Aureolic Acid Group of Agents as Potential Antituberculosis Drugs
Abstract
1. Introduction
2. Results and Discussion
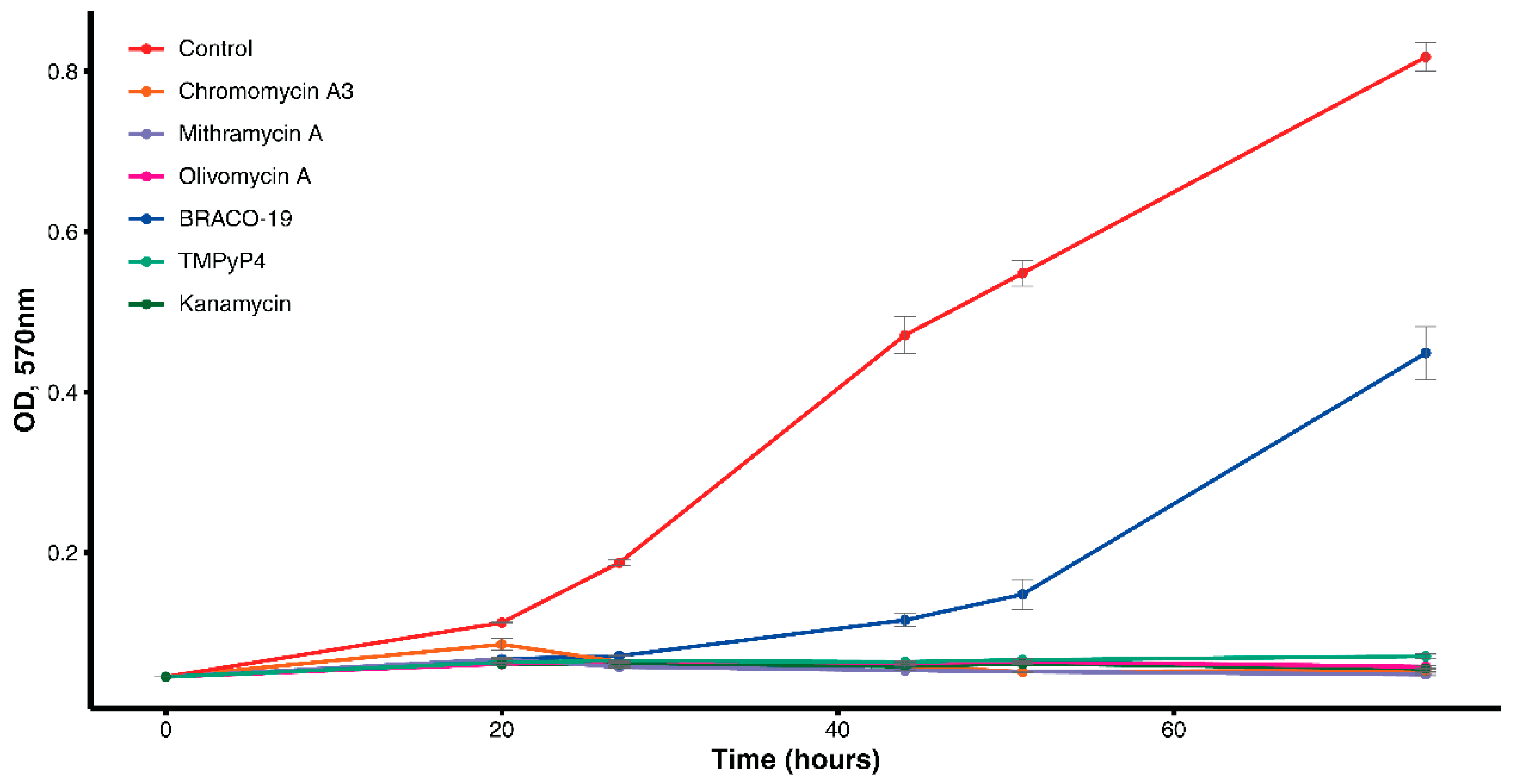
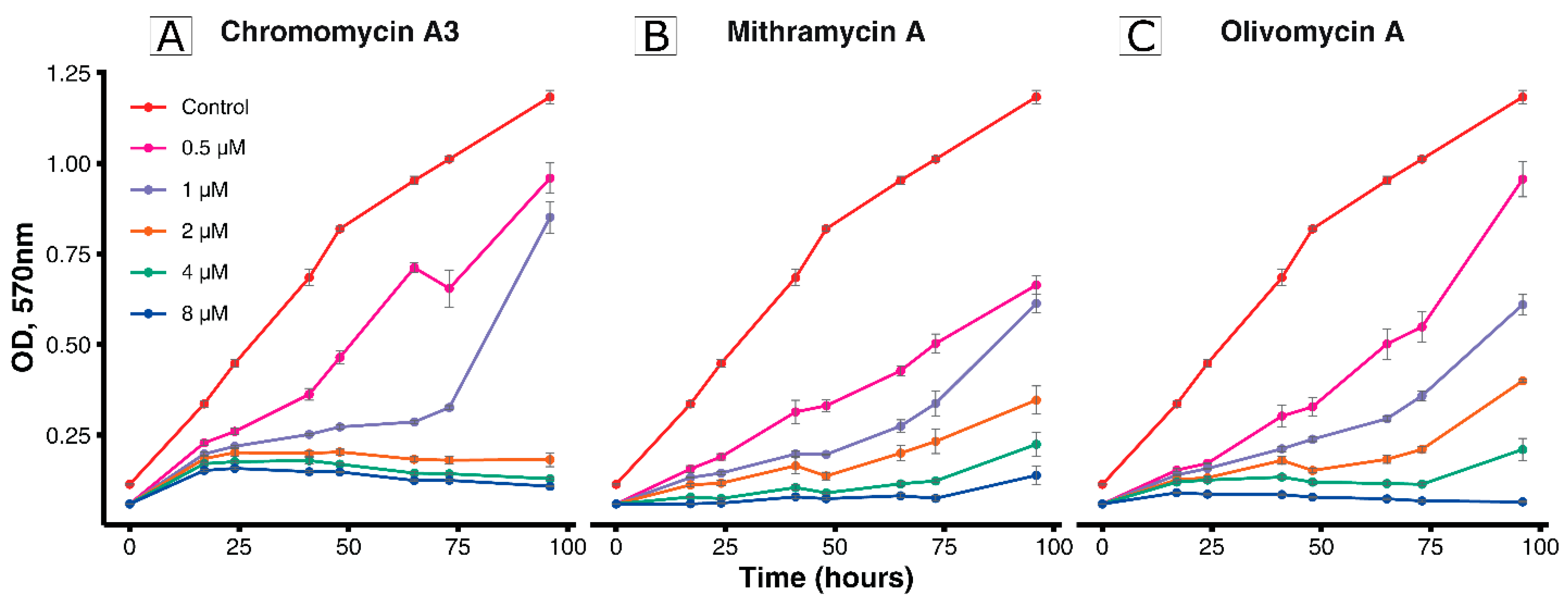
2.1. Inhibiting Effect of the Aureolic Acid Group Compounds
2.2. Identification of Putative G4 Motifs and Their Interaction with Aureolic Acid Derivatives
2.3. Transcriptomic Analysis and Correlation with CG Genome Composition
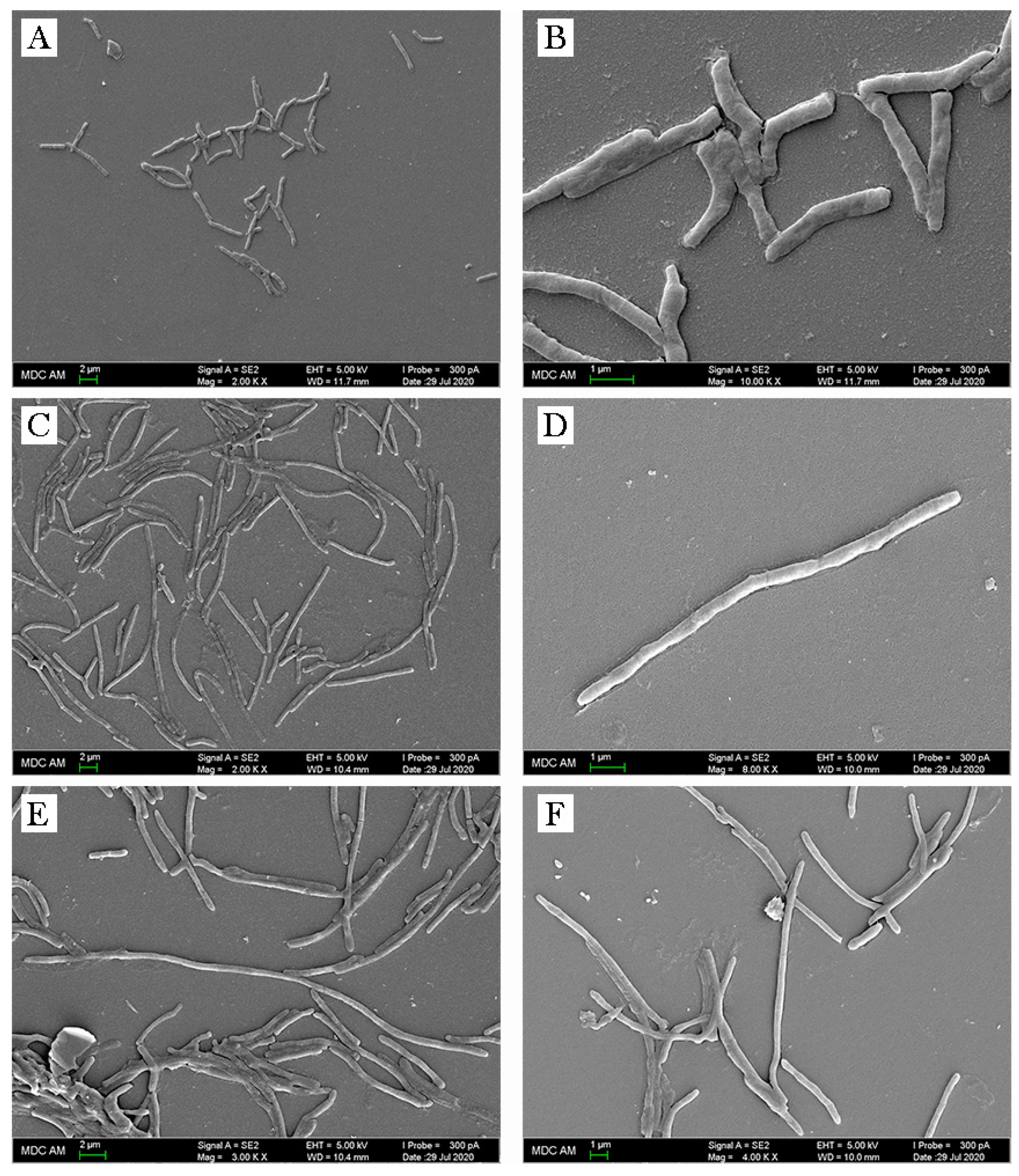
2.4. Non-Specific Changes of M. smegmatis in Response to Olivomycin A
2.5. Specific Changes of M. smegmatis in Response to Olivomycin A
3. Materials and Methods
3.1. Bacterial Strain, Growth Conditions, and Inhibition Assay
3.2. Microscopy
3.3. Transcriptomic Analysis
3.4. Bioinformatics Analysis of M. tuberculosis and M. smegmatis
3.5. Circular Dichroism Spectroscopy and FRET-Melting Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CHR | Chromomycin A3 |
| MTR | Mithramycin A |
| TB | Tuberculosis |
| G4 | G-quadruplexes |
| CD | Circular dichroism |
| PGQ | Putative G-quadruplexe |
| ODN | Oligodeoxyribonucleotide |
| OD | Optical density |
| FDR | False discovery rate |
| FC | Fold change |
| SEM | Scanning electron microscopy |
References
- WHO. Weekly Epidemiological Record; WHO: Geneva, Switzerland, 2019; Volume 95, pp. 1–12. [Google Scholar]
- Parida, S.K.; Axelsson-Robertson, R.; Rao, M.V.; Singh, N.; Master, I.; Lutckii, A.; Keshavjee, S.; Andersson, J.; Zumla, A.; Maeurer, M. Totally drug-resistant tuberculosis and adjunct therapies. J. Intern. Med. 2015, 277, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; Gumbo, T.; Maartens, G.; Dooley, K.E.; McNerney, R.; Murray, M.; Furin, J.; Nardell, E.A.; London, L.; Lessem, E.; et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir. Med. 2017, 5, 291–360. [Google Scholar] [CrossRef]
- Salfinger, M.; Migliori, G.B. Bedaquiline: Finding the pores on the pot. Eur. Respir. J. 2015, 46, 289–291. [Google Scholar] [CrossRef]
- Pontali, E.; Sotgiu, G.; D’ambrosio, L.; Centis, R.; Migliori, G.B. Bedaquiline and multidrug-resistant tuberculosis: A systematic and critical analysis of the evidence EDITORIAL TUBERCULOSIS. Eur. Respir. J. 2016, 47, 394–402. [Google Scholar] [CrossRef]
- Morís, G.-S.; Pharmacol, B. Exploring Novel Opportunities for Aureolic Acids as Anticancer Drugs. Biochem. Pharmacol. 2013, 2, 1–3. [Google Scholar]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E.; et al. Deciphering the biology of mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef]
- Rawal, P.; Kummarasetti, V.B.R.; Ravindran, J.; Kumar, N.; Halder, K.; Sharma, R.; Mukerji, M.; Das, S.K.; Chowdhury, S. Genome-wide prediction of G4 DNA as regulatory motifs: Role in Escherichia coli global regulation. Genome Res. 2006, 16, 644–655. [Google Scholar] [CrossRef]
- Perrone, R.; Lavezzo, E.; Riello, E.; Manganelli, R.; Palù, G.; Toppo, S.; Provvedi, R.; Richter, S.N. Mapping and characterization of G-quadruplexes in Mycobacterium tuberculosis gene promoter regions. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Mishra, S.K.; Shankar, U.; Jain, N.; Sikri, K.; Tyagi, J.S.; Sharma, T.K.; Mergny, J.L.; Kumar, A. Characterization of G-Quadruplex Motifs in espB, espK, and cyp51 Genes of Mycobacterium tuberculosis as Potential Drug Targets. Mol. Ther. Nucleic Acids 2019, 16, 698–706. [Google Scholar] [CrossRef]
- Lelovic, N.; Mitachi, K.; Yang, J.; Lemieux, M.R.; Ji, Y.; Kurosu, M. Application of Mycobacterium smegmatis as a surrogate to evaluate drug leads against Mycobacterium tuberculosis. J. Antibiot. 2020, 73, 780–789. [Google Scholar] [CrossRef]
- Namouchi, A.; Cimino, M.; Favre-Rochex, S.; Charles, P.; Gicquel, B. Phenotypic and genomic comparison of Mycobacterium aurum and surrogate model species to Mycobacterium tuberculosis: Implications for drug discovery. BMC Genom. 2017, 18, 530. [Google Scholar] [CrossRef] [PubMed]
- Ahr, D.J.; Scialla, S.J.; Kimball, D.B. Acquired platelet dysfunction following mithramycin therapy. Cancer 1978, 41, 448–454. [Google Scholar] [CrossRef]
- Murray, L. Goldfrank’s Toxicologic Emergencies, 7th edition. Emerg. Med. Aust. 2004, 16, 86–90. [Google Scholar] [CrossRef]
- Belmonte-Reche, E.; Morales, J.C. G4-iM Grinder: When size and frequency matter. GQuadruplex, i-Motif and higher order structure search and analysis tool. NAR Genom. Bioinform. 2020, 2, lqz005. [Google Scholar]
- Ealand, C.S.; Asmal, R.; Mashigo, L.; Campbell, L.; Kana, B.D. Characterization of putative DD-carboxypeptidase-encoding genes in Mycobacterium smegmatis. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Titgemeyer, F.; Amon, J.; Parche, S.; Mahfoud, M.; Bail, J.; Schlicht, M.; Rehm, N.; Hillmann, D.; Stephan, J.; Walter, B.; et al. A genomic view of sugar transport in Mycobacterium smegmatis and Mycobacterium tuberculosis. J. Bacteriol. 2007, 189, 5903–5915. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, T.; Hui, P.M.; Ke, J.H. Instability in Evolutionary Games. PLoS ONE 2012, 7, e49663. [Google Scholar] [CrossRef]
- Ghosh, S.; Chandra, N.; Vishveshwara, S. Mechanism of Iron-Dependent Repressor (IdeR) Activation and DNA Binding: A Molecular Dynamics and Protein Structure Network Study. PLoS Comput. Biol. 2015, 11, e1004500. [Google Scholar] [CrossRef]
- Poole, K. Bacterial stress responses as determinants of antimicrobial resistance. J. Antimicrob. Chemother. 2012, 67, 2069–2089. [Google Scholar] [CrossRef]
- Briffotaux, J.; Liu, S.; Gicquel, B. Genome-wide transcriptional responses of Mycobacterium to antibiotics. Front. Microbiol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Kohanski, M.A.; Hayete, B.; Collins, J.J. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol. Syst. Biol. 2007, 3, 91. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.E.S.; Ducati, R.G.; Breda, A.; Rosado, L.A.; De Souza, B.M.; Palma, M.S.; Santos, D.S.; Basso, L.A. Molecular, kinetic, thermodynamic, and structural analyses of Mycobacterium tuberculosis hisD-encoded metal-dependent dimeric histidinol dehydrogenase (EC 1.1.1.23). Arch. Biochem. Biophys. 2011, 512, 143–153. [Google Scholar] [CrossRef]
- Imlay, J.A.; Fridovich, I. Superoxide production by respiring membranes of escherichia coli. Free Radic. Res. 1991, 12, 59–66. [Google Scholar]
- Huang, X.; Duan, X.; Li, J.; Niu, J.; Yuan, S.; Wang, X.; Lambert, N.; Li, X.; Xu, J.; Gong, Z.; et al. The synergistic effect of exogenous glutamine and rifampicin against mycobacterium persisters. Front. Microbiol. 2018, 9, 1625. [Google Scholar] [CrossRef]
- Hards, K.; Robson, J.R.; Berney, M.; Shaw, L.; Bald, D.; Koul, A.; Andries, K.; Cook, G.M. Bactericidal mode of action of bedaquiline. J Antimicrob Chemother. 2015, 70, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Epstein, W. The KdpD sensor kinase of Escherichia coli responds to several distinct signals to turn on expression of the Kdp transport system. J. Bacteriol. 2016, 198, 212–220. [Google Scholar] [CrossRef][Green Version]
- Haupt, M.; Bramkamp, M.; Coles, M.; Kessler, H.; Altendorf, K. Prokaryotic Kdp-ATPase: Recent insights into the structure and function of KdpB. J. Mol. Microbiol. Biotechnol. 2006, 10, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Khanduja, J.S.; Tripathi, P.; Muniyappa, K. Mycobacterium tuberculosis RuvA induces two distinct types of structural distortions between the homologous and heterologous holliday junctions. Biochemistry 2009, 48, 27–40. [Google Scholar] [CrossRef]
- Smollett, K.L.; Smith, K.M.; Kahramanoglou, C.; Arnvig, K.B.; Buxton, R.S.; Davis, E.O. Global analysis of the regulon of the transcriptional repressor LexA, a key component of SOS response in Mycobacterium tuberculosis. J. Biol. Chem. 2012, 287, 22004–22014. [Google Scholar] [CrossRef]
- Boshoff, H.I.M.; Reed, M.B.; Barry, C.E.; Mizrahi, V. DnaE2 polymerase contributes to in vivo survival and the emergence of drug resistance in Mycobacterium tuberculosis. Cell 2003, 113, 183–193. [Google Scholar] [CrossRef]
- O’Sullivan, D.M.; Hinds, J.; Butcher, P.D.; Gillespie, S.H.; McHugh, T.D. Mycobacterium tuberculosis DNA repair in response to subinhibitory concentrations of ciprofloxacin. J. Antimicrob. Chemother. 2008, 62, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Kana, B.D.; Weinstein, E.A.; Avarbock, D.; Dawes, S.S.; Rubin, H.; Mizrahi, V. Characterization of the cydAB-encoded cytochrome bd oxidase from Mycobacterium smegmatis. J. Bacteriol. 2001, 183, 7076–7086. [Google Scholar] [CrossRef] [PubMed]
- Slayden, R.A.; Knudson, D.L.; Belisle, J.T. Identification of cell cycle regulators in Mycobacterium tuberculosis by inhibition of septum formation and global transcriptional analysis. Microbiology 2006, 152, 1789–1797. [Google Scholar] [CrossRef]
- Kiran, M.; Maloney, E.; Lofton, H.; Chauhan, A.; Jensen, R.; Dziedzic, R.; Madiraju, M.; Rajagopalan, M. Mycobacterium tuberculosis ftsZ expression and minimal promoter activity. Tuberculosis 2009, 89, S60–S64. [Google Scholar] [CrossRef]
- Anurag, M.; Dash, D. Unraveling the potential of intrinsically disordered proteins as drug targets: Application to Mycobacterium tuberculosis. Mol. Biosyst. 2009, 5, 1752–1757. [Google Scholar] [CrossRef]
- Morris, R.P.; Nguyen, L.; Gatfield, J.; Visconti, K.; Nguyen, K.; Schnappinger, D.; Ehrt, S.; Liu, Y.; Heifets, L.; Pieters, J.; et al. Ancestral antibiotic resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2005, 102, 12200–12205. [Google Scholar] [CrossRef] [PubMed]
- Geiman, D.E.; Raghunand, T.R.; Agarwal, N.; Bishai, W.R. Differential gene expression in response to exposure to antimycobacterial agents and other stress conditions among seven Mycobacterium tuberculosis whiB-like genes. Antimicrob. Agents Chemother. 2006, 50, 2836–2841. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.L.; Gengenbacher, M.; Chung, J.C.S.; Chen, S.L.; Mollenkopf, H.J.; Kaufmann, S.H.E.; Dick, T. Developmental transcriptome of resting cell formation in Mycobacterium smegmatis. BMC Genom. 2016, 17, 837. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yang, W. UvrD Helicase Unwinds DNA One Base Pair at a Time by a Two-Part Power Stroke. Cell 2006, 127, 1349–1360. [Google Scholar] [CrossRef]
- Saha, T.; Shukla, K.; Thakur, R.S.; Desingu, A.; Nagaraju, G. Mycobacterium tuberculosis UvrD1 and UvrD2 helicases unwind G-quadruplex DNA. FEBS J. 2019, 286, 2062–2086. [Google Scholar] [CrossRef]
- Krishna, M.; Gole, S.G. Comparison of conventional Ziehl-Neelsen method of acid fast bacilli with modified bleach method in tuberculous lymphadenitis. J. Cytol. 2017, 34, 188–192. [Google Scholar] [PubMed]
- Lai, M.J.; Soo, P.C.; Lin, N.T.; Hu, A.; Chen, Y.J.; Chen, L.K.; Chang, K.C. Identification and characterisation of the putative phage-related endolysins through full genome sequence analysis in Acinetobacter baumannii ATCC 17978. Int. J. Antimicrob. Agents 2013, 42, 141–148. [Google Scholar] [CrossRef]
- Benjak, A.; Sala, C.; Hartkoorn, R.C. Whole-transcriptome sequencing for high-resolution transcriptomic analysis in Mycobacterium tuberculosis. Methods Mol. Biol. 2015, 1285, 17–30. [Google Scholar]
- Bespyatykh, J.; Shitikov, E.; Andrei, G.; Smolyakov, A.; Klimina, K.; Veselovsky, V.; Malakhova, M.; Georgij, A. System OMICs analysis of Mycobacterium tuberculosis Beijing B0 / W148 cluster. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Bespyatykh, J.; Shitikov, E.; Bespiatykh, D.; Guliaev, A.; Klimina, K.; Veselovsky, V.; Arapidi, G.; Dogonadze, M.; Zhuravlev, V.; Ilina, E.; et al. Metabolic changes of Mycobacterium tuberculosis during the anti-tuberculosis therapy. Pathogens 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Babraham Bioinform. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 24 January 2020).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Research 2015, 4, 1521. [Google Scholar] [CrossRef]
- Robinson, M.D.; Mccarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinform. Appl. Note 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Powell, D.R. Degust: Interactive RNA-seq Analysis. Available online: http://victorian-bioinformatics-consortium.github.io/degust/ (accessed on 20 January 2020).
| Code | Sequence of the Labeled ODN, 5’-3’ | Topology 1 | Tm, °C ± 1 | Delta Tm, °C ± 2 | ||||
|---|---|---|---|---|---|---|---|---|
| Olivomycin A | MTR | CHR | TmPyP4 | BRACO19 | ||||
| 2sG | FAM-GGGGAGGATCATGGGGCTCGGGGCGGGG-BHQ1 | h-G4, p>a | 48 | 0 | 0 | 0 | 37 | 22 |
| 9sG | FAM-GGGGCGGAGACAGGGGCGGGGTTGCCGGCGGGG-BHQ1 | h-G4, p>a | 54 | 0 | 0 | 0 | 43 | 37 |
| 11sG | FAM-GGGGAACGGGCCGGGGTGTTGGGTGGGGCGTGGGCCGGGGGGTGGGCTTGGGGG-BHQ1 | h-G4, p>a | 40 | 0 | 0 | 0 | 36 | 23 |
| 12sG | FAM-GGGGATGGGGTTGCCGAACGGGGAGGTGGTGGGG-BHQ1 | p-G4 | 42 | 0 | 0 | 0 | 40 | 0 |
| Locus_Tag | Gene | Homolog in M. tuberculosis | Product | Functional Category | COG Classification | KEGG Classification | FC (Exp/Control) |
|---|---|---|---|---|---|---|---|
| Non-Specific Changes | |||||||
| MSMEG_0615 | eccA3 | Rv0282 | ATPase AAA | cell wall and cell processes | - | - | 5.48155 |
| MSMEG_1640 | - | Rv3362c | ATP/GTP-binding protein | conserved hypotheticals | General function prediction only | Not assigned to any KEGG category | 2.32442 |
| MSMEG_2723 | recA | Rv2737c | recombinase A | information pathways | Replication, recombination and repair | Not assigned to any KEGG category | 2.11516 |
| MSMEG_2740 | lexA | Rv2720 | LexA repressor | regulatory proteins | Signal transduction mechanisms | Transcription | Not assigned to any KEGG category | 2.22158 |
| MSMEG_2943 | ruvC | Rv2594c | Holliday junction resolvase | information pathways | Replication, recombination and repair | Replication and repair | 2.02021 |
| MSMEG_2944 | ruvA | Rv2593c | Holliday junction DNA helicase RuvA | information pathways | Replication, recombination and repair | Replication and repair | 2.2836 |
| MSMEG_2945 | ruvB | Rv2592c | Holliday junction DNA helicase RuvB | information pathways | Replication, recombination and repair | Replication and repair | 2.09412 |
| MSMEG_3205 | hisD | Rv1599 | histidinol dehydrogenase | intermediary metabolism and respiration | Amino acid transport and metabolism | Amino acid metabolism | −2.10763 |
| MSMEG_3231 | cydD | Rv1621c | cysteine ABC transporter permease/ATP-binding protein | intermediary metabolism and respiration | Energy production and conversion | Post-translational modification, protein turnover, and chaperones | Membrane transport | −2.5954 |
| MSMEG_3232 | cydB | Rv1622c | cytochrome D ubiquinol oxidase subunit II | intermediary metabolism and respiration | Energy production and conversion | Energy metabolism| Signal transduction | −2.53386 |
| MSMEG_3233 | cydA | Rv1623c | cytochrome D ubiquinol oxidase subunit1 | intermediary metabolism and respiration | - | - | −2.1669 |
| MSMEG_5008 | - | Rv1273c | ABC transporter ATP-binding protein | cell wall and cell processes | Defense mechanisms | Not assigned to any KEGG category | 2.04337 |
| MSMEG_5044 | - | Rv1251c | ATPase | conserved hypotheticals | General function prediction only | Not assigned to any KEGG category | 2.1844 |
| MSMEG_5137 | narI | - | respiratory nitrate reductase subunit gamma | - | - | Nitrogen metabolism | −2.28108 |
| MSMEG_5139 | narH | Rv1162 | nitrate reductase subunit beta | intermediary metabolism and respiration | - | - | −2.0609 |
| MSMEG_5392 | kdpA | Rv1029 | potassium-transporting ATPase A | cell wall and cell processes | Inorganic ion transport and metabolism | Signal transduction | −3.84888 |
| MSMEG_5393 | kdpB | Rv1030 | potassium-transporting ATPase subunitB | cell wall and cell processes | Inorganic ion transport and metabolism | Signal transduction | −3.62873 |
| MSMEG_5394 | kdpC | Rv1031 | potassium-transporting ATPase subunitC | cell wall and cell processes | Inorganic ion transport and metabolism | Signal transduction | −8.51123 |
| MSMEG_5395 | kdpD | Rv1028c | sensor protein KdpD | regulatory proteins | Signal transduction mechanisms | Not assigned to any KEGG category | −2.399 |
| MSMEG_5396 | kdpE | Rv1027c | KDP operon transcriptional regulatory protein KdpE | regulatory proteins | Signal transduction mechanisms | Transcription | Signal transduction | −2.5025 |
| MSMEG_6046 | - | - | ABC transporter ATP-binding protein | - | Not in COGs | Not assigned to any KEGG category | 2.0133 |
| MSMEG_6052 | - | - | ABC transporter ATP-binding protein | - | Not in COGs | Not assigned to any KEGG category | 2.39154 |
| MSMEG_6058 | - | - | cadmium transporting P-type ATPase | - | Not in COGs | Not assigned to any KEGG category | 2.8424 |
| Specific Changes | |||||||
| MSMEG_0438 | - | Rv0265c | periplasmic binding protein | cell wall and cell processes | Inorganic ion transport and metabolism | Membrane transport | 2.68116 |
| MSMEG_0704 | lpqJ | Rv0344c | LpqJ protein | cell wall and cell processes | Not in COGs | Not assigned to any KEGG category | 2.27771 |
| MSMEG_0806 | lpqL | Rv0418 | hydrolase | cell wall and cell processes | General function prediction only | Not assigned to any KEGG category | 2.24391 |
| MSMEG_1831 | whiB2 | Rv3260c | transcription factor WhiB | regulatory proteins | Not in COGs | Not assigned to any KEGG category | 2.27169 |
| MSMEG_1941 | - | - | helicase, UvrD/Rep family protein | - | Not in COGs | Not assigned to any KEGG category | 2.21769 |
| MSMEG_1943 | - | Rv3201c | ATP-dependent DNA helicase | information pathways | Replication, recombination and repair | Replication and repair | 2.21528 |
| MSMEG_3743 | soj | Rv1708 | SpoOJ regulator protein | cell wall and cell processes | Cell cycle control, cell division, chromosome partitioning | Not assigned to any KEGG category | 2.05016 |
| MSMEG_4228 | ftsW | Rv2154c | cell division protein FtsW | cell wall and cell processes | Cell cycle control, cell division, chromosome partitioning | Not assigned to any KEGG category | 2.3923 |
| MSMEG_5043 | lprE | Rv1252c | LprE protein | cell wall and cell processes | Not in COGs | Not assigned to any KEGG category | 2.35097 |
| MSMEG_5879 | lpqR | Rv0838 | D-alanyl-D-alanine dipeptidase | cell wall and cell processes | Cell wall/membrane/ envelope biogenesis | Signal transduction| Drug resistance | 2.30757 |
| MSMEG_6109 | lpqG | Rv3623 | LpqG protein | cell wall and cell processes | Function unknown | Not assigned to any KEGG category | 2.19376 |
| MSMEG_6369 | rfbD | Rv3783 | O-antigen export system, permease | cell wall and cell processes | Carbohydrate transport and metabolism | Cell wall/membrane/ envelope biogenesis | Not assigned to any KEGG category | 2.11427 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bespyatykh, J.; Bespiatykh, D.; Malakhova, M.; Klimina, K.; Bespyatykh, A.; Varizhuk, A.; Tevyashova, A.; Nikolenko, T.; Pozmogova, G.; Ilina, E.; et al. Aureolic Acid Group of Agents as Potential Antituberculosis Drugs. Antibiotics 2020, 9, 715. https://doi.org/10.3390/antibiotics9100715
Bespyatykh J, Bespiatykh D, Malakhova M, Klimina K, Bespyatykh A, Varizhuk A, Tevyashova A, Nikolenko T, Pozmogova G, Ilina E, et al. Aureolic Acid Group of Agents as Potential Antituberculosis Drugs. Antibiotics. 2020; 9(10):715. https://doi.org/10.3390/antibiotics9100715
Chicago/Turabian StyleBespyatykh, Julia, Dmitry Bespiatykh, Maja Malakhova, Ksenia Klimina, Andrey Bespyatykh, Anna Varizhuk, Anna Tevyashova, Tatiana Nikolenko, Galina Pozmogova, Elena Ilina, and et al. 2020. "Aureolic Acid Group of Agents as Potential Antituberculosis Drugs" Antibiotics 9, no. 10: 715. https://doi.org/10.3390/antibiotics9100715
APA StyleBespyatykh, J., Bespiatykh, D., Malakhova, M., Klimina, K., Bespyatykh, A., Varizhuk, A., Tevyashova, A., Nikolenko, T., Pozmogova, G., Ilina, E., & Shitikov, E. (2020). Aureolic Acid Group of Agents as Potential Antituberculosis Drugs. Antibiotics, 9(10), 715. https://doi.org/10.3390/antibiotics9100715






