Geographic Variation in Antibiotic Consumption—Is It Due to Doctors’ Prescribing or Patients’ Consulting?
Abstract
1. Introduction
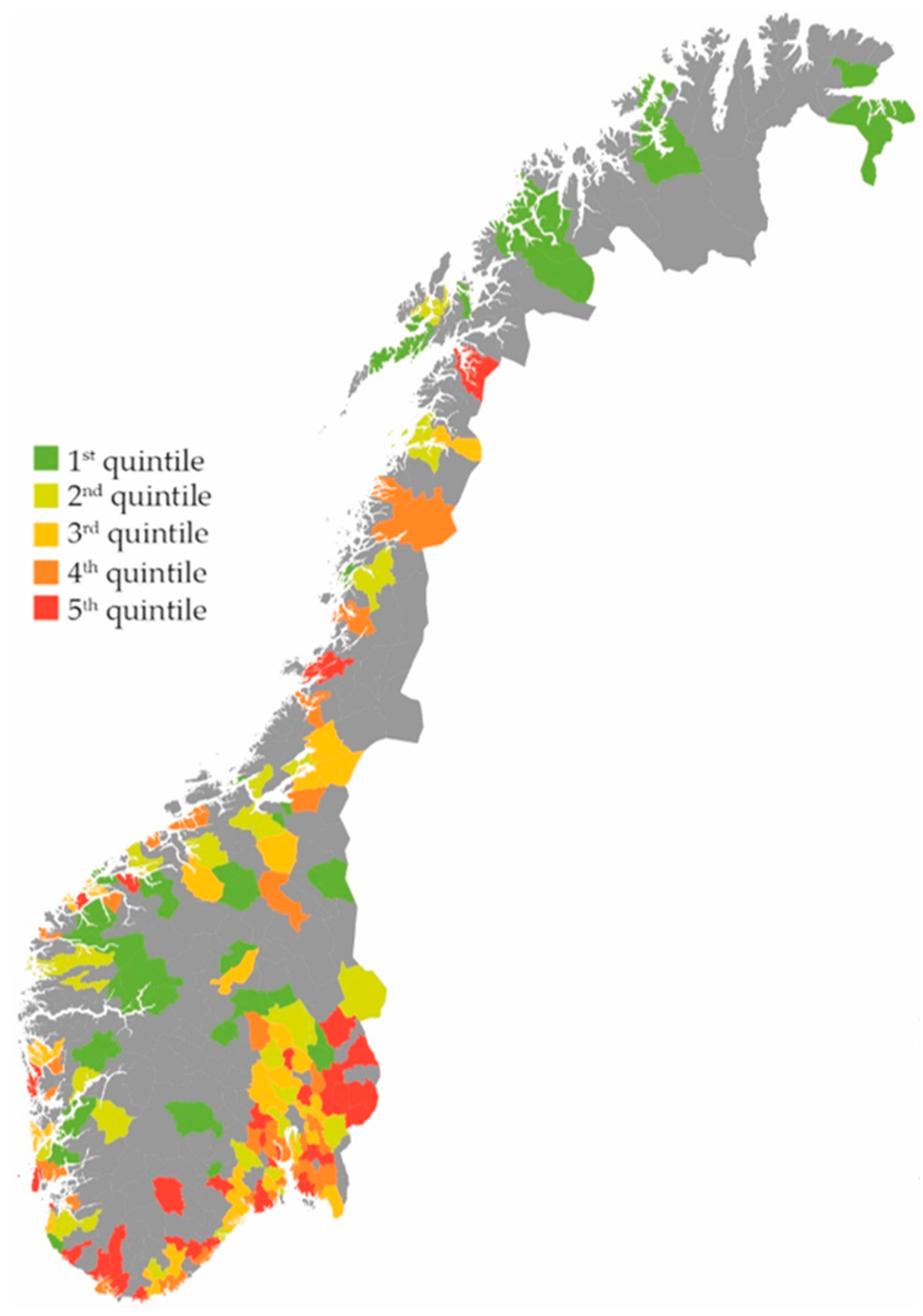
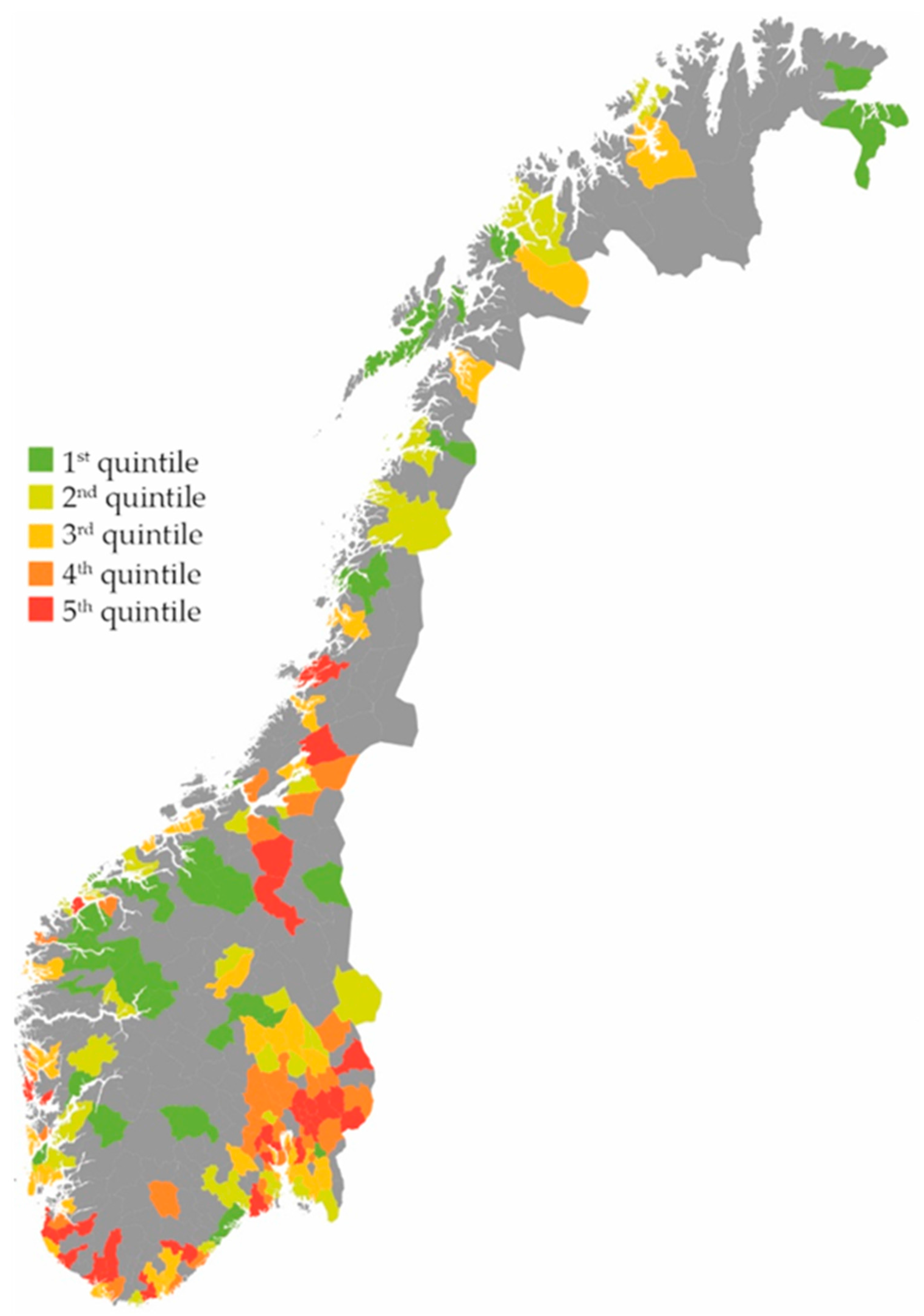
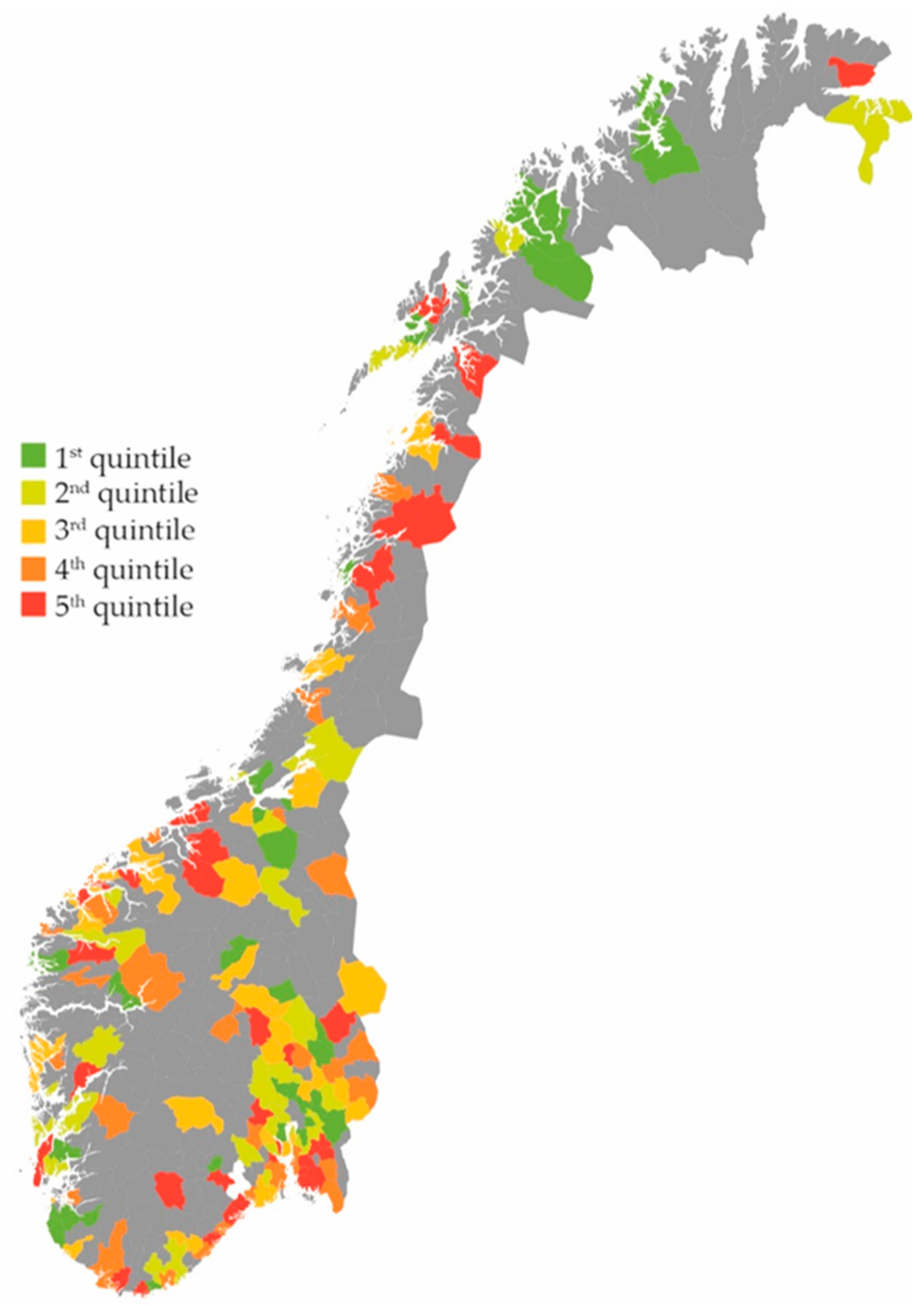
2. Results
Effect on the Total Use of Antibiotics
3. Discussion
3.1. Main Findings
3.2. Strengths
3.3. Limitations
3.4. Comparison with Existing Literature
4. Materials and Methods
4.1. Data Collection
4.2. Inclusion and Exclusion Criteria
4.3. Variable Definition
4.4. Missing Data
4.5. Modelling
4.6. Ethics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goossens, H.; Ferech, M.; Stichele, R.V.; Elseviers, M.; Grp, E.P. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Thapa, B. Antimicrobial resistance: A global threat. Available online: https://www.nepjol.info/index.php/IJIM/article/view/7405/5998 (accessed on 15 January 2017).
- Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. Available online: https://unn.no/Documents/Kompetansetjenester,%20-sentre%20og%20fagr%C3%A5d/NORM%20-%20Norsk%20overv%C3%A5kingssystem%20for%20antibiotikaresistens%20hos%20mikrober/Rapporter/NORM_NORM-VET_2016.pdf (accessed on 6 January 2017).
- WHO Collaborating Centre for Drug Statistics Methodology NIoPH. Definition and General Considerations. Available online: https://www.whocc.no/ddd/definition_and_general_considera/ (accessed on 15 January 2017).
- Sundsfjord, A.; Simonsen, G.S. Antibiotikaresistens. Available online: http://www.antibiotikaiallmennpraksis.no/index.php?action=showtopic&topic=bytMNets&j=1 (accessed on 5 May 2017).
- Van der Velden, A.W.; Pijpers, E.J.; Kuyvenhoven, M.M.; Tonkin-Crine, S.K.; Little, P.; Verheij, T.J. Effectiveness of physician-targeted interventions to improve antibiotic use for respiratory tract infections. Br. J. Gen. Pract. 2012, 62, e801–e807. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, N.; Coenen, S.; Versporten, A.; Muller, A.; Minalu, G.; Faes, C.; Vankerckhoven, V.; Aerts, M.; Hens, N.; Molenberghs, G.; et al. European Surveillance of Antimicrobial Consumption (ESAC): Outpatient antibiotic use in Europe (1997–2009). J. Antimicrob. Chemother. 2011, 66. [Google Scholar] [CrossRef] [PubMed]
- Deschepper, R.; Grigoryan, L.; Lundborg, C.S.; Hofstede, G.; Cohen, J.; Kelen, G.V.; Deliens, L.; Haaijer-Ruskamp, F.M. Are cultural dimensions relevant for explaining cross-national differences in antibiotic use in Europe? BMC Health Serv. Res. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Helsenorge.no. Forekomst av Antibiotikabehandling. Available online: https://helsenorge.no/Kvalitetsindikatorer/legemidler/forekomst-av-antibiotikabehandling (accessed on 6 January 2017).
- Haugen, P.; Skov Simonsen, G.; Primicerio, R.; Furberg, A.S.; Smabrekke, L. Antibiotics to outpatients in Norway-Assessing effect of latitude and municipality population size using quantile regression in a cross-sectional study. Pharm. Stat. 2017, 17, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, M.; Latinovic, R.; Charlton, J.; Cox, K.; Rowlands, G.; Gulliford, M. Why has antibiotic prescribing for respiratory illness declined in primary care? A longitudinal study using the General Practice Research Database. J. Public Health 2004, 26, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.M.; Ross, A.M.; Cross, K.W.; Kendall, H. The reducing incidence of respiratory tract infection and its relation to antibiotic prescribing. Br. J. Gen. Pract. 2003, 53, 778–783. [Google Scholar] [PubMed]
- Norway_Municipalities_2010_blank.svg: Kåre-Olav Derivative Work: Røed. Available online: https://creativecommons.org/licenses/by-sa/2.0 (accessed on 27 February 2018).
- Aabenhus, R.; Siersma, V.; Hansen, M.P.; Bjerrum, L. Antibiotic prescribing in Danish general practice 2004–2013. J. Antimicrob. Chemother. 2016, 71, 2286–2294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gjelstad, S.; Straand, J.; Dalen, I.; Fetveit, A.; Strom, H.; Lindbaek, M. Do general practitioners’ consultation rates influence their prescribing patterns of antibiotics for acute respiratory tract infections? J. Antimicrob. Chemother. 2011, 66, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.Y.; Seed, P.; Schofield, P.; Ibrahim, S.; Ashworth, M. Which practices are high antibiotic prescribers? A cross-sectional analysis. Br. J. Gen. Pract. 2009, 59, e315–e320. [Google Scholar] [CrossRef] [PubMed]
- Raknes, G.; Morken, T.; Hunskar, S. Travel distance and the utilisation of out-of-hours services. J. Nor. Med. Assoc. 2014, 134, 2151–2155. [Google Scholar]
- Deschepper, R.; Vander Stichele, R.H.; Haaijer-Ruskamp, F.M. Cross-cultural differences in lay attitudes and utilisation of antibiotics in a Belgian and a Dutch city. Patient Educ. Couns. 2002, 48, 161–169. [Google Scholar] [CrossRef]
- Little, P.; Gould, C.; Williamson, I.; Warner, G.; Gantley, M.; Kinmonth, A.L. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: The medicalising effect of prescribing antibiotics. BMJ 1997, 315, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Grimsmo, A.; Hagman, E.; Faikø, E.; Matthiessen, L.; Njálsson, T. Patients, diagnoses and processes in general practice in the Nordic countries. An attempt to make data from computerised medical records available for comparable statistics. Scand. J. Prim. Health Care 2001, 19, 76–82. [Google Scholar] [PubMed]
- Straand, J.; Rokstad, K.S.; Sandvik, H. Prescribing systemic antibiotics in general practice: A report from the M re & Romsdal Prescription Study. Scand. J. Prim. Health Care 1998, 16, 121–127. [Google Scholar] [PubMed]
- Gjelstad, S.; Dalen, I.; Lindbæk, M. GPs’ antibiotic prescription patterns for respiratory tract infections—Still room for improvement. Scand. J. Prim. Health Care 2009, 27, 208–215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mehta, N.; Schilder, A.; Fragaszy, E.; E R Evans, H.; Dukes, O.; Manikam, L.; Little, P.; Smith, S.C.; Hayward, A. Antibiotic prescribing in patients with self-reported sore throat. J. Antimicrob. Chemother. 2016, 72, 914–922. [Google Scholar] [CrossRef] [PubMed]
- McNulty, C.A.; Nichols, T.; French, D.P.; Joshi, P.; Butler, C.C. Expectations for consultations and antibiotics for respiratory tract infection in primary care: The RTI clinical iceberg. Br. J. Gen. Pract. 2013, 63, e429–e436. [Google Scholar] [CrossRef] [PubMed]
- Høye, S. Don’t ask your doctor. J. Nor. Med. Assoc. 2014, 134, 1341. [Google Scholar]
- The Norwegian Prescription Database. About the Norwegian Prescription Database. Available online: http://www.norpd.no/Viktig.aspx (accessed on 6 January 2017).
- The Norwegian Ministry of Health and Care Services. Proposition 106 L—Changes in the Personal Health Data Filing System Act and other regulations (Municipal Patient and User Registry) [TNMoHaC. Prop. 106 L—Endringer i Helseregisterloven m.m. (Kommunalt Pasient- og Brukerregister m.m.)]; The Norwegian Ministry of Health and Care Services: Oslo, Norway, 2015.
- Statistics Norway. About Us. Available online: http://ssb.no/en/omssb/om-oss (accessed on 6 January 2017).
- Store Norske Leksikon. Kommuner i Norge. Available online: https://snl.no/Kommuner_i_Norge (accessed on 6 January 2017).
- WHO (World Health Organization). International Classification of Primary Care, 2nd ed.; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Gjelstad, S.; Hoye, S.; Straand, J.; Brekke, M.; Dalen, I.; Lindbaek, M. Improving antibiotic prescribing in acute respiratory tract infections: Cluster randomised trial from Norwegian general practice (prescription peer academic detailing (Rx-PAD) study). BMJ 2013, 347, f4403. [Google Scholar] [CrossRef] [PubMed]
| Age | Number of Included Females | Number of Included Males | Total |
|---|---|---|---|
| 0–9 years | 201,493 | 212,574 | 414,067 |
| 10–19 years | 212,673 | 224,929 | 437,602 |
| 20–29 years | 201,200 | 214,863 | 416,063 |
| 30–79 years | 968,954 | 983,760 | 1,952,713 |
| 80 years or older | 90,299 | 53,843 | 144,142 |
| Total | 1,674,618 | 1,689,968 | 3,364,585 |
| Age | Gender | RTI Consultations per 1000 Inhabitants (SD) | RTI Antibiotic Prescriptions per 1000 RTI Consultations (SD) | RTI Antibiotic Prescriptions per 1000 Inhabitants (SD) |
|---|---|---|---|---|
| 0–9 years | Female | 604 (129) | 356 (67) | 217 (66) |
| Male | 672 (139) | 345 (67) | 234 (73) | |
| 10–19 years | Female | 295 (56) | 570 (107) | 166 (40) |
| Male | 206 (46) | 552 (134) | 110 (27) | |
| 20–29 years | Female | 352 (58) | 797 (123) | 278 (52) |
| Male | 209 (43) | 809 (177) | 165 (35) | |
| 30–79 years | Female | 307 (44) | 853 (136) | 260 (48) |
| Male | 211 (29) | 940 (146) | 197 (36) | |
| 80 years or older | Female | 282 (66) | 896 (238) | 243 (66) |
| Male | 353 (100) | 972 (293) | 325 (94) | |
| Total 1 | 311 (145) | 779 (236) | 220 (62) |
| Variable | Antibiotic Prescriptions for Treatment of RTI | ||
|---|---|---|---|
| Incidence 1 | IRR | 95% CI | |
| GPs’ prescription rates | |||
| 1st quintile | 187 | 1 | |
| 2nd quintile | 201 | 1.11 | 1.10–1.12 |
| 3rd quintile | 216 | 1.20 | 1.19–1.22 |
| 4th quintile | 233 | 1.32 | 1.30–1.33 |
| 5th quintile | 258 | 1.48 | 1.47–1.50 |
| Inhabitants’ consultation rates | |||
| 1st quintile | 184 | 1 | |
| 2nd quintile | 208 | 1.11 | 1.10–1.12 |
| 3rd quintile | 225 | 1.18 | 1.17–1.20 |
| 4th quintile | 227 | 1.29 | 1.27–1.30 |
| 5th quintile | 246 | 1.42 | 1.41–1.44 |
| Age | |||
| 0–9 years | 229 | 1 | |
| 10–19 years | 137 | 0.62 | 0.61–0.63 |
| 20–29 years | 218 | 0.99 | 0.98–1.00 |
| 30–79 years | 232 | 1.01 | 1.01–1.02 |
| ≥80 years | 277 | 1.18 | 1.16–1.19 |
| Gender | |||
| Female | 247 | 1 | |
| Male 2 | 192 | 0.78 | 0.78 |
| ICPC-2 Code | Description |
|---|---|
| R01 | Pain respiratory system |
| R02 | Shortness of breath/dyspnoea |
| R03 | Wheezing |
| R04 | Breathing problem, other |
| R05 | Cough |
| R07 | Sneezing/nasal congestion |
| R08 | Nose symptom/complaint other |
| R09 | Sinus symptom/complaint |
| R21 | Throat symptom/complaint |
| R23 | Voice symptom/complaint |
| R24 | Haemoptysis |
| R25 | Sputum/phlegm abnormal |
| R26 | Fear of cancer respiratory system |
| R27 | Fear of respiratory disease, other |
| R28 | Limited function/disability (r) |
| R29 | Respiratory symptom/complaint, other |
| R71 | Whooping cough |
| R72 | Strep throat |
| R74 | Upper respiratory infection acute |
| R75 | Sinusitis acute/chronic |
| R76 | Tonsillitis acute |
| R77 | Laryngitis/tracheitis acute |
| R78 | Acute bronchitis/bronchiolitis |
| R80 | Influenza |
| R81 | Pneumonia |
| R82 | Pleurisy/pleural effusion |
| R83 | Respiratory infection other |
| H01 | Ear pain/earache |
| H71 | Acute otitis media/myringitis |
| H72 | Serous otitis media |
| H74 | Chronic otitis media |
| ICPC-2 Code | Description |
|---|---|
| J01AA02 | Doxycycline |
| J01CA04 | Amoxicillin |
| J01CE02 | Phenoxymethylpenicillin |
| J01FA | Macrolides |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walle-Hansen, M.M.; Høye, S. Geographic Variation in Antibiotic Consumption—Is It Due to Doctors’ Prescribing or Patients’ Consulting? Antibiotics 2018, 7, 26. https://doi.org/10.3390/antibiotics7010026
Walle-Hansen MM, Høye S. Geographic Variation in Antibiotic Consumption—Is It Due to Doctors’ Prescribing or Patients’ Consulting? Antibiotics. 2018; 7(1):26. https://doi.org/10.3390/antibiotics7010026
Chicago/Turabian StyleWalle-Hansen, Marte Meyer, and Sigurd Høye. 2018. "Geographic Variation in Antibiotic Consumption—Is It Due to Doctors’ Prescribing or Patients’ Consulting?" Antibiotics 7, no. 1: 26. https://doi.org/10.3390/antibiotics7010026
APA StyleWalle-Hansen, M. M., & Høye, S. (2018). Geographic Variation in Antibiotic Consumption—Is It Due to Doctors’ Prescribing or Patients’ Consulting? Antibiotics, 7(1), 26. https://doi.org/10.3390/antibiotics7010026





