Role of Old Antibiotics in the Era of Antibiotic Resistance. Highlighted Nitrofurantoin for the Treatment of Lower Urinary Tract Infections
Abstract
:1. Introduction
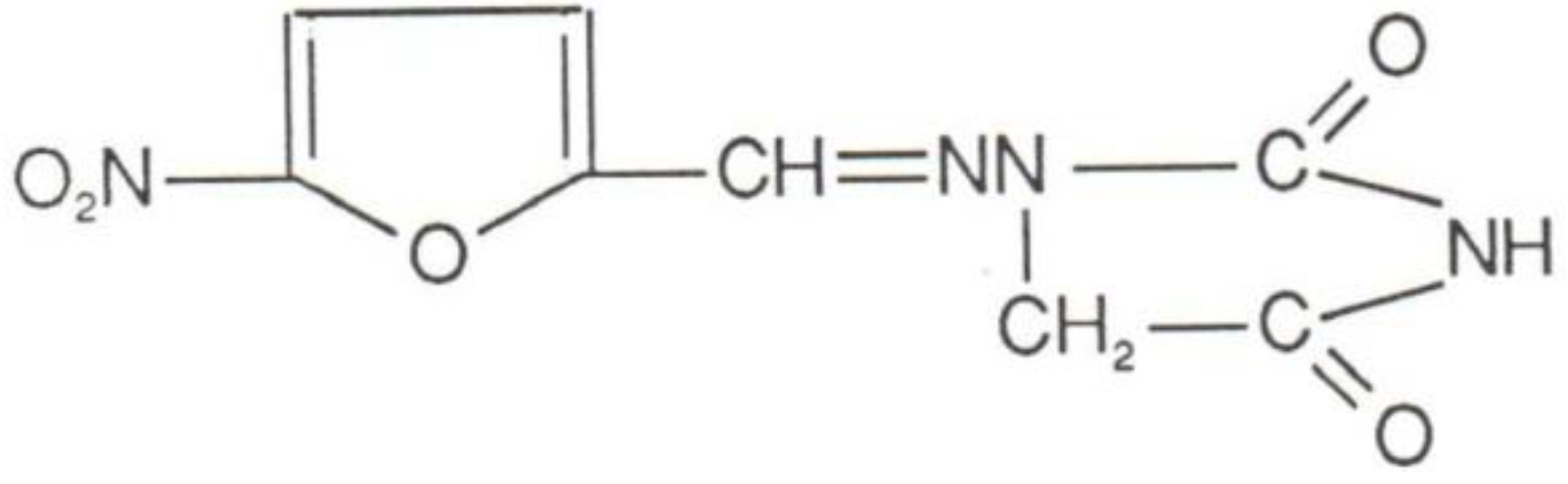
2. Mechanism of Action
3. Antimicrobial Spectrum
4. Pharmacology
4.1. Absorption
4.2. Distribution and Excretion
4.3. Excretion in Patients with Impaired Renal Function
4.4. Interactions
5. Safety Profile
6. Therapeutic Use
6.1. Classical
6.2. New Applications
7. Conclusions
Conflicts of Interest
References
- Cunha, B.A. Nitrofurantoin: An update. Obstet. Gynecol. Surv. 1989, 44, 399–406. [Google Scholar]
- Gleckman, R.; Alvarez, S.; Joubert, D.W. Drug therapy reviews: Nitrofurantoin. Am. J. Hosp. Pharm. 1979, 36, 342–351. [Google Scholar]
- Koulaouzidis, A.; Bhat, S.; Moschos, J.; Tan, C.; de Ramon, A. Nitrofurantoininduced lung- and hepatotoxicity. Ann. Hepatol. 2007, 6, 119–121. [Google Scholar]
- Hooton, T.M.; Scholes, D.; Gupta, K.; Stapleton, A.E.; Roberts, P.L.; Stamm, W.E. Amoxicillin-clavulanate vs. ciprofloxacin for the treatment of uncomplicated cystitis in women: A randomized trial. JAMA 2005, 293, 949–955. [Google Scholar] [CrossRef]
- Conklin, J.D. The pharmacokinetics of nitrofurantoin and its related bioavailability. Antibiot. Chemother. 1978, 25, 233–252. [Google Scholar]
- Mavromanolakis, E.; Maraki, S.; Samonis, G.; Tselentis, Y.; Cranidis, A. Effect of norfloxacin, trimethoprimsulfamethoxazole and nitrofurantoin on fecal flora of women with recurrent urinary tract infections. J. Chemother. 1997, 9, 203–207. [Google Scholar]
- Conklin, J.D. Biopharmaceutics of nitrofurantoin. Pharmacology 1972, 8, 178–181. [Google Scholar]
- Hosbach, R.; Foster, R.B. Absence of nitrofurantoin from human milk (letter). JAMA 1967, 202, 1057. [Google Scholar] [CrossRef]
- Varsano, I.; Fischl, J.; Shochet, S.B. The excretion of orally ingested nitrofurantoin in human milk. J. Pediatr. 1973, 82, 886–887. [Google Scholar] [CrossRef]
- Sachs, J.; Geer, J.; Noell, P.; Kunin, C.M. Effect of renal function on urinary recovery of orally administered nitrofurantoin. N. Engl. J. Med. 1968, 278, 1032–1035. [Google Scholar] [CrossRef]
- Oplinger, M.; Andrews, C.O. Nitrofurantoin contraindication in patients with a creatinine clearance below 60 mL/min: Looking for the evidence. Ann. Pharmacother. 2013, 47, 106–111. [Google Scholar] [CrossRef]
- Holmberg, L.; Boman, G.; Bottiger, L.S.; Eriksson, B.; Spross, R.; Wessling, A. Adverse reactions to nitrofurantoin. An analysis of 921 reports. Am. J. Med. 1980, 69, 733–738. [Google Scholar] [CrossRef]
- Koch-Weser, J.; Sidel, V.W.; Dexter, M.; Parish, C.; Finer, D.C.; Kanarek, P. Adverse reactions to sulfisoxazole, sulfamethoxazole, and nitrofurantoin. Arch. Intern. Med. 1971, 128, 399–404. [Google Scholar] [CrossRef]
- D’Arcy, P.F. Nitrofurantoin. Drug Intell. Clin. Pharm. 1985, 19, 540–547. [Google Scholar]
- Tan, I.L.; Polydefkis, M.J.; Ebenezer, G.J.; Hauer, P.; McArthur, J.C. Peripheral nerve toxic effects of nitrofurantoin. Arch. Neurol. 2012, 69, 265–268. [Google Scholar] [CrossRef]
- Marshall, A.D.; Dempsey, O.J. Is “nitrofurantoin lung” on the increase? Br. Med. J. 2013, 18, f3897. [Google Scholar] [CrossRef]
- Crider, K.S.; Cleves, M.A.; Reefhuis, J.; Berry, R.J.; Hobbs, C.A.; Hu, D.J. Antibacterial medication use during pregnancy and risk of birth defects: National birth defects prevention study. Arch. Pediatr. Adolesc. Med. 2009, 163, 978–985. [Google Scholar]
- Gardner, J.S.; Guyard-Boileau, B.; Alderman, B.W.; Fernbach, S.K.; Greene, C.; Mangione, E.J. Maternal exposure to prescription and non-prescription pharmaceuticals or drugs of abuse and risk of craniosynostosis. Int. J. Epidemiol. 1998, 27, 64–67. [Google Scholar] [CrossRef]
- Briggs, G.G.; Freeman, R.K.; Yaffe, S.J. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonate Risk, 8th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; pp. 1310–1312. [Google Scholar]
- Prytherch, J.P.; Sutton, M.L.; Denine, E.P. General reproduction, perinatal-postnatal, and teratology studies of nitrofurantoin macrocrystals in rats and rabbits. J. Toxicol. Environ. Health 1984, 13, 811–823. [Google Scholar] [CrossRef]
- Lee, M.; Bozzo, P.; Einarson, A.; Koren, G. Urinary tract infections in pregnancy. Can. Fam. Physician 2008, 54, 853–854. [Google Scholar]
- Hailey, F.J.; Fort, H.; Williams, J.C.; Hammers, B. Foetal safety of nitrofurantoin macrocrystals therapy during pregnancy: A retrospective analysis. J. Int. Med. Res. 1983, 11, 364–369. [Google Scholar]
- Czeizel, A.E.; Rockenbauer, M.; Sørensen, H.T.; Olsen, J. Nitrofurantoin and congenital abnormalities. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 95, 119–126. [Google Scholar] [CrossRef]
- Ben David, S.; Einarson, T.; Ben David, Y.; Nulman, I.; Pastuszak, A.; Koren, G. The safety of nitrofurantoinduring the first trimester of pregnancy: Meta-analysis. Fundam. Clin. Pharmacol. 1995, 9, 503–507. [Google Scholar] [CrossRef]
- Goldberg, O.; Koren, G.; Landau, D.; Lunenfeld, E.; Matok, I.; Levy, A. Exposure to nitrofurantoin during the first trimester of pregnancy and the risk for major malformations. J. Clin. Pharmacol. 2013, 53, 991–995. [Google Scholar] [CrossRef]
- Cunha, B.A. New uses for older antibiotics: Nitrofurantoin, amikacin, colistin, polymyxin B, doxycycline, and minocycline revisited. Med. Clin. North Am. 2006, 90, 1089–1107. [Google Scholar] [CrossRef]
- Nardiello, S.; Pizzella, T.; Ariviello, R. Risks of antibacterial agents in pregnancy. Infez. Med. 2002, 10, 8–15. [Google Scholar]
- Delzell, J.E., Jr.; Lefevre, M.L. Urinary tract infections during pregnancy. Am. Fam. Physician 2000, 61, 713–721. [Google Scholar]
- Cunha, B.A.; Schoch, P.; Hage, J.E. Oral theraphy of catheter-associated bacteriuria (CAB) in the era of antibiotic resistance: Nitrofurantoin revisited. J. Chemother. 2012, 24, 122–124. [Google Scholar] [CrossRef]
- Lumbiganon, P.; Villar, J.; Laopaiboon, M.; Widmer, M.; Thinkhamrop, J.; Carroli, G.; Duc, V.N.; Mignini, L.; Festin, M.; Prasertcharoensuk, W.; et al. World Health Organization Asymptomatic Bacteriuria Trial Group. One-day compared with 7-day nitrofurantoin for asymptomatic bacteriuria in pregnancy: A randomized controlled trial. Obstet. Gynecol. 2009, 113, 339–345. [Google Scholar]
- Guinto, V.T.; de Guia, B.; Festin, M.R.; Dowswell, T. Different antibiotic regimens for treating asymptomatic bacteriuria in pregnancy. Cochrane Database Syst. Rev. 2010, 8, CD007855. [Google Scholar]
- Amábile-Cuevas, C.F.; Arredondo-García, J.L. Antimicrobial activity data in support of nitrofurantoin three times per day. J. Antimicrob. Chemother. 2011, 66, 1652–1653. [Google Scholar] [CrossRef]
- Zalmanovici Trestioreanu, A.; Green, H.; Paul, M.; Yaphe, J.; Leibovici, L. Antimicrobial agents for treating uncomplicated urinary tract infection in women. Cochrane Database Syst. Rev. 2010, 6, CD007182. [Google Scholar]
- Gupta, K.; Hooton, T.M.; Roberts, P.L.; Stamm, W.E. Short-course nitrofurantoin for the treatment of acute uncomplicated cystitis in women. Arch. Intern. Med. 2007, 12, 2207–2212. [Google Scholar]
- Schneeberger, C.; Geerlings, S.E.; Middleton, P.; Crowther, C.A. Interventionsfor preventing urinary tract infection during pregnancy. Cochrane Database Syst. Rev. 2012, 14, CD009279. [Google Scholar]
- Williams, G.; Craig, J.C. Long-term antibiotics for preventing recurrent urinary tract infection in children. Cochrane Database Syst. Rev. 2011, 16, CD001534. [Google Scholar]
- Zhanel, G.G.; Hisanaga, T.L.; Laing, N.M.; DeCorby, M.R.; Nichol, K.A.; Weshnoweski, B.; Johnson, J.; Noreddin, A.; Low, D.E.; Karlowsky, J.A.; et al. Antibiotic resistance in Escherichia coli outpatient urinary isolates: Final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int. J. Antimicrob. Agents 2006, 27, 468–475. [Google Scholar] [CrossRef]
- Honderlick, P.; Cahen, P.; Gravisse, J.; Vignon, D. Quelle sensibilité aux antibiotiques pour les bactéries responsables d´infections urinaires? Que penser de fosfomycine et nitrofuranes? Pathol. Biol. 2006, 54, 462–466. [Google Scholar] [CrossRef]
- Puerto, A.S.; Fernandez, J.G.; del Castillo, J.; Pino, M.J.; Angulo, J.P. In vitro activity of beta-lactam and non-betalactam antibiotics in extended spectrum beta-lactamase producing clinical isolates of Escherichia coli. Diagn. Microbiol. Infect. Dis. 2006, 54, 135–139. [Google Scholar] [CrossRef]
- Gales, A.C.; Sader, H.S.; Jones, R.N. SENTRY participants group (Latin America). Urinary tract infection trends in Latin American hospitals: Report from the SENTRY antimicrobial surveillance program (1997–2000). Diagn. Microbiol. Infect. Dis. 2002, 44, 289–299. [Google Scholar] [CrossRef]
- Fadda, G.; Nicoletti, G.; Schito, G.C.; Tempera, G. Antimicrobial susceptibility patterns of contemporary pathogens from uncomplicated urinary tract infection isolated in a multicenter Italian survey: Possible impact in guidelines. J. Chemother. 2005, 17, 251–257. [Google Scholar] [CrossRef]
- Procop, G.W.; Tuohy, M.J.; Wilson, D.A.; Williams, D.; Hadziyannis, E.; Hall, G.S. Cross-class resistance to non-beta-lactam antimicrobials in extended-spectrum-beta-lactamaseproducing Klebsiella pneumoniae. Am. J. Clin. Pathol. 2003, 120, 265–267. [Google Scholar] [CrossRef]
- Garau, J. Other antimicrobials of interest in the era of extended-spectrum beta-lactamases: Fosfomycin, nitrofurantoin and tigecycline. Clin. Microbiol. Infect. 2008, 14, 198–202. [Google Scholar] [CrossRef]
- Tasbakan, M.I.; Pullukcu, H.; Sipahi, O.R.; Yamazhan, T.; Ulusoy, S. Nitrofurantoin in the treatment of extended-spectrum β-lactamase-producing Escherichia coli-related lower urinary tract infection. Int. J. Antimicrob. Agents. 2012, 40, 554–556. [Google Scholar] [CrossRef]
- Chen, Y.H.; Ko, W.C.; Hsueh, P.R. Emerging resistance problems and future perspectives in pharmacotherapy for complicated urinary tract infections. Expert Opin. Pharmacother. 2013, 14, 587–596. [Google Scholar] [CrossRef]
- Kashanian, J.; Hakimian, P.; Blute, M., Jr.; Wong, J.; Khanna, H.; Wise, G.; Shabsigh, R. Nitrofurantoin: The return of an old friend in the wake of growing resistance. BJU Int. 2008, 102, 1634–1637. [Google Scholar] [CrossRef]
- Mazzulli, T.; Skulnick, M.; Small, G.; Marshall, W.; Hoban, D.J.; Zhanel, G.G.; Finn, S.; Low, D.E. Susceptibility of community Gram-negative urinary tract isolates to mecillinam and other oral agents. Can. J. Infect. Dis. 2001, 12, 289–292. [Google Scholar]
- Wagenlehner, F.M.; Wullt, B.; Perletti, G. Antimicrobials in urogenital infections. Int. J. Antimicrob. Agents 2011, 38, 3–10. [Google Scholar] [CrossRef]
- Gupta, K.; Hooton, T.M.; Stamm, W.E. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann. Intern. Med. 2001, 135, 41–50. [Google Scholar] [CrossRef]
- Kahlmeter, G. An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: The ECO.SENS project. J. Antimicrob. Chemother. 2003, 51, 69–76. [Google Scholar] [CrossRef]
- Warren, J.W.; Abrutyn, E.; Hebel, J.R.; Johnson, J.R.; Schaeffer, A.J.; Stamm, W.E. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women, Infectious Diseases Society of America (IDSA). Clin. Infect. Dis. 1999, 29, 745–758. [Google Scholar]
- Talan, D.A.; Stamm, W.E.; Hooton, T.M.; Moran, G.J.; Burke, T.; Iravani, A.; Reuning-Scherer, J.; Church, D.A. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis pyelonephritis in women: A randomized trial. JAMA 2000, 283, 1583–1590. [Google Scholar] [CrossRef]
- Naber, K.G.; Schito, G.; Botto, H.; Palou, J.; Mazzei, T. Surveillance study in Europe and Brazil on clinical aspects and antimicrobial resistance epidemiology in females with cystitis (ARESC): Implications for empiric therapy. Eur. Urol. 2008, 54, 1164–1175. [Google Scholar] [CrossRef]
- Slekovec, C.; Leroy, J.; Huttner, A.; Ruyer, O.; Talon, D.; Hocquet, D.; Bertrand, X. When the precautionary principle disrupts 3 years of antibiotic stewardship: Nitrofurantoin in the treatment of urinary tract infections. J. Antimicrob. Chemother. 2014, 69, 282–284. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Munoz-Davila, M.J. Role of Old Antibiotics in the Era of Antibiotic Resistance. Highlighted Nitrofurantoin for the Treatment of Lower Urinary Tract Infections. Antibiotics 2014, 3, 39-48. https://doi.org/10.3390/antibiotics3010039
Munoz-Davila MJ. Role of Old Antibiotics in the Era of Antibiotic Resistance. Highlighted Nitrofurantoin for the Treatment of Lower Urinary Tract Infections. Antibiotics. 2014; 3(1):39-48. https://doi.org/10.3390/antibiotics3010039
Chicago/Turabian StyleMunoz-Davila, Maria Jose. 2014. "Role of Old Antibiotics in the Era of Antibiotic Resistance. Highlighted Nitrofurantoin for the Treatment of Lower Urinary Tract Infections" Antibiotics 3, no. 1: 39-48. https://doi.org/10.3390/antibiotics3010039
APA StyleMunoz-Davila, M. J. (2014). Role of Old Antibiotics in the Era of Antibiotic Resistance. Highlighted Nitrofurantoin for the Treatment of Lower Urinary Tract Infections. Antibiotics, 3(1), 39-48. https://doi.org/10.3390/antibiotics3010039



