The Quest for Novel Antimicrobial Compounds: Emerging Trends in Research, Development, and Technologies
Abstract
1. Introduction
2. Novel Targets, Discovery Approaches, and Sources
2.1. Novel Antibiotic Targets
2.2. Novel Discovery Approaches
2.2.1. Informatics-Based Discovery Approaches
2.2.2. BGC Activation and Engineering
2.3. Novel Antimicrobial Sources
3. Novel Antimicrobial Molecules
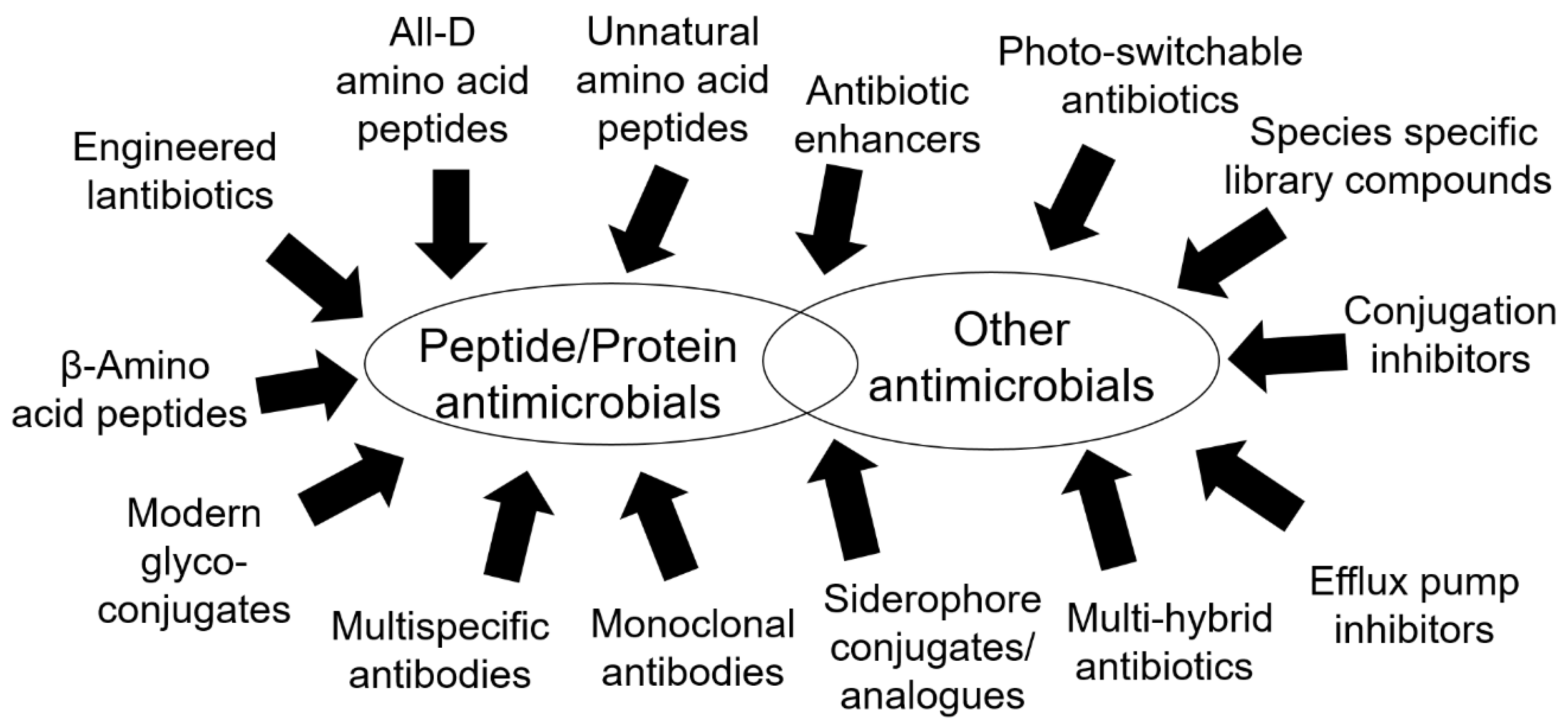
3.1. Peptides and Peptide-Related Molecules
3.2. Antibodies
3.3. Phage-Based Strategies and CRISPR
3.4. Miscellaneous Antimicrobials
4. Antimicrobial Materials
4.1. Nanomaterials
4.2. Materials and Techniques Targeting Biofilms
5. Technological Advancements in Diagnostics and Screening
5.1. Infection Monitoring Technologies
5.2. Direct in-Sample Technologies
5.3. Point-of-Care Devices
6. Ecological Management
6.1. Probiotics, Synbiotics and Prebiotics
6.2. Understanding Ecological Interactions in Health and Disease
6.2.1. Host-Microbiome Interactions
6.2.2. Inter-Microbial Interactions
6.3. Wider Ecology and One Health
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thornsberry, C.; Sahm, D.F.; Kelly, L.J.; Critchley, I.A.; Jones, M.E.; Evangelista, A.T.; Karlowsky, J.A. Regional Trends in Antimicrobial Resistance among Clinical Isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the United States: Results from the Trust Surveillance Program, 1999–2000. Clin. Infect. Dis. 2002, 34 (Suppl. S1), S4–S16. [Google Scholar] [CrossRef]
- Palumbi, S.R. Humans as the World’s Greatest Evolutionary Force. Science 2001, 293, 1786–1790. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C. Antibiotics: Actions, Origins, Resistance; ASM Press: Washington, DC, USA, 2003; ISBN 9781555817886. [Google Scholar]
- Gelband, H.; Miller-Petrie, M.; Pant, S.; Gandra, S.; Levinson, J.; Barter, D.; White, A.; Laxminarayan, R. State of the World’s Antibiotics. Wound Heal. S. Afr. 2015, 8, 30–34. [Google Scholar]
- Coates, A.; Hu, Y.; Bax, R.; Page, C. The Future Challenges Facing the Development of New Antimicrobial Drugs. Nat. Rev. Drug Discov. 2002, 1, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Outterson, K.; Gopinathan, U.; Clift, C.; So, A.D.; Morel, C.M.; Røttingen, J.A. Delinking Investment in Antibiotic Research and Development from Sales Revenues: The Challenges of Transforming a Promising Idea into Reality. PLoS Med. 2016, 13, e1002043. [Google Scholar] [CrossRef] [PubMed]
- Hudson, A.O.; Gilvarg, C.; Leustek, T. Biochemical and Phylogenetic Characterization of a Novel Diaminopimelate Biosynthesis Pathway in Prokaryotes Identifies a Diverged Form of Ll-Diaminopimelate Aminotransferase. J. Bacteriol. 2008, 190, 3256–3263. [Google Scholar] [CrossRef] [PubMed]
- Triassi, A.J.; Wheatley, M.S.; Savka, M.A.; Gan, H.M.; Dobson, R.C.; Hudson, A.O. L,L-Diaminopimelate Aminotransferase (Dapl): A Putative Target for the Development of Narrow-Spectrum Antibacterial Compounds. Front. Microbiol. 2014, 5, 509. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bello, C. Inhibition of Shikimate Kinase and Type II Dehydroquinase for Antibiotic Discovery: Structure-Based Design and Simulation Studies. Curr. Top. Med. Chem. 2016, 16, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.E.; Goodfellow, C.; Javid-Majd, F.; Baker, E.N.; Shaun Lott, J. The Crystal Structure of Trpd, a Metabolic Enzyme Essential for Lung Colonization by Mycobacterium Tuberculosis, in Complex with Its Substrate Phosphoribosylpyrophosphate. J. Mol. Biol. 2006, 355, 784–797. [Google Scholar] [CrossRef]
- Czekster, C.M.; Neto, B.A.; Lapis, A.A.; Dupont, J.; Santos, D.S.; Basso, L.A. Steady-State Kinetics of Indole-3-Glycerol Phosphate Synthase from Mycobacterium tuberculosis. Arch. Biochem. Biophys. 2009, 486, 19–26. [Google Scholar] [CrossRef]
- Shen, H.; Yang, Y.; Wang, F.; Zhang, Y.; Ye, N.; Xu, S.; Wang, H. Characterization of the Putative Tryptophan Synthase Beta-Subunit from Mycobacterium tuberculosis. Acta Biochim. Biophys. Sin. 2009, 41, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.W.; Roberts, F.; Lyons, R.E.; Kirisits, M.J.; Mui, E.J.; Finnerty, J.; Johnson, J.J.; Ferguson, D.J.; Coggins, J.R.; Krell, T.; et al. The Shikimate Pathway and Its Branches in Apicomplexan Parasites. J. Infect. Dis. 2002, 185 (Suppl. S1), S25–S36. [Google Scholar] [CrossRef]
- Campbell, S.A.; Richards, T.A.; Mui, E.J.; Samuel, B.U.; Coggins, J.R.; Mcleod, R.; Roberts, C.W. A Complete Shikimate Pathway in Toxoplasma Gondii: An Ancient Eukaryotic Innovation. Int. J. Parasitol. 2004, 34, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.W.; Skinnider, M.A.; Dejong, C.A.; Rees, P.N.; Chen, G.M.; Walker, C.G.; French, S.; Brown, E.D.; Bérdy, J.; Liu, D.Y.; et al. Assembly and Clustering of Natural Antibiotics Guides Target Identification. Nat. Chem. Biol. 2016, 12, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A New Antibiotic Kills Pathogens without Detectable Resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef]
- Srinivas, N.; Jetter, P.; Ueberbacher, B.J.; Werneburg, M.; Zerbe, K.; Steinmann, J.; Van Der Meijden, B.; Bernardini, F.; Lederer, A.; Dias, R.L.; et al. Peptidomimetic Antibiotics Target Outer-Membrane Biogenesis in Pseudomonas aeruginosa. Science 2010, 327, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Ghssein, G.; Brutesco, C.; Ouerdane, L.; Fojcik, C.; Izaute, A.; Wang, S.; Hajjar, C.; Lobinski, R.; Lemaire, D.; Richaud, P.; et al. Biosynthesis of a Broad-Spectrum Nicotianamine-Like Metallophore in Staphylococcus aureus. Science 2016, 352, 1105–1109. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum Sensing: Cell-to-Cell Communication in Bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef]
- Thoendel, M.; Horswill, A.R. Identification of Staphylococcus aureus Agrd Residues Required for Autoinducing Peptide Biosynthesis. J. Biol. Chem. 2009, 284, 21828–21838. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- Vendeville, A.; Winzer, K.; Heurlier, K.; Tang, C.M.; Hardie, K.R. Making ‘Sense’ of Metabolism: Autoinducer-2, Luxs and Pathogenic Bacteria. Nat Rev Microbiol 2005, 3, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, N.; Lee, B.; Hentzer, M.; Rasmussen, T.B.; Song, Z.; Johansen, H.K.; Givskov, M.; Høiby, N. Azithromycin Blocks Quorum Sensing and Alginate Polymer Formation and Increases the Sensitivity to Serum and Stationary-Growth-Phase Killing of Pseudomonas aeruginosa and Attenuates Chronic P. aeruginosa Lung Infection in Cftr(-/-) Mice. Antimicrob Agents Chemother 2007, 51, 3677–3687. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Coenye, T. Quorum Sensing Inhibitors as Anti-Biofilm Agents. Curr Pharm Des 2015, 21, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lu, C. Biological Activity and Identification of a Peptide Inhibitor of Luxs from Streptococcus suis Serotype 2. FEMS Microbiol Lett 2009, 294, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiao, X.D.; Hu, Y.H.; Sun, L. Attenuation of Edwardsiella tarda Virulence by Small Peptides That Interfere with LuxS/Autoinducer Type 2 Quorum Sensing. Appl Environ Microbiol 2009, 75, 3882–3890. [Google Scholar] [CrossRef]
- Lee, J.E.; Singh, V.; Evans, G.B.; Tyler, P.C.; Furneaux, R.H.; Cornell, K.A.; Riscoe, M.K.; Schramm, V.L.; Howell, P.L. Structural Rationale for the Affinity of Pico- and Femtomolar Transition State Analogues of Escherichia coli 5’-Methylthioadenosine/S-Adenosylhomocysteine Nucleosidase. J. Biol. Chem. 2005, 280, 18274–18282. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.A.; Crowder, T.; Rinaldo-Matthis, A.; Ho, M.C.; Almo, S.C.; Schramm, V.L. Transition State Analogs of 5’-Methylthioadenosine Nucleosidase Disrupt Quorum Sensing. Nat. Chem. Biol. 2009, 5, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Uroz, S.; Dessaux, Y.; Oger, P. Quorum Sensing and Quorum Quenching: The Yin and Yang of Bacterial Communication. Chembiochem 2009, 10, 205–216. [Google Scholar] [CrossRef]
- Park, J.; Jagasia, R.; Kaufmann, G.F.; Mathison, J.C.; Ruiz, D.I.; Moss, J.A.; Meijler, M.M.; Ulevitch, R.J.; Janda, K.D. Infection Control by Antibody Disruption of Bacterial Quorum Sensing Signaling. Chem. Biol. 2007, 14, 1119–1127. [Google Scholar] [CrossRef]
- Chan, A.; Lam, G.; Lee, G.; Lowe, C.; Yip, V. Effects of Antibody Induced Localized Cell Crowding on Autoinducer-2 Levels in Salmonella Typhimurium Lt2. J. Exp. Microbiol. Immunol. 2004, 5, 29–36. [Google Scholar]
- Gavrish, E.; Sit, C.S.; Cao, S.; Kandror, O.; Spoering, A.; Peoples, A.; Ling, L.; Fetterman, A.; Hughes, D.; Bissell, A.; et al. Lassomycin, a Ribosomally Synthesized Cyclic Peptide, Kills Mycobacterium tuberculosis by Targeting the Atp-Dependent Protease Clpc1p1p2. Chem. Biol. 2014, 21, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Conlon, B.P.; Nakayasu, E.S.; Fleck, L.E.; Lafleur, M.D.; Isabella, V.M.; Coleman, K.; Leonard, S.N.; Smith, R.D.; Adkins, J.N.; Lewis, K. Activated Clpp Kills Persisters and Eradicates a Chronic Biofilm Infection. Nature 2013, 503, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Cha, E.; Kim, Y.; Jeon, Y.H.; Olson, B.H.; Byun, Y.; Park, H.D. Raffinose, a Plant Galactoside, Inhibits Pseudomonas aeruginosa Biofilm Formation Via Binding to Leca and Decreasing Cellular Cyclic Diguanylate Levels. Sci. Rep. 2016, 6, 25318. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.L. Genomics Outpaces “Grind and Find” in Search for Useful Natural Products. Microbe 2015, 10, 407–408. [Google Scholar] [CrossRef]
- Weber, T.; Blin, K.; Duddela, S.; Krug, D.; Kim, H.U.; Bruccoleri, R.; Lee, S.Y.; Fischbach, M.A.; Müller, R.; Wohlleben, W.; et al. Antismash 3.0-a Comprehensive Resource for the Genome Mining of Biosynthetic Gene Clusters. Nucleic Acids Res. 2015, 43, W237–W243. [Google Scholar] [CrossRef]
- Blin, K.; Wolf, T.; Chevrette, M.G.; Lu, X.; Schwalen, C.J.; Kautsar, S.A.; Suarez Duran, H.G.; De Los Santos, E.L.C.; Kim, H.U.; Nave, M.; et al. Antismash 4.0-Improvements in Chemistry Prediction and Gene Cluster Boundary Identification. Nucleic Acids Res. 2017, 45, W36–W41. [Google Scholar] [CrossRef] [PubMed]
- Smanski, M.J.; Zhou, H.; Claesen, J.; Shen, B.; Fischbach, M.A.; Voigt, C.A. Synthetic Biology to Access and Expand Nature’s Chemical Diversity. Nat. Rev. Microbiol. 2016, 14, 135–149. [Google Scholar] [CrossRef]
- Rudolf, J.D.; Dong, L.B.; Huang, T.; Shen, B. A Genetically Amenable Platensimycin- and Platencin-Overproducer as a Platform for Biosynthetic Explorations: A Showcase of Ptmo4, a Long-Chain Acyl-Coa Dehydrogenase. Mol. Biosyst. 2015, 11, 2717–2726. [Google Scholar] [CrossRef]
- Smanski, M.J.; Schlatter, D.C.; Kinkel, L.L. Leveraging Ecological Theory to Guide Natural Product Discovery. J. Ind. Microbiol. Biotechnol. 2016, 43, 115–128. [Google Scholar] [CrossRef]
- Katz, M.; Hover, B.M.; Brady, S.F. Culture-Independent Discovery of Natural Products from Soil Metagenomes. J. Ind. Microbiol. Biotechnol. 2016, 43, 129–141. [Google Scholar] [CrossRef]
- Reddy, B.V.; Milshteyn, A.; Charlop-Powers, Z.; Brady, S.F. Esnapd: A Versatile, Web-Based Bioinformatics Platform for Surveying and Mining Natural Product Biosynthetic Diversity from Metagenomes. Chem. Biol. 2014, 21, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Hover, B.M.; Kim, S.H.; Katz, M.; Charlop-Powers, Z.; Owen, J.G.; Ternei, M.A.; Maniko, J.; Estrela, A.B.; Molina, H.; Park, S.; et al. Culture-Independent Discovery of the Malacidins as Calcium-Dependent Antibiotics with Activity against Multidrug-Resistant Gram-Positive Pathogens. Nat. Microbiol. 2018, 3, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Arango-Argoty, G.; Garner, E.; Pruden, A.; Heath, L.S.; Vikesland, P.; Zhang, L. DeepARG: A Deep Learning Approach for Predicting Antibiotic Resistance Genes from Metagenomic Data. Microbiome 2018, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. Card 2017: Expansion and Model-Centric Curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef] [PubMed]
- Nigam, A.; Almabruk, K.H.; Saxena, A.; Yang, J.; Mukherjee, U.; Kaur, H.; Kohli, P.; Kumari, R.; Singh, P.; Zakharov, L.N.; et al. Modification of Rifamycin Polyketide Backbone Leads to Improved Drug Activity against Rifampicin-Resistant Mycobacterium Tuberculosis. J. Biol. Chem. 2014, 289, 21142–21152. [Google Scholar] [CrossRef] [PubMed]
- Bentley, S.D.; Chater, K.F.; Cerdeño-Tárraga, A.M.; Challis, G.L.; Thomson, N.R.; James, K.D.; Harris, D.E.; Quail, M.A.; Kieser, H.; Harper, D.; et al. Complete Genome Sequence of the Model Actinomycete Streptomyces coelicolor A3(2). Nature 2002, 417, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Ishikawa, J.; Hanamoto, A.; Shinose, M.; Kikuchi, H.; Shiba, T.; Sakaki, Y.; Hattori, M.; Omura, S. Complete Genome Sequence and Comparative Analysis of the Industrial Microorganism Streptomyces avermitilis. Nat. Biotechnol. 2003, 21, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, P.J.; Challis, G.L. Discovery of Microbial Natural Products by Activation of Silent Biosynthetic Gene Clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef]
- Shao, Z.; Rao, G.; Li, C.; Abil, Z.; Luo, Y.; Zhao, H. Refactoring the Silent Spectinabilin Gene Cluster Using a Plug-and-Play Scaffold. ACS Synth. Biol. 2013, 2, 662–669. [Google Scholar] [CrossRef]
- Guo, F.; Xiang, S.; Li, L.; Wang, B.; Rajasärkkä, J.; Gröndahl-Yli-Hannuksela, K.; Ai, G.; Metsä-Ketelä, M.; Yang, K. Targeted Activation of Silent Natural Product Biosynthesis Pathways by Reporter-Guided Mutant Selection. Metab. Eng. 2015, 28, 134–142. [Google Scholar] [CrossRef]
- Komatsu, M.; Uchiyama, T.; Omura, S.; Cane, D.E.; Ikeda, H. Genome-Minimized Streptomyces Host for the Heterologous Expression of Secondary Metabolism. Proc. Natl. Acad. Sci. USA 2010, 107, 2646–2651. [Google Scholar] [CrossRef] [PubMed]
- Nah, H.J.; Pyeon, H.R.; Kang, S.H.; Choi, S.S.; Kim, E.S. Cloning and Heterologous Expression of a Large-Sized Natural Product Biosynthetic Gene Cluster in Streptomyces Species. Front. Microbiol. 2017, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- Pyeon, H.R.; Nah, H.J.; Kang, S.H.; Choi, S.S.; Kim, E.S. Heterologous Expression of Pikromycin Biosynthetic Gene Cluster Using Streptomyces Artificial Chromosome System. Microb. Cell Fact. 2017, 16, 96. [Google Scholar] [CrossRef]
- Castro, J.F.; Razmilic, V.; Gomez-Escribano, J.P.; Andrews, B.; Asenjo, J.A.; Bibb, M.J. Identification and Heterologous Expression of the Chaxamycin Biosynthesis Gene Cluster from Streptomyces leeuwenhoekii. Appl. Environ. Microbiol. 2015, 81, 5820–5831. [Google Scholar] [CrossRef] [PubMed]
- Shima, J.; Hesketh, A.; Okamoto, S.; Kawamoto, S.; Ochi, K. Induction of Actinorhodin Production by Rpsl (Encoding Ribosomal Protein S12) Mutations That Confer Streptomycin Resistance in Streptomyces lividans and Streptomyces coelicolor A3(2). J. Bacteriol. 1996, 178, 7276–7284. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sena Filho, J.G.; Hoover, A.R.; King, J.B.; Ellis, T.K.; Powell, D.R.; Cichewicz, R.H. Chemical Epigenetics Alters the Secondary Metabolite Composition of Guttate Excreted by an Atlantic-Forest-Soil-Derived Penicillium citreonigrum. J. Nat. Prod. 2010, 73, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, Y.Q. Genome-Guided Discovery of Diverse Natural Products from Burkholderia sp. J. Ind. Microbiol. Biotechnol. 2014, 41, 275–284. [Google Scholar] [CrossRef]
- Seyedsayamdost, M.R. High-Throughput Platform for the Discovery of Elicitors of Silent Bacterial Gene Clusters. Proc. Natl. Acad. Sci. USA 2014, 111, 7266–7271. [Google Scholar] [CrossRef]
- Pettit, R.K. Small-Molecule Elicitation of Microbial Secondary Metabolites. Microb. Biotechnol. 2011, 4, 471–478. [Google Scholar] [CrossRef]
- Hsiao, A.; Ahmed, A.M.; Subramanian, S.; Griffin, N.W.; Drewry, L.L.; Petri, W.A.; Haque, R.; Ahmed, T.; Gordon, J.I. Members of the Human Gut Microbiota Involved in Recovery from Vibrio cholerae Infection. Nature 2014, 515, 423–426. [Google Scholar] [CrossRef]
- Craney, A.; Ozimok, C.; Pimentel-Elardo, S.M.; Capretta, A.; Nodwell, J.R. Chemical Perturbation of Secondary Metabolism Demonstrates Important Links to Primary Metabolism. Chem. Biol. 2012, 19, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bimerew, M.; Ma, Y.; Müller, H.; Ovadis, M.; Eberl, L.; Berg, G.; Chernin, L. Quorum-Sensing Signaling Is Required for Production of the Antibiotic Pyrrolnitrin in a Rhizospheric Biocontrol Strain of Serratia plymuthica. FEMS Microbiol. Lett. 2007, 270, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.C.; Mori, T.; Rückert, C.; Uria, A.R.; Helf, M.J.; Takada, K.; Gernert, C.; Steffens, U.A.; Heycke, N.; Schmitt, S.; et al. An Environmental Bacterial Taxon with a Large and Distinct Metabolic Repertoire. Nature 2014, 506, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Chen, B.; Sun, C.; Ishida, K.; Hertweck, C.; Boland, W. Symbiont-Derived Antimicrobials Contribute to the Control of the Lepidopteran Gut Microbiota. Cell Chem. Biol. 2017, 24, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Vancompernolle, S.E.; Taylor, R.J.; Oswald-Richter, K.; Jiang, J.; Youree, B.E.; Bowie, J.H.; Tyler, M.J.; Conlon, J.M.; Wade, D.; Aiken, C.; et al. Antimicrobial Peptides from Amphibian Skin Potently Inhibit Human Immunodeficiency Virus Infection and Transfer of Virus from Dendritic Cells to T Cells. J Virol 2005, 79, 11598–11606. [Google Scholar] [CrossRef] [PubMed]
- M C Chung, E.; Dean, S.N.; Propst, C.N.; Bishop, B.M.; Van Hoek, M.L. Komodo Dragon-Inspired Synthetic Peptide Drgn-1 Promotes Wound-Healing of a Mixed-Biofilm Infected Wound. NPJ Biofilms Microbiomes 2017, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Zipperer, A.; Konnerth, M.C.; Laux, C.; Berscheid, A.; Janek, D.; Weidenmaier, C.; Burian, M.; Schilling, N.A.; Slavetinsky, C.; Marschal, M.; et al. Human Commensals Producing a Novel Antibiotic Impair Pathogen Colonization. Nature 2016, 535, 511–516. [Google Scholar] [CrossRef]
- Qin, Z.; Munnoch, J.T.; Devine, R.; Holmes, N.A.; Seipke, R.F.; Wilkinson, K.A.; Wilkinson, B.; Hutchings, M.I. Formicamycins, Antibacterial Polyketides Produced By Streptomyces formicae isolated from African Tetraponera plant-ants. Chem. Sci. 2017, 8, 3218–3227. [Google Scholar] [CrossRef]
- Oh, D.C.; Poulsen, M.; Currie, C.R.; Clardy, J. Dentigerumycin: A Bacterial Mediator of an Ant-Fungus Symbiosis. Nat. Chem. Biol. 2009, 5, 391–393. [Google Scholar] [CrossRef]
- Lu, C.; Shen, Y. A Novel Ansamycin, Naphthomycin K from Streptomyces sp. J. Antibiot. 2007, 60, 649–653. [Google Scholar] [CrossRef]
- Chagas, M.B.O.; Prazeres Dos Santos, I.; Nascimento Da Silva, L.C.; Correia, M.T.D.S.; Magali De Araújo, J.; Cavalcanti, M.D.S.; Lima, V.L.M. Antimicrobial Activity of Cultivable Endophytic Fungi Associated With Hancornia Speciosa Gomes Bark. Open Microbiol. J. 2017, 11, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Xing, Y.; Chen, J.; Zhang, D.; Guo, S.; Wang, C. Antimicrobial Activities of Endophytic Fungi Isolated from Ophiopogon japonicus (Liliaceae). BMC Complement. Altern. Med. 2012, 12, 238. [Google Scholar] [CrossRef]
- Verma, V.C.; Gond, S.K.; Kumar, A.; Mishra, A.; Kharwar, R.N.; Gange, A.C. Endophytic Actinomycetes from Azadirachta indica A. Juss.: Isolation, Diversity, and Anti-Microbial Activity. Microb. Ecol. 2009, 57, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Gan, H.M.; Wong, N.H.; Savka, M.A.; Steiner, K.K.; Henry, K.R.; Hudson, A.O. Isolation and Genomic Characterization of Six Endophytic Bacteria Isolated from Saccharum sp (Sugarcane): Insights into Antibiotic, Secondary Metabolite and Quorum Sensing Metabolism. J. Genom. 2018, 6, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.L. Metabolite Induction Via Microorganism Co-Culture: A Potential Way to Enhance Chemical Diversity for Drug Discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef] [PubMed]
- Tyc, O.; De Jager, V.C.L.; Van Den Berg, M.; Gerards, S.; Janssens, T.K.S.; Zaagman, N.; Kai, M.; Svatos, A.; Zweers, H.; Hordijk, C.; et al. Exploring Bacterial Interspecific Interactions for Discovery of Novel Antimicrobial Compounds. Microb. Biotechnol. 2017, 10, 910–925. [Google Scholar] [CrossRef]
- Tyc, O.; Van Den Berg, M.; Gerards, S.; Van Veen, J.A.; Raaijmakers, J.M.; De Boer, W.; Garbeva, P. Impact of Interspecific Interactions on Antimicrobial Activity among Soil Bacteria. Front. Microbiol. 2014, 5, 567. [Google Scholar] [CrossRef]
- Mearns-Spragg, A.; Bregu, M.; Boyd, K.G.; Burgess, J.G. Cross-Species Induction and Enhancement of Antimicrobial Activity Produced by Epibiotic Bacteria from Marine Algae and Invertebrates, after Exposure to Terrestrial Bacteria. Lett. Appl. Microbiol. 1998, 27, 142–146. [Google Scholar] [CrossRef]
- Burgess, J.G.; Jordan, E.M.; Bregu, M.; Mearns-Spragg, A.; Boyd, K.G. Microbial Antagonism: A Neglected Avenue of Natural Products Research. J. Biotechnol. 1999, 70, 27–32. [Google Scholar] [CrossRef]
- Cueto, M.; Jensen, P.R.; Kauffman, C.; Fenical, W.; Lobkovsky, E.; Clardy, J. Pestalone, a New Antibiotic Produced by a Marine Fungus in Response to Bacterial Challenge. J. Nat. Prod. 2001, 64, 1444–1446. [Google Scholar] [CrossRef]
- Ahmed, S.; Craney, A.; Pimentel-Elardo, S.M.; Nodwell, J.R. A Synthetic, Species-Specific Activator of Secondary Metabolism and Sporulation in Streptomyces coelicolor. ChemBioChem 2013, 14, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Slavov, N.; Cvengroš, J.; Neudörfl, J.M.; Schmalz, H.G. Total Synthesis of the Marine Antibiotic Pestalone and Its Surprisingly Facile Conversion into Pestalalactone and Pestalachloride A. Angew. Chem. Int. Ed. Engl. 2010, 49, 7588–7591. [Google Scholar] [CrossRef] [PubMed]
- Lincke, T.; Behnken, S.; Ishida, K.; Roth, M.; Hertweck, C. Closthioamide: An Unprecedented Polythioamide Antibiotic from the Strictly Anaerobic Bacterium Clostridium cellulolyticum. Angew. Chem. Int. Ed. Engl. 2010, 49, 2011–2013. [Google Scholar] [CrossRef] [PubMed]
- Schroeckh, V.; Scherlach, K.; Nützmann, H.W.; Shelest, E.; Schmidt-Heck, W.; Schuemann, J.; Martin, K.; Hertweck, C.; Brakhage, A.A. Intimate Bacterial-Fungal Interaction Triggers Biosynthesis of Archetypal Polyketides in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 2009, 106, 14558–14563. [Google Scholar] [CrossRef]
- Nichols, D.; Cahoon, N.; Trakhtenberg, E.M.; Pham, L.; Mehta, A.; Belanger, A.; Kanigan, T.; Lewis, K.; Epstein, S.S. Use of Ichip for High-Throughput in Situ Cultivation Of "Uncultivable" Microbial Species. Appl. Environ. Microbiol. 2010, 76, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Lubke, L.L.; Garon, C.F. The Antimicrobial Agent Melittin Exhibits Powerful In Vitro Inhibitory Effects on the Lyme Disease Spirochete. Clin. Infect. Dis. 1997, 25 (Suppl. S1), S48–S51. [Google Scholar] [CrossRef]
- Samy, R.P.; Gopalakrishnakone, P.; Chow, V.T.; Ho, B. Viper Metalloproteinase (Agkistrodon halys Pallas) with Antimicrobial Activity against Multi-Drug Resistant Human Pathogens. J. Cell Physiol. 2008, 216, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ma, D.; Yu, H.; Li, Z.; Liang, J.; Lin, G.; Zhang, Y.; Lai, R. A Bactericidal Homodimeric Phospholipases A2 from Bungarus fasciatus Venom. Peptides 2007, 28, 969–973. [Google Scholar] [CrossRef]
- Pletzer, D.; Hancock, R.E. Antibiofilm Peptides: Potential as Broad-Spectrum Agents. J. Bacteriol. 2016, 198, 2572–2578. [Google Scholar] [CrossRef]
- Reinhardt, A.; Neundorf, I. Design and Application of Antimicrobial Peptide Conjugates. Int. J. Mol. Sci. 2016, 17, 701. [Google Scholar] [CrossRef]
- Xiong, M.; Bao, Y.; Xu, X.; Wang, H.; Han, Z.; Wang, Z.; Liu, Y.; Huang, S.; Song, Z.; Chen, J.; et al. Selective Killing of Helicobacter Pylori with Ph-Responsive Helix-Coil Conformation Transitionable Antimicrobial Polypeptides. Proc. Natl. Acad. Sci. USA 2017, 114, 12675–12680. [Google Scholar] [CrossRef] [PubMed]
- Saadi, M.; Duperchy, E.; Brown, P.; Dawson, M.J.; Wadman, N.S. Polymyxin Compounds. U.S. Patent 20,150,031,602, 29 January 2015. [Google Scholar]
- Heinis, C.; Rutherford, T.; Freund, S.; Winter, G. Phage-Encoded Combinatorial Chemical Libraries Based on Bicyclic Peptides. Nat. Chem. Biol. 2009, 5, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Tally, F.P.; Debruin, M.F. Development of Daptomycin for Gram-Positive Infections. J. Antimicrob. Chemother. 2000, 46, 523–526. [Google Scholar] [CrossRef]
- Baltz, R.H. Daptomycin: Mechanisms of Action and Resistance, and Biosynthetic Engineering. Curr. Opin. Chem. Biol. 2009, 13, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Snydman, D.R.; Jacobus, N.V.; Mcdermott, L.A. Activity of a Novel Cyclic Lipopeptide, Cb-183,315, against Resistant Clostridium difficile and Other Gram-Positive Aerobic and Anaerobic Intestinal Pathogens. Antimicrob. Agents Chemother. 2012, 56, 3448–3452. [Google Scholar] [CrossRef] [PubMed]
- Citron, D.M.; Tyrrell, K.L.; Merriam, C.V.; Goldstein, E.J. In Vitro Activities of Cb-183,315, Vancomycin, and Metronidazole against 556 Strains of Clostridium difficile, 445 Other Intestinal Anaerobes, and 56 Enterobacteriaceae Species. Antimicrob. Agents Chemother. 2012, 56, 1613–1615. [Google Scholar] [CrossRef] [PubMed]
- Boix, V.; Fedorak, R.N.; Mullane, K.M.; Pesant, Y.; Stoutenburgh, U.; Jin, M.; Adedoyin, A.; Chesnel, L.; Guris, D.; Larson, K.B.; et al. Primary Outcomes from a Phase 3, Randomized, Double-Blind, Active-Controlled Trial of Surotomycin in Subjects with in Subjects with Clostridium difficile Infection. Open Forum Infect. Dis. 2017, 4, ofw275. [Google Scholar] [CrossRef]
- Bierbaum, G.; Sahl, H.-G. Lantibiotics: Mode of Action, Biosynthesis and Bioengineering. Curr. Pharm. Biotechnol. 2009, 10, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Froseth, B.R.; Mckay, L.L. Molecular Characterization of the Nisin Resistance Region of Lactococcus lactis Subsp. Lactis Biovar Diacetylactis Drc3. Appl. Environ. Microbiol. 1991, 57, 804–811. [Google Scholar] [PubMed]
- Khosa, S.; Frieg, B.; Mulnaes, D.; Kleinschrodt, D.; Hoeppner, A.; Gohlke, H.; Smits, S.H. Structural Basis of Lantibiotic Recognition by the Nisin Resistance Protein from Streptococcus agalactiae. Sci. Rep. 2016, 6, 18679. [Google Scholar] [CrossRef]
- Bierbaum, G.; Szekat, C.; Josten, M.; Heidrich, C.; Kempter, C.; Jung, G.; Sahl, H.G. Engineering of a Novel Thioether Bridge and Role of Modified Residues in the Lantibiotic Pep5. Appl. Environ. Microbiol. 1996, 62, 385–392. [Google Scholar] [PubMed]
- Levengood, M.R.; Van Der Donk, W.A. Use of Lantibiotic Synthetases for the Preparation of Bioactive Constrained Peptides. Bioorg. Med. Chem. Lett. 2008, 18, 3025–3028. [Google Scholar] [CrossRef] [PubMed]
- Rink, R.; Kuipers, A.; De Boef, E.; Leenhouts, K.J.; Driessen, A.J.; Moll, G.N.; Kuipers, O.P. Lantibiotic Structures as Guidelines for the Design of Peptides That Can Be Modified by Lantibiotic Enzymes. Biochemistry 2005, 44, 8873–8882. [Google Scholar] [CrossRef] [PubMed]
- Kluskens, L.D.; Kuipers, A.; Rink, R.; De Boef, E.; Fekken, S.; Driessen, A.J.; Kuipers, O.P.; Moll, G.N. Post-Translational Modification of Therapeutic Peptides by Nisb, the Dehydratase of the Lantibiotic Nisin. Biochemistry 2005, 44, 12827–12834. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Costantino, P.; Adamo, R. Potential Targets for Next Generation Antimicrobial Glycoconjugate Vaccines. FEMS Microbiol. Rev. 2018, 42, 388–423. [Google Scholar] [CrossRef] [PubMed]
- Berti, F.; Adamo, R. Antimicrobial Glycoconjugate Vaccines: An Overview of Classic and Modern Approaches for Protein Modification. Chem. Soc. Rev. 2018, 47, 9015–9025. [Google Scholar] [CrossRef] [PubMed]
- Behera, A.; Kulkarni, S.S. Chemical Synthesis of Rare, Deoxy-Amino Sugars Containing Bacterial Glycoconjugates as Potential Vaccine Candidates. Molecules 2018, 23, 1997. [Google Scholar] [CrossRef]
- Garton, M.; Nim, S.; Stone, T.A.; Wang, K.E.; Deber, C.M.; Kim, P.M. Method to Generate Highly Stable D-Amino Acid Analogs of Bioactive Helical Peptides Using a Mirror Image of the Entire Pdb. Proc. Natl. Acad. Sci. USA 2018, 115, 1505–1510. [Google Scholar] [CrossRef]
- Morinaka, B.I.; Lakis, E.; Verest, M.; Helf, M.J.; Scalvenzi, T.; Vagstad, A.L.; Sims, J.; Sunagawa, S.; Gugger, M.; Piel, J. Natural Noncanonical Protein Splicing Yields Products with Diverse Β-Amino Acid Residues. Science 2018, 359, 779–782. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Anandamma, S.K.; Kalesh, K.A. The Medicinal Chemistry of Therapeutic Peptides: Recent Developments in Synthesis and Design Optimizations. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef]
- Zhang, Y.; Ptacin, J.L.; Fischer, E.C.; Aerni, H.R.; Caffaro, C.E.; San Jose, K.; Feldman, A.W.; Turner, C.R.; Romesberg, F.E. A Semi-Synthetic Organism That Stores and Retrieves Increased Genetic Information. Nature 2017, 551, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Digiandomenico, A.; Sellman, B.R. Antibacterial Monoclonal Antibodies: The Next Generation? Curr. Opin. Microbiol. 2015, 27, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Rossmann, F.S.; Laverde, D.; Kropec, A.; Romero-Saavedra, F.; Meyer-Buehn, M.; Huebner, J. Isolation of Highly Active Monoclonal Antibodies against Multiresistant Gram-Positive Bacteria. PLoS ONE 2015, 10, e0118405. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Pegu, A.; Rao, E.; Doria-Rose, N.; Beninga, J.; Mckee, K.; Lord, D.M.; Wei, R.R.; Deng, G.; Louder, M.; et al. Trispecific Broadly Neutralizing Hiv Antibodies Mediate Potent Shiv Protection in Macaques. Science 2017, 358, 85–90. [Google Scholar] [CrossRef] [PubMed]
- León, M.; Bastías, R. Virulence Reduction in Bacteriophage Resistant Bacteria. Front. Microbiol. 2015, 6, 343. [Google Scholar] [CrossRef] [PubMed]
- Seed, K.D.; Faruque, S.M.; Mekalanos, J.J.; Calderwood, S.B.; Qadri, F.; Camilli, A. Phase Variable O Antigen Biosynthetic Genes Control Expression of the Major Protective Antigen and Bacteriophage Receptor in Vibrio cholerae O1. PLoS Pathog. 2012, 8, e1002917. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, F.; Piccardi, P.; Mancini, S.; Gabard, J.; Moreillon, P.; Entenza, J.M.; Resch, G.; Que, Y.A. Synergistic Interaction between Phage Therapy and Antibiotics Clears Pseudomonas aeruginosa Infection in Endocarditis and Reduces Virulence. J. Infect. Dis. 2017, 215, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Diard, M.; Bakkeren, E.; Cornuault, J.K.; Moor, K.; Hausmann, A.; Sellin, M.E.; Loverdo, C.; Aertsen, A.; Ackermann, M.; De Paepe, M.; et al. Inflammation Boosts Bacteriophage Transfer Between Salmonella spp. Science 2017, 355, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Lemire, S.; Pires, D.P.; Lu, T.K. Engineering Modular Viral Scaffolds for Targeted Bacterial Population Editing. Cell Syst. 2015, 1, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Briers, Y.; Walmagh, M.; Van Puyenbroeck, V.; Cornelissen, A.; Cenens, W.; Aertsen, A.; Oliveira, H.; Azeredo, J.; Verween, G.; Pirnay, J.P.; et al. Engineered Endolysin-Based “Artilysins” To Combat Multidrug-Resistant Gram-Negative Pathogens. Mbio 2014, 5. [Google Scholar] [CrossRef]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. Crispr Provides Acquired Resistance against Viruses in Prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Kim, S.; Kim, J.M.; Kim, J.S. Targeted Genome Engineering in Human Cells with the Cas9 Rna-Guided Endonuclease. Nat. Biotechnol. 2013, 31, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using Crispr/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Garneau, J.E.; Dupuis, M.; Villion, M.; Romero, D.A.; Barrangou, R.; Boyaval, P.; Fremaux, C.; Horvath, P.; Magadán, A.H.; Moineau, S. The Crispr/Cas Bacterial Immune System Cleaves Bacteriophage and Plasmid DNA. Nature 2010, 468, 67–71. [Google Scholar] [CrossRef]
- Sorek, R.; Lawrence, C.M.; Wiedenheft, B. Crispr-Mediated Adaptive Immune Systems in Bacteria and Archaea. Annu. Rev. Biochem. 2013, 82, 237–266. [Google Scholar] [CrossRef]
- Vercoe, R.B.; Chang, J.T.; Dy, R.L.; Taylor, C.; Gristwood, T.; Clulow, J.S.; Richter, C.; Przybilski, R.; Pitman, A.R.; Fineran, P.C. Cytotoxic Chromosomal Targeting by Crispr/Cas Systems Can Reshape Bacterial Genomes and Expel or Remodel Pathogenicity Islands. PLoS Genet. 2013, 9, e1003454. [Google Scholar] [CrossRef] [PubMed]
- Bikard, D.; Hatoum-Aslan, A.; Mucida, D.; Marraffini, L.A. Crispr Interference Can Prevent Natural Transformation and Virulence Acquisition During In Vivo Bacterial Infection. Cell Host Microbe 2012, 12, 177–186. [Google Scholar] [CrossRef]
- Citorik, R.J.; Mimee, M.; Lu, T.K. Sequence-Specific Antimicrobials Using Efficiently Delivered RNA-Guided Nucleases. Nat. Biotechnol. 2014, 32, 1141–1145. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Klumpe, H.E.; Luo, M.L.; Selle, K.; Barrangou, R.; Beisel, C.L. Programmable Removal of Bacterial Strains by Use of Genome-Targeting Crispr-Cas Systems. Mbio 2014, 5. [Google Scholar] [CrossRef]
- Negus, D.; Vipond, J.; Hatch, G.J.; Rayner, E.L.; Taylor, P.W. Parenteral Administration of Capsule Depolymerase Envd Prevents Lethal Inhalation Anthrax Infection. Antimicrob. Agents Chemother. 2015, 59, 7687–7692. [Google Scholar] [CrossRef]
- Dias, C.; Pais, J.P.; Nunes, R.; Blázquez-Sánchez, M.T.; Marquês, J.T.; Almeida, A.F.; Serra, P.; Xavier, N.M.; Vila-Viçosa, D.; Machuqueiro, M.; et al. Sugar-Based Bactericides Targeting Phosphatidylethanolamine-Enriched Membranes. Nat. Commun. 2018, 9, 4857. [Google Scholar] [CrossRef] [PubMed]
- Maffioli, S.I.; Zhang, Y.; Degen, D.; Carzaniga, T.; Del Gatto, G.; Serina, S.; Monciardini, P.; Mazzetti, C.; Guglierame, P.; Candiani, G.; et al. Antibacterial Nucleoside-Analog Inhibitor of Bacterial RNA Polymerase. Cell 2017, 169, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Velema, W.A.; Hansen, M.J.; Lerch, M.M.; Driessen, A.J.; Szymanski, W.; Feringa, B.L. Ciprofloxacin-Photoswitch Conjugates: A Facile Strategy for Photopharmacology. Bioconjug. Chem. 2015, 26, 2592–2597. [Google Scholar] [CrossRef]
- Babii, O.; Afonin, S.; Berditsch, M.; Reiβer, S.; Mykhailiuk, P.K.; Kubyshkin, V.S.; Steinbrecher, T.; Ulrich, A.S.; Komarov, I.V. Controlling Biological Activity with Light: Diarylethene-Containing Cyclic Peptidomimetics. Angew. Chem. Int. Ed. Engl. 2014, 53, 3392–3395. [Google Scholar] [CrossRef] [PubMed]
- Wegener, M.; Hansen, M.J.; Driessen, A.J.M.; Szymanski, W.; Feringa, B.L. Photocontrol of Antibacterial Activity: Shifting from Uv to Red Light Activation. J. Am. Chem. Soc. 2017, 139, 17979–17986. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, P.; Andrews, S.C. (Eds.) Iron Uptake and Homeostasis in Microorganisms; Caister Academic Press: Norfolk, UK, 2010; ISBN 978-1-904455-65-3. [Google Scholar]
- Corbin, B.D.; Seeley, E.H.; Raab, A.; Feldmann, J.; Miller, M.R.; Torres, V.J.; Anderson, K.L.; Dattilo, B.M.; Dunman, P.M.; Gerads, R.; et al. Metal Chelation and Inhibition of Bacterial Growth in Tissue Abscesses. Science 2008, 319, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.I.; Skaar, E.P. Nutritional Immunity: Transition Metals at the Pathogen-Host Interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Miller, P.A.; Miller, M.J. Iron Transport-Mediated Drug Delivery: Practical Syntheses and in Vitro Antibacterial Studies of Tris-Catecholate Siderophore-Aminopenicillin Conjugates Reveals Selectively Potent Antipseudomonal Activity. J. Am. Chem. Soc. 2012, 134, 9898–9901. [Google Scholar] [CrossRef]
- Cabezón, E.; De La Cruz, F.; Arechaga, I. Conjugation Inhibitors and Their Potential Use to Prevent Dissemination of Antibiotic Resistance Genes in Bacteria. Front. Microbiol. 2017, 8, 2329. [Google Scholar] [CrossRef]
- Tegos, G.P.; Haynes, M.; Strouse, J.J.; Khan, M.M.; Bologa, C.G.; Oprea, T.I.; Sklar, L.A. Microbial Efflux Pump Inhibition: Tactics and Strategies. Curr. Pharm. Des. 2011, 17, 1291–1302. [Google Scholar] [CrossRef]
- Hernandez, V.; Crépin, T.; Palencia, A.; Cusack, S.; Akama, T.; Baker, S.J.; Bu, W.; Feng, L.; Freund, Y.R.; Liu, L.; et al. Discovery of a Novel Class of Boron-Based Antibacterials with Activity against Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2013, 57, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Bologa, C.G.; Ursu, O.; Oprea, T.I.; Melançon, C.E.; Tegos, G.P. Emerging Trends in the Discovery of Natural Product Antibacterial. Curr. Opin. Pharmacol. 2013, 13, 678–687. [Google Scholar] [CrossRef]
- Getino, M.; Sanabria-Ríos, D.J.; Fernández-López, R.; Campos-Gómez, J.; Sánchez-López, J.M.; Fernández, A.; Carballeira, N.M.; De La Cruz, F. Synthetic Fatty Acids Prevent Plasmid-Mediated Horizontal Gene Transfer. Mbio 2015, 6. [Google Scholar] [CrossRef]
- Fernandez-Lopez, R.; Machón, C.; Longshaw, C.M.; Martin, S.; Molin, S.; Zechner, E.L.; Espinosa, M.; Lanka, E.; De La Cruz, F. Unsaturated Fatty Acids Are Inhibitors of Bacterial Conjugation. Microbiology 2005, 151, 3517–3526. [Google Scholar] [CrossRef]
- Smith, M.A.; Coinçon, M.; Paschos, A.; Jolicoeur, B.; Lavallée, P.; Sygusch, J.; Baron, C. Identification of the Binding Site of Brucella Virb8 Interaction Inhibitors. Chem. Biol. 2012, 19, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Trefzer, A.; Pelzer, S.; Schimana, J.; Stockert, S.; Bihlmaier, C.; Fiedler, H.P.; Welzel, K.; Vente, A.; Bechthold, A. Biosynthetic Gene Cluster of Simocyclinone, a Natural Multihybrid Antibiotic. Antimicrob. Agents Chemother. 2002, 46, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Woerly, E.M.; Roy, J.; Burke, M.D. Synthesis of Most Polyene Natural Product Motifs Using Just 12 Building Blocks and One Coupling Reaction. Nat. Chem. 2014, 6, 484–491. [Google Scholar] [CrossRef]
- Zheng, K.; Xie, C.; Hong, R. Bioinspired Iterative Synthesis of Polyketides. Front. Chem. 2015, 3, 32. [Google Scholar] [CrossRef]
- Akagawa, K.; Kudo, K. Biomimetic Iterative Method for Polyketide Synthesis. Chem. Commun. 2017, 53, 8645–8648. [Google Scholar] [CrossRef]
- Akagawa, K.; Kudo, K. Iterative Polyketide Synthesis Via a Consecutive Carbonyl-Protecting Strategy. J. Org. Chem. 2018, 83, 4279–4285. [Google Scholar] [CrossRef]
- Ahmadipour, S.; Miller, G.J. Recent Advances in the Chemical Synthesis of Sugar-Nucleotides. Carbohydr. Res. 2017, 451, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Hwu, J.R.; Chandrasekhar, D.B.; Hwang, K.C.; Lin, C.C.; Horng, J.C.; Shieh, F.K. Reductive Deamination by Benzyne for Deoxy Sugar Synthesis through a Domino Reaction. ChemistryOpen 2017, 6, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Farha, M.A.; French, S.; Stokes, J.M.; Brown, E.D. Bicarbonate Alters Bacterial Susceptibility to Antibiotics by Targeting the Proton Motive Force. ACS Infect. Dis. 2018, 4, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Ejim, L.; Farha, M.A.; Falconer, S.B.; Wildenhain, J.; Coombes, B.K.; Tyers, M.; Brown, E.D.; Wright, G.D. Combinations of Antibiotics and Nonantibiotic Drugs Enhance Antimicrobial Efficacy. Nat. Chem. Biol. 2011, 7, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Duncan, B.; Li, X.; Landis, R.F.; Kim, S.T.; Gupta, A.; Wang, L.S.; Ramanathan, R.; Tang, R.; Boerth, J.A.; Rotello, V.M. Nanoparticle-Stabilized Capsules for the Treatment of Bacterial Biofilms. ACS Nano 2015, 9, 7775–7782. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver Nanoparticles as Antimicrobial Agent: A Case Study on E. Coli as a Model for Gram-Negative Bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and Effect of Silver Nanoparticles on the Antibacterial Activity of Different Antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine 2007, 3, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Cameron, P.; Gaiser, B.K.; Bhandari, B.; Bartley, P.M.; Katzer, F.; Bridle, H. Silver Nanoparticles Decrease the Viability of Cryptosporidium parvum Oocysts. Appl. Environ. Microbiol. 2016, 82, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Vilela, D.; Stanton, M.M.; Parmar, J.; Sánchez, S. Microbots Decorated with Silver Nanoparticles Kill Bacteria in Aqueous Media. ACS Appl. Mater. Interfaces 2017, 9, 22093–22100. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Zhu, C.; Song, Y.; Lu, Q.; Ge, X.; Yang, X.; Zhu, M.J.; Du, D.; Li, H.; Lin, Y. Bioinspired Synthesis of All-in-One Organic-Inorganic Hybrid Nanoflowers Combined with a Handheld pH Meter for on-Site Detection of Food Pathogen. Small 2016, 12, 3094–3100. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zheng, W.; Kuang, L.; Ma, H.; Liang, H. Hydrophilic Phage-Mimicking Membrane Active Antimicrobials Reveal Nanostructure-Dependent Activity and Selectivity. ACS Infect. Dis. 2017, 3, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Pornpattananangkul, D.; Zhang, L.; Olson, S.; Aryal, S.; Obonyo, M.; Vecchio, K.; Huang, C.M. Bacterial Toxin-Triggered Drug Release from Gold Nanoparticle-Stabilized Liposomes for the Treatment of Bacterial Infection. J. Am. Chem. Soc. 2011, 133, 4132–4139. [Google Scholar] [CrossRef] [PubMed]
- Harvey, D.; Bardelang, P.; Goodacre, S.L.; Cockayne, A.; Thomas, N.R. Antibiotic Spider Silk: Site-Specific Functionalization of Recombinant Spider Silk Using “Click” Chemistry. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Assimacopoulos, D. Low Intensity Negative Electric Current in the Treatment of Ulcers of the Leg Due to Chronic Venous Insufficiency. Preliminary Report of Three Cases. Am. J. Surg. 1968, 115, 683–687. [Google Scholar] [CrossRef]
- Carley, P.J.; Wainapel, S.F. Electrotherapy for Acceleration of Wound Healing: Low Intensity Direct Current. Arch. Phys. Med. Rehabil. 1985, 66, 443–446. [Google Scholar] [PubMed]
- Rowley, B.A.; Mckenna, J.M.; Chase, G.R.; Wolcott, L.E. The Influence of Electrical Current on an Infecting Microorganism in Wounds. Ann. N. Y. Acad. Sci. 1974, 238, 543–551. [Google Scholar] [CrossRef]
- Wolcott, L.E.; Wheeler, P.C.; Hardwicke, H.M.; Rowley, B.A. Accelerated Healing of Skin Ulcer by Electrotherapy: Preliminary Clinical Results. South. Med. J. 1969, 62, 795–801. [Google Scholar] [CrossRef]
- Ojingwa, J.C.; Isseroff, R.R. Electrical Stimulation of Wound Healing. J. Investig. Dermatol. 2003, 121, 1–12. [Google Scholar] [CrossRef]
- Isseroff, R.R.; Dahle, S.E. Electrical Stimulation Therapy and Wound Healing: Where Are We Now? Adv. Wound Care 2012, 1, 238–243. [Google Scholar] [CrossRef]
- Sultana, S.T.; Atci, E.; Babauta, J.T.; Falghoush, A.M.; Snekvik, K.R.; Call, D.R.; Beyenal, H. Electrochemical Scaffold Generates Localized, Low Concentration of Hydrogen Peroxide That Inhibits Bacterial Pathogens and Biofilms. Sci. Rep. 2015, 5, 14908. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.M.; Park, B.W.; Vilela, D.; Bente, K.; Faivre, D.; Sitti, M.; Sánchez, S. Magnetotactic Bacteria Powered Biohybrids Target E. coli Biofilms. ACS Nano 2017, 11, 9968–9978. [Google Scholar] [CrossRef] [PubMed]
- Koek, M.M.; Jellema, R.H.; Van Der Greef, J.; Tas, A.C.; Hankemeier, T. Quantitative Metabolomics Based on Gas Chromatography Mass Spectrometry: Status and Perspectives. Metabolomics 2011, 7, 307–328. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Alam, M.A. Evaporation-Induced Stimulation of Bacterial Osmoregulation for Electrical Assessment of Cell Viability. Proc. Natl. Acad. Sci. USA 2011, 113, 7059–7064. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.H.; Ren, L.; Nama, N.; Li, S.; Li, P.; Yao, X.; Cuento, R.A.; Wei, C.H.; Chen, Y.; Xie, Y.; et al. An Acoustofluidic Sputum Liquefier. Lab Chip 2015, 15, 3125–3131. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Li, H.; Collins, J.J.; Ingber, D.E. Contributions of Microbiome and Mechanical Deformation to Intestinal Bacterial Overgrowth and Inflammation in a Human Gut-on-a-Chip. Proc. Natl. Acad. Sci. USA 2016, 113, E7–E15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Banaei, N.; Ren, K. Microfluidics for Combating Antimicrobial Resistance. Trends Biotechnol. 2017, 35, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Lasken, R.S. Genomic Sequencing of Uncultured Microorganisms from Single Cells. Nat. Rev. Microbiol. 2012, 10, 631–640. [Google Scholar] [CrossRef]
- Welling, M.M.; Bunschoten, A.; Kuil, J.; Nelissen, R.G.; Beekman, F.J.; Buckle, T.; Van Leeuwen, F.W. Development of a Hybrid Tracer for Spect and Optical Imaging of Bacterial Infections. Bioconjug. Chem. 2015, 26, 839–849. [Google Scholar] [CrossRef]
- Schmidt, K.; O’grady, J. Minion Nanopore Sequencing to Identify Pathogens and Resistance Genes Directly from Urine Specimens. In Proceedings of the ICAAC-ICC Conference of the American Society for Microbiology, San Diego, CA, USA, 17–21 September 2015. [Google Scholar]
- Yeh, E.C.; Fu, C.C.; Hu, L.; Thakur, R.; Feng, J.; Lee, L.P. Self-Powered Integrated Microfluidic Point-of-Care Low-Cost Enabling (Simple). Chip. Sci. Adv. 2017, 3, e1501645. [Google Scholar] [CrossRef]
- Cui, X.; Das, A.; Dhawane, A.N.; Sweeney, J.; Zhang, X.; Chivukula, V.; Iyer, S.S. Highly Specific and Rapid Glycan Based Amperometric Detection of Influenza Viruses. Chem. Sci. 2017, 8, 3628–3634. [Google Scholar] [CrossRef] [PubMed]
- Metchnikoff, E.; Mitchell, P.C. The Prolongation of Life: Optimistic Studies; Springer Classics in Longevity and Aging; Springer: New York, NY, USA, 2004; ISBN 0826118763 9780826118769. [Google Scholar]
- Araya, M.; Stanton, C.; Morelli, L.; Reid, G.; Pineiro, M. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation. 2006. Available online: http://www.fao.org/3/a-a0512e.pdf (accessed on 15 January 2019).
- FAO/WHO. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, ON, Canada, 2013; Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 15 January 2019).
- Ripert, G.; Racedo, S.M.; Elie, A.M.; Jacquot, C.; Bressollier, P.; Urdaci, M.C. Secreted Compounds of the Probiotic Bacillus clausii Strain O/C Inhibit the Cytotoxic Effects Induced by Clostridium difficile and Bacillus cereus Toxins. Antimicrob. Agents Chemother. 2016, 60, 3445–3454. [Google Scholar] [CrossRef] [PubMed]
- Hudcovic, T.; Kolinska, J.; Klepetar, J.; Stepankova, R.; Rezanka, T.; Srutkova, D.; Schwarzer, M.; Erban, V.; Du, Z.; Wells, J.M.; et al. Protective Effect of Clostridium tyrobutyricum in Acute Dextran Sodium Sulphate-Induced Colitis: Differential Regulation of Tumour Necrosis Factor-A and Interleukin-18 in Balb/C and Severe Combined Immunodeficiency Mice. Clin. Exp. Immunol. 2012, 167, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Updating the Concept of Prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M. Prebiotics: The Concept Revisited. J. Nutr. 2007, 137, 830S–837S. [Google Scholar] [CrossRef] [PubMed]
- Niness, K.R. Inulin and Oligofructose: What Are They? J. Nutr. 1999, 129, 1402S–1406S. [Google Scholar] [CrossRef]
- Przemyslaw, J.T.; Piotr, T. Probiotics and Prebiotics. Cereal. Chem. 2003, 80, 113–117. [Google Scholar] [CrossRef]
- Pourghassem Gargari, B.; Dehghan, P.; Aliasgharzadeh, A.; Asghari Jafar-Abadi, M. Effects of High Performance Inulin Supplementation on Glycemic Control and Antioxidant Status in Women with Type 2 Diabetes. Diabetes Metab. J. 2013, 37, 140–148. [Google Scholar] [CrossRef]
- Fooks, L.J.; Gibson, G.R. In Vitro Investigations of the Effect of Probiotics and Prebiotics on Selected Human Intestinal Pathogens. FEMS Microbiol. Ecol. 2002, 39, 67–75. [Google Scholar] [CrossRef]
- Wong, J.M.; De Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Feuillet, L.; Carvajal, J.; Sudre, I.; Pelletier, J.; Thomassin, J.M.; Drancourt, M.; Cherif, A.A. First Isolation of Bacteroides thetaiotaomicron from a Patient with a Cholesteatoma and Experiencing Meningitis. J. Clin. Microbiol. 2005, 43, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Marriott, H.M.; Mitchell, T.J.; Dockrell, D.H. Pneumolysin: A Double-Edged Sword During the Host-Pathogen Interaction. Curr. Mol. Med. 2008, 8, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Kluytmans, J.; Van Belkum, A.; Verbrugh, H. Nasal Carriage of Staphylococcus aureus: Epidemiology, Underlying Mechanisms, and Associated Risks. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G. Clinical Practice. Antibiotic-Associated Diarrhea. N. Engl. J. Med. 2002, 346, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Mcfarland, L.V. Meta-Analysis of Probiotics for the Prevention of Antibiotic Associated Diarrhea and the Treatment of Clostridium difficile Disease. Am. J. Gastroenterol. 2006, 101, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Mcdonald, L.; Lessa, F.; Sievert, D.; Wise, M.; Herrera, R.; Gould, C.; Al, E. Vital Signs: Preventing Clostridium Difficile Infections. Morbidity Mortal. Weely. Rep. (MMWR) 2012, 61, 157–162. [Google Scholar]
- Bakken, J.S.; Borody, T.; Brandt, L.J.; Brill, J.V.; Demarco, D.C.; Franzos, M.A.; Kelly, C.; Khoruts, A.; Louie, T.; Martinelli, L.P.; et al. Treating Clostridium difficile Infection with Fecal Microbiota Transplantation. Clin. Gastroenterol. Hepatol. 2011, 9, 1044–1049. [Google Scholar] [CrossRef]
- Brandt, L.J.; Aroniadis, O.C.; Mellow, M.; Kanatzar, A.; Kelly, C.; Park, T.; Stollman, N.; Rohlke, F.; Surawicz, C. Long-Term Follow-up of Colonoscopic Fecal Microbiota Transplant for Recurrent Clostridium difficile Infection. Am. J. Gastroenterol. 2012, 107, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Youngster, I.; Russell, G.H.; Pindar, C.; Ziv-Baran, T.; Sauk, J.; Hohmann, E.L. Oral, Capsulized, Frozen Fecal Microbiota Transplantation for Relapsing Clostridium difficile Infection. JAMA 2014, 312, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; Taur, Y.; Jenq, R.R.; Equinda, M.J.; Son, T.; Samstein, M.; Viale, A.; Socci, N.D.; Van Den Brink, M.R.; Kamboj, M.; et al. Vancomycin-Resistant Enterococcus Domination of Intestinal Microbiota Is Enabled by Antibiotic Treatment in Mice and Precedes Bloodstream Invasion in Humans. J. Clin. Investig. 2010, 120, 4332–4341. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; Bucci, V.; Caballero, S.; Djukovic, A.; Toussaint, N.C.; Equinda, M.; Lipuma, L.; Ling, L.; Gobourne, A.; No, D.; et al. Intestinal Microbiota Containing Barnesiella Species Cures Vancomycin-Resistant Enterococcus faecium Colonization. Infect. Immun. 2013, 81, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.E.; Hobbs, S.J. Conjugal Transfer of Plasmid-Borne Multiple Antibiotic Resistance in Streptococcus faecalis Var. Zymogenes. J. Bacteriol. 1974, 117, 360–372. [Google Scholar] [PubMed]
- Montealegre, M.C.; Singh, K.V.; Murray, B.E. Gastrointestinal Tract Colonization Dynamics by Different Enterococcus faecium Clades. J. Infect. Dis. 2016, 213, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Wurster, J.I.; Saavedra, J.T.; Gilmore, M.S. Impact of Antibiotic Use on the Evolution of Enterococcus faecium. J. Infect. Dis. 2016, 213, 1862–1865. [Google Scholar] [CrossRef]
- Stecher, B.; Berry, D.; Loy, A. Colonization Resistance and Microbial Ecophysiology: Using Gnotobiotic Mouse Models and Single-Cell Technology to Explore the Intestinal Jungle. FEMS Microbiol. Rev. 2013, 37, 793–829. [Google Scholar] [CrossRef]
- Deriu, E.; Liu, J.Z.; Pezeshki, M.; Edwards, R.A.; Ochoa, R.J.; Contreras, H.; Libby, S.J.; Fang, F.C.; Raffatellu, M. Probiotic Bacteria Reduce Salmonella typhimurium Intestinal Colonization by Competing for Iron. Cell Host Microbe 2013, 14, 26–37. [Google Scholar] [CrossRef]
- Fu, Y.; Waldor, M.K.; Mekalanos, J.J. Tn-Seq Analysis of Vibrio cholerae Intestinal Colonization Reveals a Role for T6ss-Mediated Antibacterial Activity in the Host. Cell Host Microbe 2013, 14, 652–663. [Google Scholar] [CrossRef]
- Van Rensburg, J.J.; Lin, H.; Gao, X.; Toh, E.; Fortney, K.R.; Ellinger, S.; Zwickl, B.; Janowicz, D.M.; Katz, B.P.; Nelson, D.E.; et al. The Human Skin Microbiome Associates with the Outcome of and Is Influenced by Bacterial Infection. Mbio 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Diggle, S.P.; Griffin, A.S.; Campbell, G.S.; West, S.A. Cooperation and Conflict in Quorum-Sensing Bacterial Populations. Nature 2007, 450, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Sandoz, K.M.; Mitzimberg, S.M.; Schuster, M. Social Cheating in Pseudomonas aeruginosa Quorum Sensing. Proc. Natl. Acad. Sci. USA 2007, 104, 15876–15881. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, B.E.; Dutton, R.J. Fermented Foods as Experimentally Tractable Microbial Ecosystems. Cell 2015, 161, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Guillouzouic, A.; Bemer, P.; Gay-Andrieu, F.; Bretonnière, C.; Lepelletier, D.; Mahé, P.J.; Villers, D.; Jarraud, S.; Reynaud, A.; Corvec, S. Fatal Coinfection with Legionella pneumophila Serogroup 8 and Aspergillus fumigatus. Diagn. Microbiol. Infect. Dis. 2008, 60, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Haque, A.; Mukhopadhyay, G.; Narayan, R.P.; Prasad, R. Interactions between Bacteria and Candida in the Burn Wound. Burns 2005, 31, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Hermann, C.; Hermann, J.; Munzel, U.; Rüchel, R. Bacterial Flora Accompanying Candida Yeasts in Clinical Specimens. Mycoses 1999, 42, 619–627. [Google Scholar] [CrossRef]
- Mangan, A. Interactions between Some Aural Aspergillus Species and Bacteria. J. Gen. Microbiol. 1969, 58, 261–266. [Google Scholar] [CrossRef]
- Pate, J.C.; Jones, D.B.; Wilhelmus, K.R. Prevalence and Spectrum of Bacterial Co-Infection During Fungal Keratitis. Br. J. Ophthalmol. 2006, 90, 289–292. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Hogan, D.A.; Mylonakis, E. Medically Important Bacterial-Fungal Interactions. Nat. Rev. Microbiol. 2010, 8, 340–349. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Peters, B.M.; Jabra-Rizk, M.A. Cross-Kingdom Interactions: Candida albicans and Bacteria. FEMS Microbiol Lett 2009, 299, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wargo, M.J.; Hogan, D.A. Fungal-Bacterial Interactions: A Mixed Bag of Mingling Microbes. Curr. Opin. Microbiol. 2006, 9, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.M.; Seidler, M. Characteristics of Pathogenic Fungi and Antifungal Therapy in Cystic Fibrosis. Expert Rev. Anti-Infect. Ther. 2010, 8, 957–964. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Harriott, M.M.; Noverr, M.C. Ability of Candida albicans Mutants to Induce Staphylococcus aureus Vancomycin Resistance During Polymicrobial Biofilm Formation. Antimicrob. Agents Chemother. 2010, 54, 3746–3755. [Google Scholar] [CrossRef] [PubMed]
- Harriott, M.M.; Noverr, M.C. Candida albicans and Staphylococcus aureus Form Polymicrobial Biofilms: Effects on Antimicrobial Resistance. Antimicrob. Agents Chemother. 2009, 53, 3914–3922. [Google Scholar] [CrossRef]
- Harriott, M.M.; Noverr, M.C. Importance of Candida-Bacterial Polymicrobial Biofilms in Disease. Trends Microbiol. 2011, 19, 557–563. [Google Scholar] [CrossRef]
- Carlson, E. Effect of Strain of Staphylococcus aureus on Synergism with Candida albicans Resulting in Mouse Mortality and Morbidity. Infect. Immun. 1983, 42, 285–292. [Google Scholar]
- Carlson, E. Enhancement by Candida albicans of Staphylococcus aureus, Serratia marcescens, and Streptococcus faecalis in the Establishment of Infection in Mice. Infect. Immun. 1983, 39, 193–197. [Google Scholar]
- Klaerner, H.G.; Uknis, M.E.; Acton, R.D.; Dahlberg, P.S.; Carlone-Jambor, C.; Dunn, D.L. Candida albicans and Escherichia coli Are Synergistic Pathogens During Experimental Microbial Peritonitis. J. Surg. Res. 1997, 70, 161–165. [Google Scholar] [CrossRef]
- Noverr, M.C.; Huffnagle, G.B. Regulation of Candida albicans Morphogenesis by Fatty Acid Metabolites. Infect. Immun. 2004, 72, 6206–6210. [Google Scholar] [CrossRef] [PubMed]
- Joyner, P.M.; Liu, J.; Zhang, Z.; Merritt, J.; Qi, F.; Cichewicz, R.H. Mutanobactin a from the Human Oral Pathogen Streptococcus mutans Is a Cross-Kingdom Regulator of the Yeast-Mycelium Transition. Org. Biomol. Chem. 2010, 8, 5486–5489. [Google Scholar] [CrossRef] [PubMed]
- Cugini, C.; Calfee, M.W.; Farrow, J.M.; Morales, D.K.; Pesci, E.C.; Hogan, D.A. Farnesol, a Common Sesquiterpene, Inhibits Pqs Production in Pseudomonas aeruginosa. Mol. Microbiol. 2007, 65, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Cugini, C.; Morales, D.K.; Hogan, D.A. Candida albicans-Produced Farnesol Stimulates Pseudomonas Quinolone Signal Production in Lasr-Defective Pseudomonas aeruginosa Strains. Microbiology 2010, 156, 3096–3107. [Google Scholar] [CrossRef] [PubMed]
- Frases, S.; Salazar, A.; Dadachova, E.; Casadevall, A. Cryptococcus neoformans Can Utilize the Bacterial Melanin Precursor Homogentisic Acid for Fungal Melanogenesis. Appl. Environ. Microbiol. 2007, 73, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Frases, S.; Chaskes, S.; Dadachova, E.; Casadevall, A. Induction by Klebsiella aerogenes of a Melanin-Like Pigment in Cryptococcus neoformans. Appl. Environ. Microbiol. 2006, 72, 1542–1550. [Google Scholar] [CrossRef]
- Nikawa, H.; Egusa, H.; Makihira, S.; Yamashiro, H.; Fukushima, H.; Jin, C.; Nishimura, M.; Pudji, R.R.; Hamada, T. Alteration of the Coadherence of Candida albicans with Oral Bacteria by Dietary Sugars. Oral Microbiol. Immunol. 2001, 16, 279–283. [Google Scholar] [CrossRef]
- Peters, B.M.; Jabra-Rizk, M.A.; Scheper, M.A.; Leid, J.G.; Costerton, J.W.; Shirtliff, M.E. Microbial Interactions and Differential Protein Expression in Staphylococcus aureus-Candida albicans Dual-Species Biofilms. FEMS Immunol. Med. Microbiol. 2010, 59, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Hogan, D.; Wargo, M.; Beck, N. Bacterial biofilms on fungal surfaces. In The Biofilm Mode of Life: Mechanisms and Adaptations; Kjelleberg, S., Givskov, M., Eds.; Horizon Scientific Press: Norfolk, UK, 2007; pp. 235–245. ISBN 9781904933335. [Google Scholar]
- Baena-Monroy, T.; Moreno-Maldonado, V.; Franco-Martínez, F.; Aldape-Barrios, B.; Quindós, G.; Sánchez-Vargas, L.O. Candida albicans, Staphylococcus aureus and Streptococcus mutans Colonization in Patients Wearing Dental Prosthesis. Med. Oral Patol. Oral Cir. Bucal 2005, 10 (Suppl. S1), E27–E39. [Google Scholar]
- Pierce, G.E. Pseudomonas aeruginosa, Candida albicans, and Device-Related Nosocomial Infections: Implications, Trends, and Potential Approaches for Control. J. Ind. Microbiol. Biotechnol. 2005, 32, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Zanello, G.; Meurens, F.; Berri, M.; Salmon, H. Saccharomyces boulardii Effects on Gastrointestinal Diseases. Curr. Issues Mol. Biol. 2009, 11, 47–58. [Google Scholar] [PubMed]
- Castagliuolo, I.; Lamont, J.T.; Nikulasson, S.T.; Pothoulakis, C. Saccharomyces boulardii Protease Inhibits Clostridium difficile Toxin a Effects in the Rat Ileum. Infect. Immun. 1996, 64, 5225–5232. [Google Scholar] [PubMed]
- Castagliuolo, I.; Riegler, M.F.; Valenick, L.; Lamont, J.T.; Pothoulakis, C. Saccharomyces boulardii Protease Inhibits the Effects of Clostridium difficile Toxins a and B in Human Colonic Mucosa. Infect. Immun. 1999, 67, 302–307. [Google Scholar] [CrossRef]
- Buts, J.P.; De Keyser, N. Effects of Saccharomyces boulardii on Intestinal Mucosa. Dig. Dis. Sci. 2006, 51, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Vallance, B.A.; Boyer, L.; Bergstrom, K.S.; Walker, J.; Madsen, K.; O’kusky, J.R.; Buchan, A.M.; Jacobson, K. Saccharomyces boulardii Ameliorates Citrobacter Rodentium-Induced Colitis through Actions on Bacterial Virulence Factors. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G295–G306. [Google Scholar] [CrossRef] [PubMed]
- Koval, S.F.; Hynes, S.H.; Flannagan, R.S.; Pasternak, Z.; Davidov, Y.; Jurkevitch, E. Bdellovibrio exovorus Sp. Nov., a Novel Predator of Caulobacter crescentus. Int. J. Syst. Evol. Microbiol. 2013, 63, 146–151. [Google Scholar] [CrossRef]
- Snyder, A.R.; Williams, H.N.; Baer, M.L.; Walker, K.E.; Stine, O.C. 16S rDNA Sequence Analysis of Environmental Bdellovibrio-and-Like Organisms (Balo) Reveals Extensive Diversity. Int. J. Syst. Evol. Microbiol. 2002, 52, 2089–2094. [Google Scholar] [CrossRef]
- Starr, M.P.; Seidler, R.J. The Bdellovibros. Annu. Rev. Microbiol. 1971, 25, 649–678. [Google Scholar] [CrossRef]
- Strauch, E.; Beck, S.; Appel, B. Predatory Prokaryotes: Biology, Ecology and Evolution; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-38582-0. [Google Scholar]
- Guerrini, F.; Romano, V.; Valenzi, M.; Di Giulio, M.; Mupo, M.R.; Sacco, M. Molecular Parasitism in the Escherichia coli-Bdellovibrio bacteriovorus System: Translocation of the Matrix Protein from the Host to the Parasite Outer Membrane. EMBO J. 1982, 1, 1439–1444. [Google Scholar] [CrossRef]
- Cao, H.; An, J.; Zheng, W.; He, S. Vibrio cholerae Pathogen from the Freshwater-Cultured Whiteleg Shrimp Penaeus vannamei and Control with Bdellovibrio bacteriovorus. J. Invertebr. Pathol 2015, 130, 13–20. [Google Scholar] [CrossRef] [PubMed]
- George, A.S.; Salas González, I.; Lorca, G.L.; Teplitski, M. Contribution of the Salmonella enterica Kdgr Regulon to Persistence of the Pathogen in Vegetable Soft Rots. Appl. Environ. Microbiol. 2016, 82, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Velasco, G.; Tydings, H.A.; Boyer, R.R.; Falkinham, J.O.; Ponder, M.A. Characterization of Interactions between Escherichia coli O157:H7 with Epiphytic Bacteria in Vitro and on Spinach Leaf Surfaces. Int. J. Food Microbiol. 2012, 153, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Swearingen, M.C.; Porwollik, S.; Desai, P.T.; Mcclelland, M.; Ahmer, B.M. Virulence of 32 Salmonella Strains in Mice. PLoS ONE 2012, 7, e36043. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Daghrir, R.; Drogui, P. Tetracycline Antibiotics in the Environment: A Review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Proia, L.; Von Schiller, D.; Sànchez-Melsió, A.; Sabater, S.; Borrego, C.M.; Rodríguez-Mozaz, S.; Balcázar, J.L. Occurrence and Persistence of Antibiotic Resistance Genes in River Biofilms after Wastewater Inputs in Small Rivers. Environ. Pollut. 2016, 210, 121–128. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of Antibiotics and Antibiotic Resistance Genes in Hospital and Urban Wastewaters and Their Impact on the Receiving River. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef]
- Kotwani, A.; Holloway, K. Trends in Antibiotic Use among Outpatients in New Delhi, India. BMC Infect. Dis. 2011, 11, 99. [Google Scholar] [CrossRef]
- Langford, B.J.; Seah, J.; Chan, A.; Downing, M.; Johnstone, J.; Matukas, L.M. Antimicrobial Stewardship in the Microbiology Laboratory: Impact of Selective Susceptibility Reporting on Ciprofloxacin Utilization and Susceptibility of Gram-Negative Isolates to Ciprofloxacin in a Hospital Setting. J. Clin. Microbiol. 2016, 54, 2343–2347. [Google Scholar] [CrossRef]
- Pivotal Study in Nosocomial Pneumonia Suspected or Confirmed to be Due to Pseudomonas (PRISM-UDR). Available online: https://www.clinicaltrials.gov/ct2/show/NCT03582007?term=POL7080&rank=5 (accessed on 15 January 2019).
- A Study of CB-183,315 in Participants with Clostridium difficile Associated Diarrhea (MK-4261-006). Available online: https://www.clinicaltrials.gov/ct2/show/NCT01598311?term=surotomycin&rank=4 (accessed on 15 January 2019).
- Study Comparing the Safety and Efficacy of Cethromycin to Clarithromycin for the Treatment of Community-Acquired Pneumonia (CAP). Available online: https://www.clinicaltrials.gov/ct2/show/NCT00336505?term=Cethromycin&rank=2 (accessed on 15 January 2019).
- Safety and Efficacy Study of Single-Dose Oral CEM-101 in Patients with Uncomplicated Urogenital Gonorrhea. Available online: https://www.clinicaltrials.gov/ct2/show/NCT01591447?term=Solithromycin&rank=10 (accessed on 15 January 2019).
- Efficacy and Safety Study of Oral CEM-101 Compared to Oral Levofloxacin in Treatment of Patients With Community-Acquired Bacterial Pneumonia. Available online: https://www.clinicaltrials.gov/ct2/show/NCT01168713?term=Solithromycin&rank=11 (accessed on 15 January 2019).
| Novel Targets Identified with References | Discovery Approaches | Sources |
|---|---|---|
| Essential amino acid biosynthesis [7,8,9,10,11,12,13,14] | Informatics-based mining | Medicinal plants |
| Cell wall lipid biosynthesis [15,16] | Cryptic Biosynthetic Gene Clusters (BGC) activation
| Marine invertebrates |
| Lipid insertion enzymes [17] | Insect and vertebrate symbionts | |
| Metal chelator biosynthesis [18] | Microbial co-cultures | |
| Quorum sensing metabolism [19,20,21,22,23,24,25,26,27,28,29,30,31] | BGC engineering
| Endophytic fungi and bacteria |
| Clp proteases [32,33] | Uncultured microbes | |
| Cyclic-di- Guanosine monophosphate (GMP) levels [34] | Skin, blood, venoms |
| Prevention | Preclinical | Antibiotic Delivery |
|---|---|---|
| Graphene and silver-based nanomaterials Organic-inorganic hybrid nanoparticles Microbots for water treatment | Phage-patterned nanoparticles | Silica nanoparticles Nanoparticle-liposome conjugates |
| Engineered spider silk | Electrochemical H2O2 generation | Hybrid bacteria-nanoparticle swimmers |
| Monitoring Technology Types | Direct in-Sample Methods | Point-of-Care Devices |
|---|---|---|
| Mass spectrometry-based | Magnetic resonance-based | Microfluidic blood serum separator |
| Automated imaging | Smarticles | Portable influenza tester |
| Microfluidics-based | DNA sequencing-based | - |
| Label-based | - | - |
| Drug Molecule with Reference | Clinical Phase | Medical Condition |
|---|---|---|
| POL70780/Murepavadin [270] | Phase 3 | Pneumonia |
| Surotomycin [271] | Phase 3 | Diarrhea |
| Cethromycin (semi-synthetic) [272] | Phase 3 | Pneumonia |
| Solithromycin (semi-synthetic) [273,274] | Phase 2 | Uncomplicated urogenital gonorrhea, Pneumonia |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantravadi, P.K.; Kalesh, K.A.; Dobson, R.C.J.; Hudson, A.O.; Parthasarathy, A. The Quest for Novel Antimicrobial Compounds: Emerging Trends in Research, Development, and Technologies. Antibiotics 2019, 8, 8. https://doi.org/10.3390/antibiotics8010008
Mantravadi PK, Kalesh KA, Dobson RCJ, Hudson AO, Parthasarathy A. The Quest for Novel Antimicrobial Compounds: Emerging Trends in Research, Development, and Technologies. Antibiotics. 2019; 8(1):8. https://doi.org/10.3390/antibiotics8010008
Chicago/Turabian StyleMantravadi, Pavan K., Karunakaran A. Kalesh, Renwick C. J. Dobson, André O. Hudson, and Anutthaman Parthasarathy. 2019. "The Quest for Novel Antimicrobial Compounds: Emerging Trends in Research, Development, and Technologies" Antibiotics 8, no. 1: 8. https://doi.org/10.3390/antibiotics8010008
APA StyleMantravadi, P. K., Kalesh, K. A., Dobson, R. C. J., Hudson, A. O., & Parthasarathy, A. (2019). The Quest for Novel Antimicrobial Compounds: Emerging Trends in Research, Development, and Technologies. Antibiotics, 8(1), 8. https://doi.org/10.3390/antibiotics8010008






