Contributions of Net Charge on the PlyC Endolysin CHAP Domain
Abstract
1. Introduction
2. Results
2.1. Library of PlyC CHAP Mutants
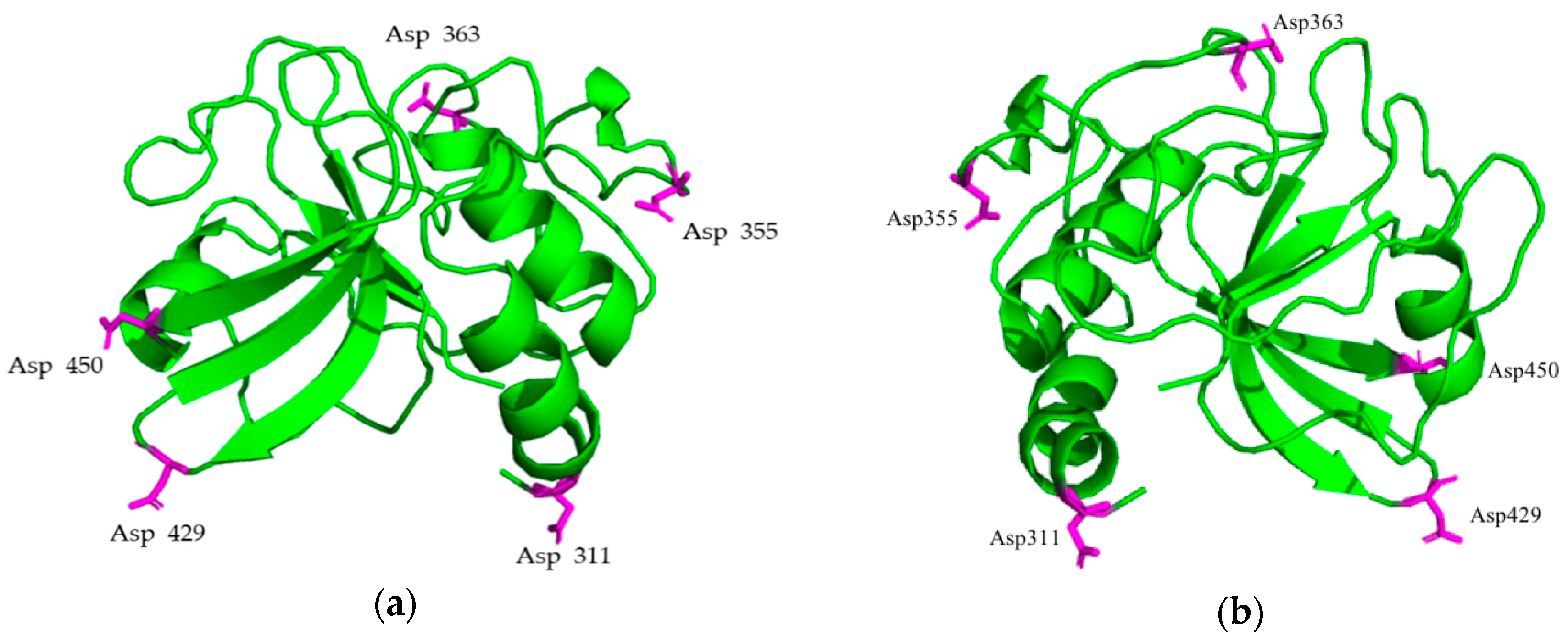
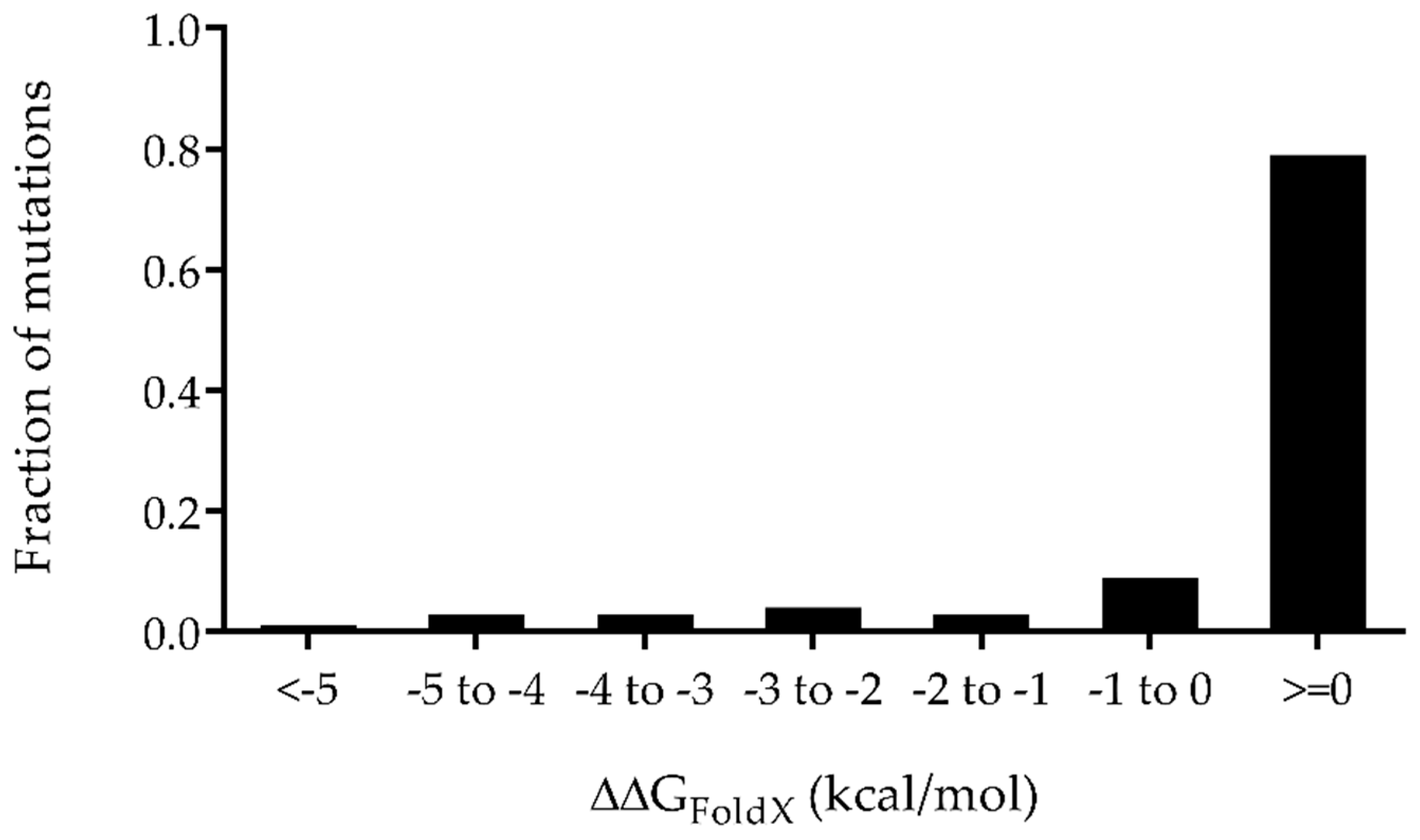
2.2. Prediction of the Properly Folded PlyC CHAP Mutants via ΔΔGFoldX
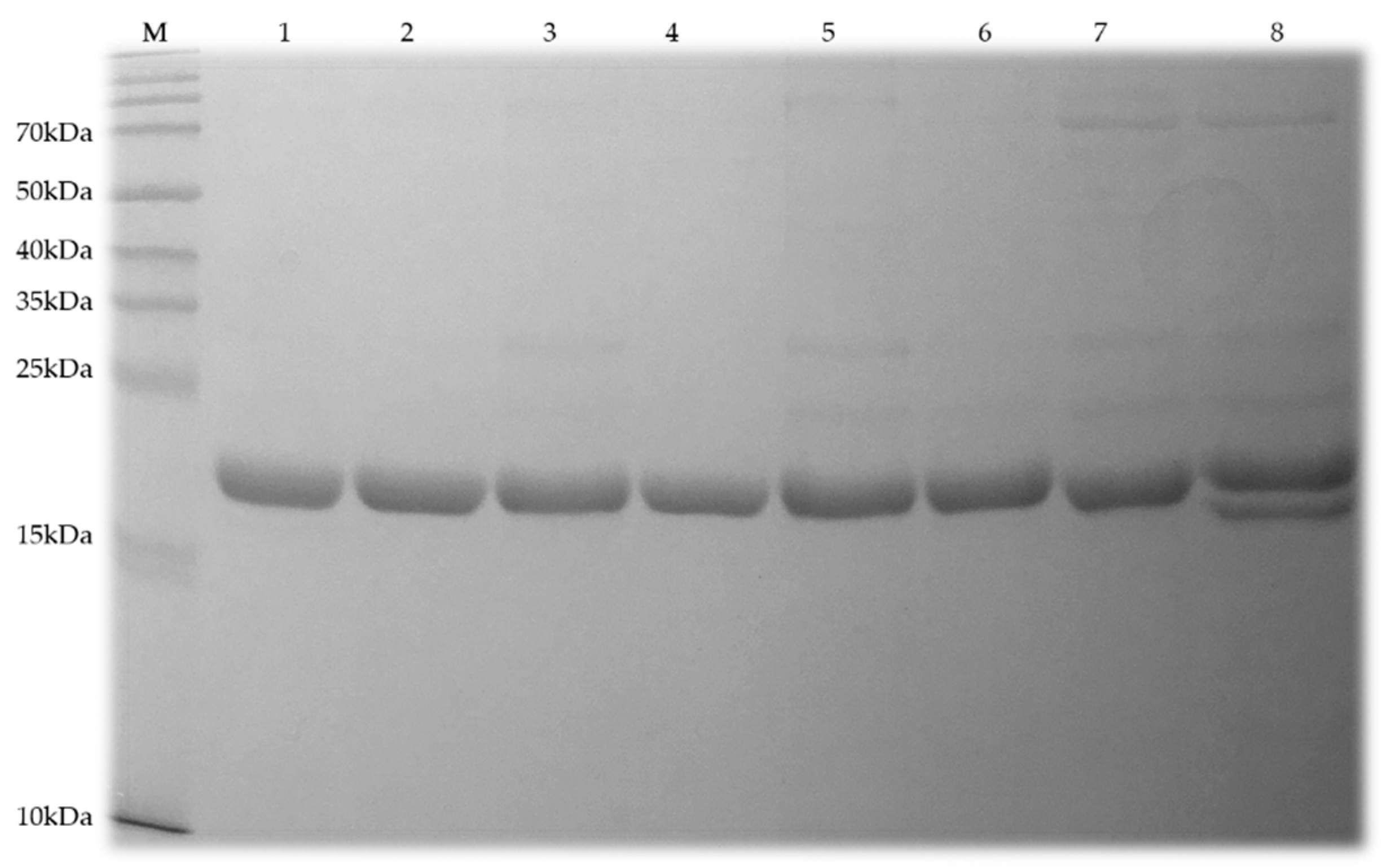
2.3. Protein Solubility and Purity
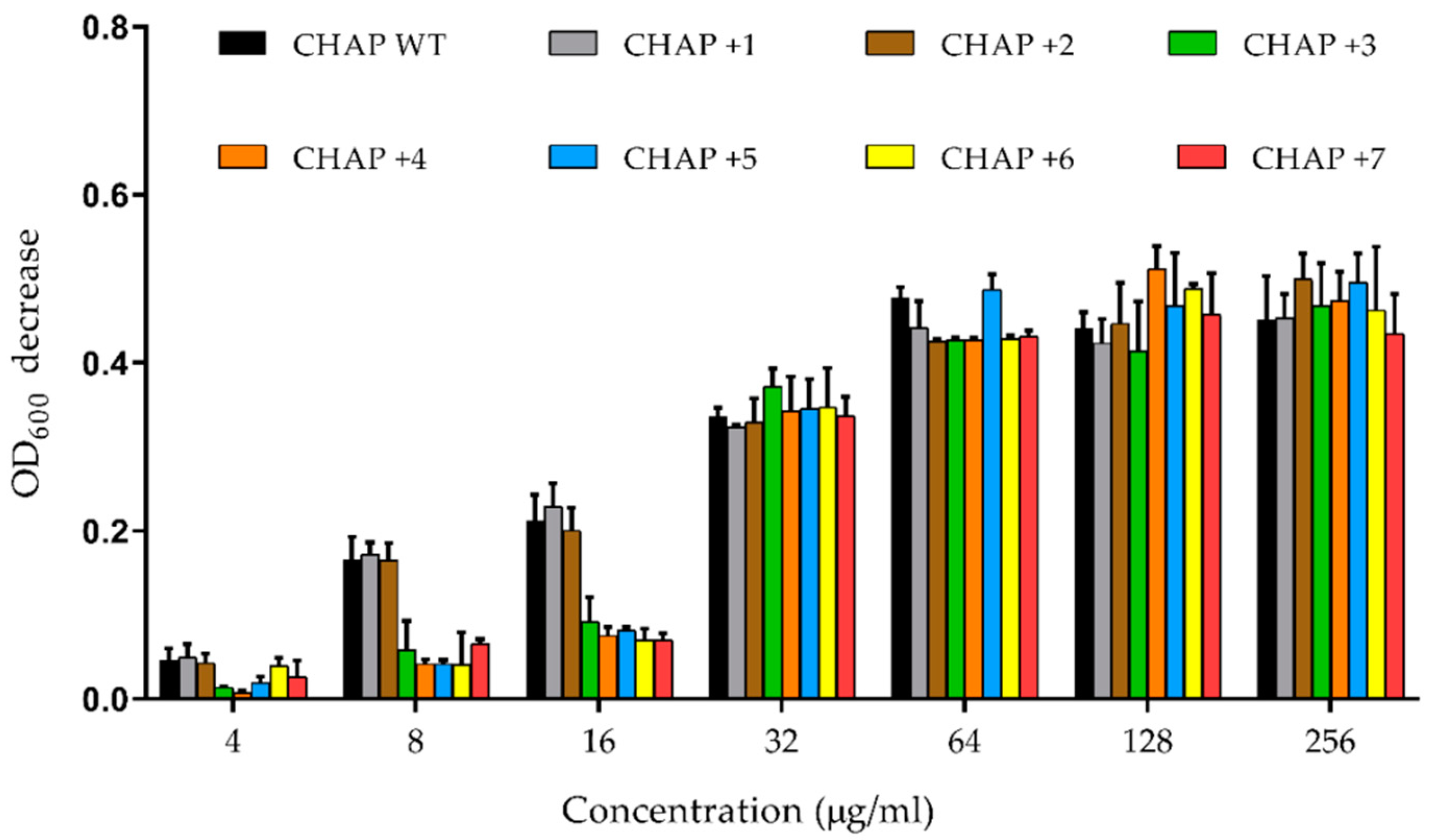
2.4. In Vitro PlyC CHAP Mutants’ Activity
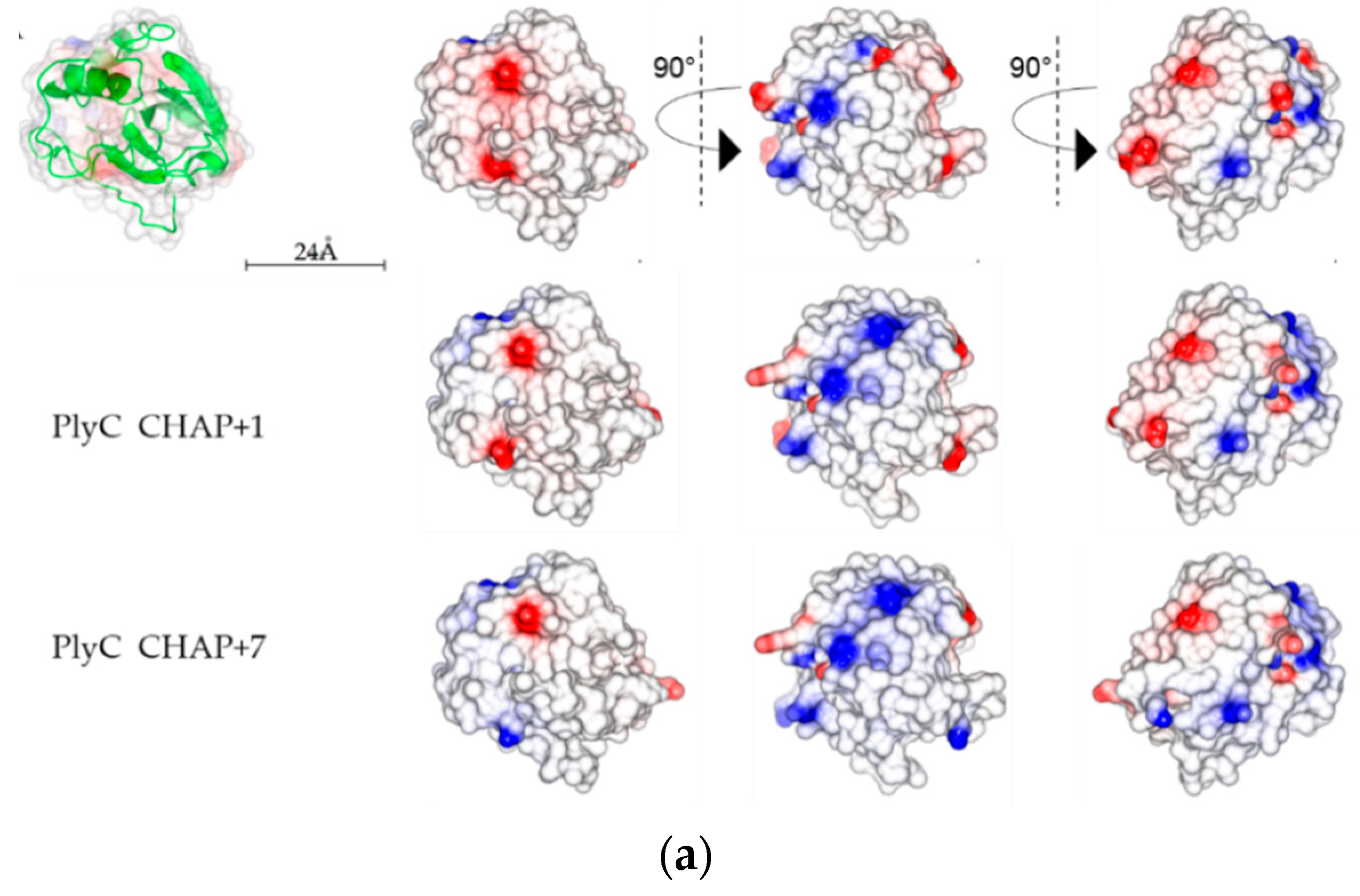
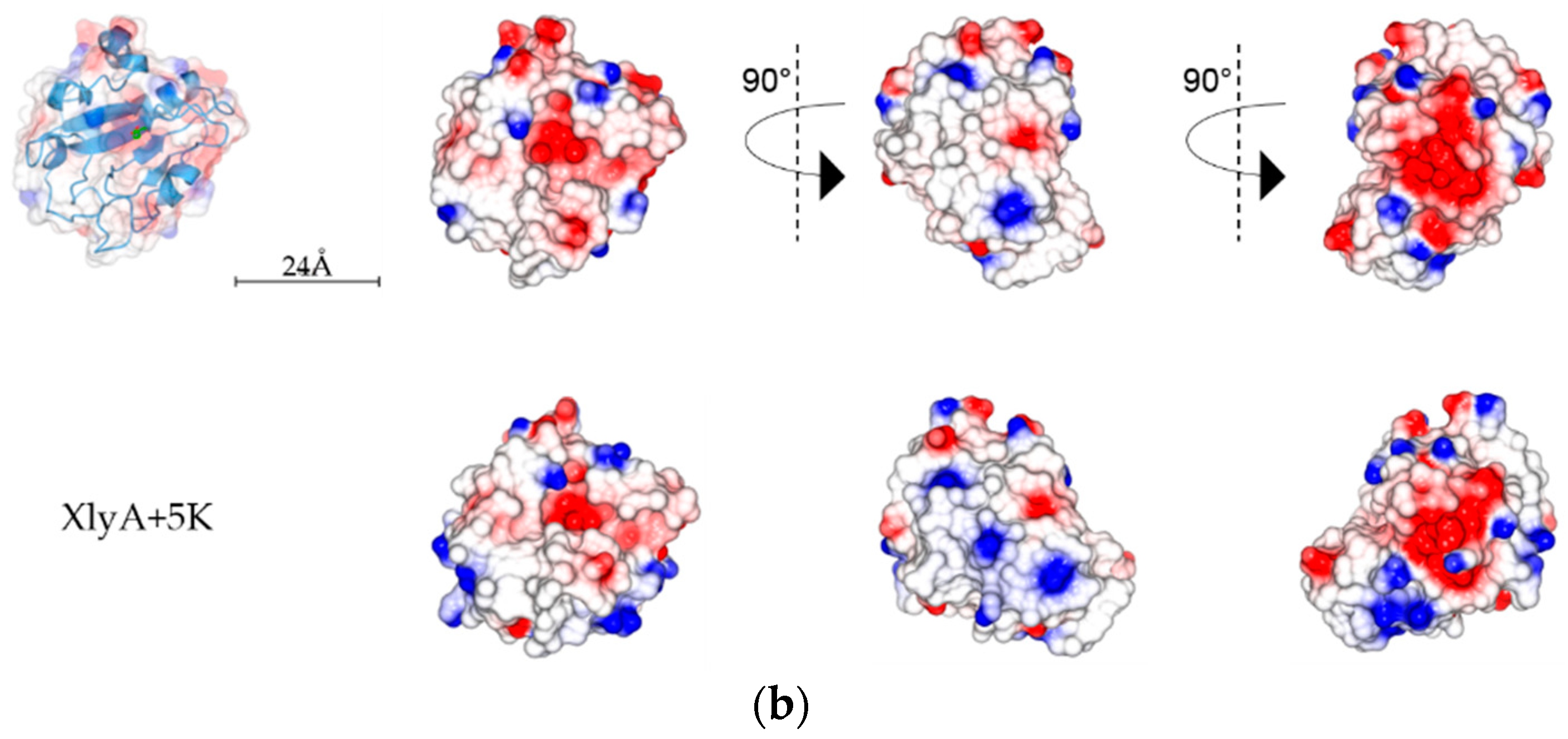
2.5. Analysis of PlyC CHAP Electrostatic Surface Potential
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. In Silico Modeling of PlyC CHAP Mutants
4.3. Cloning and Site-Directed Mutagenesis
4.4. Protein Expression and Purification
4.5. In Vitro PlyC CHAP Activity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fischetti, V.A. Exploiting what phage have evolved to control gram-positive pathogens. Bacteriophage 2011, 1, 188–194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Young, R. Bacteriophage lysis: mechanism and regulation. Microbiol. Rev. 1992, 56, 430–481. [Google Scholar]
- Fischetti, V.A.; Nelson, D.; Schuch, R. Reinventing phage therapy: are the parts greater than the sum? Nat. Biotechnol. 2006, 24, 1508–1511. [Google Scholar] [CrossRef] [PubMed]
- Briers, Y.; Walmagh, M.; Grymonprez, B.; Biebl, M.; Pirnay, J.P.; Defraine, V.; Michiels, J.; Cenens, W.; Aertsen, A.; Miller, S.; et al. Art-175 is a highly efficient antibacterial against multidrug-resistant strains and persisters of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 3774–3784. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Melo, L.D.; Santos, S.B.; Nobrega, F.L.; Ferreira, E.C.; Cerca, N.; Azeredo, J.; Kluskens, L.D. Molecular aspects and comparative genomics of bacteriophage endolysins. J. Virol. 2013, 87, 4558–4570. [Google Scholar] [CrossRef]
- Nelson, D.C.; Schmelcher, M.; Rodriguez-Rubio, L.; Klumpp, J.; Pritchard, D.G.; Dong, S.; Donovan, D.M. Endolysins as antimicrobials. Adv. Virus Res. 2012, 83, 299–365. [Google Scholar] [CrossRef] [PubMed]
- Broendum, S.S.; Buckle, A.M.; McGowan, S. Catalytic diversity and cell wall binding repeats in the phage-encoded endolysins. Mol. Microbiol. 2018, 110, 879–896. [Google Scholar] [CrossRef]
- Linden, S.B.; Zhang, H.; Heselpoth, R.D.; Shen, Y.; Schmelcher, M.; Eichenseher, F.; Nelson, D.C. Biochemical and biophysical characterization of PlyGRCS, a bacteriophage endolysin active against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2015, 99, 741–752. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, H.; Yu, J.; Wei, H. Molecular dissection of phage lysin PlySs2: integrity of the catalytic and cell wall binding domains is essential for its broad lytic activity. Virol. Sin. 2015, 30, 45–51. [Google Scholar] [CrossRef]
- Porter, C.J.; Schuch, R.; Pelzek, A.J.; Buckle, A.M.; McGowan, S.; Wilce, M.C.; Rossjohn, J.; Russell, R.; Nelson, D.; Fischetti, V.A.; et al. The 1.6 A crystal structure of the catalytic domain of PlyB, a bacteriophage lysin active against Bacillus anthracis. J. Mol. Biol. 2007, 366, 540–550. [Google Scholar] [CrossRef]
- Sanz, J.M.; Diaz, E.; Garcia, J.L. Studies on the structure and function of the N-terminal domain of the pneumococcal murein hydrolases. Mol. Microbiol. 1992, 6, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Donovan, D.M.; Lardeo, M.; Foster-Frey, J. Lysis of staphylococcal mastitis pathogens by bacteriophage phi11 endolysin. FEMS Microbiol. Lett. 2006, 265, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Horgan, M.; O’Flynn, G.; Garry, J.; Cooney, J.; Coffey, A.; Fitzgerald, G.F.; Ross, R.P.; McAuliffe, O. Phage lysin LysK can be truncated to its CHAP domain and retain lytic activity against live antibiotic-resistant staphylococci. Appl. Environ. Microbiol. 2009, 75, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Fischetti, V.A. Mutagenesis of a bacteriophage lytic enzyme PlyGBS significantly increases its antibacterial activity against group B streptococci. Appl. Microbiol. Biotechnol. 2007, 74, 1284–1291. [Google Scholar] [CrossRef]
- Low, L.Y.; Yang, C.; Perego, M.; Osterman, A.; Liddington, R. Role of net charge on catalytic domain and influence of cell wall binding domain on bactericidal activity, specificity, and host range of phage lysins. J. Biol. Chem. 2011, 286, 34391–34403. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.J.; Garefalaki, V.; Spoerl, R.; Narbad, A.; Meijers, R. Structure-based modification of a Clostridium difficile-targeting endolysin affects activity and host range. J. Bacteriol. 2011, 193, 5477–5486. [Google Scholar] [CrossRef]
- Rodriguez-Rubio, L.; Chang, W.L.; Gutierrez, D.; Lavigne, R.; Martinez, B.; Rodriguez, A.; Govers, S.K.; Aertsen, A.; Hirl, C.; Biebl, M.; et al. ’Artilysation’ of endolysin lambdaSa2lys strongly improves its enzymatic and antibacterial activity against streptococci. Sci. Rep. 2016, 6, 35382. [Google Scholar] [CrossRef]
- McGowan, S.; Buckle, A.M.; Mitchell, M.S.; Hoopes, J.T.; Gallagher, D.T.; Heselpoth, R.D.; Shen, Y.; Reboul, C.F.; Law, R.H.; Fischetti, V.A.; et al. X-ray crystal structure of the streptococcal specific phage lysin PlyC. Proc. Natl. Acad. Sci. USA 2012, 109, 12752–12757. [Google Scholar] [CrossRef]
- Heselpoth, R.D.; Yin, Y.; Moult, J.; Nelson, D.C. Increasing the stability of the bacteriophage endolysin PlyC using rationale-based FoldX computational modeling. Protein Eng. Des. Sel. 2015, 28, 85–92. [Google Scholar] [CrossRef]
- Yang, H.; Linden, S.B.; Wang, J.; Yu, J.; Nelson, D.C.; Wei, H. A chimeolysin with extended-spectrum streptococcal host range found by an induced lysis-based rapid screening method. Sci. Rep. 2015, 5, 17257. [Google Scholar] [CrossRef]
- Yang, H.; Gong, Y.; Zhang, H.; Etobayeva, I.; Miernikiewicz, P.; Luo, D.; Li, X.; Zhang, X.; Dabrowska, K.; Nelson, D.C.; et al. ClyJ, a novel pneumococcal chimeric lysin with a CHAP catalytic domain. Antimicrob. Agents Chemother. 2019. [Google Scholar] [CrossRef] [PubMed]
- Schymkowitz, J.; Borg, J.; Stricher, F.; Nys, R.; Rousseau, F.; Serrano, L. The FoldX web server: an online force field. Nucleic Acids Res. 2005, 33, W382–W388. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Loomis, L.; Fischetti, V.A. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA 2001, 98, 4107–4112. [Google Scholar] [CrossRef] [PubMed]
- McNicholas, S.; Potterton, E.; Wilson, K.S.; Noble, M.E. Presenting your structures: the CCP4mg molecular-graphics software. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef] [PubMed]
- Heselpoth, R.D.; Swift, S.M.; Linden, S.B.; Mitchell, M.S.; Nelson, D.C. Enzybiotics: Endolysins and Bacteriocins. In Bacteriophages—Biology, Technology, Therapy; Harper, D., Abedon, S.T., Burrowes, B., McConvill, M., Eds.; Springer International Publishing: Dordrecht, The Netherlands, 2018. [Google Scholar]
- Sheehan, M.M.; Garcia, J.L.; Lopez, R.; Garcia, P. The lytic enzyme of the pneumococcal phage Dp-1: a chimeric lysin of intergeneric origin. Mol. Microbiol. 1997, 25, 717–725. [Google Scholar] [CrossRef]
- Yang, H.; Bi, Y.; Shang, X.; Wang, M.; Linden, S.B.; Li, Y.; Li, Y.; Nelson, D.C.; Wei, H. Antibiofilm activities of a novel chimeolysin against Streptococcus mutans under physiological and cariogenic conditions. Antimicrob. Agents Chemother. 2016, 60, 7436–7443. [Google Scholar] [CrossRef]
- Daniel, A.; Euler, C.; Collin, M.; Chahales, P.; Gorelick, K.J.; Fischetti, V.A. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2010, 54, 1603–1612. [Google Scholar] [CrossRef]
- Diez-Martinez, R.; De Paz, H.D.; Garcia-Fernandez, E.; Bustamante, N.; Euler, C.W.; Fischetti, V.A.; Menendez, M.; Garcia, P. A novel chimeric phage lysin with high in vitro and in vivo bactericidal activity against Streptococcus pneumoniae. J. Antimicrob. Chemother. 2015, 70, 1763–1773. [Google Scholar] [CrossRef]
- Vazquez, R.; Domenech, M.; Iglesias-Bexiga, M.; Menendez, M.; Garcia, P. Csl2, a novel chimeric bacteriophage lysin to fight infections caused by Streptococcus suis, an emerging zoonotic pathogen. Sci. Rep. 2017, 7, 16506. [Google Scholar] [CrossRef]
- Blazquez, B.; Fresco-Taboada, A.; Iglesias-Bexiga, M.; Menendez, M.; Garcia, P. PL3 amidase, a tailor-made lysin constructed by domain shuffling with potent killing activity against pneumococci and related species. Front. Microbiol. 2016, 7, 1156. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Powell, A.M.; Becker, S.C.; Camp, M.J.; Donovan, D.M. Chimeric phage lysins act synergistically with lysostaphin to kill mastitis-causing Staphylococcus aureus in murine mammary glands. Appl. Environ. Microbiol. 2012, 78, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.C.; Foster-Frey, J.; Stodola, A.J.; Anacker, D.; Donovan, D.M. Differentially conserved staphylococcal SH3b_5 cell wall binding domains confer increased staphylolytic and streptolytic activity to a streptococcal prophage endolysin domain. Gene 2009, 443, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Leaver-Fay, A.; Tyka, M.; Lewis, S.M.; Lange, O.F.; Thompson, J.; Jacak, R.; Kaufman, K.; Renfrew, P.D.; Smith, C.A.; Sheffler, W.; et al. ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Methods Enzymol. 2011, 487, 545–574. [Google Scholar] [CrossRef] [PubMed]
- Guerois, R.; Nielsen, J.E.; Serrano, L. Predicting changes in the stability of proteins and protein complexes: a study of more than 1000 mutations. J. Mol. Biol. 2002, 320, 369–387. [Google Scholar] [CrossRef]
- Diez-Martinez, R.; de Paz, H.; Bustamante, N.; Garcia, E.; Menendez, M.; Garcia, P. Improving the lethal effect of cpl-7, a pneumococcal phage lysozyme with broad bactericidal activity, by inverting the net charge of its cell wall-binding module. Antimicrob. Agents Chemother. 2013, 57, 5355–5365. [Google Scholar] [CrossRef]
- PROTEIN CALCULATOR v3.4. Available online: http://protcalc.sourceforge.net (accessed on 4 January 2019).
- Nelson, D.; Schuch, R.; Chahales, P.; Zhu, S.; Fischetti, V.A. PlyC: a multimeric bacteriophage lysin. Proc. Natl. Acad Sci. USA 2006, 103, 10765–10770. [Google Scholar] [CrossRef] [PubMed]
| Mutant Name | pI (Isoelectric Point) | Net Charge (Z) at pH 7.4 | Point Mutations | ΔΔGFoldX (kcal/mol) = ΔGMut-ΔGWT |
|---|---|---|---|---|
| CHAP WT | 6.11 | −3 | N/A | 0 |
| CHAP +1 | 7.89 | +1 | D311K:D355K | −5.32 |
| CHAP +2 | 8.29 | +2 | D311K:D355K:D429A | −4.61 |
| CHAP +3 | 8.59 | +3 | D311K:D355K:D363K | −4.32 |
| CHAP +4 | 8.88 | +4 | D311K:D355K:D363K:D429A | −4.13 |
| CHAP +5 | 9.11 | +5 | D311K:D355K:D363K:D429K | −2.49 |
| CHAP +6 | 9.30 | +6 | D311K:D355K:D363K:D429A:D450K | 0.01 |
| CHAP +7 | 9.43 | +7 | D311K:D355K:D363K:D429K:D450K | 1.11 |
| Plasmid | Template | Primer | Sequence |
|---|---|---|---|
| CHAP D311K | pET28a::chap | XS3 | 5′-ATGGGGTCTAAAAGAGTTGCAGCAAAC-3′ |
| CHAP D355K | pET28a::chap | XS4 | 5′-TCATACTCAACAGGTAAACCAATGCTACCGTTA-3′ |
| CHAP D363K | pET28a::chap | XS5 | 5′-CTACCGTTAATTGGTAAAGGTATGAACGCTCAT-3′ |
| CHAP D429K | pET28a::chap | XS6 | 5′-ATTGAAAGCTGGTCAAAAACTACCGTTACAGTC-3′ |
| CHAP D429A | pET28a::chap | XS8 | 5′-ATTGAAAGCTGGTCAGCGACTACCGTTACAGTC-3′ |
| CHAP D450K | pET28a::chap | XS7 | 5′-ATACGCAGCACCTATAAACTTAACACATTCCTA-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, X.; Nelson, D.C. Contributions of Net Charge on the PlyC Endolysin CHAP Domain. Antibiotics 2019, 8, 70. https://doi.org/10.3390/antibiotics8020070
Shang X, Nelson DC. Contributions of Net Charge on the PlyC Endolysin CHAP Domain. Antibiotics. 2019; 8(2):70. https://doi.org/10.3390/antibiotics8020070
Chicago/Turabian StyleShang, Xiaoran, and Daniel C. Nelson. 2019. "Contributions of Net Charge on the PlyC Endolysin CHAP Domain" Antibiotics 8, no. 2: 70. https://doi.org/10.3390/antibiotics8020070
APA StyleShang, X., & Nelson, D. C. (2019). Contributions of Net Charge on the PlyC Endolysin CHAP Domain. Antibiotics, 8(2), 70. https://doi.org/10.3390/antibiotics8020070





