Abstract
Bartonella henselae can cause various infections in humans, ranging from benign and self-limiting diseases to severe and life-threatening diseases as well as persistent infections that are difficult to treat. To develop more effective treatments for persistent Bartonella infections, in this study, we performed a high-throughput screen of an FDA-approved drug library against stationary phase B. henselae using the SYBR Green I/propidium iodide (PI) viability assay. We identified 110 drug candidates that had better activity against stationary phase B. henselae than ciprofloxacin, and among the top 52 drug candidates tested, 41 drugs were confirmed by microscopy to have higher activity than the current frontline antibiotic erythromycin. The identified top drug candidates include pyrvinium pamoate, daptomycin, methylene blue, azole drugs (clotrimazole, miconazole, sulconazole, econazole, oxiconazole, butoconazole, bifonazole), aminoglycosides (gentamicin and streptomycin, amikacin, kanamycin), amifostine (Ethyol), antiviral Lopinavir/ritonavir, colistin, nitroxoline, nitrofurantoin, verteporfin, pentamidine, berberine, aprepitant, olsalazine, clinafloxacin, and clofoctol. Pyrvinium pamoate, daptomycin, methylene blue, clotrimazole, and gentamicin and streptomycin at their respective maximum drug concentration in serum (Cmax) had the capacity to completely eradicate stationary phase B. henselae after 3-day drug exposure in subculture studies. While the currently used drugs for treating bartonellosis, including rifampin, erythromycin, azithromycin, doxycycline, and ciprofloxacin, had very low minimal inhibitory concentration (MIC) against growing B. henselae, they had relatively poor activity against stationary phase B. henselae, except aminoglycosides. The identified FDA-approved agents with activity against stationary phase B. henselae should facilitate development of more effective treatments for persistent Bartonella infections.
1. Introduction
Bartonella species are fastidious Gram-negative intracellular bacteria that are highly adapted to their mammalian reservoir hosts. Being the predominant cause of cat scratch disease in people and the second most common species causing endocarditis and bacillary angiomatosis, they have the ability to cause either acute or chronic infections and are responsible for different clinical conditions affecting humans [1]. The bacterium can be transmitted by cat’s scratch, and blood-sucking arthropod vectors such as fleas, sandflies, mosquitoes, or ticks and is considered to be an important opportunistic pathogen [2]. The bacterium can persist in the bloodstream of the host as the results of intraerythrocytic parasitism [3,4], and can cause a wide range of systemic disease such as bacteremia and central nervous system pathologies, especially in immunocompromised individuals [5]. B. henselae is sometimes a co-infection pathogen of Lyme disease transmitted by ticks carrying multiple pathogens causing more severe and protracted clinical manifestations [6]. It is very difficult to isolate and grow B. henselae in liquid media especially from clinical samples [7], as it is extremely fastidious, impeding progress in research of this organism. Thus, clinically, serology, and PCR are often used instead of culture to confirm diagnosis [7]. Treatment of systemic B. henselae infections has been difficult with poor clinical outcomes despite antibiotic treatment for weeks and months [8]. The first-line antibiotics for treatment of Bartonella infections include doxycycline, erythromycin, azithromycin, tetracyclines, gentamicin, rifampin, ciprofloxacin, and sulfa drugs, but most investigators have observed no benefit of antibiotic therapy for the treatment of typical, uncomplicated cat scratch disease [9,10], and effective treatment for Bartonella persistent infections remains a challenge. One possibility of the difficulty of eradicating B. henselae infections is due to bacterial persistence, a phenomenon that is well known to underlie persistent infections like tuberculosis, brucellosis, and Q-fever [11]. Previously, we successfully used the SYBR Green/PI viability assay for rapid high throughput drug screens for stationary phase Borrelia burgdorferi [12,13,14], which has been shown to be a good surrogate model of persister bacteria [15]. In this study, we adapted the same SYBR Green/PI methodology and performed a high throughput drug screen using stationary phase culture of B. henselae and identified a range of drug candidates from the FDA-drug library that have much better activity against the non-replicating B. henselae than the current antibiotics used to treat bartonellosis. The implication of finding such persister drugs for more effective treatment of persistent Bartonella infection is discussed.
2. Materials and Methods
2.1. Bacterial Strain, Culture Media, and Culture Conditions
The following reagent was obtained through BEI Resources (ATCC), NIAID, NIH: Bartonella henselae strain JK53, NR-31834. B. henselae was cultured in Schneider’s medium supplemented with 10% fetal bovine serum (FBS) as described [16,17]. Cultures were incubated without shaking at 37 °C, 5% CO2 at all times. The colony forming unit (CFU) counting was performed on Columbia sheep blood agar (BD Biosciences, California, USA) after serial dilutions.
2.2. Standard Curve of SYBR Green I/PI Assay for B. henselae
The SYBR Green I/PI assay for rapid viability assessment of B. henselae was carried out as we described previously, which we have successfully used for high throughput drug screens against stationary phase B. burgdorferi [12]. Briefly, we used 70% isopropyl alcohol killed B. henselae dead cells and live cells mixed in different proportions (107 bacteria/mL) to validate the SYBR Green I/PI assay using a B. henselae 5-day stationary phase culture. The cells were added in 96-well plate, stained with SYBR Green I/PI mixture, and incubated at room temperature in the dark for 15 min. With excitation wavelength at 485 nm, the fluorescence intensities from green emission (535, 538, and 565 nm) and red emission (612, 635, and 650 nm) were measured using HTS 7000 plus Bioassay Reader (Perkin Elmer Inc., Waltham, MA, USA). The green/red fluorescence ratios were measured for each proportion of live/dead B. henselae as described [12].
2.3. Antibiotics and the FDA Drug Library
Antibiotics, including doxycycline, cefuroxime, miconazole, metronidazole, rifampin, and fluconazole, were purchased from Sigma Aldrich and were dissolved in appropriate solvents [18] to form stock solutions. All the antibiotic stocks were filter-sterilized by a 0.2 μm filter except the DMSO stocks. Then the stocks were diluted into 500 μM and stored at −20 °C. The JHCCL FDA-approved drug library was prepared in 10 mM stock solutions with DMSO. Drugs in the master plates were diluted with PBS to make 500 μM pre-diluted working plates. The pre-diluted drug plates were sealed and stored at −20 °C.
2.4. Microscopy Techniques
The SYBR Green I/PI dye was added to drug-treated or control B. henselae cell suspensions for assessing the viability by ratio of green/red fluorescence as described previously [12,13]. Samples on specimens or 96-well plates were examined by BZ-X710 All-in-One fluorescence microscope (KEYENCE, Inc., Osaka, Japan). The residual bacteria viability was confirmed by analyzing three representative images of the same bacterial cell suspension using fluorescence microscopy. BZ-X Analyzer and Image Pro-Plus software were used to quantitatively determine the fluorescence intensity.
2.5. Screening of FDA-Approved Drug Library against Stationary Phase B. henselae
For the high-throughput drug screen, 100 μL B. henselae cell suspension from a 7-day old stationary phase culture was added in 96-well plates. Each compound (10 μL, final concentration 50 μM) from the pre-diluted plate or pre-diluted stock was added to the cell suspension. Plates were sealed and placed in 37 °C incubator over of a period of 5 days. SYBR Green I/ PI viability assay was used to assess the live and dead cells after antibiotic exposure as described [13]. Briefly, 10 μL SYBR Green I (100× stock, Invitrogen, Waltham, MA, USA) and propidium iodide (PI, 600 μM, Sigma, St. Louis MO, USA) staining mixture was added to each well of the 96-well plate and mixed thoroughly. The plates were incubated at room temperature in the dark for 15 minutes followed by plate reading using microplate reader (HTS 7000 plus Bioassay Reader, Perkin Elmer Inc., Waltham MA, USA). The green/red (535 nm/635 nm) fluorescence ratio of each well was used for calculating the residual viability percentage with least-square fitting analysis as described previously [12]. All tests were run in triplicate.
2.6. Drug Exposure Assay
A 5-day old B. henselae stationary phase culture was used for drug exposure experiments. The antibiotic exposure was carried out in 1.5 mL Eppendorf tubes over the course of 5 days at 37 °C without shaking. Then the cells were collected by centrifugation and rinsed twice with fresh Schneider’s medium followed by resuspension in 1 mL fresh Schneider’s medium. The cell suspension was serially diluted and plated on Columbia blood agar plates for viable bacterial counts (colony forming unit, CFU).
2.7. Minimum inhibitory concentration (MIC) Determination
The standard microdilution method was used to determine the minimum inhibitory concentration (MIC) needed to inhibit visible growth of B. henselae after a 5-day incubation period. B. henselae cells (1 × 106) were inoculated into each well of 96-well microplate containing 90 μL fresh modified Schneider’s medium per well. Each diluted drug (10 μL) was added to the culture. All experiments were run in triplicate. The 96-well plates were sealed and incubated at 37 °C with 5% CO2 for 5 days. Cell proliferation was assessed using the SYBR Green I/PI assay and a Petroff-Hausser counting chamber after the incubation.
3. Results
3.1. Growth Behavior of B. henselae in Modified Schneider’s Medium
As the growth curve shows in Figure 1, B. henselae grew to logarithmic growth phase in 1 day and reached a growth peak at 2 days, with the highest CFU count reaching above 109 per mL, followed by a decline to 108 per mL at 5–7 days and a sharper decline to 106 per mL at 11 days. However, when we checked the live and dead B. henselae cells after staining with SYBR Green I/ PI, all the visible cells in 1-day-old and 5-day-old B. henselae cultures fluoresced green under fluorescence microscope (Figure 2), indicating intact cell membranes. Interestingly, compared to the single, free-living cells in 1-day-old cultures (Figure 2A–C), many cells from 5-day-old cultures formed aggregated clusters (Figure 2D–F), which could partly contribute to the gradual drop of CFU count. Based on the growth curve (Figure 1) and the morphology of B. henselae, we considered 1-day-old culture as log phase culture and 5-day-old culture as stationary phase culture.
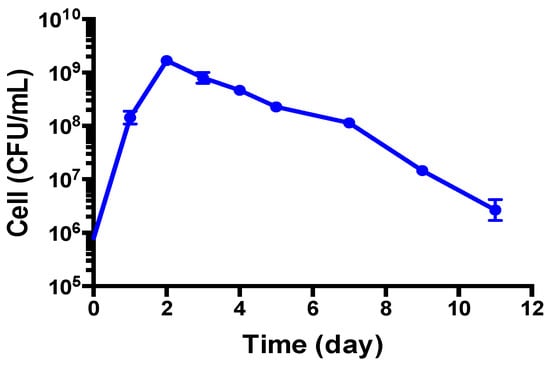
Figure 1.
Growth of B. henselae (colony forming unit (CFU)/mL) in modified Schneider’s medium at 37 °C measured over a period of 11 days.
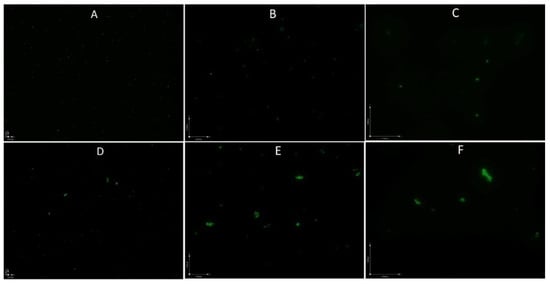
Figure 2.
Fluorescence microscopic images of 1-day-old log phase cells (A–C) and 5-day-old stationary phase B. henselae cells (D–F). (A,D): 100 × magnification; (B,E): 400 × magnification; (C,F): 600 × magnification.
3.2. Development of a SYBR Green/PI Viability Assay for B. henselae
We previously developed a SYBR Green I/PI assay for rapid viability assessment of Borrelia burgdorferi [12] and have successfully used this assay for high throughput drug screens against non-growing stationary phase B. burgdorferi [13,14]. To perform high throughput drug screens on stationary phase B. henselae, here we adapted the SYBR Green I/PI methodology to B. henselae. As in our previous study [12], we used 70% isopropyl alcohol killed B. henselae dead cells and live cells mixed in different proportions (107 bacteria/mL) to validate the SYBR Green I/PI assay using a B. henselae stationary phase culture. The results showed that the percentage of residual viability of different live and dead cell mixtures correlated well in linear relationships with the ratios of green/red fluorescence intensities of B. henselae, especially the ratio pairs of fluorescence intensities at 535 nm/650 nm and 538 nm/650 nm (Figure 3). This result indicates that the SYBR Green/PI assay could also be used for high throughput drug screens on B. henselae, and fluorescence intensities at 535 nm and 650 nm were used to calculate the ratio of green/red fluorescence in the SYBR Green I/PI assay of B. henselae.
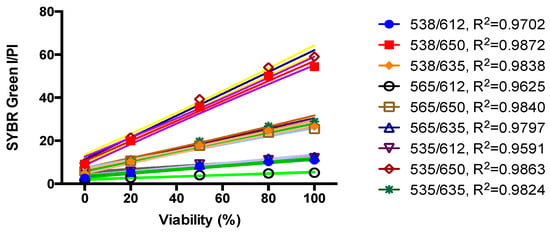
Figure 3.
Linear relationship between the B. henselae viability and Green/Red fluorescence ratios of the SYBR Green I/PI assay. Various proportions of live and isopropyl alcohol-killed B. henselae were used to validate the SYBR Green I/PI assay on the 5-day old B. henselae stationary phase culture. Cell density was about 107 bacteria/mL. The line is a least-squares fit of the relationship between the percentages of live bacteria and the green/red fluorescence ratios.
3.3. Screening FDA Drug Library to Identify Drugs Active against Non-Growing Stationary Phase B. henselae Using the SYBR Green/PI Assay
As shown in Figure 1, the 5-day old B. henselae culture in modified Schneider’s medium could be considered to be in stationary phase and was thus used to identify active drugs against stationary phase B. henselae. We used the FDA drug library at 50 μM in the screen to increase the chance of obtaining hits as described previously [13]. Meanwhile, the currently known effective antibiotics used to treat bartonellosis, such as doxycycline, azithromycin, rifampin, ciprofloxacin, etc.; were included as control drugs for comparison (Table 1). In the primary screen, 110 of the 1581 drugs in the FDA drug library were found to have higher activity against stationary phase B. henselae than the control drug ciprofloxacin. According to our previous experience, some compounds can interfere with the SYBR Green I/PI assay because of color or autofluorescence. Thus, we selected 52 top active candidates for further validation by microscopic counting to confirm the SYBR Green I/PI plate reader results. Doxycycline and erythromycin, the most frequently used antibiotics for the treatment of Bartonella infection in humans, showed poor activity against stationary phase B. henselae (residual viability above 60%) (Table 1). Gentamicin, streptomycin, azithromycin, tetracycline, and rifampin, which are clinically used for treating Bartonella infections [19], had relatively better activity (residual viability between 32% and 59%) against the stationary phase B. henselae than doxycycline and erythromycin. Other control drugs proposed for Bartonella treatment, including penicillin, chloramphenicol, cefuroxime, and ciprofloxacin, did not show higher activity compared with the most frequently used doxycycline and erythromycin, with residual viability ranging from 60% to 74%.

Table 1.
Activity of top 41 active hits that had good activity against stationary phase B. henselae a.
From the 52 selected candidates that showed good activity against stationary phase B. henselae in the primary screen, we confirmed that 41 drugs showed higher activity than erythromycin (Table 1). Among the 41 active drugs, 7 azole drugs including sulconazole, econazole, oxiconazole, butoconazole, clotrimazole, bifonazole, and miconazole, showed high activity against stationary phase B. henselae. We found daptomycin, a powerful anti-persister drug against B. burgdorferi that we identified in our previous study [13], also showed strong activity (residual viability 21%) against stationary phase B. henselae (Table 1). In addition, methylene blue, another active drug against B. burgdorferi we identified before [14], was found to have good activity (residual viability 25%) against stationary phase B. henselae (Table 1). Furthermore, amifostine (Ethyol), Lopinavir/ritonavir, colistin, nitroxoline, berberine, amikacin, kanamycin, verteporfin, pentamidine, aprepitant, clinafloxacin, and clofoctol also showed relatively high activity against stationary phase B. henselae (Table 1). Pyrvinium pamoate exhibited excellent plate reader results in the primary screening, yet microscopic checking indicated this was due to strong autofluorescence background. The MIC measurement and subculture test of pyrvinium pamoate together with other representative drugs were carried out below to further confirm their activity against both growing and non-growing stationary phase B. henselae.
3.4. MIC Determination of Active Hits
While we found agents that had good activity against the non-growing stationary phase B. henselae (Table 1), it is also necessary to determine the MICs of the active hits against growing B. henselae. The MICs for B. henselae were determined by the standard microdilution method as described in our previous study [13]. As shown in Table 2, rifampin was the most active agent, capable of inhibiting B. henselae proliferation at the lowest concentration of rifampin tested (0.01 μg/mL).

Table 2.
Minimal inhibitory concentrations (MICs) of select drug candidates against B. henselae a.
The growth of B. henselae was efficiently suppressed by azithromycin and pyrvinium pamoate at a concentration of 0.04–0.08 μg/mL, by methylene blue, doxycycline and erythromycin at 0.08–0.16 μg/mL, and by clinafloxacin at 0.16–0.31 μg/mL. Nitrofurantoin, nitroxoline, and pentamidine were active with MIC values of 0.31–0.63 μg/mL. B. henselae growing cells were also susceptible to clotrimazole, gentamicin, and berberine with MICs of 0.63–1.25 μg/mL, ciprofloxacin with MIC of 1.25–2.5 μg/mL, streptomycin and miconazole with MIC of 3.13–6.25 μg/mL, and amikacin with MIC of 6.25–12.5 μg/mL. Aprepitant and clofoctol could inhibit growth of B. henselae with MIC of 10–20 μg/mL and 70–140 μg/mL, respectively, which are much higher than those corresponding maximum drug concentration in serum (Cmax) in human. Interestingly, daptomycin, which is highly active against non-growing stationary phase B. henselae (Table 1), had relatively poor activity against growing B. henselae with a high MIC of 12.5–25 μg/mL. Colistin and amifostine were the least effective agents in inhibiting the growth of B. henselae, with MICs higher than 80 μg/mL, which is more than 10 times higher than Cmax for each agent.
3.5. Subculture Study of Stationary Phase B. henselae After Drug Exposure
Having obtained many candidates from the FDA drug library, we performed a time-kill drug exposure assay against a 5-day-old B. henselae stationary phase culture at concentrations of their respective Cmax. As shown in Table 3, pyrvinium pamoate, methylene blue and daptomycin were the most active agents, which rapidly killed B. henselae with no detectable CFU after 1-day exposure. Other active hits, including clotrimazole, gentamicin, and streptomycin, could lead to eradication of B. henselae cells without viable cells being recovered after exposure for 3 days. Nitroxoline also showed excellent activity, reducing 4 log10 CFU/mL after 3-day exposure. Clinafloxacin and nitrofurantoin also had the capability of killing stationary phase B. henselae and reduced the bacterial count by approximately 2 log10 CFU/mL in 3 days. However, although rifampin was the most effective agent against the growing B. henselae (Table 2), it failed to eradicate stationary phase B. henselae cells after treatment for 3 days, leaving about 8 × 103 per mL CFU remaining (Table 3). Compared with control without drug exposure, clinically used antibiotics for treatment of Bartonella infections, including azithromycin, doxycycline, and erythromycin, had poor activity in killing B. henselae, achieving approximately 1 log10 decrease of CFU/mL after 1-day drug exposure and no obvious further decrease even after 3-day exposure. Other drugs, including pentamidine, berberine, ciprofloxacin, aprepitant, clofoctol, colistin, and amifostine, at their Cmax values had poor activity against stationary phase B. henselae, with viable cells barely reduced.

Table 3.
Evaluation of select drug candidates against a 5-day old stationary phase B. henselae culture at their respective maximum drug concentration in serum (Cmax) values.
3.6. Comparison of Susceptibility of Log Phase B. henselae and Stationary Phase B. henselae in Drug Exposure Assay
To further demonstrate the divergent drug tolerance of B. henselae at different growth stages, we tested the efficacy of the top 7 drug hits screened from subculture test of 5-day-old stationary phase B. henselae after drug exposure, including pyrvinium pamoate, methylene blue, daptomycin, clotrimazole, gentamicin, streptomycin, and nitroxoline, against 1-day-old log phase and 5-day-old stationary phase B. henselae and compared the survival fraction of B. henselae cells within these two different growth stages. Similar to the results of subculture test for stationary phase B. henselae drug exposure experiment, pyrvinium pamoate, methylene blue, and daptomycin were the most active agents against log phase B. henselae, with no colony being detected on agar plate after treatment for 1 day and 3 days (data not shown). While gentamicin and streptomycin could lead to no viable cells in the log phase B. henselae culture after drug treatment for 1 day and 3 days (Figure 4), the survival fractions of the 5-day-old stationary phase B. henselae were around 10−3 and 10−2 after 1-day treatment with gentamicin and streptomycin, respectively. Clotrimazole could effectively kill stationary phase B. henselae cells, with 10−4 of 5-day-old stationary phase B. henselae cells and 10−6 of 1-day-old log phase B. henselae cells surviving after 1-day treatment. Although nitroxoline showed limited efficacy in killing stationary phase B. henselae, with around 10% of cells surviving after treatment, it could efficiently kill log phase B. henselae, with surviving fraction lower than 10−6. Furthermore, erythromycin and azithromycin used for treating Bartonella related diseases, did not show strong activity against stationary phase B. henselae cells with 10% of surviving fraction, yet both drugs could eliminate 99% of log phase B. henselae cells. Doxycycline had poor activity against both log phase and stationary phase B. henselae cells (Figure 4).
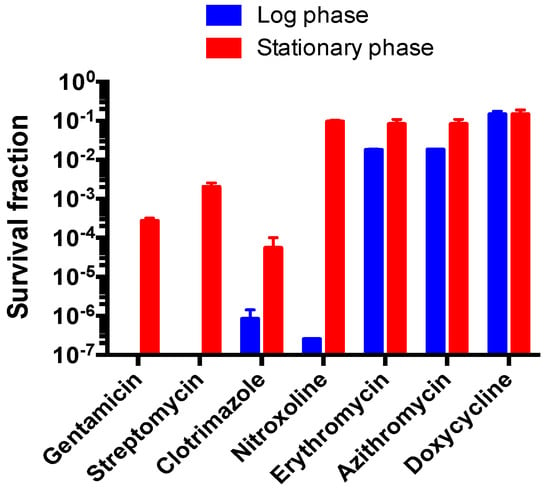
Figure 4.
Comparison of survival fraction of a 1-day-old log phase B. henselae culture and a 5-day-old stationary phase B. henselae culture after drug exposure for 1 day. Survival fractions were calculated as: CFU/mL after drug exposure divided by CFU/mL in the control without drug. Drug concentrations used in this experiment are listed as follows: 10 μg/mL gentamicin, 25 μg/mL streptomycin, 25 μg/mL clotrimazole, 5 μg/mL nitroxoline, 1 μg/mL erythromycin, 2 μg/mL azithromycin and 5 μg/mL doxycycline.
In the time-kill experiment with 5-day old stationary phase B. henselae culture, the top 8 drug candidates (daptomycin, methylene blue, clotrimazole, gentamicin, nitrofurantoin, nitroxoline, clofoctol, amifostine) along with control drugs (rifampin, doxycycline, azithromycin, amikacin) at their Cmax were added to the culture for different times of drug exposure (Day 1, Day 3, and Day 5) followed by CFU count. Daptomycin was found to be most active at killing all bacteria by Day 1, followed by methylene blue and gentamicin which killed all bacteria by Day 3 (Figure 5). Nitrofurantoin and rifampin were also quite active and killed all bacteria by Day 5, while clotrimazole at (5 ug/mL, close to Cmax) still had about 103 bacteria remaining by Day 5. Amikacin, azithromycin, and doxycycline had some activity, whereas clofoctol and amifostine had poor or no activity (Figure 5).
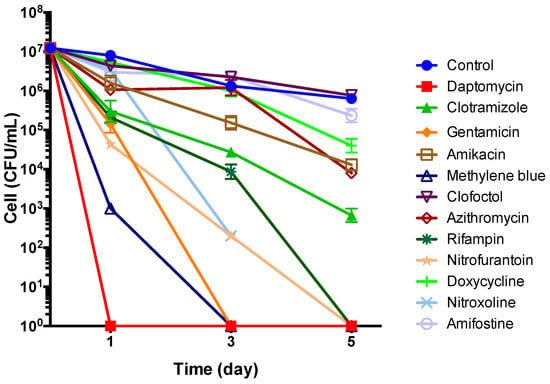
Figure 5.
Time-kill curves for drug treatment of 5-day old stationary phase B. henselae. The antibiotics were added to the culture at time point 0, and at different times of drug exposure (Day 1, Day 3, and Day 5), portions of bacteria were removed and washed and plated on Columbia blood agar for CFU counts. Drug concentrations used in this experiment were based on their Cmax (Table 2) and are as follows: 60 μg/mL daptomycin, 5 μg/mL clotrimazole, 5 μg/mL gentamicin, 5 μg/mL amikacin, 10 μg/mL methylene blue, 2 μg/mL clofoctol, 2 μg/mL azithromycin, 10 μg/mL rifampin, 5 μg/mL nitrofurantoin, 5 μg/mL doxycycline, 5 μg/mL nitroxoline, and 5 μg/mL amifostine.
4. Discussion
In this study, we were able to culture B. henselae in a reliable and consistent manner using a modified Schneider’s medium, which allowed 1-day-old culture to grow as log phase and 5-day-old culture as stationary phase (Figure 1). We then successfully adapted the SYBR Green/PI viability assay to B. henselae and performed a high throughput screen with FDA-approved drug library for activity against stationary phase B. henselae. We found that a significant number of drugs have better activity than the current drugs used to treat bartonellosis. These include seven azole drugs (sulconazole, econazole, oxiconazole, butoconazole, clotrimazole, bifonazole, and miconazole), daptomycin, methylene blue, amifostine (Ethyol), Lopinavir/ritonavir, colistin, amikacin, nitroxoline, berberine, verteporfin, pentamidine, aprepitant, clinafloxacin, and clofoctol. We then chose some of these drugs from different categories at appropriate concentrations to determine their MICs and capability of killing 5-day-old stationary phase B. henselae. The results demonstrated that B. henselae was highly susceptible to most antibiotics tested, which is consistent with previous reports [19,42]. However, the MICs did not correlate well with results of drug exposure against 5-day-old stationary phase B. henselae. Rifampin, which is the most effective agent in inhibiting growing B. henselae with the lowest MIC (Table 2), could not kill all stationary phase B. henselae cells. Furthermore, clinically used antibiotics for Bartonella infections, including azithromycin, doxycycline, and erythromycin, while exhibiting effective inhibition of growing B. henselae cells (Table 2), had very limited activity against aggregated B. henselae cells in stationary phase (Table 3).
Fortunately, 6 agents, pyrvinium pamoate, methylene blue, daptomycin, clotrimazole, gentamicin, and streptomycin, could efficiently kill stationary phase B. henselae, without CFU detected after drug exposure with drug concentrations close to Cmax (Table 3). Among them, aminoglycosides gentamicin and streptomycin have been reported to have clinical improvement for treating Bartonella infection. Bartonella species have the capability to reside and propagate in erythrocytes in humans and animals [43], which probably provide a shelter for Bartonella sp.; protecting them from the host immune responses and exposure to antibiotics. Gentamicin is documented to enter erythrocytes slowly and poorly, with a peak level of 0.26 μg/mL [44], which is much lower than its MIC of 0.63–1.25 μg/mL. Thus, Bartonellae residing within erythrocytes are protected from gentamicin, and its clinical effect may be due to its activity against extracellular organisms.
Pyrvinium pamoate, an antihelmintic drug, had good activity against stationary phase B. henselae. While it is safe to take orally even with a high dose, pyrvinium pamoate could be barely detected in plasma and urine due to poor bioavailability [22]. One limitation to pyrvinium pamoate’s application is its poor absorption for humans. Attempts of structural modification should be taken to improve pyrvinium pamoate’s bioavailability, which could make it a potentially useful agent for targeting Bartonella infections due to its high activity against both growing and non-growing form of the organism.
Surprisingly, methylene blue showed great activity against B. henselae. It is interesting to note that we have previously found methylene blue has good activity against stationary phase Borrelia burgdorferi [14]. Methylene blue is a dye and a medication, which was used as an antimalarial agent and has received renewed attention in the management of other diseases, such as urinary tract infections (UTIs) and methemoglobinemia [45]. Methylene blue was recently shown to have antifungal effect through its effect on redox and membrane disruption [46]. It is interesting to note that membrane is a target of persister drugs, and our previous finding that methylene blue also had activity against Borrelia burgdorferi stationary phase cells [14] is consistent with its activity on the membrane. Further studies are needed to determine if methylene blue could disrupt membranes of B. henselae.
It is worth noting that we found daptomycin had excellent activity against stationary phase B. henselae (Table 3), although it had a high MIC (12.5–25 µg/mL) against growing organisms (Table 2). Interestingly, the powerful killing action of daptomycin was also observed in drug treatment of spirochete B. burgdorferi in our previous studies [13,47]. Daptomycin is a cyclic lipopeptide antibiotics with a broad spectrum of activity against Gram-positive bacteria and has been used to treat severe infections caused by antibiotic-resistant strains. It has calcium-dependent bactericidal activity by creating pores on bacterial cell membranes, leading to release of potassium, membrane potential dissipation, and ultimately cell death [48]. It is well known that daptomycin has no activity against Gram-negative bacteria due to the less anionic phospholipids in the Gram-negative cytoplasmic membrane compared to Gram-positive bacteria, which would result in insufficient sites for calcium-mediated insertion of daptomycin [49]. As far as we know, this is the first time that daptomycin has been shown to exhibit excellent activity against stationary phase B. henselae, a Gram-negative bacterium. The high activity of daptomycin against both Borrelia and Bartonella indicates it may serve as a promising drug candidate in treatment of both persistent Borrelia infections and Bartonella infections, which clinically may be present as coinfections.
It is interesting to note we found several antifungal azoles to have good activity against B. henselae. Clotrimazole at a therapeutically relevant concentration had excellent activity against both growing and non-growing B. henselae and could kill stationary phase B. henselae without viable cells detected on agar plates. In addition, it is of interest that clotrimazole has been shown to be clinically useful for treating other diseases, such as sickle cell anemia, malaria, beriberi, tinea pedis, Chagas disease, and cancer [50]. Clotrimazole acts as an anti-malarial agent by causing heme-induced membrane damage in the malaria parasite [51]. As a well-known antifungal agent, clotrimazole works by altering the permeability and fluidity of the fungal cell membrane through inhibition of ergosterol synthesis, with ergosterol depleted and replaced with unusual sterols [52]. There is no ergosterol synthesis in B. henselae, and the mechanisms of action of azoles, including clotrimazole, against B. henselae remains to be determined.
It is worth noting that discrepant efficacies of antibiotics exist between in vitro testing based on MIC data and clinical data in patients with Bartonella-related infections [8]. One contributing factor could be the existence of bacterial aggregates or biofilm in patients [43,53] or the aggregated clusters or biofilm structures or persisters we found in stationary phase cultures. While clinically used antibiotics for Bartonella infections, including rifampin, azithromycin, doxycycline, and erythromycin, could effectively inhibit the growth of the B. henselae strain (Table 2), they showed poor capability in eradicating stationary phase B. henselae cells in aggregated form (Table 3). In addition, gentamicin, streptomycin, clotrimazole, and nitroxoline, capable of wiping out or efficiently eliminating log phase B. henselae cells, had less activity against stationary phase B. henselae cells (Figure 4). Erythromycin and azithromycin frequently used in treatment of Bartonella-related infections, had limited activity against stationary phase B. henselae cells, but it had high activity against log phase B. henselae cells (Figure 4). The poor activity of the current clinically used drugs for treating bartonellosis against non-growing stationary phase B. henselae could partly be responsible for the treatment failure and relapse of bartonellosis [8]. Future studies are needed to determine if the drugs we identified in this study with activity against non-growing stationary phase B. henselae cells could be more effective at treating persistent infections in vivo in animal models and in patients in the context of drug combinations.
Another important factor that could lead to the discrepancies could be the localization of Bartonella in the host during the infection. The bacteria are able to reside and propagate inside erythrocytes and/or endothelial cells [43,53]. The intracellular localization of Bartonella provides a shelter for them to evade exposure to antibiotics, such as gentamicin, which is mainly active against extracellular bacteria but not intracellular bacteria. Thus, antibiotics with good activity against Bartonella in vitro should also be tested for their activity against intracellular bacteria in future.
While we found many promising drug candidates with good activity against stationary phase B. henselae, they may not be used alone due to possible resistance development and need to be used in the context of drug combinations. Future studies are needed to evaluate drug combinations using the newly identified drug candidates with the current drugs used in clinic to better target diverse bacterial populations in different niches that can happen in the host as in the Yin-Yang model [11]. In addition, our findings in vitro will require further validation using appropriate animal models of bartonellosis to assess the utility of the identified drug candidates for more effective treatment of persistent Bartonella infections in vivo and in the clinic. While our study was performed with B. henselae, the findings may apply to other closely related pathogenic Bartonella species, such as B. quintana and B. bacilliformis. Future studies are needed to confirm this possibility.
5. Conclusions
In summary, this is the first study of a high throughput drug screen against stationary phase B. henselae using the FDA-approved drug library where we identified a range of promising drug candidates that may improve the treatment of Bartonella infections. Since these drug candidates are FDA-approved they could be more readily adopted clinically for treating Bartonella infections as long as they meet appropriate safety, PK, and efficacy requirement. Further studies are needed to determine the activity of persister drug combinations with the identified drug candidates against Bartonella persisters and biofilm bacteria in vitro and in animal models of Bartonella infection.
Author Contributions
Conceptualization, Y.Z.; Data curation, T.L.; J.F.; S.X.; and W.S.; Formal analysis, T.L.; J.F. Funding acquisition, Y.Z.; Resources, D.S.; Writing—original draft, T.L.; Writing—review editing, J.F. and Y.Z.
Funding
This research was funded in part by Steven Alexandra Cohen Foundation, NatCapLyme, and the Einstein-Sim Family Charitable Fund.
Acknowledgments
We acknowledge the support by Steven Alexandra Cohen Foundation, NatCapLyme, and the Einstein-Sim Family Charitable Fund. We thank Ed Breitschtwerdt and Ricardo Maggi for discussions on Bartonella culture. We thank BEI Resources (ATCC) for providing Bartonella henselae strain JK53 used in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Okaro, U.; Addisu, A.; Casanas, B.; Anderson, B. Bartonella species, an emerging cause of blood-culture-negative endocarditis. Clin. Microbiol. Rev. 2017, 30, 709–746. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B. Bartonellosis, One Health and all creatures great and small. Vet. Dermatol. 2017, 28, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Jacomo, V.; Kelly, P.; Raoult, D. Natural history of Bartonella infections (an exception to Koch’s postulate). Clin. Diagn. Lab. Immunol. 2002, 9, 8–18. [Google Scholar] [CrossRef]
- Rolain, J.; La Scola, B.; Liang, Z.; Davoust, B.; Raoult, D. Immunofluorescent Detection of IntraerythrocyticBartonella henselae in Naturally Infected Cats. J. Clin. Microbiol. 2001, 39, 2978–2980. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mosepele, M.; Mazo, D.; Cohn, J. Bartonella infection in immunocompromised hosts: Immunology of vascular infection and vasoproliferation. Clin. Dev. Immunol. 2012. [Google Scholar] [CrossRef]
- Eskow, E.; Rao, R.-V.S.; Mordechai, E. Concurrent infection of the central nervous system by Borrelia burgdorferi and Bartonella henselae: Evidence for a novel tick-borne disease complex. Arch. Neurol. 2001, 58, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Chomel, B.B.; Boulouis, H.-J.; Maruyama, S.; Breitschwerdt, E.B. Bartonella spp. in pets and effect on human health. Emerg. Infect. Dis. 2006, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Raoult, D. Pathogenicity and treatment of Bartonella infections. Int. J. Antimicrob. Agents 2014, 44, 16–25. [Google Scholar] [CrossRef]
- Biswas, S.; Rolain, J.-M. Bartonella infection: Treatment and drug resistance. Future Microbiol. 2010, 5, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.W.; Vincent, J.M.; Person, D.A. The expanding spectrum of Bartonella infections: II. Cat-scratch disease. Pediatr. Infect. Dis. J. 1997, 16, 163–179. [Google Scholar] [CrossRef]
- Zhang, Y. Persisters, Persistent Infections and the Yin-Yang Model. Emerg. Microb. Infect. 2014, 3, e3. [Google Scholar] [CrossRef]
- Feng, J.; Wang, T.; Zhang, S.; Shi, W.; Zhang, Y. An optimized SYBR Green I/PI assay for rapid viability assessment and antibiotic susceptibility testing for Borrelia burgdorferi. PLoS ONE 2014, 9, e111809. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, T.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. Identification of Novel Activity against Borrelia burgdorferi Persisters Using an FDA Approved Drug Library. Emerg. Microb. Infect. 2014, 3, e49. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Weitner, M.; Shi, W.; Zhang, S.; Sullivan, D.; Zhang, Y. Identification of Additional Anti-Persister Activity against Borrelia burgdorferi from an FDA Drug Library. Antibiotics 2015, 4, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. A Drug Combination Screen Identifies Drugs Active against Amoxicillin-Induced Round Bodies of In Vitro Borrelia burgdorferi Persisters from an FDA Drug Library. Front. Microbiol. 2016, 7, 743. [Google Scholar] [CrossRef]
- Lynch, T.; Iverson, J.; Kosoy, M. Combining culture techniques for Bartonella: The best of both worlds. J. Clin. Microbiol. 2011, 49, 1363–1368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Riess, T.; Dietrich, F.; Schmidt, K.V.; Kaiser, P.O.; Schwarz, H.; Schäfer, A.; Kempf, V.A. Analysis of a Novel Insect Cell Culture Medium-Based Growth Medium for Bartonella Species. Appl. Environ. Microbiol. 2008, 74, 5224–5227. [Google Scholar] [CrossRef] [PubMed]
- Performance Standards for Antimicrobial Susceptibility Testing. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 13 December 2018).
- Rolain, J.; Brouqui, P.; Koehler, J.; Maguina, C.; Dolan, M.; Raoult, D. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob. Agents Chemother. 2004, 48, 1921–1933. [Google Scholar] [CrossRef] [PubMed]
- Ruslami, R.; Nijland, H.M.; Alisjahbana, B.; Parwati, I.; van Crevel, R.; Aarnoutse, R.E. Pharmacokinetics and tolerability of a higher rifampin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrob. Agents Chemother. 2007, 51, 2546–2551. [Google Scholar] [CrossRef]
- Matzneller, P.; Krasniqi, S.; Kinzig, M.; Sörgel, F.; Hüttner, S.; Lackner, E.; Müller, M.; Zeitlinger, M. Blood, tissue and intracellular concentrations of Azithromycin during and after end of therapy. Antimicrob. Agents Chemother. 2013, 57, 1736–1742. [Google Scholar] [CrossRef]
- Smith, T.; Kinkei, A.; Gryczko, C.; Goulet, J. Absorption of pyrvinium pamoate. Clin. Pharmacol. Therap. 1976, 19, 802–806. [Google Scholar] [CrossRef]
- Walter-Sack, I.; Rengelshausen, J.; Oberwittler, H.; Burhenne, J.; Mueller, O.; Meissner, P.; Mikus, G. High absolute bioavailability of methylene blue given as an aqueous oral formulation. Eur. J. Clin. Pharmacol. 2009, 65, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Welling, P.G.; Koch, P.A.; Lau, C.C.; Craig, W.A. Bioavailability of tetracycline and doxycycline in fasted and nonfasted subjects. Antimicrob. Agents Chemother. 1977, 11, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Geerdes-Fenge, H.; Goetschi, B.; Rau, M.; Borner, K.; Koeppe, P.; Wettich, K.; Lode, H. Comparative pharmacokinetics of dirithromycin and erythromycin in normal volunteers with special regard to accumulation in polymorphonuclear leukocytes and in saliva. Eur. J. Clin. Pharmacol. 1997, 53, 127–133. [Google Scholar] [CrossRef]
- Randinitis, E.J.; Brodfuehrer, J.I.; Eiseman, I.; Vassos, A.B. Pharmacokinetics of clinafloxacin after single and multiple doses. Antimicrob. Agents Chemother. 2001, 45, 2529–2535. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Novelli, A.; Rosi, E. Pharmacological properties of oral antibiotics for the treatment of uncomplicated urinary tract infections. J. Chemother. 2017, 29, 10–18. [Google Scholar] [CrossRef]
- Bergogne-Berezin, E.; Berthelot, G.; Muller-Serieys, C. Present status of nitroxoline. Pathol. Biol. (Paris) 1987, 35, 873–878. [Google Scholar] [PubMed]
- Girard, P.-M.; Clair, B.; Certain, A.; Bidault, R.; Matheron, S.; Regnier, B.; Farinotti, R. Comparison of plasma concentrations of aerosolized pentamidine in nonventilated and ventilated patients with pneumocystosis. Am. Rev. Respir. Dis. 1989, 140, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Plempel, M. Pharmacokinetics of imidazole antimycotics. Postgrad Med. J. 1979, 55, 662–666. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Demczar, D.J.; Nafziger, A.N.; Bertino, J. Pharmacokinetics of gentamicin at traditional versus high doses: Implications for once-daily aminoglycoside dosing. Antimicrob. Agents Chemother. 1997, 41, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Ding, L.; Chen, Y.; Gong, B.; He, J.; Xu, G. Determination of berberine in human plasma by liquid chromatography–electrospray ionization–mass spectrometry. J. Pharma Biomed. Anal. 2007, 44, 931–937. [Google Scholar] [CrossRef]
- Kays, M.B.; Overholser, B.R.; Mueller, B.A.; Moe, S.M.; Sowinski, K.M. Effects of sevelamer hydrochloride and calcium acetate on the oral bioavailability of ciprofloxacin. Am. J. Kidney Dis. 2003, 42, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Shen, J.-Y.; Yin, W.-L.; Yao, J.-Y.; Xu, Y.; Pan, X.-Y.; Hao, G.-J. Pharmacokinetic Disposition of Streptomycin Sulfate in Japanese Eel (Anguilla japonica) after Oral and Intramuscular Administrations. Pharmacol. Pharma. 2012, 3, 195. [Google Scholar] [CrossRef]
- Mikamo, H.; Kawazoe, K.; Sato, Y.; Ito, K.; Tamaya, T. Pharmacokinetics of miconazole in serum and exudate of pelvic retroperitoneal space after radical hysterectomy and pelvic lymphadenectomy. Int. J. Antimicrob. Agents 1998, 9, 207–211. [Google Scholar] [CrossRef]
- White, B.P.; Lomaestro, B.; Pai, M.P. Optimizing the initial amikacin dosage in adults. Antimicrob. Agents Chemother. 2015, 59, 7094–7096. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Nakamura, Y.; Tsuya, A.; Murakami, H.; Endo, M.; Yamamoto, N. Pharmacokinetics of aprepitant and dexamethasone after administration of chemotherapeutic agents and effects of plasma substance P concentration on chemotherapy-induced nausea and vomiting in Japanese cancer patients. Cancer Chemother. Pharmacol. 2011, 68, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Dvorchik, B.H.; Brazier, D.; DeBruin, M.F.; Arbeit, R.D. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 2003, 47, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Danesi, R.; Gasperini, M.; Senesi, S.; Freer, G.; Angeletti, C.; Del, M.T. A pharmacokinetic study of clofoctol in human plasma and lung tissue by using a microbiological assay. Drugs Exp. Clin. Res. 1988, 14, 39–43. [Google Scholar] [PubMed]
- Koomanachai, P.; Landersdorfer, C.B.; Chen, G.; Lee, H.J.; Jitmuang, A.; Wasuwattakul, S.; Sritippayawan, S.; Li, J.; Nation, R.L.; Thamlikitkul, V. Pharmacokinetics of colistin methanesulfonate and formed colistin in end-stage renal disease patients receiving continuous ambulatory peritoneal dialysis. Antimicrob. Agents Chemother. 2014, 58, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Souid, A.-K.; Dubowy, R.L.; Blaney, S.M.; Hershon, L.; Sullivan, J.; McLeod, W.D.; Bernstein, M.L. Phase I clinical and pharmacologic study of weekly cisplatin and irinotecan combined with amifostine for refractory solid tumors. Clin. Cancer Res. 2003, 9, 703–710. [Google Scholar]
- Dörbecker, C.; Sander, A.; Oberle, K.; Schülin-Casonato, T. In vitro susceptibility of Bartonella species to 17 antimicrobial compounds: Comparison of Etest and agar dilution. J. Antimicrob. Chemother. 2006, 58, 784–788. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B.; Kordick, D.L. Bartonella infection in animals: Carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin. Microbiol. Rev. 2000, 13, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.-M.; Maurin, M.; Mallet, M.-N.; Parzy, D.; Raoult, D. Culture and antibiotic susceptibility of Bartonella quintana in human erythrocytes. Antimicrob. Agents Chemother. 2003, 47, 614–619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schirmer, R.H.; Adler, H.; Pickhardt, M.; Mandelkow, E. Lest we forget you—methylene blue. Neurobiol. Aging 2011, 32, 2325. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Fatima, Z.; Hameed, S. Antifungal Action of Methylene Blue Involves Mitochondrial Dysfunction and Disruption of Redox and Membrane Homeostasis in C. albicans. Open Microbiol. J. 2016, 10, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Auwaerter, P.G.; Zhang, Y. Drug Combinations against Borrelia burgdorferi Persisters In Vitro: Eradication Achieved by Using Daptomycin, Cefoperazone and Doxycycline. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Perlmutter, N.G.; Shapiro, H.M. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Randall, C.P.; Mariner, K.R.; Chopra, I.; O’Neill, A.J. The target of daptomycin is absent from Escherichia coli and other gram-negative pathogens. Antimicrob. Agents Chemother. 2013, 57, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Kadavakollu, S.; Stailey, C.; Kunapareddy, C.; White, S. Clotrimazole as a cancer drug: A short review. Med. Chem. 2014, 4, 722. [Google Scholar]
- Huy, N.T.; Takano, R.; Hara, S.; Kamei, K. Enhancement of heme-induced membrane damage by the anti-malarial clotrimazole: The role of colloid-osmotic forces. Biol. Pharmacol. Bull. 2004, 27, 361–365. [Google Scholar] [CrossRef][Green Version]
- Odds, F.C.; Brown, A.J.; Gow, N.A. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Pulliainen, A.T.; Dehio, C. Persistence of Bartonella spp. stealth pathogens: From subclinical infections to vasoproliferative tumor formation. FEMS Microbiol. Rev. 2012, 36, 563–599. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).