Impetigo Animal Models: A Review of Their Feasibility and Clinical Utility for Therapeutic Appraisal of Investigational Drug Candidates
Abstract
1. Introduction
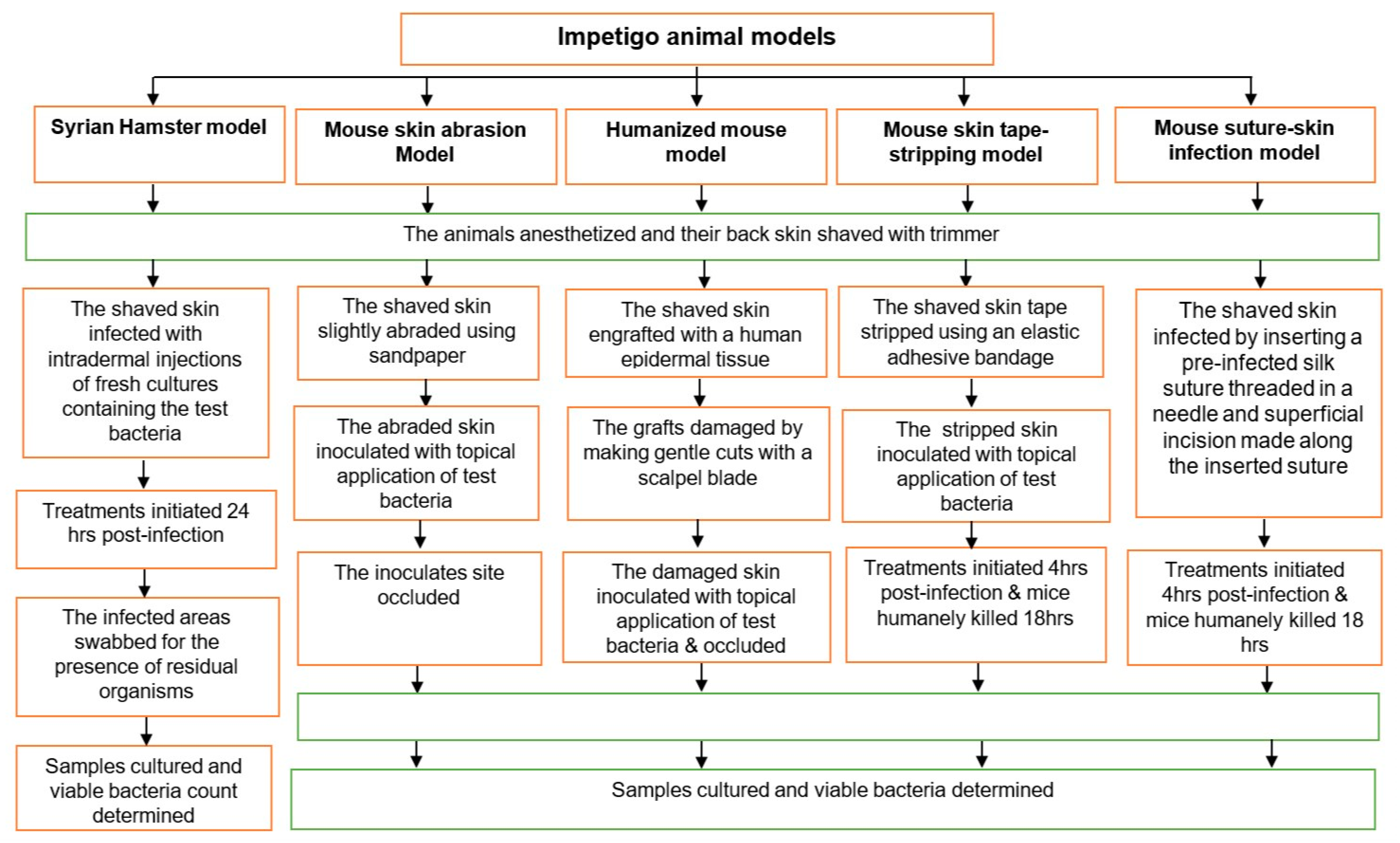
1.1. Syrian Hamster Impetigo Model
1.2. Mouse Skin Abrasion Impetigo Model
1.3. Humanized Mouse Model
1.4. Mouse Skin Tape-Stripping Model
1.5. Mouse Suture-Superficial Skin Infection Model
2. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The Global Burden of Skin Disease in 2010: An Analysis of the Prevalence and Impact of Skin Conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.C.; Carapetis, J.R.; Currie, B.J.; Fowler, V.; Chambers, H.F.; Tong, S.Y.C. Sulfamethoxazole-Trimethoprim (Cotrimoxazole) for Skin and Soft Tissue Infections Including Impetigo, Cellulitis, and Abscess. Open Forum Infect. Dis. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.C.; Mahe, A.; Hay, R.J.; Andrews, R.M.; Steer, A.C.; Tong, S.Y.C.; Carapetis, J.R. The Global Epidemiology of Impetigo: A Systematic Review of the Population Prevalence of Impetigo and Pyoderma. PLoS ONE 2015, 10, e0136789. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.B. Impetigo—Review. An. Bras. Dermatol. 2014, 89, 293–299. [Google Scholar] [CrossRef]
- May, P.J.; Tong, S.Y.C.; Steer, A.C.; Currie, B.J.; Andrews, R.M.; Carapetis, J.R.; Bowen, A.C. Treatment, prevention and public health management of impetigo, scabies, crusted scabies and fungal skin infections in endemic populations: A systematic review. Trop. Med. Int. Heal. 2019, 24, 280–293. [Google Scholar] [CrossRef]
- Koning, S.; Van Der Sande, R.; Verhagen, A.P.; A Van Suijlekom-Smit, L.W.; Morris, A.D.; Butler, C.C.; Berger, M.; Van Der Wouden, J.C. Interventions for impetigo. Cochrane Database Syst. Rev. 2012, 1, CD003261. [Google Scholar] [CrossRef]
- D’Cunha, N.M.; Peterson, G.M.; Baby, K.E.; Thomas, J. Impetigo: A need for new therapies in a world of increasing antimicrobial resistance. J. Clin. Pharm. Ther. 2017, 43, 150–153. [Google Scholar] [CrossRef]
- Steer, A.C.; Danchin, M.H.; Carapetis, J.R. Group A streptococcal infections in children. J. Paediatr. Child Health 2007, 43, 203–213. [Google Scholar] [CrossRef]
- Dajani, A.S.; Wannamaker, L.W. Experimental Infection of the Skin in the Hamster Simulating Human Impetigo. I. Natural History of the Infection. J. Infect. Dis. 1970, 122, 196–204. [Google Scholar] [CrossRef]
- Vila, J.; Hebert, A.A.; Torrelo, A.; López, Y.; Tato, M.; García-Castillo, M.; Cantón, R. Ozenoxacin: A review of preclinical and clinical efficacy. Expert Rev. Anti Infect. Ther. 2019, 17, 159–168. [Google Scholar] [CrossRef]
- Cole, C.; Gazewood, J. Diagnosis and treatment of impetigo. Am. Fam. Physician 2007, 75, 859–864. [Google Scholar] [PubMed]
- WHO. Antimicrobial Resistance: Global Report on Surveillance Geneva; WHO Press, World Health Organization: Geneva, Switzerland, 2014. Available online: https://www.who.int/drugresistance/documents/surveillancereport/en/ (accessed on 5 July 2019).
- Bowen, A.C.; Tong, S.Y.C.; Chatfield, M.; Carapetis, J.R. The microbiology of impetigo in indigenous children: Associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect. Dis. 2014, 14, 727. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, D.K.; Anderson, A.; Cleland, G.; Bowen, A.C. Are scabies and impetigo “normalised”? A cross-sectional comparative study of hospitalised children in northern Australia assessing clinical recognition and treatment of skin infections. PLoS Negl. Trop. Dis. 2017, 11, e0005726. [Google Scholar] [CrossRef] [PubMed]
- Pangilinan, R.; Tice, A.; Tillotson, G. Topical antibiotic treatment for uncomplicated skin and skin structure infections: Review of the literature. Expert Rev. Anti Infect. Ther. 2009, 7, 957–965. [Google Scholar] [CrossRef]
- Bohaty, B.R.; Choi, S.; Cai, C.; Hebert, A.A. Clinical and bacteriological efficacy of twice daily topical retapamulin ointment 1% in the management of impetigo and other uncomplicated superficial skin infections. Int. J. Women’s Dermatol. 2015, 1, 13–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonev, B.B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef]
- WHO. WHO Publishes List of Bacteria for Which New Antibiotics are Urgently Needed Geneva. 2017. Available online: https://www.who.int/en/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 1 October 2019).
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- The Pew Charitable Trusts. Antibiotics Currently in Clinical Development 2017 (cited 2019 October 17). Available online: http://www.pewtrusts.org/~/media/assets/2017/05/antibiotics-currently-in-clinical-development-03-2017.pdf?la=en (accessed on 17 October 2019).
- FDA. Guideline for Clinical Evaluation of Anti-Nfective Drugs for Systemic Administration USA: U.S Department of Health and Human Service, Food and Drug Adminstration 1977. Available online: https://catalog.hathitrust.org/Record/000090753 (accessed on 10 July 2020).
- Zak, O.; O’Reilly, T. Animal models in the evaluation of antimicrobial agents. Antimicrob. Agents Chemother. 1991, 35, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.K. In vivo Pharmacological Disease Models for Psoriasis and Atopic Dermatitis in Drug Discovery. Basic Clin. Pharmacol. Toxicol. 2006, 99, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Sadasivam, M.; Gupta, A.; De Melo, W.C.; Huang, Y.-Y.; Yin, R.; Chandran, R.; Kumar, R.; Otufowora, A.; Nyame, T.; et al. Animal models of skin disease for drug discovery. Expert Opin. Drug Discov. 2013, 8, 331–355. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D. The use of animal models in studying genetic disease: Transgenesis and induced mutation. Nat. Educ. 2008, 1, 70. [Google Scholar]
- Barré-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Futur. Sci. OA 2015, 1, 1–3. [Google Scholar] [CrossRef]
- Craig, W. Relevance of animal models for clinical treatment. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12 (Suppl. 1), S55–S57. [Google Scholar] [CrossRef]
- Beam, J.T.R.; Gilbert, D.N.; Kunin, C.M. General Guidelines for the Clinical Evaluation of Anti-Infective Drug Products. Clin. Infect. Dis. 1992, 15 (Suppl. 1), S5–S32. [Google Scholar] [CrossRef] [PubMed]
- Soriano, F.; Aguilar, L.; Ponte, C. In vitro antibiotic sensitivity testing breakpoints and therapeutic activity in induced infections in animal models. J. Chemother. 1997, 9 (Suppl. 1), 36–46. [Google Scholar]
- Watson, M.E.J.; Neely, M.N.; Caparon, M.G. Animal models of Streptococcus pyogenes infection. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2016. [Google Scholar]
- Zhao, M.; Lepak, A.J.; Andes, D. Animal models in the pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Bioorg. Med. Chem. 2016, 24, 6390–6400. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, R.; Cirioni, O.; Giacometti, A.; Scalise, A.; Simonetti, O.; Mocchegiani, F.; Orlando, F.; Goteri, G.; Della Vittoria, A.; Filosa, A.; et al. Comparative Efficacy of Topical Versus Systemic Teicoplanin in Experimental Model of Wound Infections. J. Surg. Res. 2008, 144, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Van Der Staay, F.J.; Arndt, S.S.; Nordquist, R.E. Evaluation of animal models of neurobehavioral disorders. Behav. Brain Funct. 2009, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- McRipley, R.J.; Whitney, R.R. Characterization and Quantitation of Experimental Surgical-Wound Infections Used to Evaluate Topical Antibacterial Agents. Antimicrob. Agents Chemother. 1976, 10, 38–44. [Google Scholar] [CrossRef] [PubMed]
- McRipley, R.J.; Whitney, R.R. Responsiveness of Experimental Surgical-Wound Infections to Topical Chemotherapy. Antimicrob. Agents Chemother. 1976, 10, 45–51. [Google Scholar] [CrossRef][Green Version]
- Kim, H.K.; Missiakas, D.; Schneewind, O. Mouse models for infectious diseases caused by Staphylococcus aureus. J. Immunol. Methods 2014, 410, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Malachowa, N.; Kobayashi, S.D.; Braughton, K.R.; DeLeo, F.R. Mouse Model of Staphylococcus aureus Skin Infection. Recent Results Cancer Res. 2013, 1031, 109–116. [Google Scholar] [CrossRef]
- Yamakawa, T.; Mitsuyama, J.; Hayashi, K. In vitro and in vivo antibacterial activity of T-3912, a novel non-fluorinated topical quinolone. J. Antimicrob. Chemother. 2002, 49, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Kharkwal, G.B.; Tanaka, M.; Huang, Y.-Y.; De Arce, V.J.B.; Hamblin, M.R. Animal models of external traumatic wound infections. Virulence 2011, 2, 296–315. [Google Scholar] [CrossRef]
- Cushing, A.H.; Mortimer, E.A. A Hamster Model for Streptococcal Impetigo. J. Infect. Dis. 1970, 122, 224–226. [Google Scholar] [CrossRef]
- Dajani, A.S.; Hill, P.L.; Wannamaker, L.W. Experimental infection of the skin in the hamster simulating human impetigo. II. Assessment of various therapeutic regimens. Pediatrics 1971, 48, 83–90. [Google Scholar]
- Dajani, A.S.; Wannamaker, L.W. Experimental infection of the skin in the hamster simulating human impetigo. III. Interaction between Staphylococci and group A Streptococci. J. Exp. Med. 1971, 134, 588–599. [Google Scholar] [CrossRef]
- Dajani, A.S.; Wannamaker, L.W. Experimental infection of the skin in the hamster simulating human impetigo. IV. Cellular responses after Streptococcal and Staphylococcal infections. Infect. Immun. 1972, 5, 942–946. [Google Scholar] [CrossRef]
- Gisby, J.; Bryant, J. Efficacy of a New Cream Formulation of Mupirocin: Comparison with Oral and Topical Agents in Experimental Skin Infections. Antimicrob. Agents Chemother. 2000, 44, 255–260. [Google Scholar] [CrossRef]
- Abe, Y.; Akiyama, H.; Arata, J. Production of experimental Staphylococcal impetigo in mice. J. Dermatol. Sci. 1992, 4, 42–48. [Google Scholar] [CrossRef][Green Version]
- Scaramuzzino, D.A.; McNiff, J.M.; Bessen, D.E. Humanized In Vivo Model for Streptococcal Impetigo. Infect. Immun. 2000, 68, 2880–2887. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.D.; Scaramuzzino, D.A.; Sjobring, U.; Olsen, A.; Frank, C.; Bessen, D.E. Role for a secreted cysteine proteinase in the establishment of host tissue tropism by group A streptococci. Mol. Microbiol. 2000, 38, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.D.; Sjöbring, U.; Luo, F.; Bessen, D.E. Roles of the plasminogen activator streptokinase and the plasminogen-associated M protein in an experimental model for streptococcal impetigo. Microbiology 2002, 148, 3933–3945. [Google Scholar] [CrossRef] [PubMed]
- Kugelberg, E.; Norström, T.; Petersen, T.K.; Duvold, T.; Andersson, D.I.; Hughes, D. Establishment of a Superficial Skin Infection Model in Mice by Using Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 2005, 49, 3435–3441. [Google Scholar] [CrossRef]
- Imanishi, I.; Hattori, S.; Hisatsune, J.; Ide, K.; Sugai, M.; Nishifuji, K. Staphylococcus aureus penetrate the interkeratinocyte spaces created by skin-infiltrating neutrophils in a mouse model of impetigo. Vet. Dermatol. 2016, 28, 126-e27. [Google Scholar] [CrossRef]
- Hahn, B.L.; Onunkwo, C.C.; Watts, C.J.; Sohnle, P.G. Systemic dissemination and cutaneous damage in a mouse model of Staphylococcal skin infections. Microb. Pathog. 2009, 47, 16–23. [Google Scholar] [CrossRef]
- Tarragó, C.; Esquirol, L.P.; Arañó, A.; Lachamp, L.; D’Aniello, F.; Zsolt, I. Therapeutic efficacy of ozenoxacin in animal models of dermal infection with Staphylococcus aureus. Futur. Microbiol. 2018, 13, 21–30. [Google Scholar] [CrossRef]
- Håkansson, J.; Björn, C.; Lindgren, K.; Sjöström, E.; Sjöstrand, V.; Mahlapuu, M. Efficacy of the Novel Topical Antimicrobial Agent PXL150 in a Mouse Model of Surgical Site Infections. Antimicrob. Agents Chemother. 2014, 58, 2982–2984. [Google Scholar] [CrossRef]
- Rittenhouse, S.; Singley, C.; Hoover, J.; Page, R.; Payne, D. Use of the Surgical Wound Infection Model to Determine the Efficacious Dosing Regimen of Retapamulin, a Novel Topical Antibiotic. Antimicrob. Agents Chemother. 2006, 50, 3886–3888. [Google Scholar] [CrossRef]
- Boon, R.J.; Beale, A.S. Response of Streptococcus pyogenes to therapy with amoxicillin or amoxicillin-clavulanic acid in a mouse model of mixed infection caused by Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 1987, 31, 1204–1209. [Google Scholar] [CrossRef]
- Boon, R.J.; Beale, A.S.; Sutherland, R. Efficacy of topical mupirocin against an experimental Staphylococcus aureus surgical wound infection. J. Antimicrob. Chemother. 1985, 16, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Berry, V.; Page, R.; Satterfield, J.; Singley, C.; Straub, R.; Woodnutt, G. Comparative efficacy of gemifloxacin in experimental models of pyelonephritis and wound infection. J. Antimicrob. Chemother. 2000, 45, 87. [Google Scholar] [CrossRef]
- Håkansson, J.; Ringstad, L.; Umerska, A.; Johansson, J.; Andersson, T.; Boge, L.; Rozenbaum, R.T.; Sharma, P.K.; Tollbäck, P.; Björn, C.; et al. Characterization of the in vitro, ex vivo, and in vivo Efficacy of the Antimicrobial Peptide DPK-060 Used for Topical Treatment. Front. Cell. Infect. Microbiol. 2019, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Lengeling, A. Genetic regulation of host responses to Group A Streptococcus in mice. Briefings Funct. Genom. Proteom. 2005, 4, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Sladden, M.J.; Johnston, A.G. Common skin infections in children. BMJ 2004, 329, 95–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bangert, S.; Levy, M.; Hebert, A.A. Bacterial Resistance and Impetigo Treatment Trends: A Review. Pediatr. Dermatol. 2012, 29, 243–248. [Google Scholar] [CrossRef]
- Leyden, J.J.; Stewart, R.; Kligman, A.M. Experimental Infections with Group A Streptococci in Humans. J. Investig. Dermatol. 1980, 75, 196–201. [Google Scholar] [CrossRef]
- Duncan, W.C.; McBride, M.E.; Knox, J.M. Experimental Production of Infections in Humans. J. Investig. Dermatol. 1970, 54, 319–323. [Google Scholar] [CrossRef]
- Singh, G.; Marples, R.R.; Kligman, A.M. Experimental Staphylococcus aureus Infections in Humans. J. Investig. Dermatol. 1971, 57, 149–162. [Google Scholar] [CrossRef]
- Ikeda, M.; Arata, J.; Kashiwa, N. Antibiotic effects on bacterial counts in skin lesions of experimental Staphylococcal skin infections in the hamster. J. Dermatol. 1984, 11, 67–72. [Google Scholar] [CrossRef]
- Baxter, V.K.; Griffin, D.E. Chapter 10—Animal models: No model is perfect, but many are useful. In Viral Pathogenesis, 3rd ed.; Katze, M.G., Korth, M.J., Law, G.L., Nathanson, N., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 125–138. [Google Scholar]
- Justice, M.J.; Dhillon, P. Using the mouse to model human disease: Increasing validity and reproducibility. Dis. Model. Mech. 2016, 9, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, A.C.; Crim, M.J.; Franklin, C.L. A Brief History of Animal Modeling. Mo. Med. 2013, 110, 201–205. [Google Scholar] [PubMed]
- Bunce, C.; Wheeler, L.; Reed, G.; Musser, J.; Barg, N. Murine model of cutaneous infection with gram-positive cocci. Infect. Immun. 1992, 60, 2636–2640. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Akiyama, H.; Arata, J. Production of Staphylococcal impetigo-like lesion on human skin explants in culture. J. Dermatol. Sci. 1993, 5, 150–164. [Google Scholar] [CrossRef]
- Marples, R.R.; Kligman, A.M. Methods for Evaluating Topical Antibacterial Agents on Human Skin. Antimicrob. Agents Chemother. 1974, 5, 323–329. [Google Scholar] [CrossRef]
- Sumitomo, T.; Mori, Y.; Nakamura, Y.; Honda-Ogawa, M.; Nakagawa, S.; Yamaguchi, M.; Matsue, H.; Terao, Y.; Nakata, M.; Kawabata, S. Streptococcal Cysteine Protease-Mediated Cleavage of Desmogleins Is Involved in the Pathogenesis of Cutaneous Infection. Front. Cell. Infect. Microbiol. 2018, 8, 10. [Google Scholar] [CrossRef]
- Iwatsuki, K.; Yamasaki, O.; Morizane, S.; Oono, T. Staphylococcal cutaneous infections: Invasion, evasion and aggression. J. Dermatol. Sci. 2006, 42, 203–214. [Google Scholar] [CrossRef]
- Mempel, M.; Schnopp, C.; Hojka, M.; Fesq, H.; Weidinger, S.; Schaller, M.; Korting, H.; Ring, J.; Abeck, D. Invasion of human keratinocytes by Staphylococcus aureus and intracellular bacterial persistence represent haemolysin-independent virulence mechanisms that are followed by features of necrotic and apoptotic keratinocyte cell death. Br. J. Dermatol. 2002, 146, 943–951. [Google Scholar] [CrossRef]
- Onunkwo, C.C.; Hahn, B.L.; Sohnle, P.G. Clearance of experimental cutaneous Staphylococcus aureus infections in mice. Arch. Dermatol. Res. 2010, 302, 375–382. [Google Scholar] [CrossRef][Green Version]
- Hu, Y.; Shamaei-Tousi, A.; Liu, Y.; Coates, A.R.M. A New Approach for the Discovery of Antibiotics by Targeting Non-Multiplying Bacteria: A Novel Topical Antibiotic for Staphylococcal Infections. PLoS ONE 2010, 5, e11818. [Google Scholar] [CrossRef]
- Hu, Y.; Coates, A.R.M. Enhancement by novel anti-methicillin-resistant Staphylococcus aureus compound HT61 of the activity of neomycin, gentamicin, mupirocin and chlorhexidine: In vitro and in vivo studies. J. Antimicrob. Chemother. 2012, 68, 374–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Russell, E.H.; Gutekunst, D.P.; Chamberlain, E.R. Evaluation of furazolium chloride in topical treatment of model infections in laboratory animals. Antimicrob. Agents Chemother. 1967, 7, 497–501. [Google Scholar]
- Alexander, J.W.; Kaplan, J.Z.; Altemeier, W.A. Role of Suture Materials in the Development of Wound Infection. Ann. Surg. 1967, 165, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Elek, S.D. Experimental Staphylococcal infections in the skin of man. Ann. N. Y. Acad. Sci. 1956, 65, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Hillman, E.M.; Amoozegar, C.B.; Wang, T.; McCaslin, A.F.H.; Bouchard, M.B.; Mansfield, J.; Levenson, R.M. In vivo optical imaging and dynamic contrast methods for biomedical research. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 4620–4643. [Google Scholar] [CrossRef]
- Cho, J.S.; Zussman, J.; Donegan, N.P.; Ramos, R.I.; Garcia, N.C.; Uslan, D.Z.; Iwakura, Y.; Simon, S.I.; Cheung, A.L.; Modlin, R.L.; et al. Noninvasive In Vivo Imaging to Evaluate Immune Responses and Antimicrobial Therapy against Staphylococcus aureus and USA300 MRSA Skin Infections. J. Investig. Dermatol. 2011, 131, 907–915. [Google Scholar] [CrossRef]
- Guo, Y.; Ramos, R.I.; Cho, J.S.; Donegan, N.P.; Cheung, A.L.; Miller, L.S. In Vivo Bioluminescence Imaging to Evaluate Systemic and Topical Antibiotics against Community-Acquired Methicillin-Resistant Staphylococcus aureus—Infected Skin Wounds in Mice. Antimicrob. Agents Chemother. 2012, 57, 855–863. [Google Scholar] [CrossRef]
- Albareda, N.; Zeichner, J.; Rosenberg, N. A Randomized Vehicle-Controlled Trial to Assess the Efficacy, Safety, and Tolerability of Ozenoxacin 1% Cream in 412 Patients 2 Months and Older with Impetigo. Ski. J. Cutan. Med. 2017, 1, s103. [Google Scholar] [CrossRef]
- Gropper, S.; Albareda, N.; Chelius, K.; Kruger, D.; Mitha, I.; Vahed, Y.; Gani, M.; García-Alonso, F. Ozenoxacin 1% cream in the treatment of impetigo: A multicenter, randomized, placebo- and retapamulin-controlled clinical trial. Futur. Microbiol. 2014, 9, 1013–1023. [Google Scholar] [CrossRef]
- Koning, S.; Van Der Wouden, J.; Chosidow, O.; Twynholm, M.; Singh, K.; Scangarella, N.; Oranje, A.; Van Der Wouden, J.C. Efficacy and safety of retapamulin ointment as treatment of impetigo: Randomized double-blind multicentre placebo-controlled trial. Br. J. Dermatol. 2008, 158, 1077–1082. [Google Scholar] [CrossRef]
- Goldfarb, J.; Crenshaw, D.; O’Horo, J.; Lemon, E.; Blumer, J.L. Randomized clinical trial of topical mupirocin versus oral erythromycin for impetigo. Antimicrob. Agents Chemother. 1988, 32, 1780–1783. [Google Scholar] [CrossRef] [PubMed]
- Rist, T.; Parish, L.C.; Capin, L.R.; Sulica, V.; Bushnell, W.D.; Cupo, M.A. A comparison of the efficacy and safety of mupirocin cream and cephalexin in the treatment of secondarily infected eczema. Clin. Exp. Dermatol. 2002, 27, 14–20. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| Model | Bacteria Tested | Host Animal | Anaesthetic Agent | Inoculation Route | Inoculum Dose | Time for Infection Occurrence | Antimicrobial Agents Tested | Sampling Method |
|---|---|---|---|---|---|---|---|---|
| Hamster impetigo model [9,40,41,42,43,44] | S. aureus or S. pyogenes | Hamster (Golden Syrian type, 6–8 weeks, 80–120 g, n = 4–75) | Isoflurane (3%, inhalation) | Intradermal injections | 1.2 × 107 CFU | 24 h post-inoculation | Gentamicin ointment, Bacitracin ointment, Benzathine penicillin G injection, Procaine penicillin injection | Swabbing the lesion surface |
| Mouse skin abrasion impetigo model [45] | S. aureus | Mouse (ddY type, female, 5 weeks old, ~20 g, n = 5) | Not reported | Topical/epicutaneous inoculation to slightly damaged skin by sandpaper | 7 × 104 CFU | 24 h post-inoculation | No antimicrobial agent used | Biopsy of infected skin area |
| Humanized mouse impetigo Model [46,47,48] | S. pyogenes | Mouse (SCID type, female, 4–6-week-old, n = no report) | Ketamine-Xylazine (Intraperitoneal injection) | Topical/epicutaneous inoculation to slightly damaged skin by sandpaper, scalpel, or tape stripping | 50 CFU | 1 week post-inoculation | No antimicrobial agent used | Biopsy of infected skin area |
| Mouse skin tape-stripping model [49,50,51] | S. aureus or S. pyogenes | Mouse (BALB/c type, female, 6–8-weeks-old, n = no report) | 1:1:2 v/v mixture of hypnorm (fentanyl, fluanisone), dormicum (midazolam) and distilled water, Intraperitoneal injection) | Topical/epicutaneous inoculation to slightly damaged skin by tape stripping | 107 cells | 4 h post-inoculation | Fusidic acid ointment | Biopsy of infected skin area |
| Mouse suture-superficial skin infection model [34,35,44,52,53,54,55,56,57,58] | S. aureus and/or S. pyogenes | Mouse (CF-1, CD1, and MF1 type, female and male, 18–20 g, n = 10–50) | Sodium pentobarbital (30 mg/kg, Intraperitoneal injection) Or Diazepam plus fentanyl Fluanisone (1.25 mg/kg plus 0.5 mL/kg Intramuscular injection) | Topical/epicutaneous inoculation by insertion of an infected suture | 103–105 cells | 6 h post-inoculation | Gentamicin cream, Polymyxin B-bacitracin-neomycin ointment, Retapamulin ointment, Fusidic acid cream, Muprocin ointment and cream, Ozenoxacin cream, amoxicillin or amoxicillin-clavulanic acid oral, Gemifloxacin oral | Swabbing the lesion surface Or Biopsy of infected skin area |
| Models | General Strengths | General Limitations |
|---|---|---|
| Hamster impetigo model [9,40,41,42,43,44] |
|
|
| Mouse skin abrasion impetigo model [45] |
|
|
| Humanized mouse impetigo Model [46,47,48] |
|
|
| Mouse skin tape-stripping model [49,50,51] |
|
|
| Mouse suture-superficial skin infection model [34,35,44,52,53,54,55,56,57,58] |
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrha, S.; Bartholomaeus, A.; Tesfaye, W.; Thomas, J. Impetigo Animal Models: A Review of Their Feasibility and Clinical Utility for Therapeutic Appraisal of Investigational Drug Candidates. Antibiotics 2020, 9, 694. https://doi.org/10.3390/antibiotics9100694
Abrha S, Bartholomaeus A, Tesfaye W, Thomas J. Impetigo Animal Models: A Review of Their Feasibility and Clinical Utility for Therapeutic Appraisal of Investigational Drug Candidates. Antibiotics. 2020; 9(10):694. https://doi.org/10.3390/antibiotics9100694
Chicago/Turabian StyleAbrha, Solomon, Andrew Bartholomaeus, Wubshet Tesfaye, and Jackson Thomas. 2020. "Impetigo Animal Models: A Review of Their Feasibility and Clinical Utility for Therapeutic Appraisal of Investigational Drug Candidates" Antibiotics 9, no. 10: 694. https://doi.org/10.3390/antibiotics9100694
APA StyleAbrha, S., Bartholomaeus, A., Tesfaye, W., & Thomas, J. (2020). Impetigo Animal Models: A Review of Their Feasibility and Clinical Utility for Therapeutic Appraisal of Investigational Drug Candidates. Antibiotics, 9(10), 694. https://doi.org/10.3390/antibiotics9100694





