The Antibacterial and Anti-Biofilm Activity of Metal Complexes Incorporating 3,6,9-Trioxaundecanedioate and 1,10-Phenanthroline Ligands in Clinical Isolates of Pseudomonas aeruginosa from Irish Cystic Fibrosis Patients
Abstract
1. Introduction
2. Results
2.1. The Inhibition of Planktonic Bacterial Growth by Metal-Tdda-Phen Complexes
2.2. Metal-Tdda-Phen Complexes Are Able to Inhibit Biofilm Formation and Disrupt Established Biofilm
2.3. Metal-Tdda-Phen Complexes Enhanced the Anti-Biofilm Activity of Gentamicin
2.4. Metal-Tdda-Phen Complexes Reduce Components in a Biofilm; This Activity Is Enhanced When Combined with Gentamicin
3. Discussion
4. Materials and Methods
4.1. Test Complexes
4.2. Bacterial Strains and Culture Conditions
4.3. Antimicrobial Susceptibility Testing of Complexes on Pseudomonas aeruginosa Strains
4.3.1. Effects of Test Compounds on Planktonic Bacteria
4.3.2. Effects of Test Compounds on Biofilm Formation (Pre-Treatment)
4.3.3. Effects of Test Compounds on the Mature Biofilm (Post-Treatment)
4.3.4. Checkerboard Assay for Mature Biofilms
4.4. Effects of Test Complexes on Individual Components of Biofilm
4.4.1. Extraction and Quantification of Exopolysaccharide
4.4.2. Extraction and Quantification of Extracellular DNA (eDNA)
4.4.3. Quantification of Pyocyanin
4.4.4. Quantification of Pyoverdine
4.4.5. Quantification of Bacteria
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brandenburg, K.S.; Weaver, A.J.; Karna, S.L.R.; You, T.; Chen, P.; Van Stryk, S.; Qian, L.; Pineda, U.; Abercrombie, J.; Leung, K. Formation of Pseudomonas aeruginosa Biofilms in Full-thickness Scald Burn Wounds in Rats. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Estrada, S.; Borgatta, B.; Rello, J. Pseudomonas aeruginosa ventilator-associated pneumonia management. Infect. Drug Resist. 2016, 9, 7–18. [Google Scholar] [PubMed]
- Fenker, D.E.; Mcdaniel, C.T.; Panmanee, W.; Panos, R.J.; Sorscher, E.J.; Sabusap, C.; Clancy, J.P.; Hassett, D. A Comparison between Two Pathophysiologically Different yet Microbiologically Similar Lung Diseases: Cystic Fibrosis and Chronic Obstructive Pulmonary Disease. Int. J. Respir. Pulm. Med. 2018, 5, 1–33. [Google Scholar]
- Farrell, P.M. The prevalence of cystic fibrosis in the European Union. J. Cyst. Fibros. 2008, 7, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Reece, E.; Segurado, R.; Jackson, A.; McClean, S.; Renwick, J.; Greally, P. Co-colonisation with Aspergillus fumigatus and Pseudomonas aeruginosa is associated with poorer health in cystic fibrosis patients: An Irish registry analysis. BMC Pulm. Med. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Christophersen, L.; Schwartz, F.A.; Lerche, C.J.; Svanekjær, T.; Kragh, K.N.; Laulund, A.S.; Thomsen, K.; Henneberg, K.; Sams, T.; Høiby, N.; et al. In vivo demonstration of Pseudomonas aeruginosa biofilms as independent pharmacological microcompartments. J. Cyst. Fibros. 2020, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Seviour, T.; Derlon, N.; Dueholm, M.S.; Flemming, H.C.; Girbal-Neuhauser, E.; Horn, H.; Kjelleberg, S.; van Loosdrecht, M.; Lotti, T.; Malpei, M.F.; et al. Extracellular polymeric substances of biofilms: Suffering from an identity crisis. Water Res. 2019, 151, 1–7. [Google Scholar] [CrossRef]
- De la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E.W. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Hughes, G.; Webber, M.A. Novel approaches to the treatment of bacterial biofilm infections. Br. J. Pharm. 2017, 174, 2237–2246. [Google Scholar] [CrossRef]
- Stefani, S.; Campana, S.; Cariani, L.; Carnovale, V.; Colombo, C.; Lleo, M.M.; Iula, V.D.; Minicucci, L.; Morelli, P.; Pizzamiglio, G.; et al. Relevance of multidrug-resistant Pseudomonas aeruginosa infections in cystic fibrosis. Int. J. Med. Microbiol. 2017, 307, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Kellett, A.; O’Connor, M.; McCann, M.; Howe, O.; Casey, A.; McCarron, P.; Kavanagh, K.; McNamara, M.; Kennedy, S.; May, D.; et al. Water-soluble bis(1,10-phenanthroline) octanedioate Cu2+ and Mn2+ complexes with unprecedented nano and picomolar in vitro cytotoxicity: Promising leads for chemotherapeutic drug development. Medchemcomm 2011, 2, 579–584. [Google Scholar] [CrossRef]
- Rochford, G.; Molphy, Z.; Browne, N.; Surlis, C.; Devereux, M.; McCann, M.; Kellett, A.; Howe, O.; Kavanagh, K. In-vivo evaluation of the response of Galleria mellonella larvae to novel copper(II) phenanthroline-phenazine complexes. J. Inorg. Biochem. 2018, 186, 135–146. [Google Scholar] [CrossRef]
- McCann, M.; Geraghty, M.; Devereux, M.; O’Shea, D.; Mason, J.; O’Sullivan, L. Insights into the mode of action of the anti-candida activity of 1,10-phenanthroline and its metal chelates. Met. Based Drugs 2000, 7, 185–193. [Google Scholar] [CrossRef]
- Gandra, R.M.; Carron, P.M.; Fernandes, M.F.; Ramos, L.S.; Mello, T.P.; Aor, A.C.; Branquinha, M.H.; McCann, M.; Devereux, M.; Santos, A.L.S. Antifungal potential of copper(II), manganese(II) and silver(I) 1,10-phenanthroline chelates against multidrug-resistant fungal species forming the Candida haemulonii Complex: Impact on the planktonic and biofilm lifestyles. Front. Microbiol. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Viganor, L.; Galdino, A.C.M.; Nunes, A.P.F.; Santos, K.R.N.; Branquinha, M.H.; Devereux, M.; Kellett, A.; McCann, M.; Santos, A.L.S. Anti-Pseudomonas aeruginosa activity of 1,10-phenanthroline-based drugs against both planktonic- and biofilm-growing cells. J. Antimicrob. Chemother. 2016, 71, 128–134. [Google Scholar] [CrossRef]
- McCarron, P.; McCann, M.; Devereux, M.; Kavanagh, K.; Skerry, C.; Karakousis, P.C.; Aor, A.C.; Mello, T.P.; Santos, A.L.S.; Campos, D.L. Unprecedented in vitro antitubercular activitiy of manganese(II) complexes containing 1,10-phenanthroline and dicarboxylate ligands: Increased activity, superior selectivity, and lower toxicity in comparison to their copper(II) analogs. Front. Microbiol. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Papadia, P.; Margiotta, N.; Bergamo, A.; Sava, G.; Natile, G. Platinum(II) complexes with antitumoral/antiviral aromatic heterocycles: Effect of glutathione upon in vitro cell growth inhibition. J. Med. Chem. 2005, 48, 3364–3371. [Google Scholar] [CrossRef]
- Viganor, L.; Howe, O.; McCarron, P.; McCann, M.; Devereux, M. The Antibacterial Activity of Metal Complexes Containing 1,10-phenanthroline: Potential as Alternative Therapeutics in the Era of Antibiotic Resistance. Curr. Top. Med. Chem. 2016, 17, 1280–1302. [Google Scholar] [CrossRef]
- Galdino, A.C.M.; Viganor, L.; De Castro, A.A.; Da Cunha, E.F.F.; Mello, T.P.; Mattos, L.M.; Pereira, M.D.; Hunt, M.C.; O’Shaughnessy, M.; Howe, O.; et al. Disarming Pseudomonas aeruginosa virulence by the inhibitory action of 1,10-phenanthroline-5,6-dione-based compounds: Elastase B (lasB) as a chemotherapeutic target. Front. Microbiol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ventura, R.F.; Galdino, A.C.M.; Viganor, L.; Schuenck, R.P.; Devereux, M.; McCann, M.; Santos, A.L.S.; Nunes, A.P.F. Antimicrobial action of 1,10-phenanthroline-based compounds on carbapenemase-producing Acinetobacter baumannii clinical strains: Efficacy against planktonic- and biofilm-growing cells. Braz. J. Microbiol. 2020, 1–8. [Google Scholar] [CrossRef]
- Gandra, R.M.; McCarron, P.; Viganor, L.; Fernandes, M.F.; Kavanagh, K.; McCann, M.; Branquinha, M.H.; Santos, A.L.S.; Howe, O.; Devereux, M. In vivo Activity of Copper(II), Manganese(II), and Silver(I) 1,10-Phenanthroline Chelates Against Candida haemulonii Using the Galleria mellonella Model. Front. Microbiol. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 7.1. 2017. Available online: http://www.eucast.org (accessed on 20 June 2017).
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents—How, P. aeruginosa can escape antibiotics. Front. Microbiol. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Chiang, W.C.; Nilsson, M.; Jensen, P.Ø.; Høiby, N.; Nielsen, T.E.; Givskov, M.; Tolker-Nielsen, T. Extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2013, 57, 2352–2361. [Google Scholar] [CrossRef]
- Kang, D.; Turner, K.E.; Kirienko, N.V. PqsA promotes pyoverdine production via biofilm formation. Pathogens 2018, 7, 3. [Google Scholar] [CrossRef]
- Cystic Fibrosis Ireland. Cystic Fibrosis Ireland Annual Report for 2017. Available online: https://cfri.ie/annual-reports (accessed on 15 August 2018).
- Anderson, E.T.; Young, L.S.; Hewitt, W.L. Antimicrobial synergism in the therapy of gram-negative rod bacteremia. Chemotherapy 1978, 24, 45–54. [Google Scholar] [CrossRef]
- Tang, S.Y.; Zhang, S.W.; Wu, J.D.; Wu, F.; Zhang, J.; Dong, J.T.; Guo, P.; Zhang, D.L.; Yang, J.T.; Zhang, W.J. Comparison of mono- and combination antibiotic therapy for the treatment of Pseudomonas aeruginosa bacteraemia: A cumulative meta-analysis of cohort studies. Exp. Med. 2018, 15, 2418–2428. [Google Scholar]
- Smith, W.D.; Bardin, E.; Cameron, L.; Edmondson, C.L.; Farrant, K.V.; Martin, I.; Murphy, R.A.; Soren, O.; Turnball, A.R.; Wierre-Gore, N.; et al. Current and future therapies for Pseudomonas aeruginosa infection in patients with cystic fibrosis. FEMS Microbiol. Lett. 2017, 364, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bonchi, C.; Frangipani, E.; Imperi, F.; Visca, P. Pyoverdine and proteases affect the response of Pseudomonas aeruginosa to gallium in human serum. Antimicrob. Agents Chemother. 2015, 59, 5641–5646. [Google Scholar] [CrossRef] [PubMed]
- DeLeon, K.; Balldin, F.; Watters, C.; Hamood, A.; Griswold, J.; Sreedharan, S.; Rumbaugh, K.P. Gallium maltolate treatment eradicates Pseudomonas aeruginosa infection in thermally injured mice. Antimicrob. Agents Chemother. 2009, 53, 1331–1337. [Google Scholar] [CrossRef]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef]
- Goss, C.H.; Kaneko, Y.; Khuu, L.; Anderson, G.D.; Ravishankar, S.; Aitken, M.L.; Lechtzin, N.; Zhou, G.; Czyz, D.M.; McLean, K.; et al. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018, 10, 1–12. [Google Scholar] [CrossRef]
- Ross-Gillespie, A.; Weigert, M.; Brown, S.P.; Kümmerli, R. Gallium-mediated siderophore quenching as an evolutionarily robust antibacterial treatment. Evol. Med. Public Health 2014, 1, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Halwani, M.; Yebio, B.; Suntres, Z.E.; Alipour, M.; Azghani, A.O.; Omri, A. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas Aeruginosa. J. Antimicrob. Chemother. 2008, 62, 1291–1297. [Google Scholar] [CrossRef]
- Kapoor, P.; Murphy, P. Combination antibiotics against Pseudomonas aeruginosa, representing common and rare cystic fibrosis strains from different Irish clinics. Heliyon 2018, 4, 1–4. [Google Scholar] [CrossRef]
- Hentzer, M.; Teitzel, G.M.; Balzer, G.J.; Heydorn, A.; Givskov, M.; Parsek, M.R. Alginate Overproduction Affects. Society 2001, 183, 5395–5401. [Google Scholar]
- Billings, N.; Ramirez Millan, M.; Caldara, M.; Rusconi, R.; Tarasova, Y.; Stocker, R.; Ribbeck, K. The Extracellular Matrix Component Psl Provides Fast-Acting Antibiotic Defense in Pseudomonas aeruginosa Biofilms. PLoS Pathog. 2013, 9, e1003526. [Google Scholar] [CrossRef]
- Colvin, K.M.; Gordon, V.D.; Murakami, K.; Borlee, B.R.; Wozniak, D.J.; Wong, G.C.L.; Parsek, M.R. The Pel Polysaccharide Can Serve a Structural and Protective Role in the Biofilm Matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011, 7, e1001264. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, X.; Liu, H.; Zhang, L.; Guo, Y.; Yu, S.; Wozniak, D.J.; Ma, L.Z. The exopolysaccharide Psl-eDNA interaction enables the formation of a biofilm skeleton in Pseudomonas aeruginosa. Environ. Microbiol. Rep. 2015, 7, 330–340. [Google Scholar] [PubMed]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, H.; Charron-Mazenod, L.; Lewenza, S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008, 4, e1000213. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Sehar, S.; Koop, L.; Wong, Y.K.; Ahmed, S.; Siddiqui, K.S.; Manefield, M. Influence of calcium in extracellular DNA mediated bacterial aggregation and biofilm formation. PLoS ONE 2014, 9, e91935. [Google Scholar] [CrossRef]
- Banin, E.; Brady, K.M.; Greenberg, E.P. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl. Env. Microbiol. 2006, 72, 2064–2069. [Google Scholar] [CrossRef]
- Tetz, G.V.; Artemenko, N.K.; Tetz, V.V. Effect of DNase and antibiotics on biofilm characteristics. Antimicrob. Agents Chemother. 2009, 53, 1204–1209. [Google Scholar] [CrossRef]
- Sigman, D.S.; Graham, D.R.; D’Aurora, V.; Stern, A.M. Oxygen-dependent Cleavage of DNA by the 1,10-Phenantroline Cuprous Complex. J. Biol. Chem. 1979, 254, 12269–12272. [Google Scholar]
- Kellett, A.; O’Connor, M.; McCann, M.; McNamara, M.; Lynch, P.; Rosair, G.; McKee, V.; Creaven, N.; Walsh, M.; McClean, S.; et al. Bis-phenanthroline copper(II) phthalate complexes are potent in vitro antitumour agents with “self-activating” metallo-nuclease and DNA binding properties. Dalton Trans. 2011, 40, 1024–1027. [Google Scholar] [CrossRef]
- McCann, S.; McCann, M.; Casey, T.; Devereux, M.; McKee, V.; McMichael, P.; McCrea, J. Manganese(II) complexes of 3,6,9-Trioxaundecanedioic Acid (3,6,9-tdaH2): X-ray Crystal Structures of [Mn(3,6,9-tda)(H2O)2]·2H2O and [Mn(3,6,9-tda)(phen)2]·3H2O·EtOH. Polyhedron 1997, 16, 4247–4252. [Google Scholar] [CrossRef]
- Medeiros, A.A.; O’Brien, T.F.; Wacker, W.E.C.; Yulug, N.F. Effect of salt concentration on the apparent in-vitro susceptibility of pseudomonas and other gram-negative bacilli to gentamicin. J. Infect. Dis. 1971, 124, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Holloway, B.W. Genetic Recombination in Pseudomonas aeruginosa. J. Gen. Microbiol. 1955, 13, 572–581. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing Routine and Extended Internal Quality Control as Recommended by EUCAST. Version 7.0. Available online: http://www.eucast.org (accessed on 20 June 2017).
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Skogman, M.E.; Vuorela, P.M.; Fallarero, A. Combining biofilm matrix measurements with biomass and viability assays in susceptibility assessments of antimicrobials against Staphylococcus aureus biofilms. J. Antibiot. (Tokyo) 2012, 65, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tribedi, P.; Sil, A.K. Cell surface hydrophobicity: A key component in the degradation of polyethylene succinate by Pseudomonas sp. AKS2. J. Appl. Microbiol. 2014, 116, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 350–356. [Google Scholar] [CrossRef]
- Wu, J.; Xi, C. Evaluation of different methods for extracting extracellular DNA from the biofilm matrix. Appl. Environ. Microbiol. 2009, 75, 5390–5395. [Google Scholar] [CrossRef]
- Loiselle, M.; Anderson, K.W. The use of cellulase in inhibiting biofilm formation from organisms commonly found on medical implants. Biofouling 2003, 19, 77–85. [Google Scholar] [CrossRef]
- Fleming, D.; Chahin, L.; Rumbaugh, K. Glycoside hydrolases degrade polymicrobial bacterial biofilms in wounds. Antimicrob. Agents Chemother. 2017, 61, 1–9. [Google Scholar] [CrossRef]
- Zipper, H. Mechanisms underlying the impact of humic acids on DNA quantification by SYBR Green I and consequences for the analysis of soils and aquatic sediments. Nucleic Acids Res. 2003, 31, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Essar, D.W.; Eberly, L.; Hadero, A.; Crawford, I.P. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: Interchangeability of the two anthranilate synthase and evolutionary implications. J. Bacteriol. 1990, 172, 884–900. [Google Scholar] [CrossRef] [PubMed]
- Adonizio, A.; Kong, K.F.; Mathee, K. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by south Florida plant extracts. Antimicrob. Agents Chemother. 2008, 52, 198–203. [Google Scholar] [CrossRef] [PubMed]
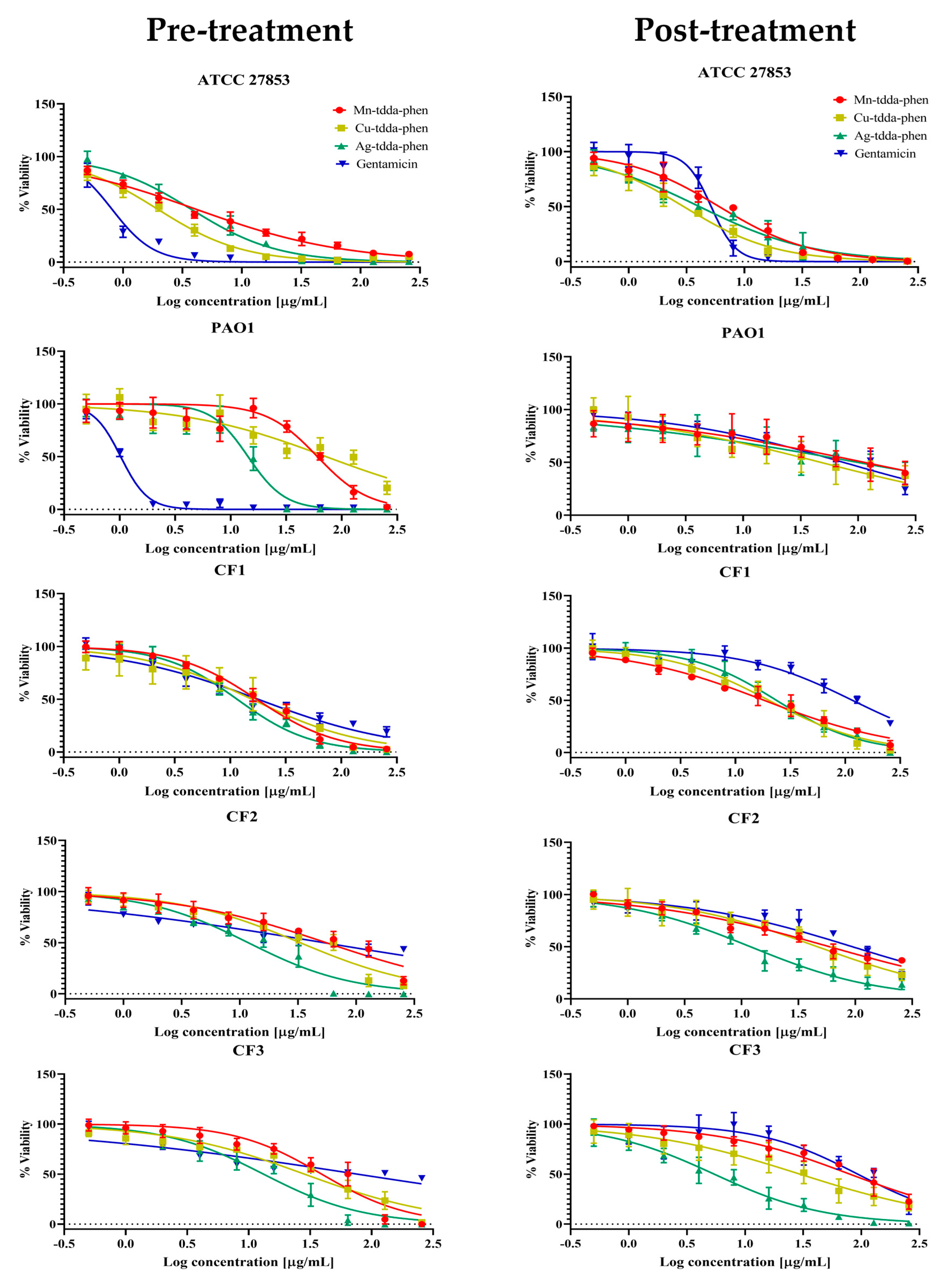
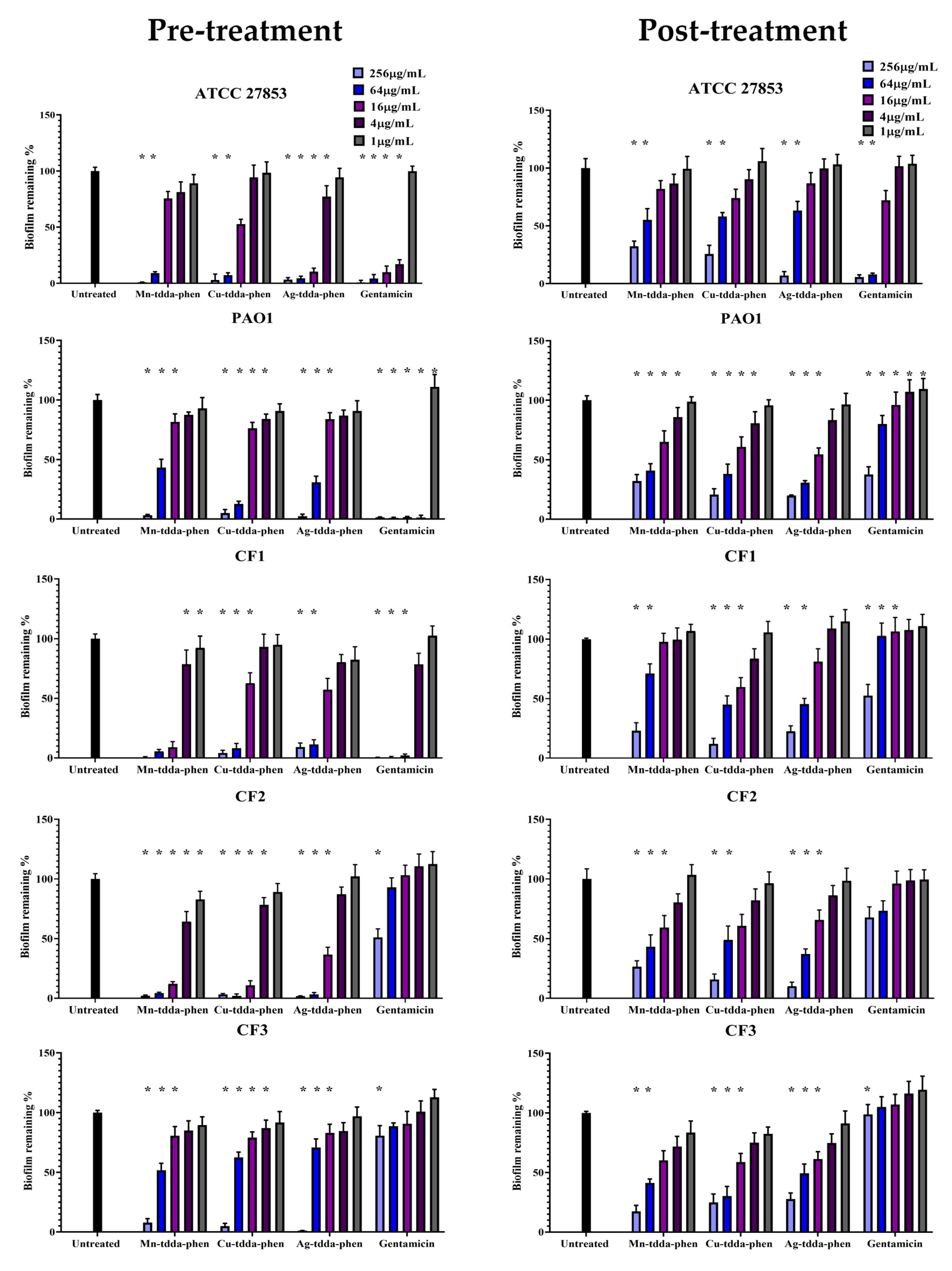

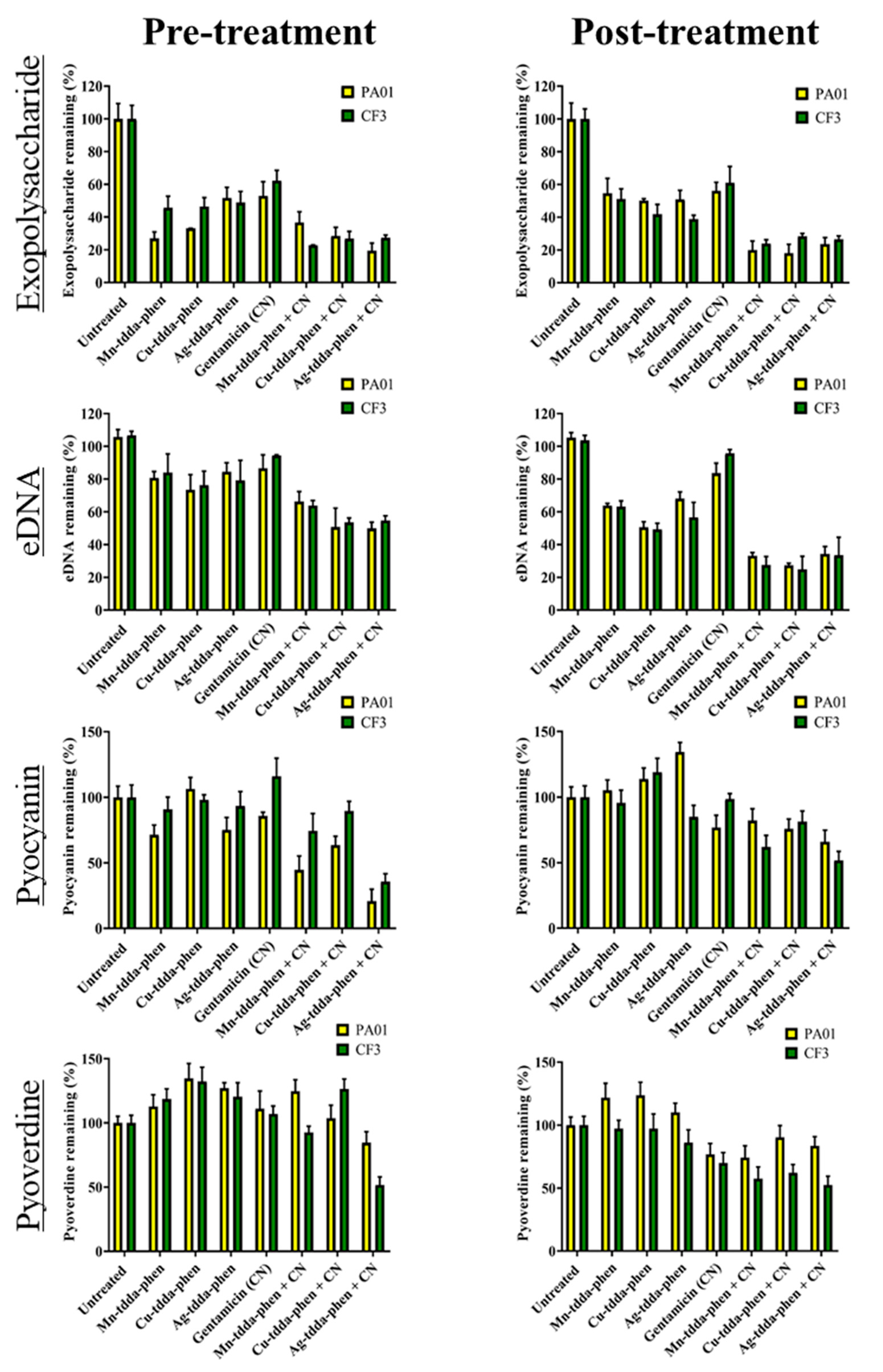

| Test Compound | MIC µg/mL and (µM) | ||||
|---|---|---|---|---|---|
| ATCC 27853 | PAO1 | CF1 | CF2 | CF3 | |
| Mn-tdda | >256 (737) | >256 (737) | >256 (737) | >256 (737) | >256 (737) |
| Cu-tdda | >256 (848) | 128 (424) | >256 (848) | >256 (848) | 256 (848) |
| Ag-tdda | >256 (542) | >256 (542) | >256 (542) | >256 (542) | >256 (542) |
| Mn-tdda-phen | 16 (21.7) | 32 (43.5) | 8 (10.9) | 8 (10.9) | 128 (174) |
| Cu-tdda-phen | 16 (21.5) | 32 (43) | 8 (10.7) | 8 (10.7) | 64 (86) |
| Ag-tdda-phen | 8 (6.6) | 32 (26.6) | 16(13.3) | 16 (13.3) | 64 (53.2) |
| Phen | 128 (710) | >256 (1420) | 128 (710) | 128 (710) | >256 (1420) |
| tddaH2 | >256 (1152) | >256 (1152) | >256 (1152) | >256 (1152) | >256 (1152) |
| MnCl2 | >256 (1294) | >256 (1294) | >256 (1294) | >256 (1294) | >256 (1294) |
| CuCl2 | >256 (1502) | >256 (1502) | >256 (1502) | >256 (1502) | >256 (1502) |
| AgNO3 | 128 (753) | 256 (1507) | >256 (1507) | >256 (1507) | 256 (1507) |
| Gentamicin | 1 (1.7) | 2 (3.5) | 8 (13.9) | 128 (222) | >256 (445) |
| Test Complexes | Organism | FIC Index | Interpretation |
|---|---|---|---|
| Mn-tdda-phen + Gentamicin | PAO1 | 0.141 | Synergy |
| CF3 | 0.133 | Synergy | |
| Cu-tdda-phen + Gentamicin | PAO1 | 0.313 | Synergy |
| CF3 | 0.156 | Synergy | |
| Ag-tdda-phen + Gentamicin | PAO1 | 0.141 | Synergy |
| CF3 | 0.133 | Synergy |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Shaughnessy, M.; McCarron, P.; Viganor, L.; McCann, M.; Devereux, M.; Howe, O. The Antibacterial and Anti-Biofilm Activity of Metal Complexes Incorporating 3,6,9-Trioxaundecanedioate and 1,10-Phenanthroline Ligands in Clinical Isolates of Pseudomonas aeruginosa from Irish Cystic Fibrosis Patients. Antibiotics 2020, 9, 674. https://doi.org/10.3390/antibiotics9100674
O’Shaughnessy M, McCarron P, Viganor L, McCann M, Devereux M, Howe O. The Antibacterial and Anti-Biofilm Activity of Metal Complexes Incorporating 3,6,9-Trioxaundecanedioate and 1,10-Phenanthroline Ligands in Clinical Isolates of Pseudomonas aeruginosa from Irish Cystic Fibrosis Patients. Antibiotics. 2020; 9(10):674. https://doi.org/10.3390/antibiotics9100674
Chicago/Turabian StyleO’Shaughnessy, Megan, Pauraic McCarron, Livia Viganor, Malachy McCann, Michael Devereux, and Orla Howe. 2020. "The Antibacterial and Anti-Biofilm Activity of Metal Complexes Incorporating 3,6,9-Trioxaundecanedioate and 1,10-Phenanthroline Ligands in Clinical Isolates of Pseudomonas aeruginosa from Irish Cystic Fibrosis Patients" Antibiotics 9, no. 10: 674. https://doi.org/10.3390/antibiotics9100674
APA StyleO’Shaughnessy, M., McCarron, P., Viganor, L., McCann, M., Devereux, M., & Howe, O. (2020). The Antibacterial and Anti-Biofilm Activity of Metal Complexes Incorporating 3,6,9-Trioxaundecanedioate and 1,10-Phenanthroline Ligands in Clinical Isolates of Pseudomonas aeruginosa from Irish Cystic Fibrosis Patients. Antibiotics, 9(10), 674. https://doi.org/10.3390/antibiotics9100674






