Differential Susceptibility of Bacteria to Mouse Paneth Cell a-Defensins under Anaerobic Conditions
Abstract
:1. Introduction
2. Results and Discussion
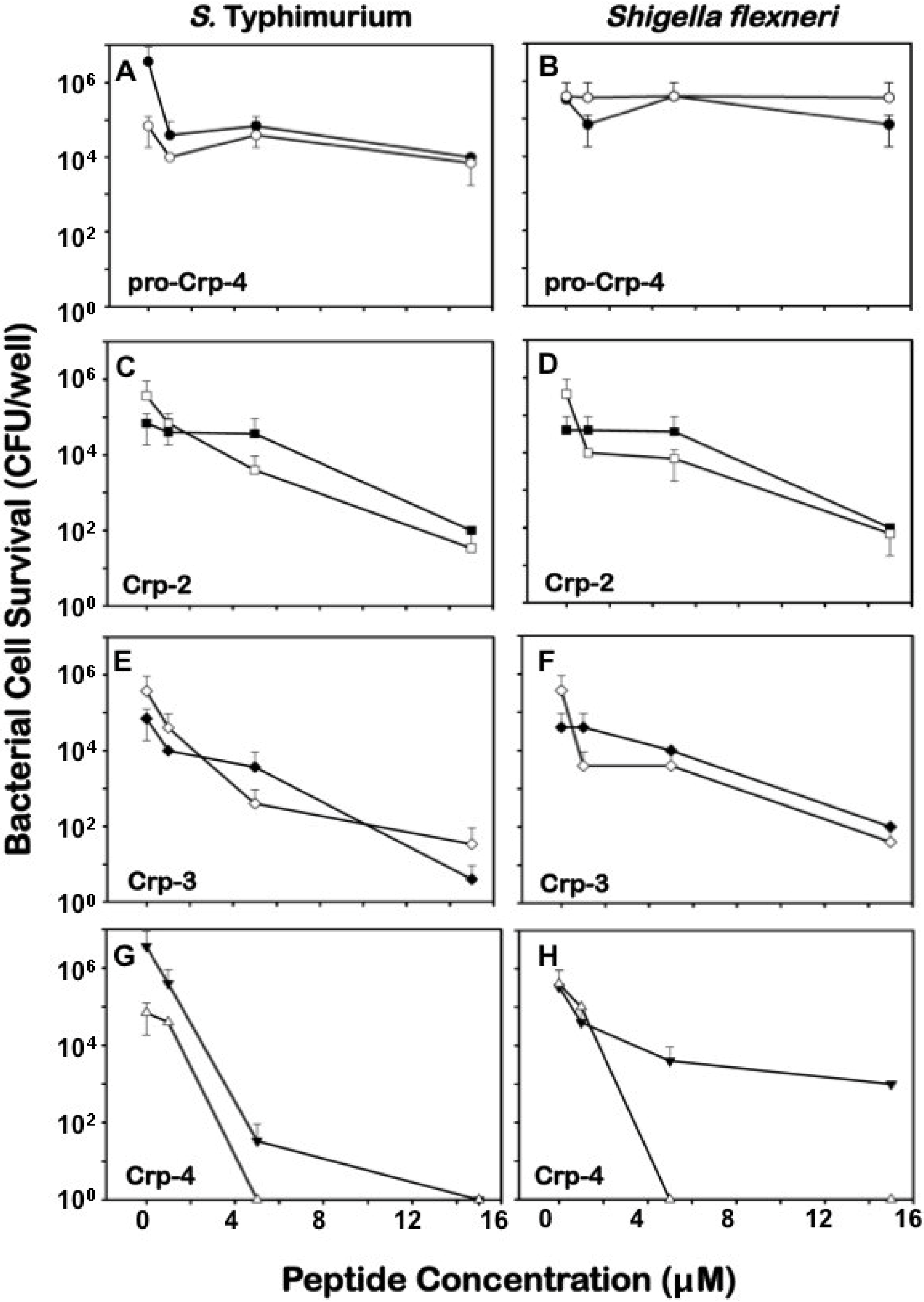
2.1. α-Defensin Activities against Facultative Bacteria under Aerobic and Anaerobic Conditions
2.2. Sensitivity of E. coli to Mouse α-Defensins under Aerobic and Anaerobic Conditions
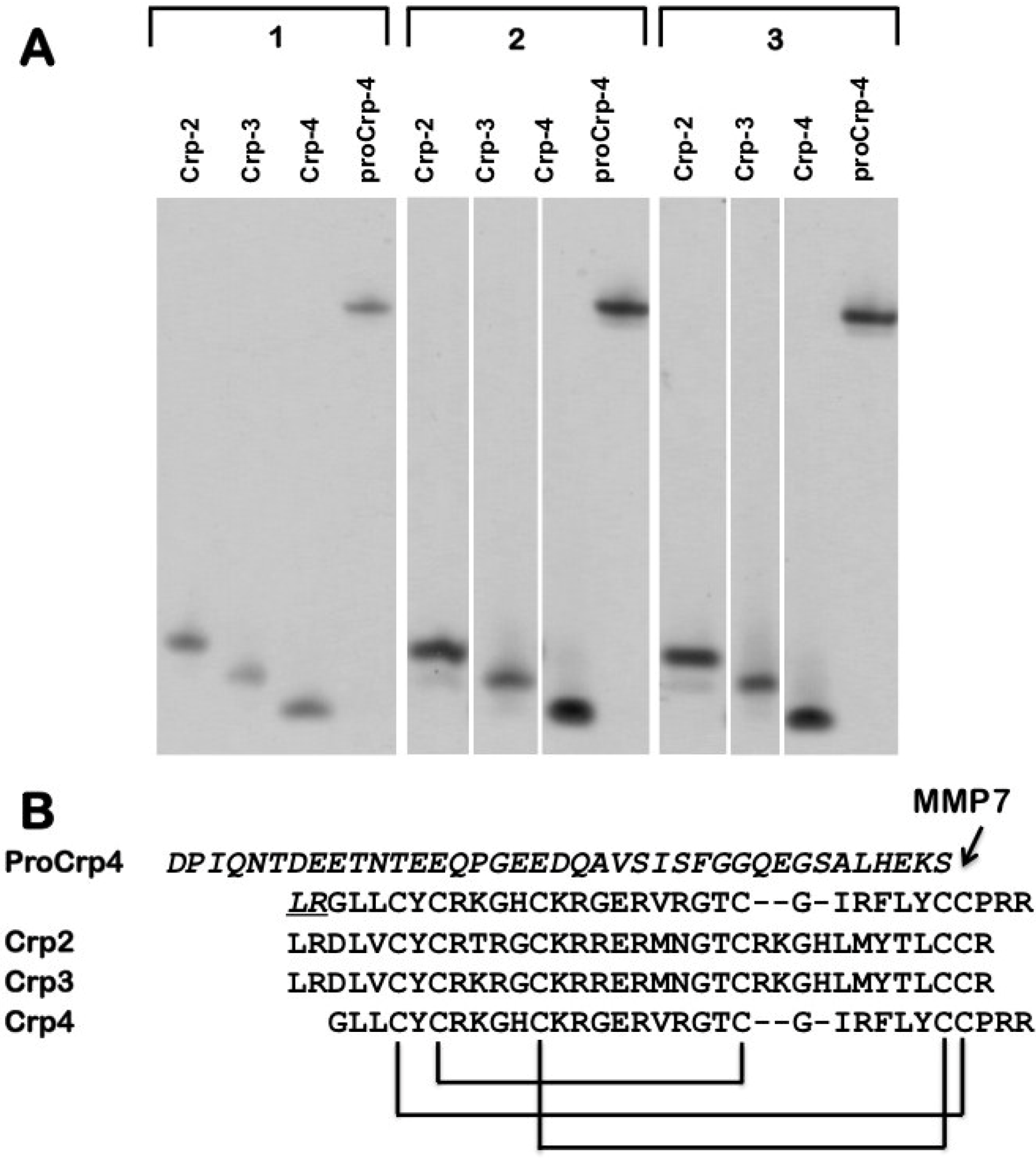

| Bacteria | Oxygen Status | Crp2 MBC (μM) | Crp3 MBC (μM) | Crp4 MBC (μM) |
|---|---|---|---|---|
| Shigella flexneri BS497 | aerobic | 5–15 | 1–5 | 1–5 |
| anaerobic | 90% a | 99% a | 99% a | |
| Salmonella Typhimurium WT | aerobic | 5–15 | 5–15 | 1–5 |
| anaerobic | 99% a | 5–15 | 1–5 | |
| E. coli ML35 | aerobic | 1–5 | 5–15 | 1–5 |
| anaerobic | 5–15 | 5–15 | 1–5 | |
| E. coli clinical isolate | aerobic | 1–5 | 1 | 1 |
| anaerobic | 5–15 | 5–15 | 1–5 | |
| EPEC | aerobic | 1–5 | 1–5 | 1–5 |
| anaerobic | 5–15 | 5–15 | 1–5 | |
| Clostridium difficile | anaerobic | 1–5 | 1–5 | 5–15 |
| Bacteroides fragilis | anaerobic | 5–15 | 5–15 | 0%a |
| Fusobacterium necrophorum | anaerobic | 0% a | 60% a | 1–5 |
| Enterococcus faecalis | anaerobic | 5–15 | 5–15 | 90% a |
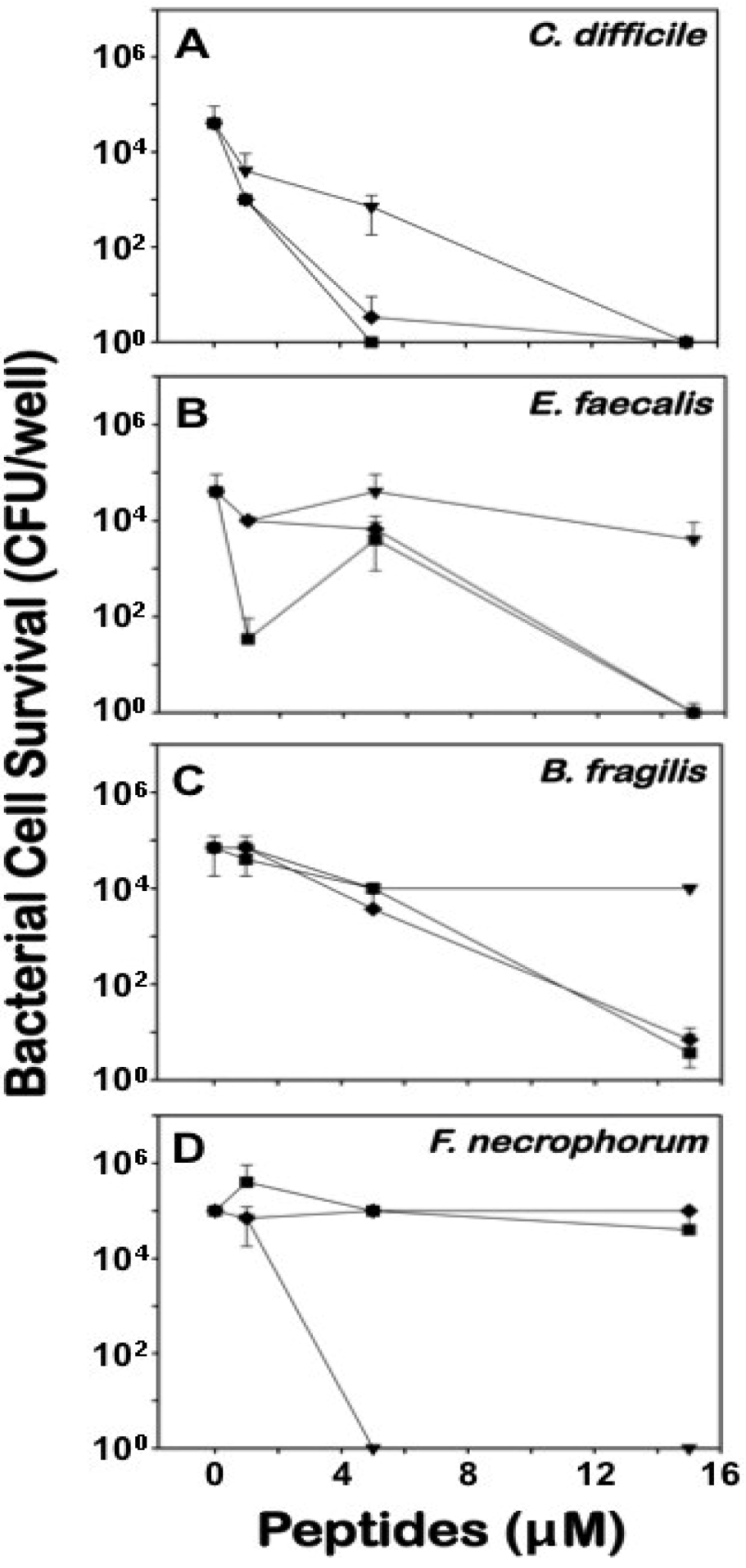
2.3. Differential Effects of Crps 2–4 against Anaerobic Bacteria
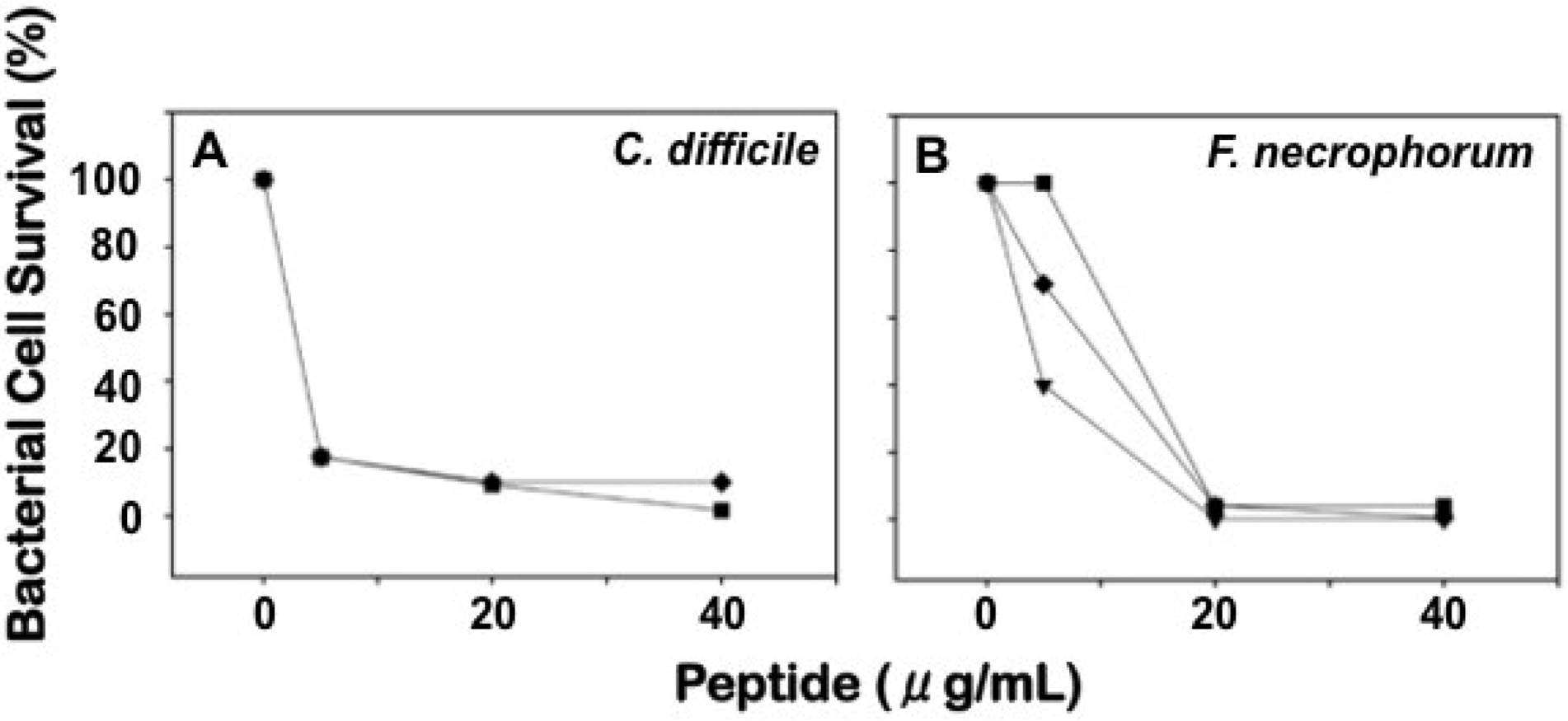
2.4. Comparative Bactericidal Activities of Mouse α-Defensin Mixtures
2.5. Discussion
3. Experimental Section
3.1. Preparation of Recombinant and Synthetic Peptides
3.2. Purification of Tissue-Derived Mouse Enteric α-Defensins
3.3. Bacterial Species and Culture Conditions
3.4. Bactericidal Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Selsted, M.E.; Ouellette, A.J. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005, 6, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, M.T.; Tanabe, H.; Ouellette, A.J. Strain-specific polymorphisms in Paneth cell alpha-defensins of C57BL/6 mice and evidence of vestigial myeloid alpha-defensin pseudogenes. Infect. Immun. 2011, 79, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Pazgier, M.; Jung, G.; Nuccio, S.P.; Castillo, P.A.; de Jong, M.F.; Winter, M.G.; Winter, S.E.; Wehkamp, J.; Shen, B.; et al. Human alpha-defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science 2012, 337, 477–481. [Google Scholar] [CrossRef]
- White, S.H.; Wimley, W.C.; Selsted, M.E. Structure, function, and membrane integration of defensins. Curr. Opin. Struct. Biol. 1995, 5, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Hristova, K.; Selsted, M.E.; White, S.H. Critical role of lipid composition in membrane permeabilization by rabbit neutrophil defensins. J. Biol. Chem. 1997, 272, 24224–24233. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.W.; Mishra, A.; Lai, G.H.; Davis, M.; Sanders, L.K.; Tran, D.; Garcia, A.; Tai, K.P.; McCray, P.B.; Ouellette, A.J.; et al. Criterion for amino acid composition of defensins and antimicrobial peptides based on geometry of membrane destabilization. J. Am. Chem. Soc. 2011, 133, 6720–6727. [Google Scholar] [CrossRef] [PubMed]
- Sahl, H.G.; Pag, U.; Bonness, S.; Wagner, S.; Antcheva, N.; Tossi, A. Mammalian defensins: Structures and mechanism of antibiotic activity. J. Leukocyte Biol. 2005, 77, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Sass, V.; Schneider, T.; Wilmes, M.; Korner, C.; Tossi, A.; Novikova, N.; Shamova, O.; Sahl, H.G. Human beta-defensin 3 inhibits cell wall biosynthesis in staphylococci. Infect. Immun. 2010, 78, 2793–2800. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, T.; Satchell, D.P.; Wilson, C.L.; Parks, W.C.; Selsted, M.E.; Ouellette, A.J. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 2000, 1, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Habara, Y.; Ono, K.; Kanno, T. Carbamylcholine- and catecholamine-induced intracellular calcium dynamics of epithelial cells in mouse ileal crypts. Gastroenterology 1995, 108, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Ishikawa, K.; Oomori, Y.; Takeda, S.; Ono, K. Bethanechol and a G-protein activator, NaF/AlCl3, induce secretory response in Paneth cells of mouse intestine. Cell Tissue Res. 1992, 269, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.L.; Ouellette, A.J.; Satchell, D.P.; Ayabe, T.; Lopez-Boado, Y.S.; Stratman, J.L.; Hultgren, S.J.; Matrisian, L.M.; Parks, W.C. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 1999, 286, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, A.J. Paneth cell alpha-defensins: Peptide mediators of innate immunity in the small intestine. Springer Semin. Immunopathol. 2005, 27, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Salzman, N.H.; Hung, K.; Haribhai, D.; Chu, H.; Karlsson-Sjoberg, J.; Amir, E.; Teggatz, P.; Barman, M.; Hayward, M.; Eastwood, D.; et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat. Immunol. 2010, 11, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.L.; Gawenis, L.R.; Bradford, E.M.; Judd, L.M.; Boyle, K.T.; Simpson, J.E.; Shull, G.E.; Tanabe, H.; Ouellette, A.J.; Franklin, C.L.; et al. Abnormal Paneth cell granule dissolution and compromised resistance to bacterial colonization in the intestine of CF mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G1050–G1058. [Google Scholar] [CrossRef]
- Garabedian, E.M.; Roberts, L.J.; McNevin, M.S.; Gordon, J.I. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J. Biol. Chem. 1997, 272, 23729–23740. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, E.E.; Matsumoto, T.; Lindenbergh, D.; Willemsen, R.; Kaser, A.; Simons-Oosterhuis, Y.; Brugman, S.; Yamaguchi, K.; Ishikawa, H.; Aiba, Y.; et al. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J. Clin. Invest. 2009, 119, 1241–1250. [Google Scholar] [CrossRef]
- De Lisle, R.C. Altered transit and bacterial overgrowth in the cystic fibrosis mouse small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G104–G111. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, J.R.; Ouellette, A.J. Alpha-defensins in enteric innate immunity: Functional Paneth cell alpha-defensins in mouse colonic lumen. J. Biol. Chem. 2009, 284, 27848–27856. [Google Scholar] [CrossRef] [PubMed]
- Bevins, C.L.; Salzman, N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, B.E. The physiological importance of the colonic microflora. Scand. J. Gastroenterol. Suppl. 1982, 77, 117–131. [Google Scholar] [PubMed]
- Salzman, N.H.; Underwood, M.A.; Bevins, C.L. Paneth cells, defensins, and the commensal microbiota: A hypothesis on intimate interplay at the intestinal mucosa. Semin. Immunol. 2007, 19, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, A.J.; Hsieh, M.M.; Nosek, M.T.; Cano-Gauci, D.F.; Huttner, K.M.; Buick, R.N.; Selsted, M.E. Mouse Paneth cell defensins: Primary structures and antibacterial activities of numerous cryptdin isoforms. Infect. Immun. 1994, 62, 5040–5047. [Google Scholar] [PubMed]
- Ayabe, T.; Satchell, D.P.; Pesendorfer, P.; Tanabe, H.; Wilson, C.L.; Hagen, S.J.; Ouellette, A.J. Activation of Paneth cell alpha-defensins in mouse small intestine. J. Biol. Chem. 2002, 277, 5219–5228. [Google Scholar] [CrossRef] [PubMed]
- Ericksen, B.; Wu, Z.; Lu, W.; Lehrer, R.I. Antibacterial activity and specificity of the six human alpha-defensins. Antimicrob. Agents Chemother. 2005, 49, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Miyasaki, K.T.; Bodeau, A.L.; Ganz, T.; Selsted, M.E.; Lehrer, R.I. In vitro sensitivity of oral, Gram-negative, facultative bacteria to the bactericidal activity of human neutrophil defensins. Infect. Immun. 1990, 58, 3934–3940. [Google Scholar] [PubMed]
- Miyasaki, K.T.; Lehrer, R.I. Beta-sheet antibiotic peptides as potential dental therapeutics. Int. J. Antimicrob. Agents 1998, 9, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Nuding, S.; Zabel, L.T.; Enders, C.; Porter, E.; Fellermann, K.; Wehkamp, J.; Mueller, H.A.; Stange, E.F. Antibacterial activity of human defensins on anaerobic intestinal bacterial species: A major role of HBD-3. Microbes Infect. 2009, 11, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Sakai, N.; Nakamura, K.; Yoshioka, S.; Ayabe, T. Bactericidal activity of mouse alpha-defensin cryptdin-4 predominantly affects noncommensal bacteria. J. Innate. Immun. 2010, 3, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Salzman, N.H. Microbiota-immune system interaction: An uneasy alliance. Curr. Opin. Microbiol. 2010, 14, 99–105. [Google Scholar] [PubMed]
- Selsted, M.E. Investigational approaches for studying the structures and biological functions of myeloid antimicrobial peptides. Genet. Eng. (N Y) 1993, 15, 131–147. [Google Scholar]
- Shirafuji, Y.; Tanabe, H.; Satchell, D.P.; Henschen-Edman, A.; Wilson, C.L.; Ouellette, A.J. Structural determinants of procryptdin recognition and cleavage by matrix metalloproteinase-7. J. Biol. Chem. 2003, 278, 7910–7919. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef]
- Handler, M.Z.; Miriovsky, B.; Gendelman, H.E.; Sandkovsky, U. Fusobacterium necrophorum causing infective endocarditis and liver and splenic abscesses. Rev. Inst. Med. Trop. Sao Paulo 2011, 53, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Selsted, M.E.; Miller, S.I.; Henschen, A.H.; Ouellette, A.J. Enteric defensins: Antibiotic peptide components of intestinal host defense. J. Cell. Biol. 1992, 118, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, J.R.; Costales, J.K.; Zaksheske, J.; Selsted, M.E.; Salzman, N.H.; Ouellette, A.J. Alternative luminal activation mechanisms for Paneth cell alpha-defensins. J. Cell. Biol. 2012, 287, 11205–11212. [Google Scholar]
- Takahashi, N.; Vanlaere, I.; de Rycke, R.; Cauwels, A.; Joosten, L.A.; Lubberts, E.; van den Berg, W.B.; Libert, C. Il-17 produced by Paneth cells drives tnf-induced shock. J. Exp. Med. 2008, 205, 1755–1761. [Google Scholar]
- Porter, E.M.; Bevins, C.L.; Ghosh, D.; Ganz, T. The multifaceted Paneth cell. Cell. Mol. Life Sci. 2002, 59, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, A.J. Paneth cells and innate mucosal immunity. Curr. Opin. Gastroen. 2010, 26, 547–553. [Google Scholar] [CrossRef]
- Salzman, N.H.; Ghosh, D.; Huttner, K.M.; Paterson, Y.; Bevins, C.L. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 2003, 422, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, A.J. Paneth cell alpha-defensins in enteric innate immunity. Cell. Mol. Life Sci. 2011, 68, 2215–2229. [Google Scholar] [CrossRef] [PubMed]
- Hadjicharalambous, C.; Sheynis, T.; Jelinek, R.; Shanahan, M.T.; Ouellette, A.J.; Gizeli, E. Mechanisms of alpha-defensin bactericidal action: Comparative membrane disruption by cryptdin-4 and its disulfide-null analogue. Biochemistry 2008, 47, 12626–12634. [Google Scholar] [CrossRef] [PubMed]
- Satchell, D.P.; Sheynis, T.; Shirafuji, Y.; Kolusheva, S.; Ouellette, A.J.; Jelinek, R. Interactions of mouse Paneth cell alpha-defensins and alpha-defensin precursors with membranes. Prosegment inhibition of peptide association with biomimetic membranes. J. Biol. Chem. 2003, 278, 13838–13846. [Google Scholar] [CrossRef] [PubMed]
- Kagan, B.L.; Selsted, M.E.; Ganz, T.; Lehrer, R.I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc. Natl. Acad. Sci. USA 1990, 87, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lim, K.B.; Poduje, C.M.; Daniel, M.; Gunn, J.S.; Hackett, M.; Miller, S.I. Lipid a acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 1998, 95, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Gunn, J.S.; Lim, K.B.; Krueger, J.; Kim, K.; Guo, L.; Hackett, M.; Miller, S.I. Pmra-pmrb-regulated genes necessary for 4-aminoarabinose lipid a modification and polymyxin resistance. Mol. Microbiol. 1998, 27, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 2002, 10, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Boulette, M.L.; Payne, S.M. Anaerobic regulation of Shigella flexneri virulence: Arca regulates fur and iron acquisition genes. J. Bacteriol. 2007, 189, 6957–6967. [Google Scholar] [CrossRef] [PubMed]
- Marteyn, B.; Scorza, F.B.; Sansonetti, P.J.; Tang, C. Breathing life into pathogens: The influence of oxygen on bacterial virulence and host responses in the gastrointestinal tract. Cell. Microbiol. 2011, 13, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.D.; Khullar, M.; Ganguly, N.K. Role of anaerobiosis in virulence of Salmonella typhimurium. Mol. Cell. Biochem. 2000, 215, 39–46. [Google Scholar] [CrossRef] [PubMed]
- James, B.W.; Keevil, C.W. Influence of oxygen availability on physiology, verocytotoxin expression and adherence of Escherichia coli o157. J. Appl. Microbiol. 1999, 86, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Bevins, C.L.; Salzman, N.H. The potter’s wheel: The host’s role in sculpting its microbiota. Cell. Mol. Life Sci. 2011, 68, 3675–3685. [Google Scholar] [CrossRef] [PubMed]
- Daw, M.A.; Falkiner, F.R. Bacteriocins: Nature, function and structure. Micron 1996, 27, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Harder, J.; Weichenthal, M.; Schwab, M.; Schaffeler, E.; Schlee, M.; Herrlinger, K.R.; Stallmach, A.; Noack, F.; Fritz, P.; et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut 2004, 53, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Salzman, N.H.; Porter, E.; Nuding, S.; Weichenthal, M.; Petras, R.E.; Shen, B.; Schaeffeler, E.; Schwab, M.; Linzmeier, R.; et al. Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc. Natl. Acad. Sci. USA 2005, 102, 18129–18134. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.; Su, J.; Koprowski, S.; Hsu, A.; Tweddell, J.S.; Rafiee, P.; Gross, G.J.; Salzman, N.H.; Baker, J.E. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012, 26, 1727–1735. [Google Scholar]
- Loscalzo, J. Lipid metabolism by gut microbes and atherosclerosis. Circ. Res. 2011, 109, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Wilson, I.D. Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. Microbiol. 2005, 3, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Maemoto, A.; Qu, X.; Rosengren, K.J.; Tanabe, H.; Henschen-Edman, A.; Craik, D.J.; Ouellette, A.J. Functional analysis of the alpha-defensin disulfide array in mouse cryptdin-4. J. Biol. Chem. 2004, 279, 44188–44196. [Google Scholar] [CrossRef] [PubMed]
- Satchell, D.P.; Sheynis, T.; Kolusheva, S.; Cummings, J.; Vanderlick, T.K.; Jelinek, R.; Selsted, M.E.; Ouellette, A.J. Quantitative interactions between cryptdin-4 amino terminal variants and membranes. Peptides 2003, 24, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, H.; Qu, X.; Weeks, C.S.; Cummings, J.E.; Kolusheva, S.; Walsh, K.B.; Jelinek, R.; Vanderlick, T.K.; Selsted, M.E.; Ouellette, A.J. Structure-activity determinants in Paneth cell alpha-defensins: Loss-of-function in mouse cryptdin-4 by charge-reversal at arginine residue positions. J. Biol. Chem. 2004, 279, 11976–11983. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Powell, R.; Lu, W. Productive folding of human neutrophil alpha-defensins in vitro without the pro-peptide. J. Am. Chem. Soc. 2003, 125, 2402–2403. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ericksen, B.; Tucker, K.; Lubkowski, J.; Lu, W. Synthesis and characterization of human alpha-defensins 4-6. J. Pept. Res. 2004, 64, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Prahl, A.; Powell, R.; Ericksen, B.; Lubkowski, J.; Lu, W. From pro defensins to defensins: Synthesis and characterization of human neutrophil pro alpha-defensin-1 and its mature domain. J. Pept. Res. 2003, 62, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Sandlin, R.C.; Goldberg, M.B.; Maurelli, A.T. Effect of O side-chain length and composition on the virulence of Shigella flexneri 2a. Mol. Microbiol. 1996, 22, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Savkovic, S.D.; Villanueva, J.; Turner, J.R.; Matkowskyj, K.A.; Hecht, G. Mouse model of enteropathogenic Escherichia coli infection. Infect. Immun. 2005, 73, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastroianni, J.R.; Lu, W.; Selsted, M.E.; Ouellette, A.J. Differential Susceptibility of Bacteria to Mouse Paneth Cell a-Defensins under Anaerobic Conditions. Antibiotics 2014, 3, 493-508. https://doi.org/10.3390/antibiotics3040493
Mastroianni JR, Lu W, Selsted ME, Ouellette AJ. Differential Susceptibility of Bacteria to Mouse Paneth Cell a-Defensins under Anaerobic Conditions. Antibiotics. 2014; 3(4):493-508. https://doi.org/10.3390/antibiotics3040493
Chicago/Turabian StyleMastroianni, Jennifer R., Wuyuan Lu, Michael E. Selsted, and André J. Ouellette. 2014. "Differential Susceptibility of Bacteria to Mouse Paneth Cell a-Defensins under Anaerobic Conditions" Antibiotics 3, no. 4: 493-508. https://doi.org/10.3390/antibiotics3040493
APA StyleMastroianni, J. R., Lu, W., Selsted, M. E., & Ouellette, A. J. (2014). Differential Susceptibility of Bacteria to Mouse Paneth Cell a-Defensins under Anaerobic Conditions. Antibiotics, 3(4), 493-508. https://doi.org/10.3390/antibiotics3040493




