Braz. J. Med. Biol. Res. 2019, 52. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Al Aboody, M.S.; Alturaiki, W.; Alsagaby, S.A.; Alfaiz, F.A.; Veeraraghavan, V.P.; Mickymaray, S. Photosynthesized gold nanoparticles from Catharanthus roseus induces caspase-mediated apoptosis in cervical cancer cells (HeLa). Artificial Cells Nanomed. Biotechnol. 2019, 47, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.; Kandaswami, C. The impact of plant flavonoids on mammalian biology: implications for immunity, inflammation and cancer. In The Flavonoids: Advances in Research since 1986; Harborne, J.B., Ed.; Chapman and Hall: London, UK, 1993; pp. 619–652. [Google Scholar] [CrossRef]
- Kumar, G.; Banu, G.S.; Kannan, V.; Pandian, M.R. Antihepatotoxic effect of beta-carotene on paracetamol induced hepatic damage in rats. Indian J. Exp. Biol. 2005, 43, 351–355. [Google Scholar]
- Kumar, G.; Banu, G.S.; Pappa, P.V.; Sundararajan, M.; Pandian, M.R. Hepatoprotective activity of Trianthema portulacastrum L. against paracetamol and thioacetamide intoxication in albino rats. J. Ethnopharmacol. 2004, 92, 37–40. [Google Scholar] [CrossRef]
- Kumar, G.; Murugesan, A.G. Hypolipidaemic activity of Helicteres isora L. bark extracts in streptozotocin induced diabetic rats. J. Ethnopharmacol. 2008, 116, 161–166. [Google Scholar] [CrossRef]
- Kumar, G.; Sharmila Banu, G.; Ganesan Murugesan, A. Effect of Helicteres isora bark extracts on heart antioxidant status and lipid peroxidation in streptozotocin diabetic rats. J. Appl. Biomed. 2008, 6, 89–95. [Google Scholar] [CrossRef]
- Kumar, G.; Banu, G.S.; Pandian, M.R. Biochemical activity of selenium and glutathione on country made liquor (CML) induced hepatic damage in rats. Indian J. Clin. Biochem. 2007, 22, 105–108. [Google Scholar] [CrossRef]
- Ziberna, L.; Fornasaro, S.; Čvorović, J.; Tramer, F.; Passamonti, S. Bioavailability of Flavonoids. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 489–511. [Google Scholar] [CrossRef]
- Kumar, G.; Sharmila Banu, G.; Murugesan, A.G.; Rajasekara Pandian, M. Effect ofHelicteres isora. Bark Extracts on Brain Antioxidant Status and Lipid Peroxidation in Streptozotocin Diabetic Rats. Pharma. Biol. 2007, 45, 753–759. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.P.; Zhang, J.D.; Li, Q.; Shen, H.; Chen, S.M.; He, L.J.; Yan, L.; Xu, G.T.; An, M.M.; et al. Synergistic Antifungal Effect of Glabridin and Fluconazole. PLoS ONE 2014, 9, e103442. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Aranda, R.; Granados-Guzmán, G.; Pérez-Meseguer, J.; González, G.; de Torres, N. Activity of Polyphenolic Compounds against Candida glabrata. Molecules 2015, 20, 17903–17912. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.R.d.; Nabavi, S.F.; Daglia, M.; Rastrelli, L.; Nabavi, S.M. Epigallocatechin gallate and mitochondria—A story of life and death. Pharmacol. Res. 2016, 104, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.M.; Carraro, E.; Auler, M.E.; Khalil, N.M. Quercetin and rutin as potential agents antifungal against Cryptococcus spp. Braz. J. Biol. 2016, 76, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-X.; An, M.-M.; Jin, Y.-S.; Chen, H.-S. Chemical constituents from the rhizome of Polygonum paleaceum and their antifungal activity. J. Asian Nat. Prod. Res. 2016, 19, 47–52. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.R.; de Andrade Neto, J.B.; de Sousa Campos, R.; Figueiredo, N.S.; Sampaio, L.S.; Magalhães, H.I.F.; Cavalcanti, B.C.; Gaspar, D.M.; de Andrade, G.M.; Lima, I.S.P.; et al. Synergistic Effect of the Flavonoid Catechin, Quercetin, or Epigallocatechin Gallate with Fluconazole Induces Apoptosis in Candida tropicalis Resistant to Fluconazole. Antimicrob. Agents Chemother. 2013, 58, 1468–1478. [Google Scholar] [CrossRef]
- Andrade, J.T.; Santos, F.R.S.; Lima, W.G.; Sousa, C.D.F.; Oliveira, L.S.F.M.; Ribeiro, R.I.M.A.; Gomes, A.J.P.S.; Araújo, M.G.F.; Villar, J.A.F.P.; Ferreira, J.M.S. Design, synthesis, biological activity and structure-activity relationship studies of chalcone derivatives as potential anti-Candida agents. J. Antibiotics 2018, 71, 702–712. [Google Scholar] [CrossRef]
- Houlihan, A.J.; Conlin, P.; Chee-Sanford, J.C. Water-soluble exudates from seeds of Kochia scoparia exhibit antifungal activity against Colletotrichum graminicola. PLoS ONE 2019, 14, e0218104. [Google Scholar] [CrossRef]
- Mohotti, S.; Rajendran, S.; Muhammad, T.; Strömstedt, A.A.; Adhikari, A.; Burman, R.; de Silva, E.D.; Göransson, U.; Hettiarachchi, C.M.; Gunasekera, S. Screening for bioactive secondary metabolites in Sri Lankan medicinal plants by microfractionation and targeted isolation of antimicrobial flavonoids from Derris scandens. J. Ethnopharmacol. 2020, 246, 112158. [Google Scholar] [CrossRef]
- Lee, J.A.; Chee, H.Y. In VitroAntifungal Activity of Equol against Candida albicans. Mycobiology 2010, 38, 328. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, T.A.; Komoto, T.T.; Massaroto, B.G.; Miranda, C.E.S.; Beleboni, R.O.; Marins, M.; Fachin, A.L. Trans-chalcone and quercetin down-regulate fatty acid synthase gene expression and reduce ergosterol content in the human pathogenic dermatophyte Trichophyton rubrum. BMC Compl. Altern. Med. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Edziri, H.; Mastouri, M.; Mahjoub, M.A.; Mighri, Z.; Mahjoub, A.; Verschaeve, L. Antibacterial, Antifungal and Cytotoxic Activities of Two Flavonoids from Retama raetam Flowers. Molecules 2012, 17, 7284–7293. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, E.N.; Sampietro, D.A.; Sgariglia, M.A.; Soberón, J.R.; Vattuone, M.A. Antimycotic activity of 5′-prenylisoflavanones of the plant Geoffroea decorticans, against Aspergillus species. Int. J. Food Microbiol. 2009, 132, 42–46. [Google Scholar] [CrossRef]
- Belofsky, G.; Kolaczkowski, M.; Adams, E.; Schreiber, J.; Eisenberg, V.; Coleman, C.M.; Zou, Y.; Ferreira, D. Fungal ABC Transporter-Associated Activity of Isoflavonoids from the Root Extract of Dalea formosa. J. Nat. Prod. 2013, 76, 915–925. [Google Scholar] [CrossRef]
- Messier, C.; Epifano, F.; Genovese, S.; Grenier, D. Inhibition of Candida albicans biofilm formation and yeast-hyphal transition by 4-hydroxycordoin. Phytomedicine 2011, 18, 380–383. [Google Scholar] [CrossRef]
- Vieira, M.L.A.; Johann, S.; Hughes, F.M.; Rosa, C.A.; Rosa, L.H. The diversity and antimicrobial activity of endophytic fungi associated with medicinal plant Baccharis trimera (Asteraceae) from the Brazilian savannah. Can. J. Microbiol. 2014, 60, 847–856. [Google Scholar] [CrossRef]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.B.; Gasson, M.J.; Narbad, A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef]
- Djouossi, M.G.; Tamokou, J.-d.-D.; Ngnokam, D.; Kuiate, J.-R.; Tapondjou, L.A.; Harakat, D.; Voutquenne-Nazabadioko, L. Antimicrobial and antioxidant flavonoids from the leaves of Oncoba spinosa Forssk. (Salicaceae). BMC Compl. Altern. Med. 2015, 15. [Google Scholar] [CrossRef]
- Gadetskaya, A.V.; Tarawneh, A.H.; Zhusupova, G.E.; Gemejiyeva, N.G.; Cantrell, C.L.; Cutler, S.J.; Ross, S.A. Sulfated phenolic compounds from Limonium caspium: Isolation, structural elucidation, and biological evaluation. Fitoterapia 2015, 104, 80–85. [Google Scholar] [CrossRef]
- Karalija, E.; Parić, A.; Dahija, S.; Bešta-Gajević, R.; Ćavar Zeljković, S. Phenolic compounds and bioactive properties of Verbascum glabratum subsp. bosnense (K. Malý) Murb., an endemic plant species. Nat. Prod. Res. 2018, 1–5. [Google Scholar] [CrossRef] [PubMed]
- da Silva Sa, F.; de Paula, J.; dos Santos, P.; de Almeida Ribeiro Oliveira, L.; de Almeida Ribeiro Oliveira, G.; Liao, L.; de Paula, J.; do Rosario Rodrigues Silva, M. Phytochemical Analysis and Antimicrobial Activity of Myrcia tomentosa (Aubl.) DC. Leaves. Molecules 2017, 22, 1100. [Google Scholar] [CrossRef] [PubMed]
- Toiu, A.; Vlase, L.; Vodnar, D.C.; Gheldiu, A.-M.; Oniga, I. Solidago graminifolia L. Salisb. (Asteraceae) as a Valuable Source of Bioactive Polyphenols: HPLC Profile, In Vitro Antioxidant and Antimicrobial Potential. Molecules 2019, 24, 2666. [Google Scholar] [CrossRef] [PubMed]
- da Costa, M.P.; Bozinis, M.C.V.; Andrade, W.M.; Costa, C.R.; da Silva, A.L.; Alves de Oliveira, C.M.; Kato, L.; Fernandes, O.d.F.L.; Souza, L.K.H.; Silva, M.d.R.R. Antifungal and cytotoxicity activities of the fresh xylem sap of Hymenaea courbaril L. and its major constituent fisetin. BMC Compl. Altern. Med. 2014, 14. [Google Scholar] [CrossRef]
- Sohn, H.-Y. Fungicidal Effect of Prenylated Flavonol, Papyriflavonol A, Isolated from Broussonetia papyrifera (L.) Vent. Against Candida albicans. J. Microbiol. Biotechnol. 2010, 20, 1397–1402. [Google Scholar] [CrossRef]
- Boeck, P.; Leal, P.C.; Yunes, R.A.; Filho, V.C.; López, S.; Sortino, M.; Escalante, A.; Furlán, R.L.E.; Zacchino, S. Antifungal Activity and Studies on Mode of Action of Novel Xanthoxyline-Derived Chalcones. Archiv. Pharmazie 2005, 338, 87–95. [Google Scholar] [CrossRef]
- Rajasekharan, S.K.; Ramesh, S.; Bakkiyaraj, D. Synergy of flavonoids with HDAC inhibitor: new approach to targetCandida tropicalisbiofilms. J. Chemother. 2014, 27, 246–249. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, M.; Wang, T.; Li, Y.; Wang, C. The roles ofCDR1,CDR2,andMDR1in kaempferol-induced suppression with fluconazole-resistant Candida albicans. Pharma. Biol. 2015, 54, 984–992. [Google Scholar] [CrossRef]
- Da, X.; Nishiyama, Y.; Tie, D.; Hein, K.Z.; Yamamoto, O.; Morita, E. Antifungal activity and mechanism of action of Ou-gon (Scutellaria root extract) components against pathogenic fungi. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Cantelli, B.A.M.; Bitencourt, T.A.; Komoto, T.T.; Beleboni, R.O.; Marins, M.; Fachin, A.L. Caffeic acid and licochalcone A interfere with the glyoxylate cycle of Trichophyton rubrum. Biomed. Pharmacother. 2017, 96, 1389–1394. [Google Scholar] [CrossRef]
- Baptista, R.; Madureira, A.M.; Jorge, R.; Adão, R.; Duarte, A.; Duarte, N.; Lopes, M.M.; Teixeira, G. Antioxidant and Antimycotic Activities of Two NativeLavandulaSpecies from Portugal. Evid. Based Compl. Altern. Med. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- González-Alamilla, E.N.; Gonzalez-Cortazar, M.; Valladares-Carranza, B.; Rivas-Jacobo, M.A.; Herrera-Corredor, C.A.; Ojeda-Ramírez, D.; Zaragoza-Bastida, A.; Rivero-Perez, N. Chemical Constituents of Salix babylonica L. and Their Antibacterial Activity Against Gram-Positive and Gram-Negative Animal Bacteria. Molecules 2019, 24, 2992. [Google Scholar] [CrossRef]
- Huang, S.; Cao, Y.Y.; Dai, B.D.; Sun, X.R.; Zhu, Z.Y.; Cao, Y.B.; Wang, Y.; Gao, P.H.; Jiang, Y.Y. In Vitro Synergism of Fluconazole and Baicalein against Clinical Isolates of Candida albicans Resistant to Fluconazole. Biol. Pharma. Bull. 2008, 31, 2234–2236. [Google Scholar] [CrossRef]
- Dai, B.D.; Cao, Y.Y.; Huang, S.; Xu, Y.G.; Gao, P.H.; Wang, Y.; Jiang, Y.Y. Baicalein induces programmed cell death in Candida albicans. J. Microbiol. Biotechnol. 2009, 19, 803–809. [Google Scholar] [PubMed]
- Kang, K.; Fong, W.-P.; Tsang, P.W.-K. Antifungal Activity of Baicalein Against Candida krusei Does Not Involve Apoptosis. Mycopathologia 2010, 170, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Serpa, R.; Franca, E.J.G.; Furlaneto-Maia, L.; Andrade, C.G.T.J.; Diniz, A.; Furlaneto, M.C. In vitro antifungal activity of the flavonoid baicalein against Candida species. J. Med. Microbiol. 2012, 61, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Tsang, P.W.-K.; Chau, K.-Y.; Yang, H.-P. Baicalein exhibits inhibitory effect on the energy-dependent efflux pump activity in non-albicans Candidafungi. J. Chemother. 2014, 27, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Woo, E.-R.; Lee, D.G. Apigenin induces cell shrinkage in Candida albicans by membrane perturbation. FEMS Yeast Res. 2018, 18. [Google Scholar] [CrossRef]
- Mangoyi, R.; Midiwo, J.; Mukanganyama, S. Isolation and characterization of an antifungal compound 5-hydroxy-7,4’-dimethoxyflavone from Combretum zeyheri. BMC Compl. Altern. Med. 2015, 15. [Google Scholar] [CrossRef]
- Katerere, D.R.; Gray, A.I.; Nash, R.J.; Waigh, R.D. Phytochemical and antimicrobial investigations of stilbenoids and flavonoids isolated from three species of Combretaceae. Fitoterapia 2012, 83, 932–940. [Google Scholar] [CrossRef]
- Perez, C.; Tiraboschi, I.N.; Ortega, M.G.; Agnese, A.M.; Cabrera, J.L. Further Antimicrobial Studies of 2’4’-dihidroxy-5’-(1?-dimethylallyl)-6-prenylpinocembrin from Dalea elegans. Pharma. Biol. 2003, 41, 171–174. [Google Scholar] [CrossRef]
- Peralta, M.A.; da Silva, M.A.; Ortega, M.G.; Cabrera, J.L.; Paraje, M.G. Antifungal activity of a prenylated flavonoid from Dalea elegans against Candida albicans biofilms. Phytomedicine 2015, 22, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Kashiwada, Y.; Shibatav, H.; Takaishi, Y. Prenylated Flavonoids from the Roots of Desmodium caudatum and Evaluation of Their Antifungal Activity. Planta Med. 2012, 78, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Lourenção Brighenti, F.; Salvador, M.J.; Vidal Lacerda Gontijo, A.; Botazzo Delbem, A.C.; Botazzo Delbem, Á.C.; Soares, C.P.; Carvalho de Oliveira, M.A.; Miorelli Girondi, C.; Koga-Ito, C.Y. Plant extracts: initial screening, identification of bioactive compounds and effect against Candida albicansbiofilms. Future Microbiol. 2017, 12, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Seleem, D.; Benso, B.; Noguti, J.; Pardi, V.; Murata, R.M. In vitro and in vivo Antifungal Activity of Lichochalcone-A against Candida albicans Biofilms. PLoS ONE 2016, 11, e0157188. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.R.; Tupe, S.G.; Gample, S.P.; Chandgude, M.G.; Sarkar, D.; Deshpande, M.V.; Joshi, S.P. Antifungal dimeric chalcone derivative kamalachalcone E fromMallotus philippinensis. Nat. Prod. Res. 2013, 28, 245–250. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, H.N.; Joshi, A.S.; Nimrod, A.C.; Walker, L.A.; Clark, A.M. Antifungal Chalcones from Maclura tinctoria. Planta Med. 2001, 67, 87–89. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Brighenti, F.L.; Delbem, A.C.B.; Delbem, Á.C.B.; Khouri, S.; Gontijo, A.V.L.; Pascoal, A.C.R.F.; Salvador, M.J.; Koga-Ito, C.Y. Antifungal activity of extracts and isolated compounds fromBuchenavia tomentosaon Candida albicans and non-albicans. Future Microbiol. 2015, 10, 917–927. [Google Scholar] [CrossRef]
- Herrera, C.L.; Alvear, M.; Barrientos, L.; Montenegro, G.; Salazar, L.A. The antifungal effect of six commercial extracts of Chilean propolis on Candida spp. Ciencia e Investigación Agraria 2010, 37. [Google Scholar] [CrossRef]
- Yoon, T.M.; Kim, J.W.; Kim, J.G.; Kim, W.G.; Suh, J.W. Talosins A and B: New Isoflavonol Glycosides with Potent Antifungal Activity from Kitasatospora kifunensis MJM341. J. Antibiotics 2006, 59, 633–639. [Google Scholar] [CrossRef][Green Version]
- Taveira, M.; Silva, L.s.R.; Vale-Silva, L.s.A.; Pinto, E.n.; Valentão, P.c.; Ferreres, F.; Guedes de Pinho, P.; Andrade, P.B. Lycopersicon esculentumSeeds: An Industrial Byproduct as an Antimicrobial Agent. J. Agric. Food Chem. 2010, 58, 9529–9536. [Google Scholar] [CrossRef] [PubMed]
- Yousefbeyk, F.; Gohari, A.R.; Hashemighahderijani, Z.; Ostad, S.N.; Sourmaghi, M.H.S.; Amini, M.; Golfakhrabadi, F.; Jamalifar, H.; Amin, G.; Amin, M. Erratum to: Bioactive terpenoids and flavonoids from Daucus littoralis Smith subsp. hyrcanicus Rech.f, an endemic species of Iran. DARU J. Pharma. Sci. 2014, 22. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, V.K.; ElSohly, H.N.; Khan, S.I.; Jacob, M.R.; Joshi, V.C.; Smillie, T.; Khan, I.A.; Walker, L.A. Constituents of Nelumbo nucifera leaves and their antimalarial and antifungal activity. Phytochem. Lett. 2008, 1, 89–93. [Google Scholar] [CrossRef]
- Awouafack, M.D.; McGaw, L.J.; Gottfried, S.; Mbouangouere, R.; Tane, P.; Spiteller, M.; Eloff, J.N. Antimicrobial activity and cytotoxicity of the ethanol extract, fractions and eight compounds isolated from Eriosema robustum (Fabaceae). BMC Compl. Altern. Med. 2013, 13. [Google Scholar] [CrossRef]
- Shakirullah, M.; Ahmad, H.; Shah, M.R.; Ahmad, I.; Ishaq, M.; Khan, N.; Badshah, A.; Khan, I. Antimicrobial activities of Conyzolide and Conyzoflavone fromConyza canadensis. J. Enzyme Inhibit. Med. Chem. 2010, 26, 468–471. [Google Scholar] [CrossRef]
- Yenjai, C.; Prasanphen, K.; Daodee, S.; Wongpanich, V.; Kittakoop, P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia 2004, 75, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shang, M.-Y.; Liu, G.-X.; Xu, F.; Wang, X.; Shou, C.-C.; Cai, S.-Q. Chemical Constituents from the Rhizomes of Smilax glabra and Their Antimicrobial Activity. Molecules 2013, 18, 5265–5287. [Google Scholar] [CrossRef] [PubMed]
- Moshi, M.; Joseph, C.; Innocent, E.; Nkunya, M. In VitroAntibacterial and Antifungal Activities of Extracts and Compounds fromUvaria scheffleri. Pharma. Biol. 2004, 42, 269–273. [Google Scholar] [CrossRef]
- Qu, J.; Xie, C.; Guo, H.; Yu, W.; Lou, H. Antifungal dibenzofuran bis(bibenzyl)s from the liverwort Asterella angusta. Phytochemistry 2007, 68, 1767–1774. [Google Scholar] [CrossRef]
- Picerno, P.; Mencherini, T.; Sansone, F.; Del Gaudio, P.; Granata, I.; Porta, A.; Aquino, R.P. Screening of a polar extract of Paeonia rockii: Composition and antioxidant and antifungal activities. J. Ethnopharmacol. 2011, 138, 705–712. [Google Scholar] [CrossRef]
- Mulaudzi, R.B.; Ndhlala, A.R.; Kulkarni, M.G.; Van Staden, J. Pharmacological properties and protein binding capacity of phenolic extracts of some Venda medicinal plants used against cough and fever. J. Ethnopharmacol. 2012, 143, 185–193. [Google Scholar] [CrossRef] [PubMed]
- De Leo, M.; Braca, A.; De Tommasi, N.; Norscia, I.; Morelli, I.; Battinelli, L.; Mazzanti, G. Phenolic Compounds fromBaseonema acuminatum Leaves: Isolation and Antimicrobial Activity. Planta Med. 2004, 70, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Mbaveng, A.T.; Kuete, V.; Ngameni, B.; Beng, V.P.; Ngadjui, B.T.; Meyer, J.J.M.; Lall, N. Antimicrobial activities of the methanol extract and compounds from the twigs of Dorstenia mannii (Moraceae). BMC Compl. Altern. Med. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Gabriela, N.; Rosa, A.M.; Catiana, Z.I.; Soledad, C.; Mabel, O.R.; Esteban, S.J.; Veronica, B.; Daniel, W.; Ines, I.M. The Effect of Zuccagnia punctata, an Argentine Medicinal Plant, on Virulence Factors from Candida Species. Nat. Prod. Comm. 2014, 9, 1934578X1400900. [Google Scholar] [CrossRef]
- Zuzarte, M.; Vale-Silva, L.; Gonçalves, M.J.; Cavaleiro, C.; Vaz, S.; Canhoto, J.; Pinto, E.; Salgueiro, L. Antifungal activity of phenolic-rich Lavandula multifida L. essential oil. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 31, 1359–1366. [Google Scholar] [CrossRef]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef]
- Wächter, G.A.; Hoffmann, J.J.; Furbacher, T.; Blake, M.E.; Timmermann, B.N. Antibacterial and antifungal flavanones from Eysenhardtia texana. Phytochemistry 1999, 52, 1469–1471. [Google Scholar] [CrossRef]
- Lopes, G.; Pinto, E.; Salgueiro, L. Natural Products: An Alternative to Conventional Therapy for Dermatophytosis? Mycopathologia 2016, 182, 143–167. [Google Scholar] [CrossRef]
- Correia, A.F.; Silveira, D.; Fonseca-Bazzo, Y.M.; Magalhães, P.O.; Fagg, C.W.; da Silva, E.C.; Gomes, S.M.; Gandolfi, L.; Pratesi, R.; de Medeiros Nóbrega, Y.K. Activity of crude extracts from Brazilian cerrado plants against clinically relevant Candida species. BMC Compl. Altern. Med. 2016, 16. [Google Scholar] [CrossRef]
- Yamaguchi, M.U.; Garcia, F.P.; Cortez, D.A.G.; Ueda-Nakamura, T.; Filho, B.P.D.; Nakamura, C.V. Antifungal effects of Ellagitannin isolated from leaves of Ocotea odorifera (Lauraceae). Antonie van Leeuwenhoek 2010, 99, 507–514. [Google Scholar] [CrossRef]
- Klewicka, E.; Sójka, M.; Klewicki, R.; Kołodziejczyk, K.; Lipińska, L.; Nowak, A. Ellagitannins from Raspberry (Rubus idaeus L.) Fruit as Natural Inhibitors of Geotrichum candidum. Molecules 2016, 21, 908. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, C.; Vargas, Á.; Fronza, N.; dos Santos, J.H.Z. Structural, textural and morphological characteristics of tannins from Acacia mearnsii encapsulated using sol-gel methods: Applications as antimicrobial agents. Colloids Surfaces B Biointerfaces 2017, 151, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J.Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Tapas, A.R.; Sakarkar, D.M.; Kakde, R.B. Flavonoids as Nutraceuticals: A Review. Trop. J. Pharma. Res. 2008, 7. [Google Scholar] [CrossRef]
- Taleb-Contini, S.H.; Salvador, M.J.; Watanabe, E.; Ito, I.Y.; Oliveira, D.C.R.d. Antimicrobial activity of flavonoids and steroids isolated from two Chromolaena species. Revista Brasileira de Ciências Farmacêuticas 2003, 39, 403–408. [Google Scholar] [CrossRef]
- Li, K.; Xing, S.; Wang, M.; Peng, Y.; Dong, Y.; Li, X. Anticomplement and Antimicrobial Activities of Flavonoids from Entada phaseoloides. Nat. Prod. Comm. 2012, 7, 1934578X1200700. [Google Scholar] [CrossRef]
- Ahmadi, F.; Sadeghi, S.; Modarresi, M.; Abiri, R.; Mikaeli, A. Chemical composition, in vitro anti-microbial, antifungal and antioxidant activities of the essential oil and methanolic extract of Hymenocrater longiflorus Benth., of Iran. Food Chem. Toxicol. 2010, 48, 1137–1144. [Google Scholar] [CrossRef]
- Montagner, C.; de Souza, S.M.; Groposo, C.; Delle Monache, F.; Smânia, E.F.A.; Smânia Jr, A. Antifungal Activity of Coumarins. Zeitschrift für Naturforschung C 2008, 63, 21–28. [Google Scholar] [CrossRef]
- Navarro-García, V.M.; Rojas, G.; Avilés, M.; Fuentes, M.; Zepeda, G. In vitro antifungal activity of coumarin extracted from Loeselia mexicana Brand. Mycoses 2011, 54, e569–e571. [Google Scholar] [CrossRef]
- Raut, J.S.; Shinde, R.B.; Chauhan, N.M.; Karuppayil, S.M. Phenylpropanoids of Plant Origin as Inhibitors of Biofilm Formation by Candida albicans. J. Microbiol. Biotechnol. 2014, 24, 1216–1225. [Google Scholar] [CrossRef]
- Salas, M.P.; Céliz, G.; Geronazzo, H.; Daz, M.; Resnik, S.L. Antifungal activity of natural and enzymatically-modified flavonoids isolated from citrus species. Food Chem. 2011, 124, 1411–1415. [Google Scholar] [CrossRef]
- Mendoza, L.; Yañez, K.; Vivanco, M.; Melo, R.; Cotoras, M. Characterization of extracts from winery by-products with antifungal activity against Botrytis cinerea. Indust. Crops Prod. 2013, 43, 360–364. [Google Scholar] [CrossRef]
- Han, Y. Synergic Anticandidal Effect of Epigallocatechin-O-gallate Combined with Amphotericin B in a Murine Model of Disseminated Candidiasis and Its Anticandidal Mechanism. Biol. Pharma.Bull. 2007, 30, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Han, Y. Synergic effect of grape seed extract with amphotericin B against disseminated candidiasis due to Candida albicans. Phytomedicine 2007, 14, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Sagdic, O.; Ozturk, I.; Ozkan, G.; Yetim, H.; Ekici, L.; Yilmaz, M.T. RP-HPLC–DAD analysis of phenolic compounds in pomace extracts from five grape cultivars: Evaluation of their antioxidant, antiradical and antifungal activities in orange and apple juices. Food Chem. 2011, 126, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Abat, J.K.; Kumar, S.; Mohanty, A. Ethnomedicinal, Phytochemical and Ethnopharmacological Aspects of Four Medicinal Plants of Malvaceae Used in Indian Traditional Medicines: A Review. Medicines 2017, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, T.A.; Macedo, C.; Franco, M.E.; Rocha, M.C.; Moreli, I.S.; Cantelli, B.A.M.; Sanches, P.R.; Beleboni, R.O.; Malavazi, I.; Passos, G.A.; et al. Trans-chalcone activity against Trichophyton rubrum relies on an interplay between signaling pathways related to cell wall integrity and fatty acid metabolism. BMC Genomics 2019, 20. [Google Scholar] [CrossRef]
- Terças, A.G.; Monteiro, A.d.S.; Moffa, E.B.; Santos, J.R.A.d.; Sousa, E.M.d.; Pinto, A.R.B.; Costa, P.C.d.S.; Borges, A.C.R.; Torres, L.M.B.; Barros Filho, A.K.D.; et al. Phytochemical Characterization of Terminalia catappa Linn. Extracts and Their antifungal Activities against Candida spp. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Bottari, N.B.; Lopes, L.Q.S.; Pizzuti, K.; Filippi dos Santos Alves, C.; Corrêa, M.S.; Bolzan, L.P.; Zago, A.; de Almeida Vaucher, R.; Boligon, A.A.; Giongo, J.L.; et al. Antimicrobial activity and phytochemical characterization of Carya illinoensis. Microb. Pathogen. 2017, 104, 190–195. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Gontijo, A.V.L.; Salvador, M.J.; Tanaka, M.H.; Brighenti, F.L.; Delbem, A.C.B.; Delbem, Á.C.B.; Koga-Ito, C.Y. Effects of Acetone Fraction From Buchenavia tomentosa Aqueous Extract and Gallic Acid on Candida albicans Biofilms and Virulence Factors. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Gupta, S.; Jacob, M.; Khan, S.; Ferreira, D. Antioxidant, Antimalarial and Antimicrobial Activities of Tannin-Rich Fractions, Ellagitannins and Phenolic Acids from Punica granatum L. Planta Med. 2007, 73, 461–467. [Google Scholar] [CrossRef]
- Shahzad, M.; Sherry, L.; Rajendran, R.; Edwards, C.A.; Combet, E.; Ramage, G. Utilising polyphenols for the clinical management of Candida albicans biofilms. Int. J. Antimicrob. Agents 2014, 44, 269–273. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.L.; Magalhães, T.F.F.; dos Santos, J.R.A.; de Paula, T.P.; Modolo, L.V.; de Fátima, A.; Buzanello Martins, C.V.; Santos, D.A.; de Resende-Stoianoff, M.A. Curcumin enhances the activity of fluconazole againstCryptococcus gattii-induced cryptococcosis infection in mice. J. Appl.Microbiol. 2015, 120, 41–48. [Google Scholar] [CrossRef]
- Alalwan, H.; Rajendran, R.; Lappin, D.F.; Combet, E.; Shahzad, M.; Robertson, D.; Nile, C.J.; Williams, C.; Ramage, G. The Anti-Adhesive Effect of Curcumin on Candida albicans Biofilms on Denture Materials. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Mohanram, K.; Kannan, I. Antifungal activity of curcumin-silver nanoparticles against fluconazole-resistant clinical isolates of Candida species. AYU (Int. Q. J. Res. Ayurveda) 2018, 39, 182. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Rice, L.B. Antifungal Agents: Mode of Action, Mechanisms of Resistance, and Correlation of These Mechanisms with Bacterial Resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M.; White, N.A. Introduction to Fungal Physiology. In Fungi; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 1–34. [Google Scholar] [CrossRef]
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009, 58, 1454–1462. [Google Scholar] [CrossRef]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: induction, repair and significance. Mutat.Res.Rev. Mutat. Res. 2004, 567, 1–61. [Google Scholar] [CrossRef]
- Wong-ekkabut, J.; Xu, Z.; Triampo, W.; Tang, I.M.; Peter Tieleman, D.; Monticelli, L. Effect of Lipid Peroxidation on the Properties of Lipid Bilayers: A Molecular Dynamics Study. Biophys. J. 2007, 93, 4225–4236. [Google Scholar] [CrossRef]
- Rautenbach, M.; Troskie, A.M.; Vosloo, J.A. Antifungal peptides: To be or not to be membrane active. Biochimie 2016, 130, 132–145. [Google Scholar] [CrossRef]
- Khan, A.; Ahmad, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Induction of oxidative stress as a possible mechanism of the antifungal action of three phenylpropanoids. FEMS Yeast Res. 2010, 11, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Sánchez, R.D.; Torrescano-Urrutia, G.R.; Acedo-Félix, E.; Carvajal-Millán, E.; González-Córdova, A.F.; Vallejo-Galland, B.; Torres-Llanez, M.J.; Sánchez-Escalante, A. Antioxidant and Antimicrobial Activity of Commercial Propolis Extract in Beef Patties. J. Food Sci. 2014, 79, C1499–C1504. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.P.C.; Carvalho, C.R.C.; Andrade, F.A.; Fernandes, O.F.L.; Arruda, W.; Silva, M.R.R. Fisetin as a promising antifungal agent againstCryptocococcus neoformansspecies complex. J. Appl. Microbiol. 2016, 121, 373–379. [Google Scholar] [CrossRef]
- Li, X.-C.; Joshi, A.S.; ElSohly, H.N.; Khan, S.I.; Jacob, M.R.; Zhang, Z.; Khan, I.A.; Ferreira, D.; Walker, L.A.; Broedel, S.E.; et al. Fatty Acid Synthase Inhibitors from Plants: Isolation, Structure Elucidation, and SAR Studies. J. Nat. Prod. 2002, 65, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Lee, H.; Ko, H.J.; Woo, E.-R.; Lee, D.G. Fungicidal effect of isoquercitrin via inducing membrane disturbance. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Moazeni, M.; Hedayati, M.T.; Nabili, M.; Mousavi, S.J.; Abdollahi Gohar, A.; Gholami, S. Glabridin triggers over-expression of MCA1 and NUC1 genes in Candida glabrata: Is it an apoptosis inducer? J. Mycol. Méd. 2017, 27, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Sangalli-Leite, F.; Scorzoni, L.; Alves de Paula e Silva, A.C.; da Silva, J.d.F.; de Oliveira, H.C.; de Lacorte Singulani, J.; Gullo, F.P.; Moraes da Silva, R.; Regasini, L.O.; Siqueira da Silva, D.H.; et al. Synergistic effect of pedalitin and amphotericin B against Cryptococcus neoformans by in vitro and in vivo evaluation. Int. J. Antimicrob. Agents 2016, 48, 504–511. [Google Scholar] [CrossRef]
- Yun, D.G.; Lee, D.G. Silymarin exerts antifungal effects via membrane-targeted mode of action by increasing permeability and inducing oxidative stress. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 467–474. [Google Scholar] [CrossRef]
- Cao, Y.; Dai, B.; Wang, Y.; Huang, S.; Xu, Y.; Cao, Y.; Gao, P.; Zhu, Z.; Jiang, Y. In vitro activity of baicalein against Candida albicans biofilms. Int. J. Antimicrob. Agents 2008, 32, 73–77. [Google Scholar] [CrossRef]
- Gibellini, L.; Bianchini, E.; De Biasi, S.; Nasi, M.; Cossarizza, A.; Pinti, M. Natural Compounds Modulating Mitochondrial Functions. Evid. Based Complement. Alternat. Med. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Guntuku, L.; Naidu, V.G.M.; Ganesh Yerra, V. Mitochondrial Dysfunction in Gliomas: Pharmacotherapeutic Potential of Natural Compounds. Curr. Neuropharmacol. 2016, 14, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Canonico, B.; Candiracci, M.; Citterio, B.; Curci, R.; Squarzoni, S.; Mazzoni, A.; Papa, S.; Piatti, E. Honey flavonoids inhibit Candida albicans morphogenesis by affecting DNA behavior and mitochondrial function. Future Microbiol. 2014, 9, 445–456. [Google Scholar] [CrossRef]
- Ning, Y.; Ling, J.; Wu, C.D. Synergistic effects of tea catechin epigallocatechin gallate and antimycotics against oral Candida species. Archiv. Oral Biol. 2015, 60, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- da Costa Cordeiro, B.M.P.; de Lima Santos, N.D.; Ferreira, M.R.A.; de Araújo, L.C.C.; Junior, A.R.C.; da Conceição Santos, A.D.; de Oliveira, A.P.; da Silva, A.G.; da Silva Falcão, E.P.; dos Santos Correia, M.T.; et al. Hexane extract from Spondias tuberosa (Anacardiaceae) leaves has antioxidant activity and is an anti-Candida agent by causing mitochondrial and lysosomal damages. BMC Compl. Altern. Med. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Reiners, J.J. Suppression of cell cycle progression by flavonoids: dependence on the aryl hydrocarbon receptor. Carcinogenesis 1999, 20, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, R.K.; Lee, J.-H.; Kim, Y.-G.; Lee, J. Alizarin and Chrysazin Inhibit Biofilm and Hyphal Formation by Candida albicans. Front. Cell. Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef]
- Sun, L.; Liao, K.; Wang, D. Effects of Magnolol and Honokiol on Adhesion, Yeast-Hyphal Transition, and Formation of Biofilm by Candida albicans. PLoS ONE 2015, 10, e0117695. [Google Scholar] [CrossRef]
- Han, B.; Chen, J.; Yu, Y.-q.; Cao, Y.-b.; Jiang, Y.-y. Antifungal activity ofRubus chingiiextract combined with fluconazole against fluconazole-resistant Candida albicans. Microbiol. Immunol. 2016, 60, 82–92. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Q.; Wu, J.; Wang, W.; Yao, G.; Li, T.; Li, X.; Li, L.; Zhang, Y.; Cui, W.; et al. A new Prenylated Flavonoid induces G0/G1 arrest and apoptosis through p38/JNK MAPK pathways in Human Hepatocellular Carcinoma cells. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Kang, K.; Fong, W.-P.; Tsang, P.W.-K. Novel antifungal activity of purpurin against Candidaspeciesin vitro. Med. Mycol. 2010, 48, 904–911. [Google Scholar] [CrossRef]
- Li, Y.; Chang, W.; Zhang, M.; Li, X.; Jiao, Y.; Lou, H. Synergistic and drug-resistant reversing effects of diorcinol D combined with fluconazole against Candida albicans. FEMS Yeast Res. 2015, 15. [Google Scholar] [CrossRef]
- Sharma, M.; Manoharlal, R.; Shukla, S.; Puri, N.; Prasad, T.; Ambudkar, S.V.; Prasad, R. Curcumin Modulates Efflux Mediated by Yeast ABC Multidrug Transporters and Is Synergistic with Antifungals. Antimicrob. Agents Chemother. 2009, 53, 3256–3265. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Prasad, R. The Quorum-Sensing Molecule Farnesol Is a Modulator of Drug Efflux Mediated by ABC Multidrug Transporters and Synergizes with Drugs in Candida albicans. Antimicrob. Agents Chemother. 2011, 55, 4834–4843. [Google Scholar] [CrossRef] [PubMed]
- Yiğit, D.; Yiğit, N.; Mavi, A. Antioxidant and antimicrobial activities of bitter and sweet apricot (Prunus armeniaca L.) kernels. Braz. J. Med. Biol. Res. 2009, 42, 346–352. [Google Scholar] [CrossRef]
- Moudgal, V.; Sobel, J. Antifungals to treat Candida albicans. Exp. Opin. Pharmacother. 2010, 11, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Tamura, M.; Imai, K.; Ishigami, T.; Ochiai, K. Catechin inhibits Candida albicans dimorphism by disrupting Cek1 phosphorylation and cAMP synthesis. Microb. Pathogen. 2013, 56, 16–20. [Google Scholar] [CrossRef]
- Cassetta, A.; Stojan, J.; Krastanova, I.; Kristan, K.; Brunskole Švegelj, M.; Lamba, D.; Lanišnik Rižner, T. Structural basis for inhibition of 17β-hydroxysteroid dehydrogenases by phytoestrogens: The case of fungal 17β-HSDcl. J. Steroid Biochem. Mol. Biol. 2017, 171, 80–93. [Google Scholar] [CrossRef]
- Qian, S.; Fan, W.; Qian, P.; Zhang, D.; Wei, Y.; Chen, H.; Li, X. Apigenin Restricts FMDV Infection and Inhibits Viral IRES Driven Translational Activity. Viruses 2015, 7, 1613–1626. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Sheehan, D.J.; Hitchcock, C.A.; Ghannoum, M.A. Combination Treatment of Invasive Fungal Infections. Clin.Microbiol. Rev. 2005, 18, 163–194. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Pippi, B.; Lana, A.J.D.; Moraes, R.C.; Güez, C.M.; Machado, M.; de Oliveira, L.F.S.; Lino von Poser, G.; Fuentefria, A.M. In vitroevaluation of the acquisition of resistance, antifungal activity and synergism of Brazilian red propolis with antifungal drugs onCandidaspp. J. Appl. Microbiol. 2015, 118, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Moraes, R.C.; Carvalho, A.R.; Lana, A.J.D.; Kaiser, S.; Pippi, B.; Fuentefria, A.M.; Ortega, G.G. In vitrosynergism of a water insoluble fraction ofUncaria tomentosacombined with fluconazole and terbinafine against resistant non-Candida albicans isolates. Pharma. Biol. 2016, 55, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Danielli, L.J.; Pippi, B.; Soares, K.D.; Duarte, J.A.; Maciel, A.J.; Machado, M.M.; Oliveira, L.F.S.; Bordignon, S.A.L.; Fuentefria, A.M.; Apel, M.A. Chemosensitization of filamentous fungi to antifungal agents using Nectandra Rol. ex Rottb. species essential oils. Ind. Crops Prod. 2017, 102, 7–15. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Dong, H.-H.; Zhao, F.; Wang, J.; Yan, F.; Jiang, Y.-Y.; Jin, Y.-S. The synthesis and synergistic antifungal effects of chalcones against drug resistant Candida albicans. Bioorg. Med. Chem. Lett. 2016, 26, 3098–3102. [Google Scholar] [CrossRef]
- Li, D.-D.; Chai, D.; Huang, X.-W.; Guan, S.-X.; Du, J.; Zhang, H.-Y.; Sun, Y.; Jiang, Y.-Y. PotentIn VitroSynergism of Fluconazole and Osthole against Fluconazole-Resistant Candida albicans. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Ahmad, A.; Wani, M.Y.; Khan, A.; Manzoor, N.; Molepo, J. Synergistic Interactions of Eugenol-tosylate and Its Congeners with Fluconazole against Candida albicans. PLoS ONE 2015, 10, e0145053. [Google Scholar] [CrossRef]
- Fatima, A.; Gupta, V.K.; Luqman, S.; Negi, A.S.; Kumar, J.K.; Shanker, K.; Saikia, D.; Srivastava, S.; Darokar, M.P.; Khanuja, S.P.S. Antifungal activity ofGlycyrrhiza glabraextracts and its active constituent glabridin. Phytother. Res. 2009, 23, 1190–1193. [Google Scholar] [CrossRef]
- Gao, M.; Wang, H.; Zhu, L. Quercetin Assists Fluconazole to Inhibit Biofilm Formations of Fluconazole-Resistant Candida albicans in In Vitro and In Vivo Antifungal Managements of Vulvovaginal Candidiasis. Cell. Physiol. Biochem. 2016, 40, 727–742. [Google Scholar] [CrossRef]

| Flavonoids (Compound Name) | Sources | Structure of the Flavonoids | Fungal Strains Inhibited | MIC * | References |
|---|---|---|---|---|---|
| Isoflavonoid glycosides (Dalpanitin) | Dalbergia scandens Roxb., Corom. | 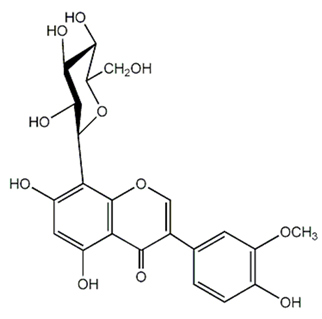 | C. albicans | 780–6250 mg/mL | [104] |
| Isoflavones (Equol) | Soybeans |  | C. albicans | 516–1032 μg/mL | [105] |
| Isoflavones (Daidzein) | Soybeans | 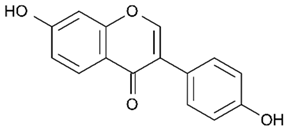 | C. albicans | 516–1032 μg/mL | [105] |
| Isoflavone (Genistein) | Soybeans | 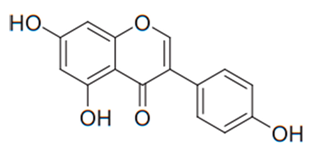 | T. rubrum | 1000 µg/mL | [106] |
| Isoflavone (Derrone) | Retama raetam | 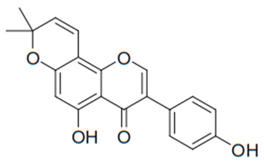 | C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. neoformans | 7.81 µg/mL | [107] |
| Isoflavanone [(3R)-7-2′-3′-trihydroxy-4′-methoxy-5′-prenylisoflavanon] | Geoffroea decorticans |  | A. flavus,A. parasiticus, A. nomius | 9–18 μg/mL | [108] |
| Isoflavanone [(3R)-5,7,2′,3′-tetrahydroxy-4′-methoxy-5′-prenylisoflavanone] | Geoffroea decorticans | 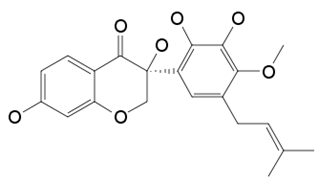 | A. flavus,A. parasiticus, A. nomius | 10–21 μg/mL | [108] |
| Isoflavanone (Sedonan A) | Dalea formosa |  | C. albicans | 7.6–15µg/mL | [109] |
| Isoflavane (Glabridin) | Glycyrrhiza glabra | 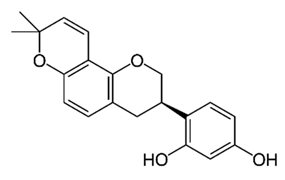 | C. albicans | 6.3–12.5 μg/mL | [110] |
| Isoflavane (Glabridin) | Glycyrrhiza glabra | 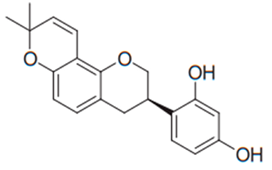 | C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, C. neoformans | 16–64 µg/mL | [96] |
| Flavonones (Naringenin) | Kochia scoparia | 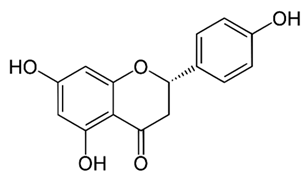 | C. graminicola, T. deformans, A. flavus, H. carbonum, C. zeae-maydis, P. innundatus, S. japonicas, P. herbarum, R. solani. | 3.125 mg/mL | [103] |
| Flavonones (Hesperetin) | Baccharis trimera |  | C. albicans, C. tropicalis, C. parapsilosis, Epicoccum sp., C. sphaerospermum, C. neoformans, P. brasiliensis, C. gatti, Pestalotiopsis sp., C. lunatus, Nigrospora sp. | 7.8–500 μg/mL | [111] |
| Flavonones (Eriodictyol) | Citrus bergamia Risso |  | A. parasiticus, A. flavus, F. semitectum and P. expansum. | 200–800 μg/mL | [112] |
| Flavonol (Vincetoxicoside B) | Polygonum paleaceum |  | C. albicans | 64 µg/mL | [100] |
| Flavonol (Rutin) | Many plants | 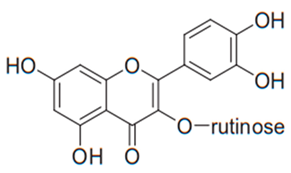 | C. albicans, C. parapsilosis, C. neoformans | 256 µg/mL | [113] |
| Flavonol (Quercitrin) | Juglans mollis |  | C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, T. rubrum, T. beigelii | 7.8–256 µg/mL | [97,113] |
| Flavonol (Quercetin) | Many plants | 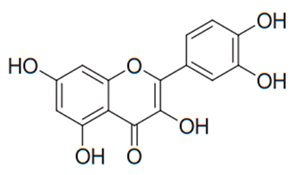 | C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, T. rubrum, T. beigelii | 31.2–125 µg/mL | [97,99,100,101,102,113] |
| Flavonol (Myricitrin) | Juglans mollis |  | C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, T. rubrum, T. beigelii | 3.9–83 µg/mL | [97] |
| Flavonol (Myricetin-3-O-β-glucoside) | Limonium caspium | 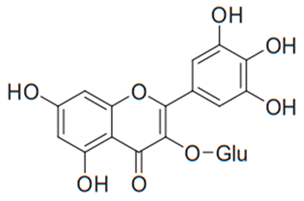 | C. glabrata | 8.53 µg/mL | [114] |
| Flavonol (Myricetin) | Myrica rubra |  | C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, | 3.9–64 µg/mL | [97,114] |
| Flavonol (Morin) | Verbascum glabratum subsp. bosnense (K. Malý) Murb | 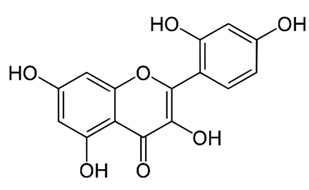 | C. albicans | 600, 1200 μg/mL | [115] |
| Flavonol (Isoquercitrin) | Aster yomena |  | C. albicans, C. parapsilosis | 2.5–5.0 µg/mL | [116] |
| Flavonol (Hyperoside) | Hypericum perforatum | 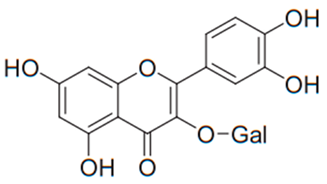 | C. albicans, C. parapsilosis, C. neoformans | 128–256 µg/mL | [113] |
| Flavonol (Hyperoside) | Solidago graminifolia L. Salisb. | 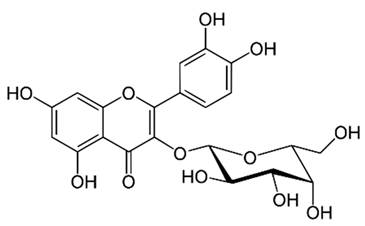 | C. albicans, C. parapsilosis. | 190–6250 μg/mL | [117] |
| Flavonol (Guaijaverin) | Myrcia tomentosa | 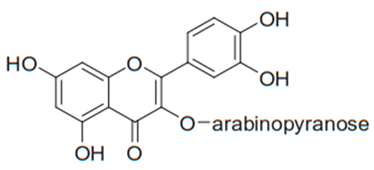 | C. albicans, C. parapsilosis | 2–32 µg/mL | [116] |
| Flavonol (Galangin) | Alpinia officinarum | 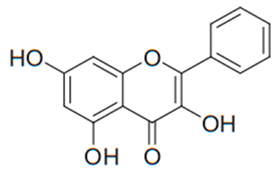 | C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, C. neoformans, C. gattii, T. rubrum | 15.6–1000 µg/mL | [97,106] |
| Flavonol (Fisetin) | Many plants | 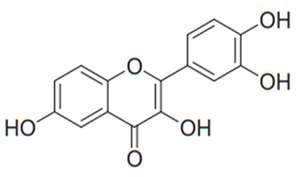 | C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, C. neoformans, C. gattii | 8–128 µg/mL | [97,118] |
| Flavonol (Avicularin) | Myrcia tomentosa | 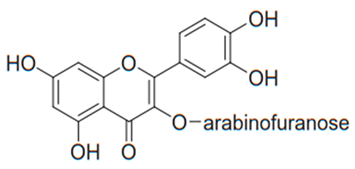 | C. albicans, C. parapsilosis | 2–32 µg/mL | [116] |
| Flavonol (5-Methylmyricetin) | Limonium caspium |  | C. glabrata | 6.79 µg/mL | [114] |
| Flavonol (Papyriflavonol A) | Broussonetia papyrifera (L.) Vent. | 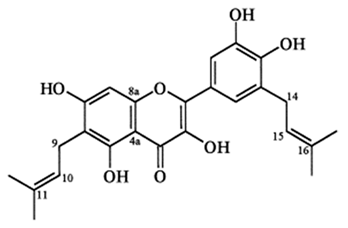 | C. albicans | 25 μg/mL | [119] |
| Flavonol (Kaempferol) | Many plants |  | C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, T. rubrum, T. beigelii | 31.2–512 µg/mL | [97,120,121,122] |
| Flavonol | Many plants | 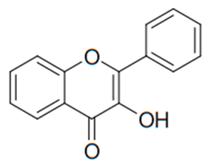 | C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, | 3.9–83 µg/mL | [97,106] |
| Flavone (Wogonin) | Scutellaria baicalensis |  | A. fumigatus, T. rubrum | 60–230 µg/mL | [123] |
| Flavone (Pedalitin) | Pterogyne nitens | 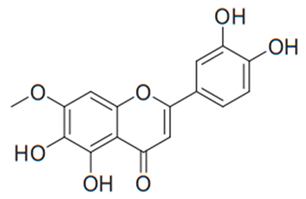 | C. neoformans | 3.9 µg/mL | [123] |
| Flavone (Luteolin) | Reseda luteola | 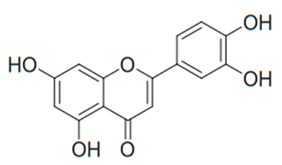 | C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, A. fumigatus, T. rubrum | 3.9–83 µg/mL | [97,124] |
| Flavone (Luteolin 7-O-β-D-glucuronide) | Lavandula stoechas Lavandula luisieri and Lavandula pedunculata, | 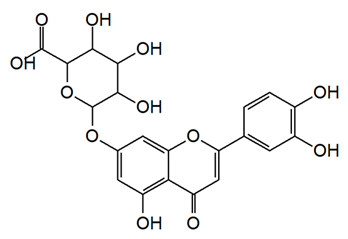 | A. niger, C. albicans, C. guilliermondii, S. cerevisiae, C. neoformans, R. rubra, and T. cutaneum | 7.5–62.5 μg/mL | [125] |
| Flavone (Luteolin 7-O-glucoside) | Salix babylonica L. | 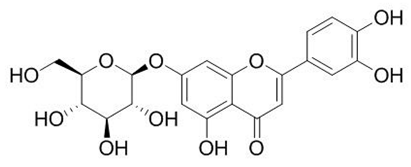 | C. albicans | 1.56–100 mg/mL | [126] |
| Flavone (Licoflavone C) | Retama raetam | 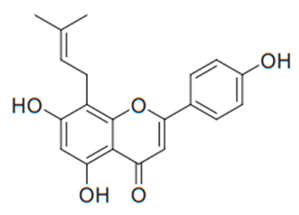 | C. albicans, C. glabrata, C. tropicalis, C. neoformans | 15.62 µg/mL | [107] |
| Flavone (Baicalin) | Scutellaria baicalensis | 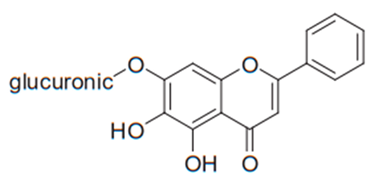 | C. albicans, C. parapsilosis | 250–500 µg/mL | [107] |
| Flavone (Baicalein) | Scutellaria baicalensis | 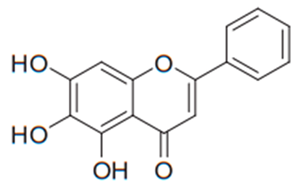 | C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. neoformans, A. fumigatus, T. rubrum | 1.9–64 µg/mL | [123,127,128,129,130,131] |
| Flavone (Apigenin-7-O-β-glucuronoside) | Oncoba spinosa |  | C. albicans, C. parapsilosis, C. neoformans | 64–256 µg/mL | [113] |
| Flavone (Apigenin) | Many plants | 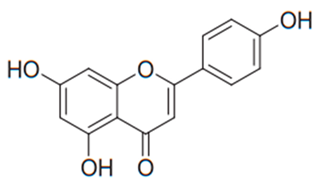 | A. fumigatus, C. parapsilosis, T. rubrum, T. beigelii | 5.0 µg/mL | [132] |
| Flavone (Apigenin 7-O-β-D-glucoside) | Lavandula stoechas Lavandula luisieri and Lavandula pedunculata, | 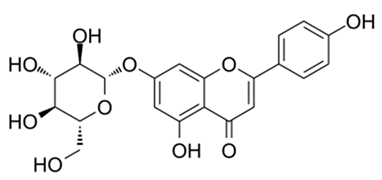 | A. niger, C. albicans, C. guilliermondii, S. cerevisiae, C. neoformans, R. rubra, and T. cutaneum | 7.5–62.5 μg/mL | [125] |
| Flavone (7,4′-dimethylapigenin) | Combretum zeyheri |  | C. albicans | 10 µg/mL | [133] |
| Flavone | - |  | C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, C. neoformans, A. fumigatus, T. rubrum | 62.5–83 µg/mL | [97] |
| Flavanone (Pinocembrin) | Combretum hereroense, Combretum apiculatum, Combretum Collinum |  | C. albicans | 6.25 μg/mL | [134] |
| Flavanone (8PP) | Dalea elegans |  | C. albicans, C. glabrata, C. krusei, C. neoformans | 10–20 µg/mL | [135,136] |
| Flavanone (Alpinetin) | Combretum hereroense, Combretum apiculatum, Combretum Collinum | 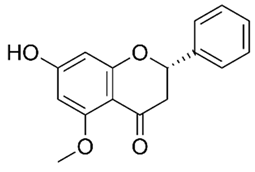 | C. albicans | 25 μg/mL | [134] |
| Flavanone | Desmodium caudatum | 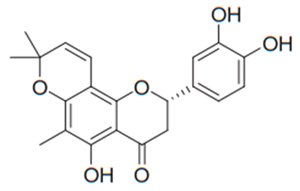 | C. albicans, C. glabrata | 1.95 µg/mL | [137] |
| Flavan-3-ol (Epicatechin) | Unonopsis lindmanii R. E. Fries |  | C. albicans | 25–250 μg/mL | [138] |
| Flavan (Epigallocatechin gallate) | Tea | 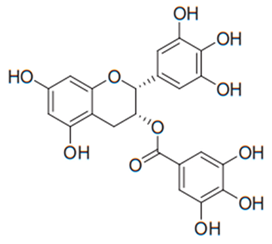 | C. albicans | 15–30 µg/mL | [101] |
| Flavan (Catechin) | Tea | 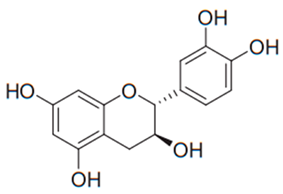 | C. albicans | 15–30 µg/mL | [101] |
| di-C-glycosylflavones (Schaftoside) | Solidago graminifolia L. Salisb. | 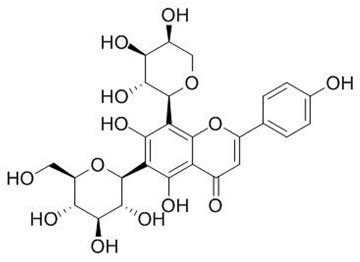 | C. albicans, C. parapsilosis. | 40–3120 μg/mL | [117] |
| Chalcone (Lico A) | Glycyrrhiza glabra |  | C. albicans, T. rubrum | 62.5–150 µg/mL | [124,139] |
| Chalcone (4-hydroxycordoin) | Lonchocarpus neuroscapha Benth. |  | C. albicans | 50–200 μg/mL | [110] |
| Chalcone | Mallotus philippinensis |  | C. neoformans, A. fumigatus | 4–16 µg/mL | [140] |
| Chalcone | Maclura tinctoria (L.) |  | C. albicans, C. neoformans | 3–15 µg/mL | [141] |
| Apigenin flavone glucoside (Vitexin) | Unonopsis lindmanii R. E. Fries |  | C. albicans | 25–250 μg/mL | [138] |
| Anthocyanidins (Peonidin) | Buchenavia tomentosa L. | 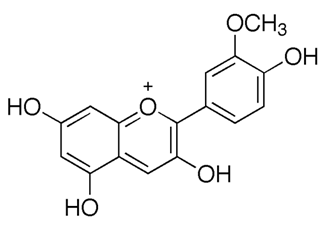 | C. albicans, C. tropicalis, C. parapsilosis, C. glabrata, C. krusei and C. dubliniensis. | 200–12500 μg/mL | [142] |
| Anthocyanidins (Pelargonidin) | Buchenavia tomentosa L. | 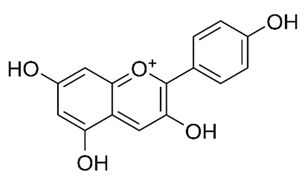 | C. albicans, C. tropicalis, C. parapsilosis, C. glabrata, C. krusei and C. dubliniensis. | 200–12500 μg/mL | [142] |
| Anthocyanidins (Malvidin) | Buchenavia tomentosa L. | 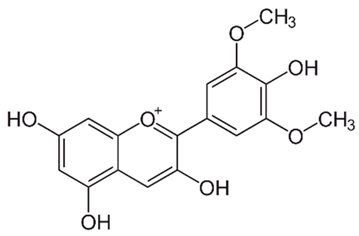 | C. albicans, C. tropicalis, C. parapsilosis, C. glabrata, C. krusei and C. dubliniensis. | 200–12,500 μg/mL | [142] |
| Anthocyanidins (Cyanidin) | Buchenavia tomentosa L. | 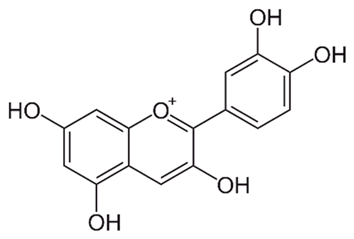 | C. albicans, C. tropicalis, C. parapsilosis, C. glabrata, C. krusei and C. dubliniensis. | 200–12,500 μg/mL | [142] |
| Flavonols (Pinocembrin) | Propolis |  | C. albicans | 197–441 mg/mL | [143] |
| Flavonols (Talosin A) | Kitasatos-pora kifunensis | 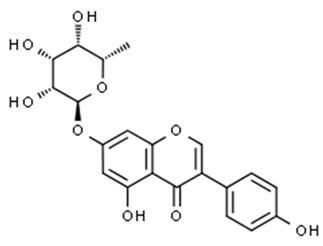 | C. albicans | 15 mg/mL | [144] |
| Flavonols (Talosin B) | Kitasatos-pora kifunensis | 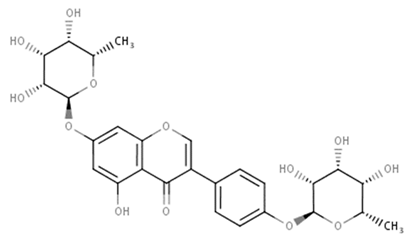 | C. albicans | 7 mg/mL | [145] |
| Quercetin 3-O-beta-glucoside | Daucus littoralis Smith | 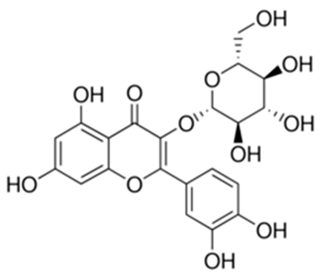 | C. albicans | 7.8 mg/mL | [146] |
| (R)-roemerine | Nelumbo nucifera | 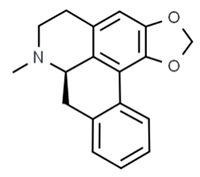 | C. albicans | 16 mg/mL | [147] |
| Flavones (Robusflavones A) | Eriosema robustum | 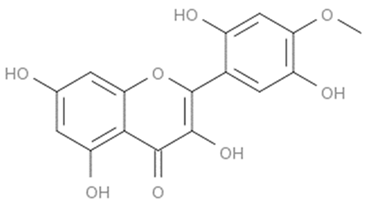 | C. albicans | 160 µg/mL | [148] |
| Flavones (Conyzoflavone) | Conyza canadensis |  | C. albicans | 10 mg/mL | [149] |
| Flavones (5,7,3’,4’-tetramethoxyflavone) | Kaempferia parviflora | 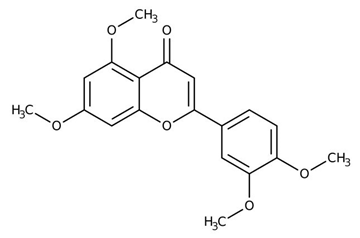 | C. albicans | 39.71 mg/mL | [150] |
| Flavones (Smiglabrone A) | Smilax glabra |  | C. albicans | 146 µg/mL | [151] |
| Flavones (5,7-dihydroxy-flavone) | Uvaria scheffleri Diel |  | C. albicans | 31.25 mg/mL | [152] |
| Flavones (Asterelin A) | Asterella angusta | 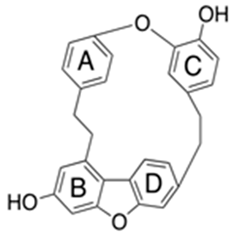 | C. albicans | 16–512 mg/mL | [153] |
| Flavanols (Gallic acid) | Paeonia rockii | 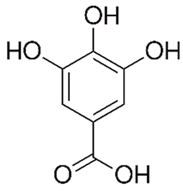 | C. albicans | 30 mg/mL | [154] |
| Flavanols (Gallotannin) | Syzygium cordatum | 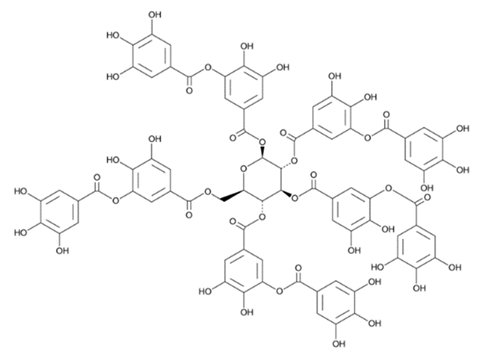 | C. albicans | 195 µg/mL | [155] |
| Flavanols (1-Galloyl-beta-D-glucopyranosyl-(1!4)-beta-D-galactopyranoside) | Baseonema acuminatum | 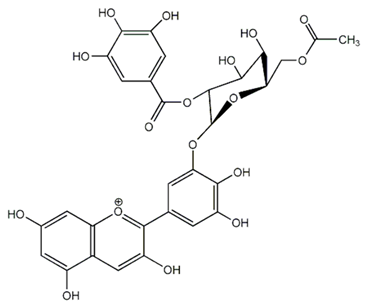 | C. albicans | 12.5 mg/mL | [156] |
| Isoflavones (Dorsmanin) | Dorstenia manni | 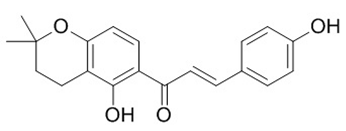 | C. albicans | 64 µg/mL | [157] |
| Chalcones (2,4-dihydroxy-3-methoxychalcone) | Zuccagnia punctata | 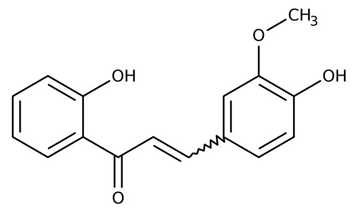 | C. albicans | 400 µg /mL | [158] |
| Chalcones (2,4-dihydrocychalcone) | Zuccagnia punctata | 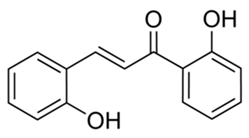 | C. albicans | 400 µg /mL | [158] |
| Chalcones (Carvacrol) | Lavandula multifida | 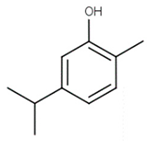 | C. albicans | 160 µg /mL | [159] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. https://doi.org/10.3390/antibiotics9020045
Al Aboody MS, Mickymaray S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics. 2020; 9(2):45. https://doi.org/10.3390/antibiotics9020045
Chicago/Turabian StyleAl Aboody, Mohammed Saleh, and Suresh Mickymaray. 2020. "Anti-Fungal Efficacy and Mechanisms of Flavonoids" Antibiotics 9, no. 2: 45. https://doi.org/10.3390/antibiotics9020045
APA StyleAl Aboody, M. S., & Mickymaray, S. (2020). Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics, 9(2), 45. https://doi.org/10.3390/antibiotics9020045




