Characterization of Multidrug Resistance Patterns of Emerging Salmonella enterica Serovar Rissen along the Food Chain in China
Abstract
1. Introduction
2. Materials and Methods
2.1. The Source of Salmonella Isolates
2.2. Identification of Salmonella Isolates
2.3. DNA Extraction by Boiling Method and PCR
2.4. PCR Amplification of stn Gene
2.5. Serotyping by Agglutination Assay
2.6. Antimicrobial Susceptibility Test
2.7. Genomic Sequencing and Bioinformatic Analysis
2.8. Ethical Approval
3. Results and Discussion
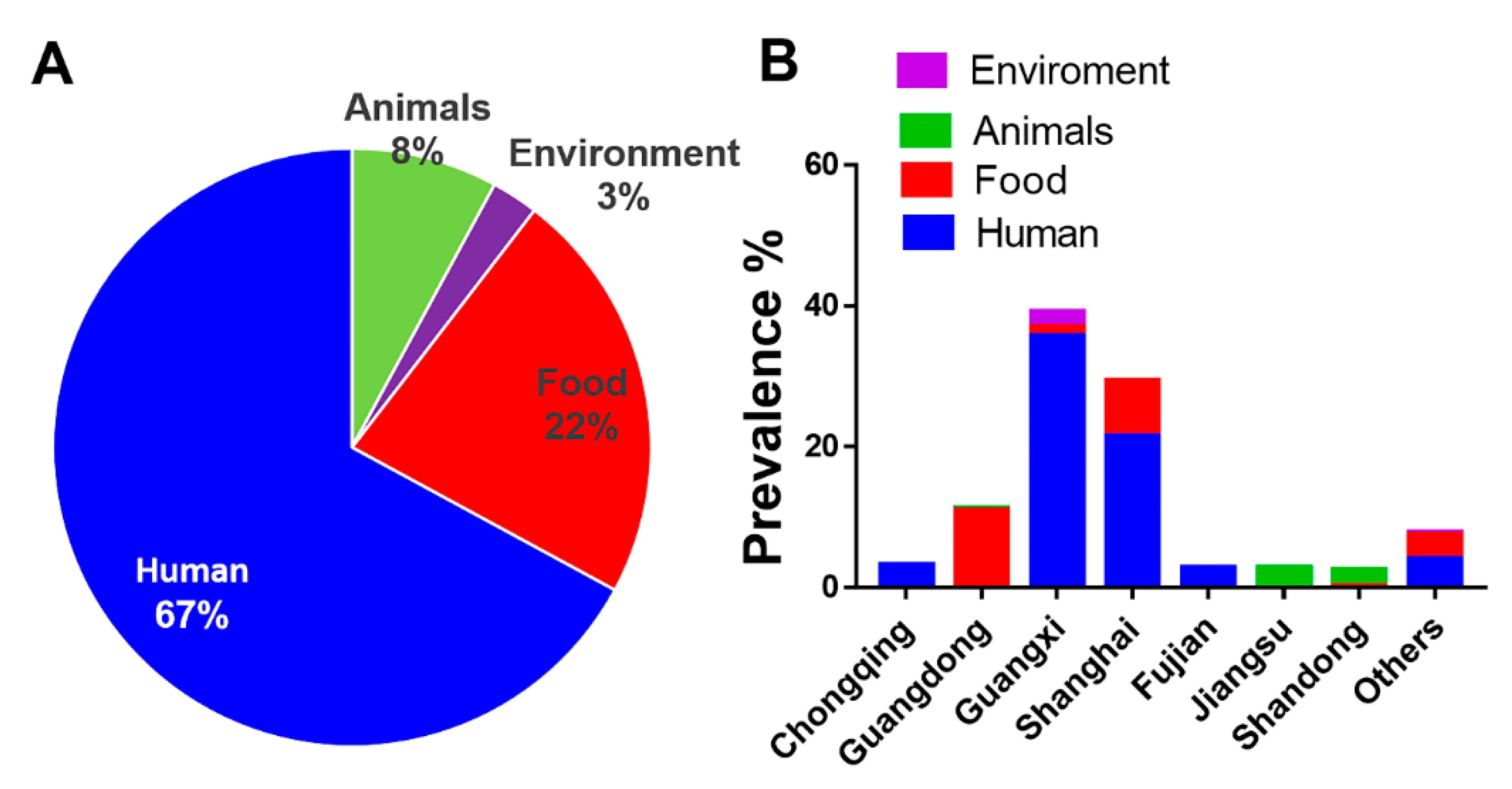
3.1. Human Isolates of S. Rissen Are Dominant
3.2. S. Rissen Showed Resistant Properties Against Important Antimicrobials
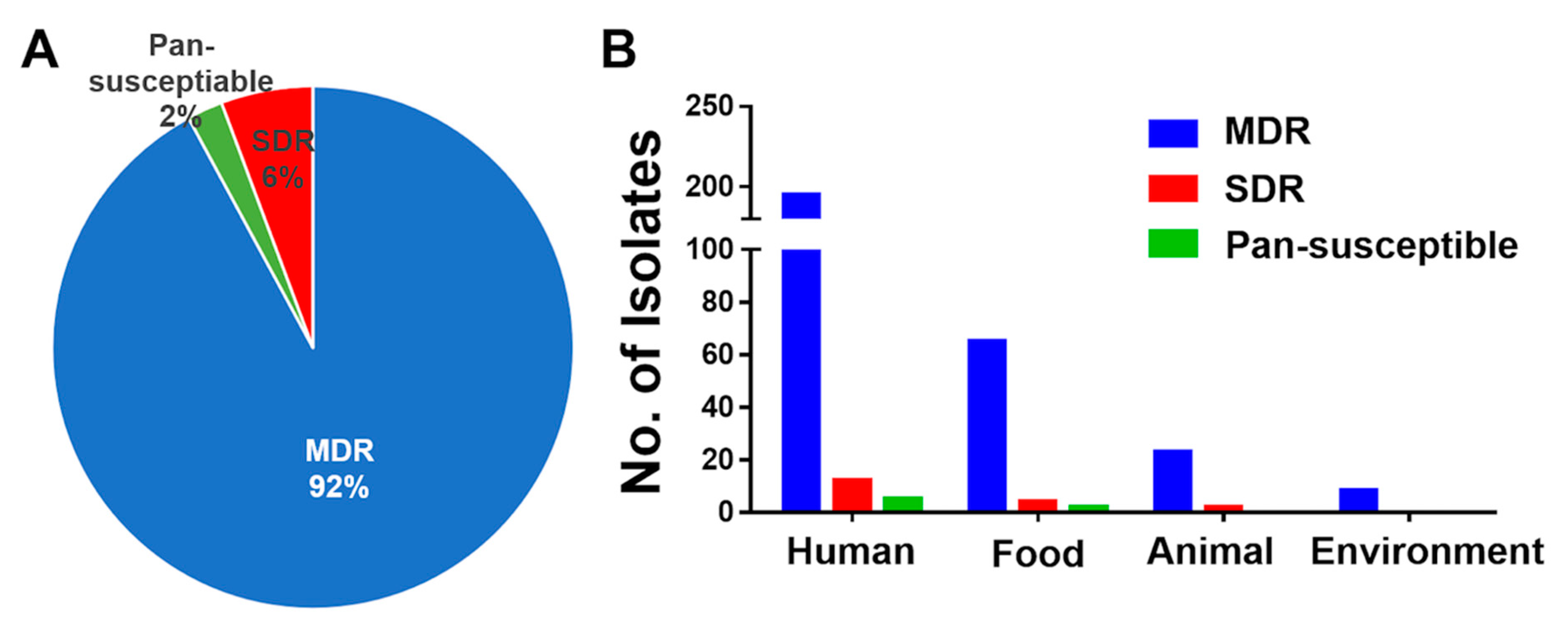
3.3. High Prevalence of MDR S. Rissen Isolates
3.4. Genomic Characterization of an Extensively Drug Resistant Salmonella Rissen
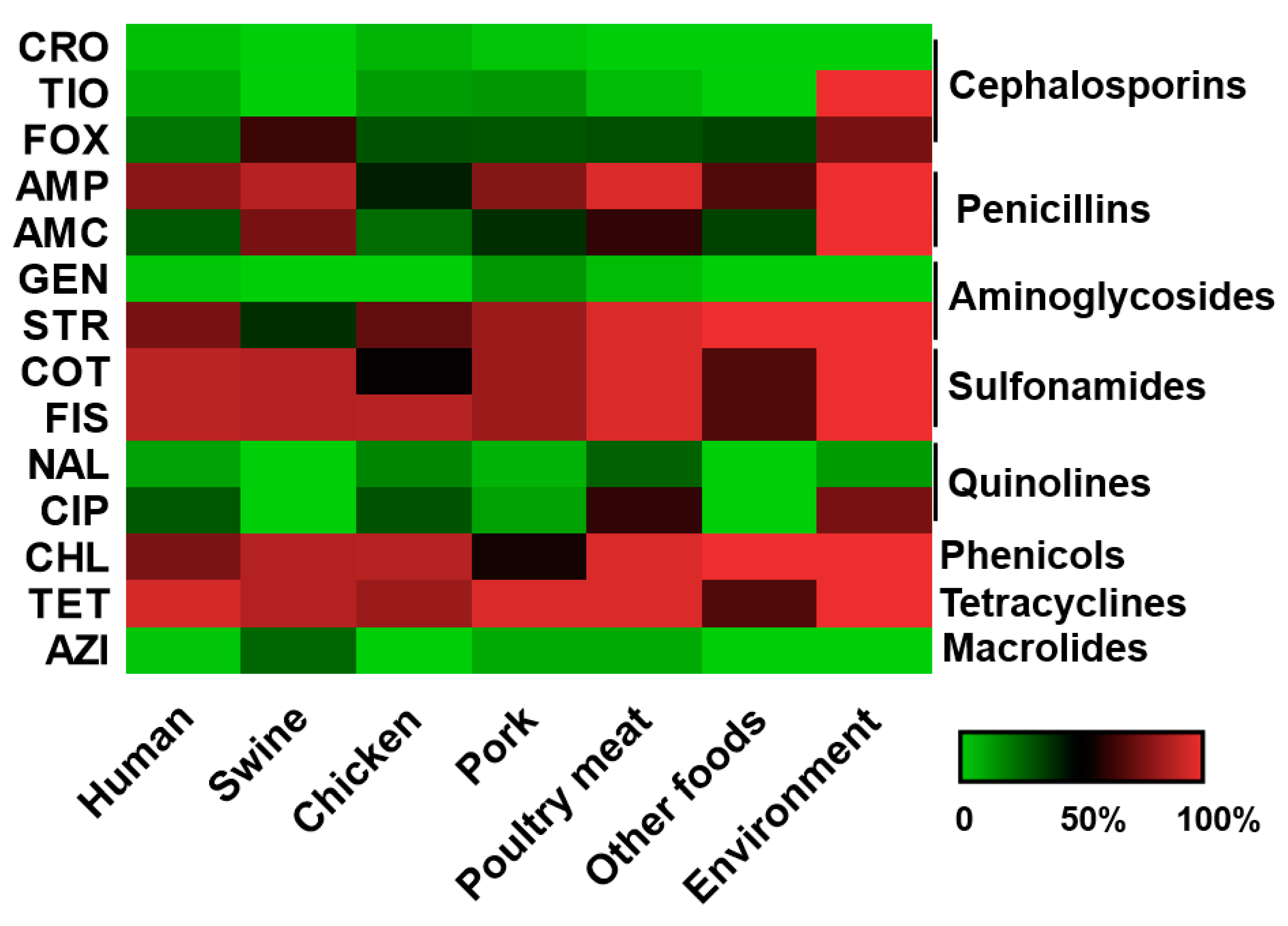
3.5. S. Rissen from Animal and Animal Products with Antimicrobial Resistance
3.6. Antimicrobial Susceptibility Pattern of the S. Rissen Isolates
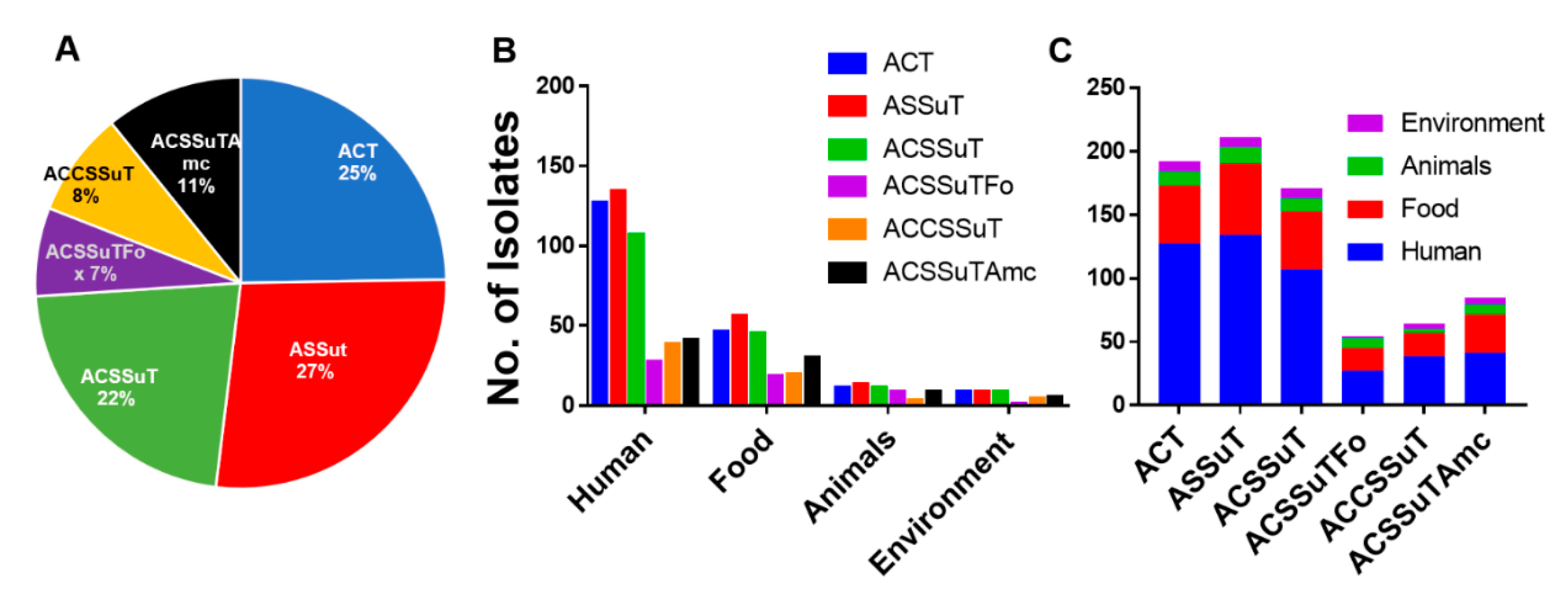
3.7. ASSuT (Ampicillin, Streptomycin, Sulphonamide, and Tetracycline), and ACSSuT (Ampicillin, Chloramphenicol, Streptomycin, Sulphonamide, and Tetracycline) Pattern of Antimicrobial Resistance
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Boyle, E.C.; Bishop, J.L.; Grassl, G.A.; Finlay, B.B. Salmonella: From pathogenesis to therapeutics. J. Bacteriol. 2007, 189, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- CDC. Incidence and trends of infection with pathogens transmitted commonly through food—Foodborne Diseases Active Sur-veillance Network, 10 U.S. Sites, 1996–2012. Wkly. Rep. 2013, 62, 283–287. [Google Scholar]
- Crump, J.A.; Sjölund-Karlsson, M.; Gordon, M.A.; Parry, C.M. Epidemiology, Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and Antimicrobial Management of Invasive Salmonella Infections. Clin. Microbiol. Rev. 2015, 28, 901–937. [Google Scholar] [CrossRef]
- Biswas, S.; Li, Y.; Elbediwi, M.; Yue, M. Emergence and Dissemination of mcr-Carrying Clinically Relevant Salmonella Typhimurium Monophasic Clone ST34. Microorganisms 2019, 7, 298. [Google Scholar] [CrossRef]
- Bengtsson, B.; Greko, C. Antibiotic resistance--consequences for animal health, welfare, and food production. Upsala J. Med. Sci. 2014, 119, 96–102. [Google Scholar] [CrossRef]
- Jajere, S.M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World 2019, 12, 504–521. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses. EFSA J. 2017, 15, e05077. [Google Scholar] [CrossRef]
- Vo, A.T.T.; van Duijkeren, E.; Fluit, A.C.; Heck, M.E.O.C.; Verbruggen, A.; Maas, H.M.E.; Gaastra, W. Distribution of Salmonella enterica serovars from humans, livestock and meat in Vietnam and the dominance of Salmonella Typhimurium phage type 90. Vet. Microbiol. 2006, 113, 153–158. [Google Scholar] [CrossRef]
- Lim, S.K.; Lee, H.S.; Nam, H.M.; Jung, S.C.; Koh, H.B.; Roh, I.S. Antimicrobial resistance and phage types of Salmonella isolates from healthy and diarrheic pigs in Korea. Foodborne Pathog. Dis. 2009, 6, 981–987. [Google Scholar] [CrossRef]
- Dalton, C.B.; Gregory, J.; Kirk, M.D.; Stafford, R.J.; Givney, R.; Kraa, E.; Gould, D. Foodborne disease outbreaks in Australia, 1995 to 2000. Commun. Dis. Intell. Q. Rep. 2004, 28, 211–224. [Google Scholar]
- Prasertsee, T.; Chuammitri, P.; Deeudom, M.; Chokesajjawatee, N.; Santiyanont, P.; Tadee, P.; Nuangmek, A.; Tadee, P.; Sheppard, S.K.; Pascoe, B.; et al. Core genome sequence analysis to characterize Salmonella enterica serovar Rissen ST469 from a swine production chain. Int. J. Food Microbiol. 2019, 304, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Elbediwi, M.; Pan, H.; Jiang, Z.; Biswas, S.; Li, Y.; Yue, M. Genomic Characterization of mcr-1-carrying Salmonella enterica Serovar 4,[5],12:i:-ST 34 Clone Isolated From Pigs in China. Front. Bioeng. Biotechnol. 2020, 8, 663. [Google Scholar] [CrossRef] [PubMed]
- Elbediwi, M.; Pan, H.; Biswas, S.; Li, Y.; Yue, M. Emerging colistin resistance in Salmonella enterica serovar Newport isolates from human infections. Emerg. Microbes Infect. 2020, 9, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, N.; Pan, H.; Elbediwi, M.; Zhou, X.; Peng, X.; Li, X.; Fang, W.; Yue, M. Characterization of Salmonella Dublin isolated from bovine and human hosts. BMC Microbiol. 2019, 19, 226. [Google Scholar] [CrossRef]
- Paudyal, N.; Pan, H.; Wu, B.; Zhou, X.; Zhou, X.; Chai, W.; Wu, Q.; Li, S.; Li, F.; Gu, G.; et al. Persistent Asymptomatic Human Infections by Salmonella enterica Serovar Newport in China. mSphere 2020, 5, e00163-20. [Google Scholar] [CrossRef]
- Elbediwi, M.; Li, Y.; Paudyal, N.; Pan, H.; Li, X.; Xie, S.; Rajkovic, A.; Feng, Y.; Fang, W.; Rankin, S.C.; et al. Global Burden of Colistin-Resistant Bacteria: Mobilized Colistin Resistance Genes Study (1980–2018). Microorganisms 2019, 7, 461. [Google Scholar] [CrossRef]
- Iwu, C.J.; Iweriebor, B.C.; Obi, L.C.; Basson, A.K.; Okoh, A.I. Multidrug-Resistant Salmonella Isolates from Swine in the Eastern Cape Province, South Africa. J. Food Prot. 2016, 79, 1234–1239. [Google Scholar] [CrossRef]
- Exner, M.; Bhattacharya, S.; Christiansen, B.; Gebel, J.; Goroncy-Bermes, P.; Hartemann, P.; Heeg, P.; Ilschner, C.; Kramer, A.; Larson, E.; et al. Antibiotic resistance: What is so special about multidrug-resistant Gram-negative bacteria? GMS Hyg. Infect. Control 2017, 12, Doc05. [Google Scholar] [CrossRef]
- Le Hello, S.; Hendriksen, R.S.; Doublet, B.; Fisher, I.; Nielsen, E.M.; Whichard, J.M.; Bouchrif, B.; Fashae, K.; Granier, S.A.; Jourdan-Da Silva, N.; et al. International spread of an epidemic population of Salmonella enterica serotype Kentucky ST198 resistant to ciprofloxacin. J. Infect. Dis. 2011, 204, 675–684. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, X.; Jiang, Z.; Qi, Y.; Ed-Dra, A.; Yue, M. Epidemiological investigation and antimicrobial resistance profiles of Salmonella isolated from breeder chicken hatcheries in Henan, China. Front. Cell Infect. Microbiol. 2020, 10, 497. [Google Scholar] [CrossRef]
- Wang, X.; Biswas, S.; Paudyal, N.; Pan, H.; Li, X.; Fang, W.; Yue, M. Antibiotic Resistance in Salmonella Typhimurium Isolates Recovered From the Food Chain Through National Antimicrobial Resistance Monitoring System Between 1996 and 2016. Front. Microbiol. 2019, 10, 985. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Song, H.; Bai, L. Call for Special Issue Papers: Food Safety in China: Current Practices and Future Needs. Foodborne Pathog. Dis. 2020, 17, 471. [Google Scholar] [CrossRef] [PubMed]
- OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2018 in Salmonellosis; Hymann, E.C., Poppe, C., Eds.; World Organization for Animal Health: Paris, France, 2016. [Google Scholar]
- Zhu, C.; Yue, M.; Rankin, S.; Weill, F.X.; Frey, J.; Schifferli, D.M. One-Step Identification of Five Prominent Chicken Salmonella Serovars and Biotypes. J. Clin. Microbiol. 2015, 53, 3881–3883. [Google Scholar] [CrossRef] [PubMed]
- Popoff, M.Y.; Le Minor, L. Antigenic Formulas of the Salmonella Serovars, 8th ed.; WHO Collaborating Centre for Reference and Research on Salmonella; Institute Pasteur: Paris, France, 2001. [Google Scholar]
- Biswas, S.; Elbediwi, M.; Gu, G.; Yue, M. Genomic Characterization of New Variant of Hydrogen Sulfide (H2S)-Producing Escherichia coli with Multidrug Resistance Properties Carrying the mcr-1 Gene in China dagger. Antibiotics 2020, 9, 80. [Google Scholar] [CrossRef]
- Yu, H.; Elbediwi, M.; Zhou, X.; Shuai, H.; Lou, X.; Wang, H.; Li, Y.; Yue, M. Epidemiological and Genomic Characterization of Campylobacter jejuni Isolates from a Foodborne Outbreak at Hangzhou, China. Int. J. Mol. Sci. 2020, 21, 3001. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Pan, H.; Pang, Z.; Li, F.; Peng, X.; Ed-Dra, A.; Li, Y.; Yue, M. Genomic characterization of Salmonella Uzaramo for human invasive infection. Microb. Genom. 2020, 6. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Antipov, D.; Hartwick, N.; Shen, M.; Raiko, M.; Lapidus, A.; Pevzner, P.A. plasmidSPAdes: Assembling plasmids from whole genome sequencing data. Bioinformatics 2016, 32, 3380–3387. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Higa, J. Outbreak of Salmonella Rissen Associated with Ground White Pepper: The Epi Investigation. 2011. Available online: http://www.cdph.ca.gov/programs/DFDRS/Documents/QSS_Presentation_SRissen_and_%20white%20pepper_010611.pdf (accessed on 20 June 2020).
- Hendriksen, R.; Bangtrakulnonth, A.; Pulsrikarn, C.; Pornreongwong, S.; Hasman, H.; Song, S.; Aarestrup, F. Antimicrobial Resistance and Molecular Epidemiology of Salmonella Rissen from Animals, Food Products, and Patients in Thailand and Denmark. Foodborne Pathog. Dis. 2008, 5, 605–619. [Google Scholar] [CrossRef]
- Irvine, N. Communicable Diseases Monthly Report, Northern Ireland Edition. The Health Protection Agency. 2009. Available online: http://www.publichealth.hscni.net/directorate-public-health/health-protection/surveillance-data (accessed on 20 June 2020).
- Giovannini, A.; Prencipe, V.; Conte, A.; Marino, L.; Petrini, A.; Pomilio, F.; Rizzi, V.; Migliorati, G. Quantitative risk assessment of Salmonella spp. infection for the consumer of pork products in an Italian region. Food Control 2004, 15, 139–144. [Google Scholar] [CrossRef]
- Mürmann, L.; Santos, M.; Cardoso, M. Prevalence, genetic characterization and antimicrobial resistance of Salmonella isolated from fresh pork sausages in Porto Alegre, Brazil. Food Control 2009, 20, 191–195. [Google Scholar] [CrossRef]
- Pires, S.M.; Vieira, A.R.; Hald, T.; Cole, D. Source attribution of human salmonellosis: An overview of methods and estimates. Foodborne Pathog. Dis. 2014, 11, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Lynne, A.; Foley, S.; Han, J. Salmonella: Properties and Occurrence. Encycl. Food Health 2016. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Zhang, Z.; Hu, Y.; Cao, C.; Wang, X.; Xi, M.; Xia, X.; Yang, B.; Meng, J. Distribution and Molecular Characterization of Salmonella enterica Hypermutators in Retail Food in China. J. Food Prot. 2015, 78, 1481–1487. [Google Scholar] [CrossRef]
- White, D.G.; Zhao, S.; Sudler, R.; Ayers, S.; Friedman, S.; Chen, S.; McDermott, P.F.; McDermott, S.; Wagner, D.D.; Meng, J. The isolation of antibiotic-resistant Salmonella from retail ground meats. N. Engl. J. Med. 2001, 345, 1147–1154. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, Y.; Xiong, Z.; Ma, Y.; Wei, Y.; Qu, X.; Zhang, H.; Zhang, J.; Liao, M. Highly Prevalent Multidrug-Resistant Salmonella From Chicken and Pork Meat at Retail Markets in Guangdong, China. Front. Microbiol. 2018, 9, 2104. [Google Scholar] [CrossRef]
- Yang, H.; Paruch, L.; Chen, X.; van Eerde, A.; Skomedal, H.; Wang, Y.; Liu, D.; Liu Clarke, J. Antibiotic Application and Resistance in Swine Production in China: Current Situation and Future Perspectives. Front. Vet. Sci. 2019, 6, 136. [Google Scholar] [CrossRef]
- Bai, L.; Hurley, D.; Li, J.; Meng, Q.; Wang, J.; Fanning, S.; Xiong, Y. Characterisation of multidrug-resistant Shiga toxin-producing Escherichia coli cultured from pigs in China: Co-occurrence of extended-spectrum beta-lactamase- and mcr-1-encoding genes on plasmids. Int. J. Antimicrob. Agents 2016, 48, 445–448. [Google Scholar] [CrossRef]
- Arguello, H.; Alvarez-Ordonez, A.; Carvajal, A.; Rubio, P.; Prieto, M. Role of slaughtering in Salmonella spreading and control in pork production. J. Food Prot. 2013, 76, 899–911. [Google Scholar] [CrossRef]
- Caleja, C.; de Toro, M.; Gonçalves, A.; Themudo, P.; Vieira-Pinto, M.; Monteiro, D.; Rodrigues, J.; Sáenz, Y.; Carvalho, C.; Igrejas, G.; et al. Antimicrobial resistance and class I integrons in Salmonella enterica isolates from wild boars and Bísaro pigs. Int. Microbiol. 2011, 14, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ye, C.; Chang, W.; Sun, S. Serotype Distribution, Antimicrobial Resistance, and Class 1 Integrons Profiles of Salmonella from Animals in Slaughterhouses in Shandong Province, China. Front. Microbiol. 2017, 8, 1049. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-H.; Zhu, Y.-H.; Ren, T.-Y.; Guo, L.; Yang, G.-Y.; Jiao, L.-G.; Wang, J.-F. Distribution and Antimicrobial Resistance of Salmonella Isolated from Pigs with Diarrhea in China. Microorganisms 2018, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Riaño, I.; Moreno, M.A.; Teshager, T.; Sáenz, Y.; Domínguez, L.; Torres, C. Detection and characterization of extended-spectrum beta-lactamases in Salmonella enterica strains of healthy food animals in Spain. J. Antimicrob. Chemother. 2006, 58, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Astorga Márquez, R.; Salaberria, A.; García, A.; Valdezate, S.; Carbonero, A.; García, A.; Arenas, A. Surveillance and Antimicrobial Resistance of Salmonella Strains Isolated from Slaughtered Pigs in Spain. J. Food Prot. 2007, 70, 1502–1506. [Google Scholar] [CrossRef]
- Li, R.; Xie, M.; Zhang, J.; Yang, Z.; Liu, L.; Liu, X.; Zheng, Z.; Chan, E.W.; Chen, S. Genetic characterization of mcr-1-bearing plasmids to depict molecular mechanisms underlying dissemination of the colistin resistance determinant. J. Antimicrob. Chemother. 2017, 72, 393–401. [Google Scholar] [CrossRef]
- García-Feliz, C.; Collazos, J.A.; Carvajal, A.; Vidal, A.; Aladueña, A.; Ramiro, R.; de la Fuente del Moral, F.; Echeita, M.A.; Rubio, P. Salmonella enterica Infections in Spanish Swine Fattening Units. Zoonoses Public Health 2007, 54, 294–300. [Google Scholar] [CrossRef]
- Pornsukarom, S.; Patchanee, P.; Erdman, M.; Cray, P.; Wittum, T.; Lee, J.; Gebreyes, W. Comparative Phenotypic and Genotypic Analyses of Salmonella Rissen that Originated from Food Animals in Thailand and United States. Zoonoses Public Health 2014, 62, 151–158. [Google Scholar] [CrossRef]
- Tadee, P.; Boonkhot, P.; Pornruangwong, S.; Patchanee, P. Comparative phenotypic and genotypic characterization of Salmonella spp. in pig farms and slaughterhouses in two provinces in northern Thailand. PLoS ONE 2015, 10, e0116581. [Google Scholar] [CrossRef]
- García-Fierro, R.; Montero, I.; Bances, M.; González-Hevia, M.Á.; Rodicio, M.R. Antimicrobial Drug Resistance and Molecular Typing of Salmonella enterica Serovar Rissen from Different Sources. Microb. Drug Resist. 2015, 22, 211–217. [Google Scholar] [CrossRef]
- Carattoli, A. Plasmids and the spread of resistance. Int. J. Med. Microbiol. 2013, 303, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Frech, G.; Schwarz, S. Molecular analysis of tetracycline resistance in Salmonella enterica subsp. enterica serovars Typhimurium, Enteritidis, Dublin, Choleraesuis, Hadar and Saintpaul: Construction and application of specific gene probes. J. Appl. Microbiol. 2000, 89, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Frye, J.G.; Jackson, C.R. Genetic mechanisms of antimicrobial resistance identified in Salmonella enterica, Escherichia coli, and Enteroccocus spp. isolated from U.S. food animals. Front. Microbiol. 2013, 4, 135. [Google Scholar] [CrossRef] [PubMed]
- de Toro, M.; Sáenz, Y.; Cercenado, E.; Rojo-Bezares, B.; García-Campello, M.; Undabeitia, E.; Torres, C. Genetic characterization of the mechanisms of resistance to amoxicillin/clavulanate and third-generation cephalosporins in Salmonella enterica from three Spanish hospitals. Int. Microbiol. 2011, 14, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Eguale, T.; Birungi, J.; Asrat, D.; Njahira, M.N.; Njuguna, J.; Gebreyes, W.A.; Gunn, J.S.; Djikeng, A.; Engidawork, E. Genetic markers associated with resistance to beta-lactam and quinolone antimicrobials in non-typhoidal Salmonella isolates from humans and animals in central Ethiopia. Antimicrob. Resist. Infect. Control 2017, 6, 13. [Google Scholar] [CrossRef]
- García, V.; Vázquez, X.; Bances, M.; Herrera-León, L.; Herrera-León, S.; Rodicio, M.R. Molecular Characterization of Salmonella enterica Serovar Enteritidis, Genetic Basis of Antimicrobial Drug Resistance and Plasmid Diversity in Ampicillin-Resistant Isolates. Microb. Drug Resist. 2018, 25, 219–226. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updates 2010, 13, 151–171. [Google Scholar] [CrossRef]
- Wang, N.; Yang, X.; Jiao, S.; Zhang, J.; Ye, B.; Gao, S. Sulfonamide-Resistant Bacteria and Their Resistance Genes in Soils Fertilized with Manures from Jiangsu Province, Southeastern China. PLoS ONE 2014, 9, e112626. [Google Scholar] [CrossRef]
- Antunes, P.; Machado, J.; Peixe, L. Dissemination of sul3-containing elements linked to class 1 integrons with an unusual 3’ conserved sequence region among Salmonella isolates. Antimicrob. Agents Chemother. 2007, 51, 1545–1548. [Google Scholar] [CrossRef]
- Wang, W.; Peng, Z.; Baloch, Z.; Hu, Y.; Xu, J.; Zhang, W.; Fanning, S.; Li, F. Genomic characterization of an extensively-drug resistance Salmonella enterica serotype Indiana strain harboring bla(NDM-1) gene isolated from a chicken carcass in China. Microbiol. Res. 2017, 204, 48–54. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Tahoun, A.; El-Gohary, A.E.-G.A.; El-Abasy, M.; El-Khayat, F.; Gillespie, T.; Kitade, Y.; Hafez, H.M.; Neubauer, H.; El-Adawy, H. Epidemiological, molecular characterization and antibiotic resistance of Salmonella enterica serovars isolated from chicken farms in Egypt. Gut Pathog. 2017, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Krauland, M.; Harrison, L.; Paterson, D.; Marsh, J. Novel integron gene cassette arrays identified in a global collection of multi-drug resistant non-typhoidal Salmonella enterica. Curr. Microbiol. 2010, 60, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Miko, A.; Pries, K.; Schroeter, A.; Helmuth, R. Molecular mechanisms of resistance in multidrug-resistant serovars of Salmonella enterica isolated from foods in Germany. J. Antimicrob. Chemother. 2005, 56, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, S.; Bruini, I.; Alpigiani, I.; Vismarra, A.; Barilli, E.; Brindani, F.; Morganti, M.; Bellotti, P.; Bolzoni, L.; Pongolini, S. Influence of Pigskin on Salmonella Contamination of Pig Carcasses and Cutting Lines in an Italian Slaughterhouse. Ital. J. Food Saf. 2016, 5, 5654. [Google Scholar] [CrossRef]
- Antunes, P.; Machado, J.; Peixe, L. Characterization of antimicrobial resistance and class 1 and 2 integrons in Salmonella enterica isolates from different sources in Portugal. J. Antimicrob. Chemother. 2006, 58, 297–304. [Google Scholar] [CrossRef]
- Clemente, L.; Manageiro, V.; Ferreira, E.; Jones-Dias, D.; Correia, I.; Themudo, P.; Albuquerque, T.; Caniça, M. Occurrence of extended-spectrum β-lactamases among isolates of Salmonella enterica subsp. enterica from food-producing animals and food products, in Portugal. Int. J. Food Microbiol. 2013, 167, 221–228. [Google Scholar] [CrossRef]
- Antunes, P.; Mourão, J.; Pestana, N.; Peixe, L. Leakage of emerging clinically relevant multidrug-resistant Salmonella clones from pig farms. J. Antimicrob. Chemother. 2011, 66, 2028–2032. [Google Scholar] [CrossRef]
- Sinwat, N.; Angkittitrakul, S.; Coulson, K.F.; Pilapil, F.; Meunsene, D.; Chuanchuen, R. High prevalence and molecular characteristics of multidrug-resistant Salmonella in pigs, pork and humans in Thailand and Laos provinces. J. Med. Microbiol. 2016, 65, 1182–1193. [Google Scholar] [CrossRef]
- Maurer, J.; Martin, G.; Hernandez, S.; Cheng, Y.; Gerner-Smidt, P.; Hise, K.; D’Angelo, M.; Cole, D.; Sanchez, S.; Madden, M.; et al. Diversity and Persistence of Salmonella enterica Strains in Rural Landscapes in the Southeastern United States. PLoS ONE 2015, 10, e0128937. [Google Scholar] [CrossRef]
- Martinez-Urtaza, J.; Liebana, E. Investigation of clonal distribution and persistence of Salmonella Senftenberg in the marine environment and identification of potential sources of contamination. FEMS Microbiol. Ecol. 2005, 52, 255–263. [Google Scholar] [CrossRef]
- Biswas, S.; Raoult, D.; Rolain, J.M. A bioinformatic approach to understanding antibiotic resistance in intracellular bacteria through whole genome analysis. Int. J. Antimicrob. Agents 2008, 32, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Samosornsuk, S. Significant increase in antibiotic resistance of Salmonella isolates from human beings and chicken meat in Thailand. Vet. Microbiol. 1998, 62, 73–80. [Google Scholar]
- Tian-Grim, S. Susceptibility patterns of clinical bacterial isolates in nineteen selected hospitals in Thailand. J. Med Assoc. Thail. = Chotmaihet Thangphaet 1994, 77, 298–307. [Google Scholar]
- Isenbarger, D.W.; Hoge, C.W.; Srijan, A.; Pitarangsi, C.; Vithayasai, N.; Bodhidatta, L.; Hickey, K.W.; Cam, P.D. Comparative antibiotic resistance of diarrheal pathogens from Vietnam and Thailand, 1996–1999. Emerg. Infect. Dis. 2002, 8, 175–180. [Google Scholar] [CrossRef]
| Antibiotic Classes | SAL02425 | SAL02454 | SAL02475 | SAL02482 | SAL02490 | SAL02560 | SAL02592 | SAL02603 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) | Related Genes | MIC (mg/L) | Related Genes | MIC (mg/L) | Related Genes | MIC (mg/L) | Related Genes | MIC (mg/L) | Related Genes | MIC (mg/L) | Related Genes | MIC (mg/L) | Related Genes | MIC (mg/L) | Related Genes | |||
| Antimicrobial Susceptibility testing | β-Lactam and β-Lactams inhibitor | AMP | >32 | blaTEM-1B blaCTX-M-14 | >32 | blaTEM-1B blaCTX-M-14 | >32 | blaCTX-M-14 | >32 | blaTEM-1B blaCTX-M-27 | >32 | blaTEM-1B blaCTX-M-55 | 16 | 32 | blaTEM-1B | 32 | blaTEM-1B | |
| AMC | >32/16 | >32/16 | >32/16 | >32/16 | >32/16 | 8/4 | >32/16 | >32/16 | ||||||||||
| Amino- glycoside | STR | 32 | aadA2, aadA1, aac(6′)-Iaa, aph(3″)-lld | 64 | aadA2, aadA1, aac(6′)-Iaa | 64 | aadA2, aac(6′)-Iaa | >64 | aadA2, aadA1, aac(6′)-Iaa | >64 | aadA2, aadA1, aac(6′)-Iaa, ant(3″)-Ia | >64 | aadA2, aac(6′)-Iaa | >64 | aadA2, aadA1, aac(6′)-Iaa, ant(3″)-Ia | >64 | aadA2, aadA1, aac(6′)-Iaa | |
| GEN | >16 | >16 | 2 | 2 | 2 | 1 | 2 | 2 | ||||||||||
| Macrolides | AZI | 8 | 8 | 8 | 8 | 8 | 4 | 8 | 8 | |||||||||
| Quinolone | CIP | 0.03 | 0.03 | 0.03 | 0.03 | 0.06 | 0.06 | 0.06 | 0.06 | |||||||||
| NAL | 4 | 4 | 4 | 4 | 4 | 8 | 4 | 4 | ||||||||||
| Phenicol | CHL | 8 | 8 | 8 | 8 | 32 | CmlA2 | 32 | CmlA2 | 32 | CmlA2 | 32 | CmlA2 | |||||
| Sulfaxisazole | FIS | >256 | sul3 | >256 | sul3 | 1 | >256 | sul3 | >256 | sul3 | 1 | >256 | sul3 | >256 | sul3 | |||
| Trimethoprim/Sulphonamide | COT | >32/608 | dfrA12, sul3 | >32/608 | dfrA12, sul3 | >32/608 | dfrA12 | >32/608 | dfrA12, sul3 | >32/608 | dfrA12, sul3 | >32/608 | dfrA12 | >32/608 | dfrA12, sul3 | >32/608 | dfrA12, sul3 | |
| Tetra-cyclines | TET | >32 | tet(A) | >32 | tet(A) | >32 | tet(A) | >32 | tet(A) | >32 | tet(A) | >32 | tet(A) | >32 | tet(A) | >32 | tet(A) | |
| Cephalo-sporines | CRO | >64 | blaTEM-1B blaCTX-M-14 | >64 | blaTEM-1B blaCTX-M-14 | 32 | blaCTX-M-14 | >64 | blaTEM-1B blaCTX-M-27 | >64 | blaTEM-1B blaCTX-M-55 | 2 | 4 | blaTEM-1B | 4 | blaTEM-1B | ||
| TIO | >8 | >8 | >8 | >8 | >8 | 1 | >8 | >8 | ||||||||||
| FOX | 8 | 16 | 16 | 16 | 16 | 1 | 32 | 32 | ||||||||||
| Host | Human | Human | Human | Human | Human | Live Swine | Chicken meat | Pork | ||||||||||
| Collection place | Fujian | Shanghai | Fujian | Chongqing | Chongqing | Jiangsu | Guangdong | Guangxi | ||||||||||
| Sequence type | ST469 | ST469 | ST469 | ST469 | ST469 | ST469 | ST469 | ST469 | ||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Biswas, S.; Gu, G.; Elbediwi, M.; Li, Y.; Yue, M. Characterization of Multidrug Resistance Patterns of Emerging Salmonella enterica Serovar Rissen along the Food Chain in China. Antibiotics 2020, 9, 660. https://doi.org/10.3390/antibiotics9100660
Xu X, Biswas S, Gu G, Elbediwi M, Li Y, Yue M. Characterization of Multidrug Resistance Patterns of Emerging Salmonella enterica Serovar Rissen along the Food Chain in China. Antibiotics. 2020; 9(10):660. https://doi.org/10.3390/antibiotics9100660
Chicago/Turabian StyleXu, Xuebin, Silpak Biswas, Guimin Gu, Mohammed Elbediwi, Yan Li, and Min Yue. 2020. "Characterization of Multidrug Resistance Patterns of Emerging Salmonella enterica Serovar Rissen along the Food Chain in China" Antibiotics 9, no. 10: 660. https://doi.org/10.3390/antibiotics9100660
APA StyleXu, X., Biswas, S., Gu, G., Elbediwi, M., Li, Y., & Yue, M. (2020). Characterization of Multidrug Resistance Patterns of Emerging Salmonella enterica Serovar Rissen along the Food Chain in China. Antibiotics, 9(10), 660. https://doi.org/10.3390/antibiotics9100660






