Measuring Appropriate Antibiotic Prescribing in Acute Hospitals: Development of a National Audit Tool Through a Delphi Consensus
Abstract
1. Introduction
2. Results
2.1. Defining Appropriateness
- Prescribing an antibiotic for a patient in the absence of (documented) evidence of bacterial infection.
- Prescribing a critical broad-spectrum antibiotic to patients in the absence of a (documented) rationale.
- Continuing an antibiotic prescription beyond the course length recommended in local or national guidelines, in the absence of a (documented) rationale.
2.2. Initial Draft of Audit Tool
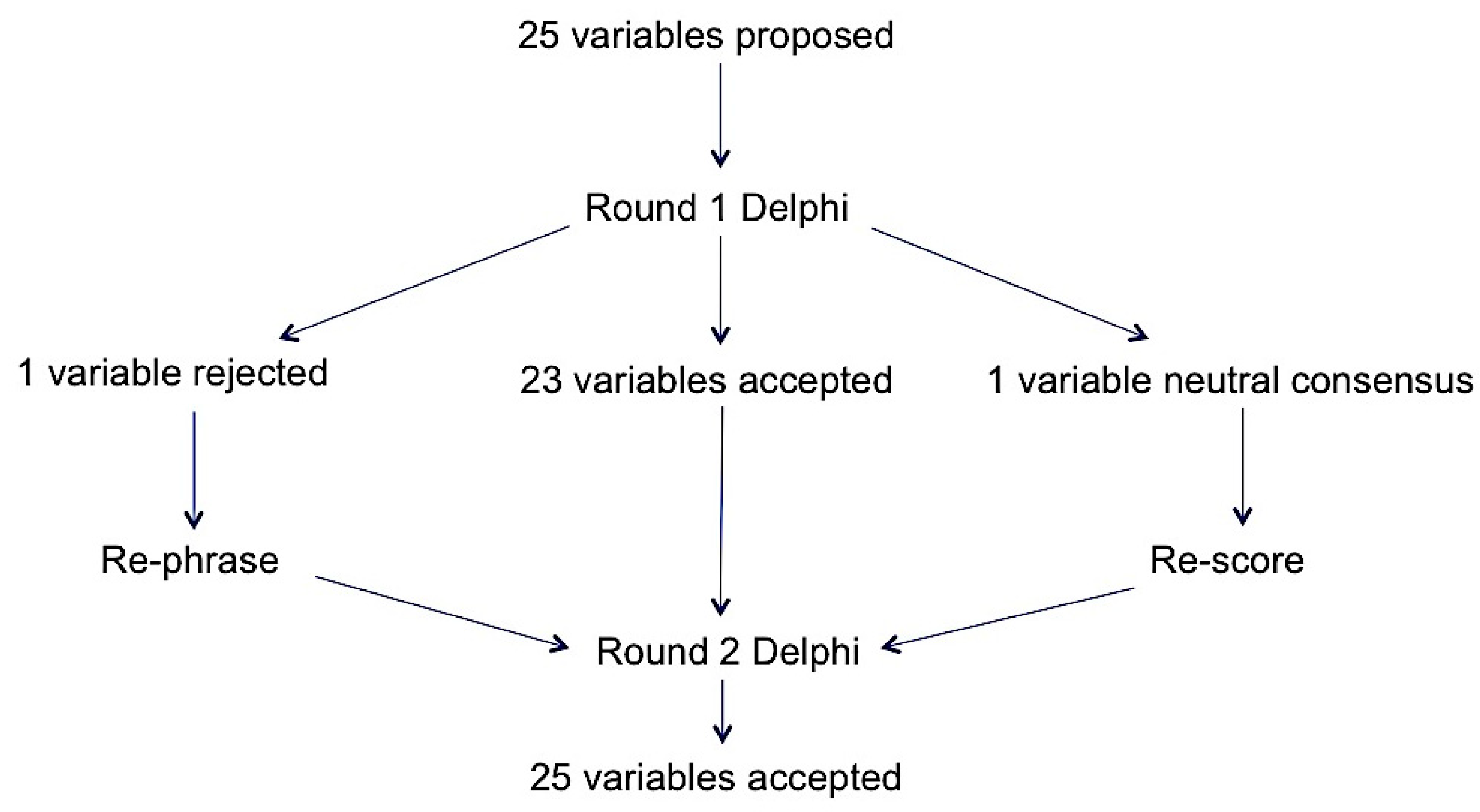
2.3. Round 1 Delphi
2.4. Round 2 Delphi
2.5. Feasibility
3. Discussion
3.1. Study Strengths and Limitations
3.2. Future Work
4. Materials and Methods
4.1. Part 1: Development of the Audit Tool
4.2. Part 2: Validation of the Audit Tool
- Initiation (was the antibiotic indicated and necessary at the start date?);
- Early post prescription review (was the antibiotic continued after infection was ruled out?);
- End of therapy (was the antibiotic continued beyond the standard duration?).
4.3. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO Antimicrobial Resistance. Available online: http://www.who.int/mediacentre/factsheets/fs194/en/ (accessed on 17 January 2019).
- Review on Antimicrobial Resistance. Available online: https://amr-review.org/background.html (accessed on 17 January 2019).
- English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) Report 2018. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/ attachment_data/file/759975/ESPAUR_2018_report.pdf (accessed on 22 March 2019).
- English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) Report 2017. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/ attachment_data/file/656611/ESPAUR_report_2017.pdf (accessed on 18 January 2019).
- Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals Surveillance Report 2011–2012. Available online: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/healthcare-associated-infections-antimicrobial-use-PPS.pdf (accessed on 18 January 2019).
- Kieran, J.A.; O’Doherty, R.G.; Hudson, B. ESAC point prevalence methodology to assess antimicrobial consumption and quality of prescribing in an Australian setting. Med. J. Aust. 2011, 194, 103–104. [Google Scholar]
- James, R.; Upjohn, L.; Cotta, M.; Luu, S.; Marshall, C.; Buising, K.; Thursky, K. Measuring antimicrobial prescribing quality in Australian hospitals: Development and evaluation of a national antimicrobial prescribing survey tool. J. Antimicrob. Chemother. 2015, 70, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Spivak, E.S.; Cosgrove, S.E.; Srinivasan, A. Measuring appropriate antimicrobial use: Attempts at opening the black box. Clin. Infect. Dis. 2016, 63, 1639–1644. [Google Scholar] [PubMed]
- Lopez-Lozano, J.M.; Monnet, D.L.; Yague, A.; Burgos, A.; Gonzalo, N.; Campillos, P.; Saez, M. Modelling and forecasting antimicrobial resistance and its dynamic relationship to antimicrobial use: A time series analysis. Int..J Antimicrob. Agents 2000, 14, 21–31. [Google Scholar] [CrossRef]
- Tacconelli, E.; Cataldo, M.A.; De Pascale, G.; Manno, D.; Spanu, T.; Cambieri, A.; Antonelli, M.; Sanguinetti, M.; Fadda, G.; Cauda, R. Prediction models to identify hospitalized patients at risk of being colonized or infected with multidrug-resistant Acinetobacter baumannii calcoaceticus complex. J. Antimicrob. Chemother. 2008, 62, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; De Angellis, G.; Cataldo, M.A.; Mantengoli, E.; Spanu, T.; Pan, A.; Corti, G.; Radice, A.; Stolzuoli, L.; Antinori, S.; et al. Antibiotic usage and risk of colonization and infection with antibiotic-resistant bacteria: A hospital population-based study. Antimicrob. Agents Chemother. 2009, 53, 4264–4269. [Google Scholar] [CrossRef] [PubMed]
- Braykov, N.P.; Morgan, D.J.; Schweizer, M.L.; Usian, D.Z.; Kelesidis, T.; Weisenberg, S.A.; Johannsson, B.; Young, H.; Cantey, J.; Scrinivason, A.; et al. Assessment of empirical antibiotic therapy optimisation in six hospitals: An observational cohort study. Lancet Infect. Dis. 2014, 14, 1220–1227. [Google Scholar] [CrossRef]
- De Sousa, A.G.; Fernandes Junior, C.J.; Santos, G.P.D.; Laselva, C.R.; Polessi, J.; Lisboa, L.F.; Akamine, N.; Silva, E. The impact of each action in the Surviving Sepsis Campaign measures on hospital mortality of patients with severe sepsis/septic shock. Einstein 2008, 6, 323–327. [Google Scholar]
- Paul, M.; Shani, V.; Muchtar, E.; Kariv, G.; Robenshtok, E.; Leibovici, L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob. Agents Chemother. 2010, 54, 4851–4863. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.D.; Perencevich, E.; Roghmann, M.C.; Morris, G.; Kaye, K.S.; Johnson, J.A. Risk factors for piperacillin-tazobactam-resistant Pseudomonas aeruginosa among hospitalized patients. Antimicrob. Agents Chemother. 2002, 46, 854–858. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, C.Y.; Chu, C.C.; Tan, C.K.; Lu, C.L.; Lee, Y.C.; Huang, Y.T.; Lee, P.I.; Hsueh, P.R. Correlation between antibiotic consumption and resistance of Gram-negative bacteria causing healthcare-associated infections at a university hospital in Taiwan from 2000 to 2009. J. Antimicrob. Chemother. 2011, 66, 1374–1382. [Google Scholar] [CrossRef]
- Pakyz, A.L.; Oinonen, M.; Polk, R.E. Relationship of carbapenem restriction in 22 university teaching hospitals to carbapenem use and carbapenem-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2009, 53, 1983–1986. [Google Scholar] [CrossRef]
- Opatowski, L.; Mandel, J.; Varon, E.; Boelle, P.Y.; Temime, L.; Guillemot, D. Antibiotic dose impact on resistance selection in the community: A mathematical model of beta-lactams and Streptococcus pneumoniae dynamics. Antimicrob. Agents Chemother. 2010, 54, 2330–2337. [Google Scholar] [CrossRef]
- Guillemot, D.; Carbon, C.; Balkau, B.; Geslin, P.; Lecoeur, H.; Vauzelle-Keroedan, F.; Bouvenot, G.; Eschwege, E. Low dosage and long treatment duration of beta-lactam: Risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA 1998, 279, 365–370. [Google Scholar] [CrossRef]
- Handel, A.; Margolis, E.; Levin, B.R. Exploring the role of the immune response in preventing antibiotic resistance. J. Theor. Biol. 2009, 256, 655–662. [Google Scholar] [CrossRef]
- Martinez, M.N.; Papich, M.G.; Drusano, G.L. Dosing regimen matters: The importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob. Agents Chemother. 2012, 56, 2795–2805. [Google Scholar] [CrossRef]
- Olofsson, S.K.; Cars, O. Optimizing drug exposure to minimize selection of antibiotic resistance. Clin. Infect. Dis. 2007, 45 (Suppl. 2), S129–S136. [Google Scholar] [CrossRef]
- Schrag, S.J.; Pena, C.; Fernandez, J.; Sanchez, J.; Gomez, V.; Perez, E.; Feris, J.M.; Besser, R.E. Effect of short-course, high-dose amoxicillin therapy on resistant pneumococcal carriage: A randomized trial. JAMA 2001, 286, 49–56. [Google Scholar] [CrossRef]
- Tam, V.H.; Louie, A.; Deziel, M.R.; Liu, W.; Drusano, G.L. The relationship between quinolone exposures and resistance amplification is characterized by an inverted U: A new paradigm for optimizing pharmacodynamics to counterselect resistance. Antimicrob. Agents Chemother. 2007, 51, 744–747. [Google Scholar] [CrossRef]
- Singh, N.; Rogers, P.; Atwood, C.W.; Wagener, M.M.; Yu, V.L. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am. J. Respir. Crit. Care Med. 2000, 162, 505–511. [Google Scholar] [CrossRef]
- Chastre, J.; Wolff, M.; Fagon, J.Y.; Chevret, S.; Thomas, F.; Wermert, D.; Clementi, E.; Gonzalez, J.; Jusserand, D.; Asfar, P.; et al. PneumA trial group. comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: A randomized trial. JAMA 2003, 290, 2588–2598. [Google Scholar] [CrossRef]
- Palacios-Baena, Z.R.; Gutierrez-Gutierrez, B.; De Cueto, M.; Viale, P.; Venditti, M.; Hernandez-Torres, A.; Oliver, A.; Martinez-Martinez, L.; Calbo, E.; Pintado, V.; et al. REIPI/ESGBIS/INCREMENT Group. Development and validation of the INCREMENT-ESBL predictive score for mortality in patients with bloodstream infections due to extended-spectrum-beta-lactamase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2017, 72, 906–913. [Google Scholar]
- Schuts, E.C.; Hulscher, M.E.; Mouton, J.W.; Verduin, C.M.; Stuart, J.W.T.C.; Overdiek, H.W.P.M.; van der Linden, P.D.; Natsch, S.; Hertogh, C.M.P.M.; Wolfs, T.F.W.; et al. Current evidence on hospital antimicrobial stewardship objectives: A systematic review and meta-analysis. Lancet Infect. Dis. 2016, 16, 847–856. [Google Scholar] [CrossRef]
- Marra, A.R.; de Almeida, S.M.; Correa, L.; Silva, M., Jr.; Martino, M.D.; Silva, C.V.; Cal, R.G.; Edmond, M.B.; dos Santos, O.F. The effect of limiting antimicrobial therapy duration on antimicrobial resistance in the critical care setting. Am. J. Infect. Control. 2009, 37, 204–209. [Google Scholar] [CrossRef]
- Boulkedid, R.; Abdoul, H.; Loustau, M.; Sibony, O.; Alberti, C. Using and reporting the delphi method for selecting healthcare quality indicators: A systematic review. PLoS ONE 2011, 6, e20476. [Google Scholar] [CrossRef]
- Corfield, A.R.; Lees, F.; Zealley, I.; Houston, G.; Dickie, S.; Ward, K.; McGuffie, C. Utility of a single early warning score in patients with sepsis in the emergency department. Emerg. Med. J. 2014, 31, 482–487. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Iwashyna, T.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; et al. Assessment of clinical criteria for sepsis: For the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]
- Freund, Y.; Lemachatti, N.; Krastinova, E.; Van Laer, M.; Claessens, Y.-E.; Avonso, A.; Occelli, C.; Feral-Pierssens, A.-L.; Truchot, J.; Ortega, M.; et al. Prognostic accuracy of sepsis-3 criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA 2017, 317, 301–308. [Google Scholar] [CrossRef]
- NHS England National CQUIN Templates 2016/17. Available online: https://www.england.nhs.uk/nhs-standard-contract/cquin/cquin-16-17/ (accessed on 18 January 2019).
- National Early Warning Score (NEWS): Standardising the Assessment of Acute Illness Severity in the NHS. Report of a Working Party. Available online: https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news (accessed on 18 January 2019).
- Keep, J.W.; Messmer, A.S.; Sladden, R.; Burrell, N.; Pinate, R.; Tunnicliff, M.; Glucksman, E. National early warning score at Emergency Department triage may allow earlier identification of patients with severe sepsis and septic shock: A retrospective observational study. Emerg. Med. J. 2016, 33, 37–41. [Google Scholar] [CrossRef]
- Vagias, W.M. Likert-Type Scale Response Anchors; Clemson International Institute for Tourism & Research Development, Department of Parks, Recreation and Tourism Management, Clemson University: Clemson, SC, USA, 2006. [Google Scholar]
- Holey, E.A.; Feeley, J.L.; Dixon, J.; Whittaker, V.J. An exploration of the use of simple statistics to measure consensus and stability in Delphi studies. BMC Med. Res. Methodol. 2007, 7, 52. [Google Scholar] [CrossRef]
| Prescribing Elements (Potential Audit Variables) | Comments | Selected for Audit |
|---|---|---|
| START SMART | ||
| No antibiotic if not indicated (no reasonable evidence of infection) | Unnecessary antibiotic exposure selects for avoidable resistance [9,10,11]. | ✓ |
| Indication documented | Good practice for continuity of care but of uncertain relevance to resistance. | ✓ |
| Appropriate specimens taken for microscopy, culture, and sensitivity (MC&S)—blood cultures and suspected site of infection | Important for establishing evidence of infection and for targeting appropriate therapy but requires manual audit and >50% of cultures are negative [12,13]. | ✓ |
| No allergy or contra-indication to treatments | Important patient safety consideration but not relevant for resistance. | ✕ |
| Prompt administration of first dose | Important patient safety consideration in cases of severe sepsis but of uncertain relevance to resistance. Already captured by national sepsis audits. | ✕ |
| Treatment regimen adequate to cover most likely pathogens | Meta-analysis of RCTs reports increased risk of mortality if initial regimen inadequate [14]. Relevance to resistance uncertain. | ✓ * |
| Treatment regimen not unnecessarily broad spectrum | Indiscriminate use of critical broad-spectrum agents unnecessarily selects for resistance [15,16,17]. | ✓ * |
| No redundant agents in treatment regimen | Unnecessary antibiotic exposure selects for avoidable resistance [9,10,11]. | ✓ |
| Treatment regimen compliant with local/national guideline or justified deviation | Validity dependent upon quality of local guideline. Relevance to resistance uncertain. | ✕ |
| Treatment regimen cost-effective | Not relevant to resistance. | ✕ |
| No underdosing | Limited evidence from modeling suggests that low doses may select resistance in pneumococci [18] but underdosing unlikely to be a problem in NHS hospitals due to pharmacist and nurse intervention. | ✕ |
| No overdosing | Important patient safety consideration but likely to reduce rather than increase risk of selecting resistance [19,20,21,22,23,24]. | ✕ |
| Correct route of administration | Relevant for efficacy, length of stay, and risk of line infection but of uncertain relevance to resistance. | ✕ |
| Prompt appropriate source control | Subjective assessment. Of uncertain relevance to resistance. | ✕ |
| No missed doses or delayed doses | Of uncertain relevance to selection of resistance. | ✕ |
| Therapeutic drug monitoring (TDM) for narrow therapeutic index drugs | Important primarily for patient safety (but also for efficacy); of uncertain relevance to resistance. | ✕ |
| THEN FOCUS | ||
| Prompt discontinuation of antibiotics if alternative diagnosis established and infection excluded | There is RCT evidence that unnecessary continuation selects for multi-resistant organisms [25,26,27]. | ✓ |
| Appropriate broadening of spectrum in response to MC&S results | This may necessitate an increase in broad-spectrum agent use if indicated by MC&S results. Failure to adjust ineffective treatment to MC&S results is associated with a higher risk of mortality [27]. | ✓ * |
| Appropriate narrowing of spectrum in response to MC&S results | Evidence largely from observational studies suggests that de-escalation to narrow-spectrum agents is safe when patients are improving clinically and a plausible pathogen has been identified [28]. | ✓ * |
| Prompt referral to outpatient parenteral antibiotic therapy OPAT services for suitable patients | Relevant for length of stay and risk of healthcare-associated infection (HCAI) but of uncertain relevance to resistance. | ✕ |
| Prompt switch from IV to oral route of administration when safe and effective | Relevant for length of stay and risk of line infection but of uncertain relevance to resistance. | ✕ |
| Antibiotic plan documented in the notes | Good practice for continuity of care but of uncertain relevance to resistance. | ✕ |
| No unjustified prolonged duration of treatment | There is evidence from RCTs and observational studies that unnecessarily prolonged duration selects for multi-resistant organisms [25,26,29]. Can only be audited at the end of therapy. | ✓ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hood, G.; Hand, K.S.; Cramp, E.; Howard, P.; Hopkins, S.; Ashiru-Oredope, D., on behalf of Antibiotic Prescribing Appropriateness Measures (APAM) subgroup of the national Advisory Committee on Antimicrobial Resistance, Prescribing and Healthcare Associated Infection (ARPHAI). Measuring Appropriate Antibiotic Prescribing in Acute Hospitals: Development of a National Audit Tool Through a Delphi Consensus. Antibiotics 2019, 8, 49. https://doi.org/10.3390/antibiotics8020049
Hood G, Hand KS, Cramp E, Howard P, Hopkins S, Ashiru-Oredope D on behalf of Antibiotic Prescribing Appropriateness Measures (APAM) subgroup of the national Advisory Committee on Antimicrobial Resistance, Prescribing and Healthcare Associated Infection (ARPHAI). Measuring Appropriate Antibiotic Prescribing in Acute Hospitals: Development of a National Audit Tool Through a Delphi Consensus. Antibiotics. 2019; 8(2):49. https://doi.org/10.3390/antibiotics8020049
Chicago/Turabian StyleHood, Graeme, Kieran S. Hand, Emma Cramp, Philip Howard, Susan Hopkins, and Diane Ashiru-Oredope on behalf of Antibiotic Prescribing Appropriateness Measures (APAM) subgroup of the national Advisory Committee on Antimicrobial Resistance, Prescribing and Healthcare Associated Infection (ARPHAI). 2019. "Measuring Appropriate Antibiotic Prescribing in Acute Hospitals: Development of a National Audit Tool Through a Delphi Consensus" Antibiotics 8, no. 2: 49. https://doi.org/10.3390/antibiotics8020049
APA StyleHood, G., Hand, K. S., Cramp, E., Howard, P., Hopkins, S., & Ashiru-Oredope, D., on behalf of Antibiotic Prescribing Appropriateness Measures (APAM) subgroup of the national Advisory Committee on Antimicrobial Resistance, Prescribing and Healthcare Associated Infection (ARPHAI). (2019). Measuring Appropriate Antibiotic Prescribing in Acute Hospitals: Development of a National Audit Tool Through a Delphi Consensus. Antibiotics, 8(2), 49. https://doi.org/10.3390/antibiotics8020049






