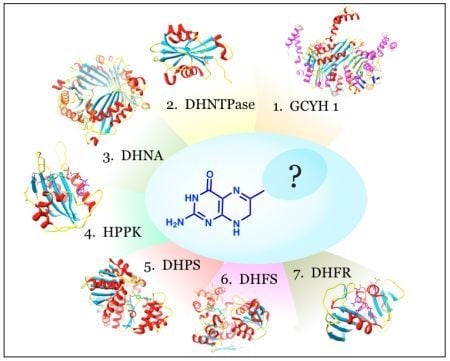
Utility of the Biosynthetic Folate Pathway for Targets in Antimicrobial Discovery
Abstract
:1. Introduction
Pathways for Folate Production and Utilization
2. Results and Discussion
2.1. Enzymes for the Biosynthesis of Folate: 1. Guanine Cyclohydrolase (GCYH I)

2.2. Enzymes for the Biosynthesis of Folate: 2. Dihydroneopterin Hydrolase (DHNTPase)

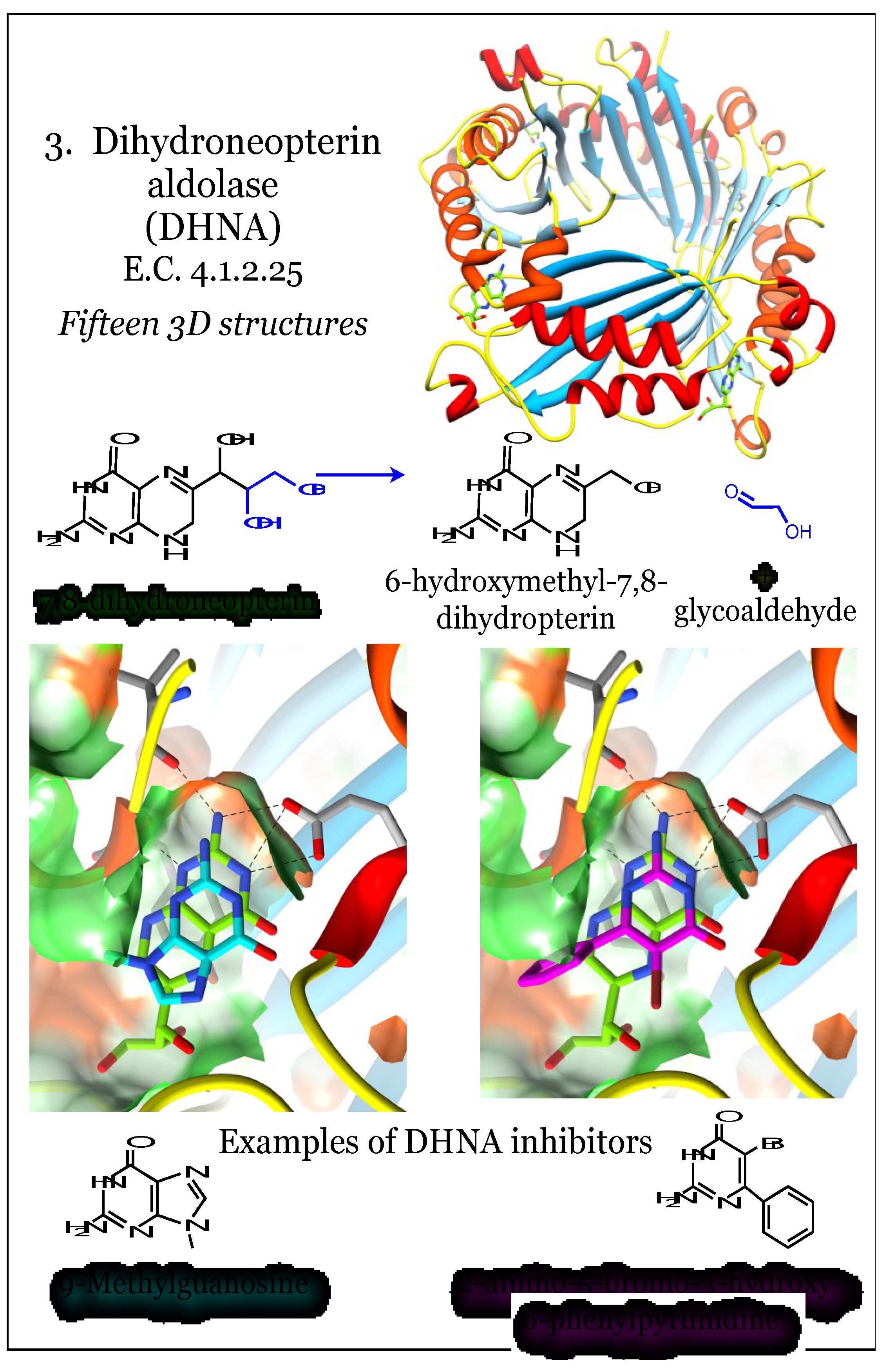
2.3. Enzymes for the Biosynthesis of Folate: 3. Dihydroneopterin Aldolase (DHNA)
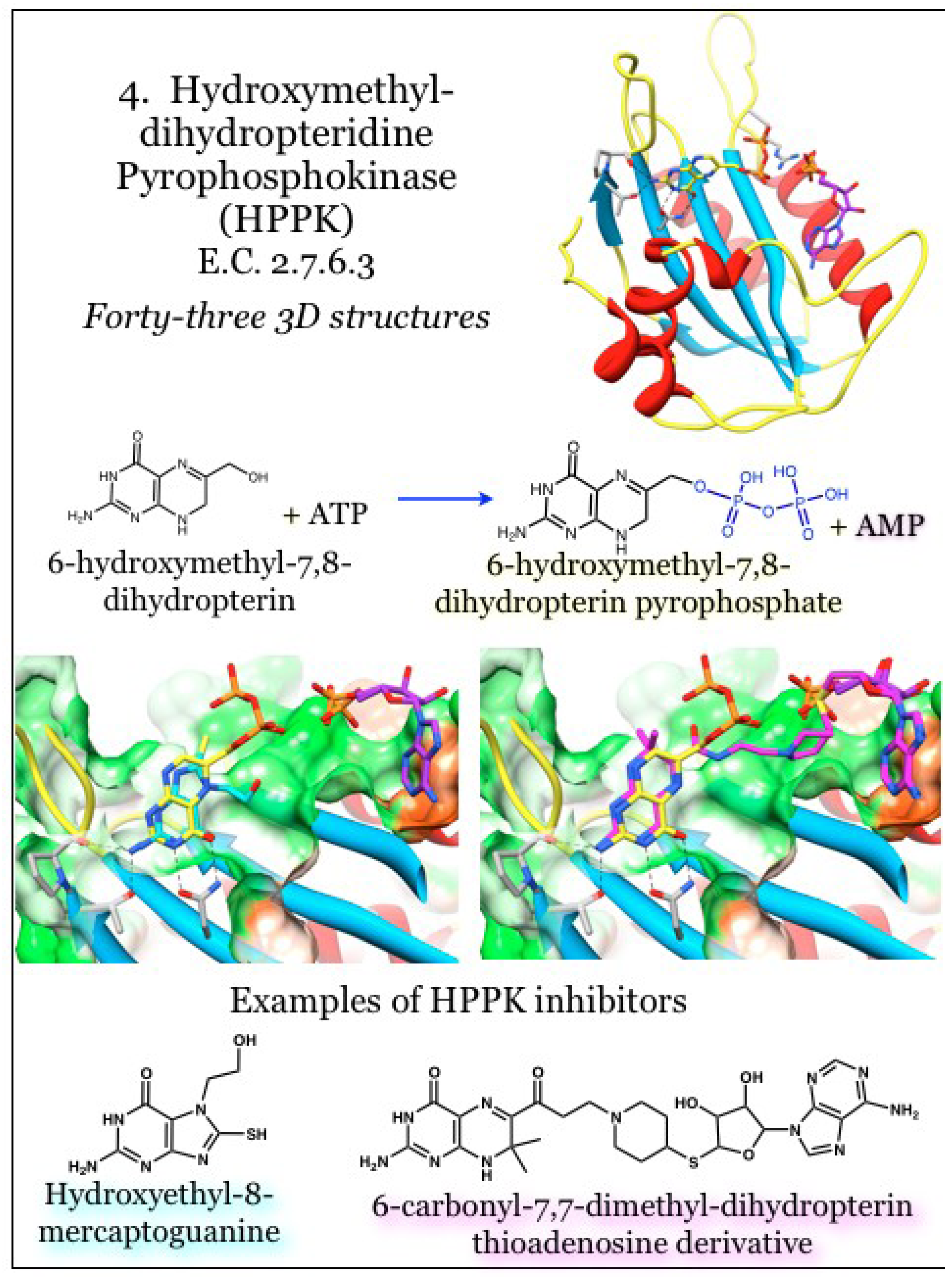
2.4. Enzymes for the Biosynthesis of Folate: 4. 2-Amino-4-hydroxy-6-hydroxymethyldihydropteridine pyrophosphokinase (HPPK)
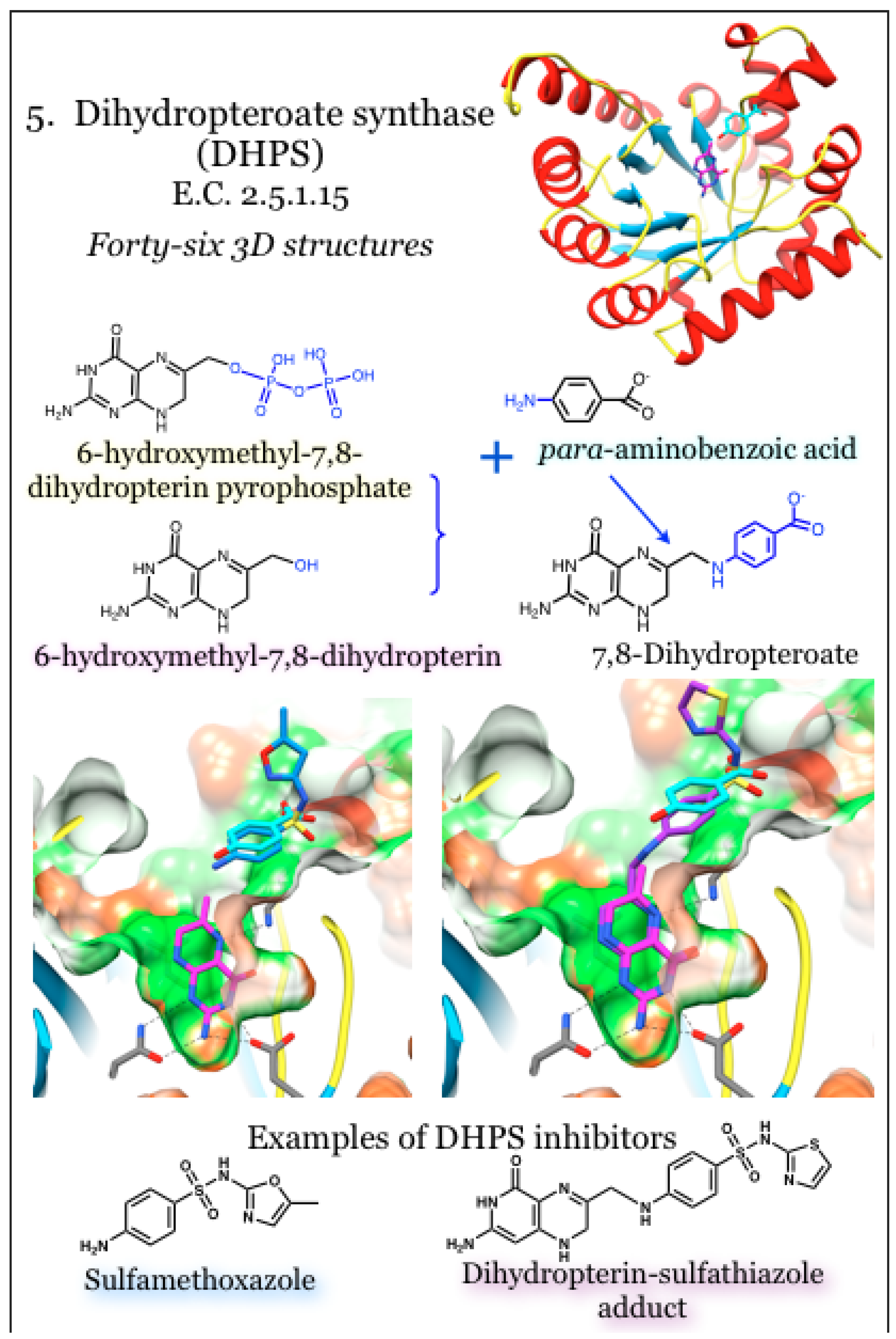
2.5. Enzymes for the Biosynthesis of Folate: 5. Dihydropteroate Synthase (DHPS)
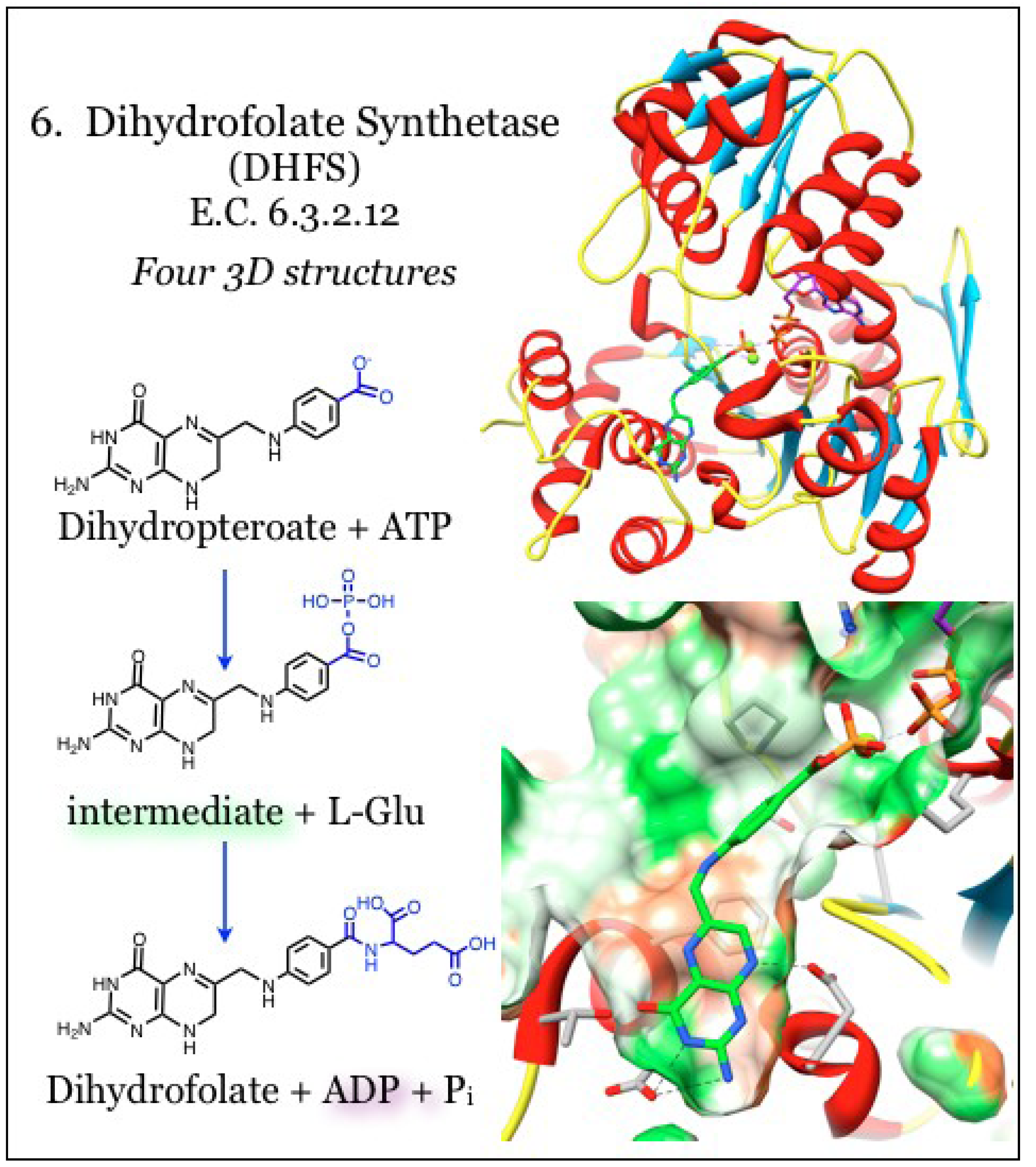
2.6. Enzymes for the Biosynthesis of Folate: 6. Dihydrofolate Synthetase (DHFS)
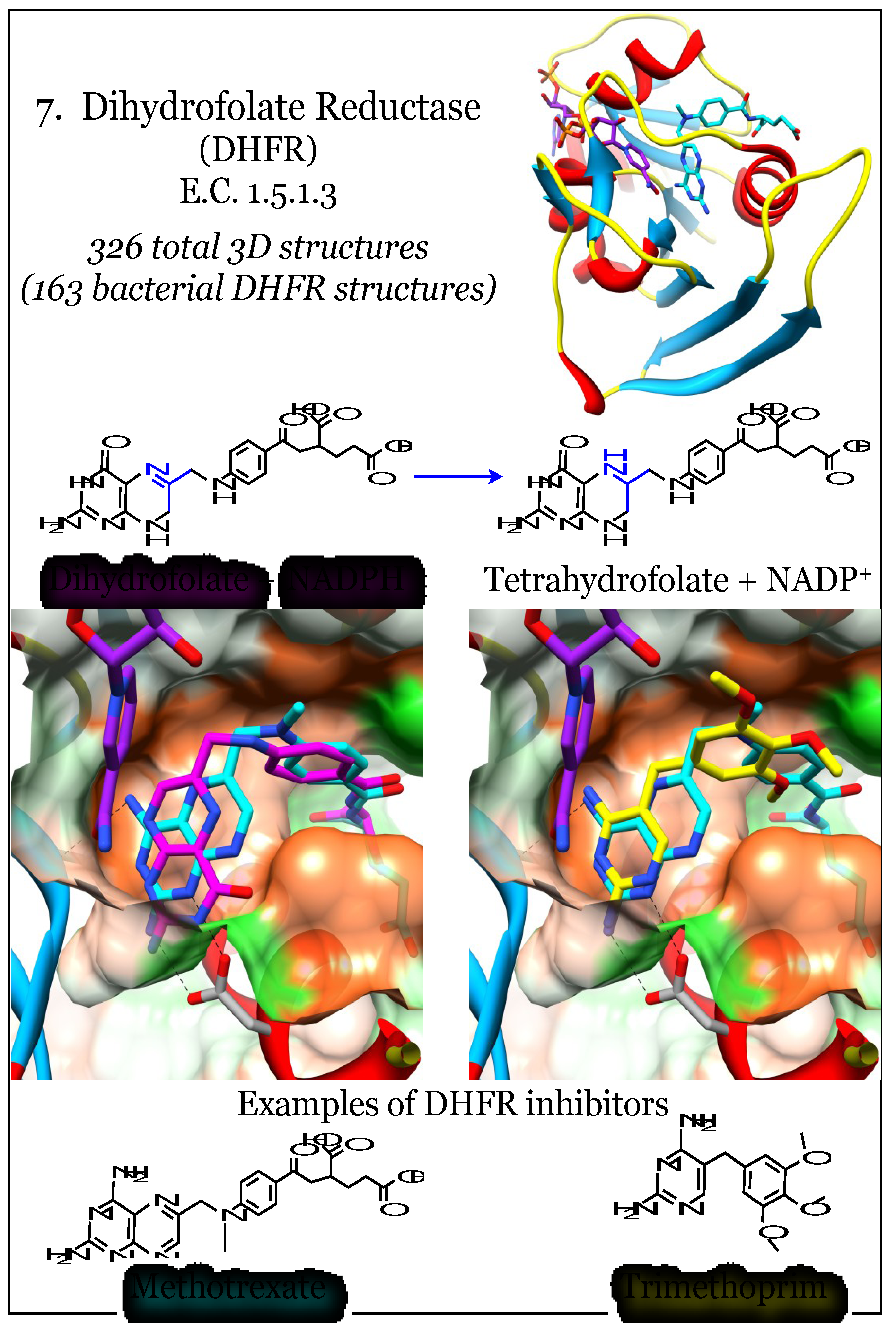
2.7. Enzymes for the Biosynthesis of Folate: 7. Dihydrofolate Reductase (DHFR)
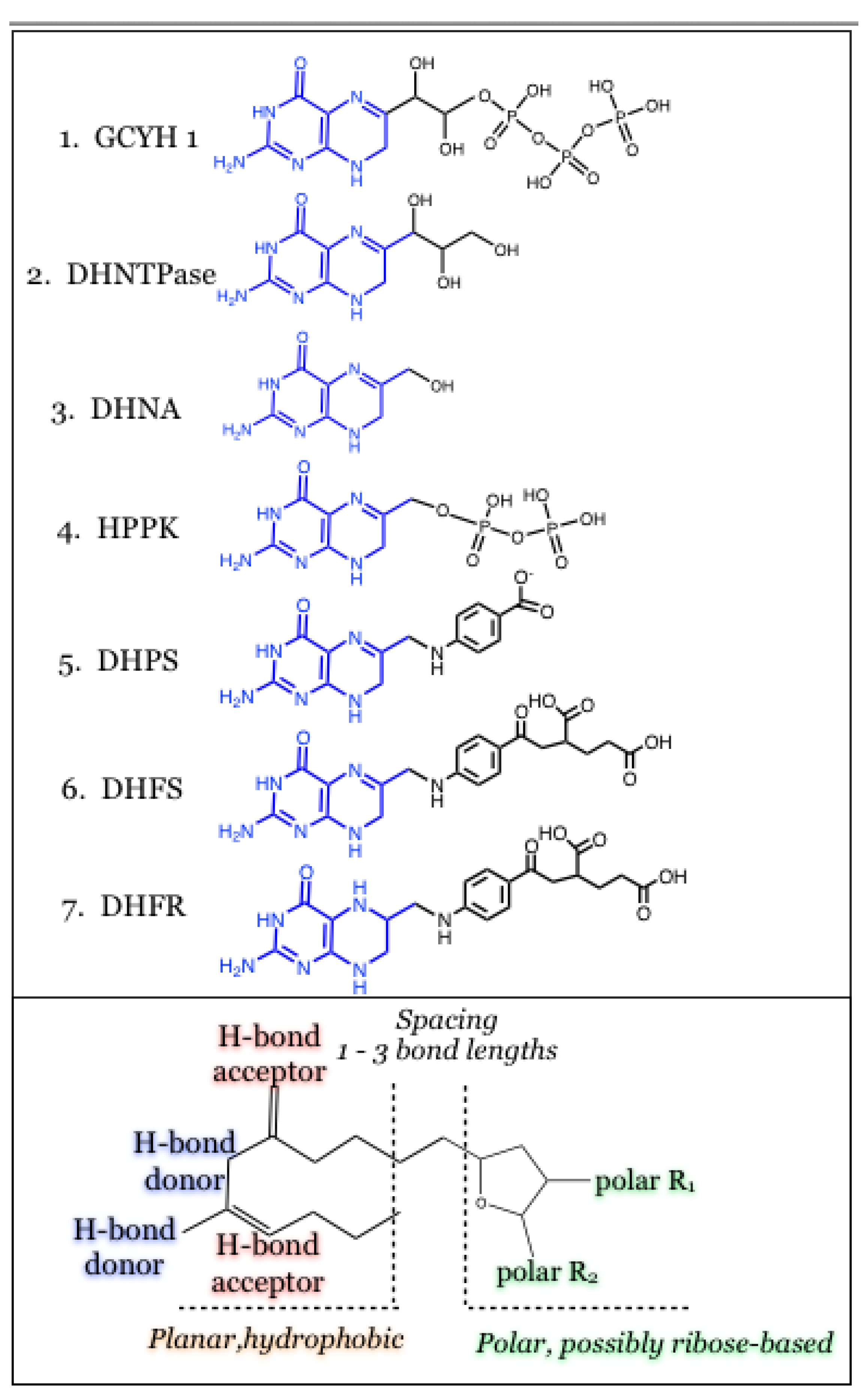
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Coates, A.R.; Hu, Y. Novel approaches to developing new antibiotics for bacterial infections. Br. J. Pharmacol. 2007, 152, 1147–1154. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for emerging pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef]
- Mills, S.D. When will the genomics investment pay off for antibacterial discovery? Biochem. Pharmacol. 2006, 71, 1096–1102. [Google Scholar] [CrossRef]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Torres, C. Up against the wall. Nat. Med. 2010, 16, 628–631. [Google Scholar] [CrossRef]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef]
- Munos, B. Lessons from 60 years of pharmaceutical innovation. Nat. Rev. Drug Discov. 2009, 8, 959–968. [Google Scholar] [CrossRef]
- Imming, P.; Sinning, C.; Meyer, A. Drugs, their targets and the nature and number of drug targets. Nat. Rev. Drug Discov. 2006, 5, 821–834. [Google Scholar] [CrossRef]
- Peters, J.U.; Schnider, P.; Mattei, P.; Kansy, M. Pharmacological promiscuity: Dependence on compound properties and target specificity in a set of recent Roche compounds. ChemMedChem 2009, 4, 680–686. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Mason, J.S.; Overington, J.P. Can we rationally design promiscuous drugs? Curr. Opin. Struct. Biol. 2006, 16, 127–136. [Google Scholar] [CrossRef]
- Hamad, B. The antibiotics market. Nat. Rev. Drug Discov. 2010, 9, 675–676. [Google Scholar] [CrossRef]
- Pathania, R.; Brown, E.D. Small and lethal: Searching for new antibacterial compounds with novel modes of action. Biochem. Cell. Biol. 2008, 86, 111–115. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, J.; Estiu, G.; Isin, B.; Ahn, Y.Y.; Lee, D.S.; Barabasi, A.L.; Kapatral, V.; Wiest, O.; Oltvai, Z.N. Blueprint for antimicrobial hit discovery targeting metabolic networks. Proc. Natl. Acad. Sci. USA 2010, 107, 1082–1087. [Google Scholar] [CrossRef]
- Hu, Y.; Wassermann, A.M.; Lounkine, E.; Bajorath, J. Systematic analysis of public domain compound potency data identifies selective molecular scaffolds across druggable target families. J. Med. Chem. 2010, 53, 752–758. [Google Scholar] [CrossRef]
- Keiser, M.J.; Setola, V.; Irwin, J.J.; Laggner, C.; Abbas, A.I.; Hufeisen, S.J.; Jensen, N.H.; Kuijer, M.B.; Matos, R.C.; Tran, T.B.; et al. Predicting new molecular targets for known drugs. Nature 2009, 462, 175–181. [Google Scholar] [CrossRef]
- Swamidass, S.J. Mining small-molecule screens to repurpose drugs. Brief. Bioinform. 2011, 12, 327–335. [Google Scholar] [CrossRef]
- Yim, G.; Wang, H.H.; Davies, J. Antibiotics as signalling molecules. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 1195–1200. [Google Scholar] [CrossRef]
- Dantas, G.; Sommer, M.O.; Oluwasegun, R.D.; Church, G.M. Bacteria subsisting on antibiotics. Science 2008, 320, 100–103. [Google Scholar] [CrossRef]
- Wei, J.R.; Krishnamoorthy, V.; Murphy, K.; Kim, J.H.; Schnappinger, D.; Alber, T.; Sassetti, C.M.; Rhee, K.Y.; Rubin, E.J. Depletion of antibiotic targets has widely varying effects on growth. Proc. Natl. Acad. Sci. USA 2011, 108, 4176–4181. [Google Scholar] [CrossRef]
- Adams, J.C.; Keiser, M.J.; Basuino, L.; Chambers, H.F.; Lee, D.S.; Wiest, O.G.; Babbitt, P.C. A mapping of drug space from the viewpoint of small molecule metabolism. PLoS Comput. Biol. 2009, 5, e1000474. [Google Scholar] [CrossRef]
- Kinnings, S.L.; Xie, L.; Fung, K.H.; Jackson, R.M.; Bourne, P.E. The Mycobacterium tuberculosis drugome and its polypharmacological implications. PLoS Comput. Biol. 2010, 6, e1000976. [Google Scholar] [CrossRef]
- Maggiora, G.M. Is there a future for computational chemistry in drug research? J. Comput. Aided Mol. Des. 2011. [Google Scholar] [CrossRef]
- Phelan, V.V.; Liu, W.T.; Pogliano, K.; Dorrestein, P.C. Microbial metabolic exchange—The chemotype-to-phenotype link. Nat. Chem. Biol. 2012, 8, 26–35. [Google Scholar]
- Keiser, M.J.; Irwin, J.J.; Shoichet, B.K. The chemical basis of pharmacology. Biochemistry 2010, 49, 10267–10276. [Google Scholar] [CrossRef]
- Bermingham, A.; Derrick, J.P. The folic acid biosynthesis pathway in bacteria: Evaluation of potential for antibacterial drug discovery. Bioessays 2002, 24, 637–648. [Google Scholar] [CrossRef]
- Cody, V.; Schwalbe, C.H. Structural characteristics of antifolate dihydrofolate reductase enzyme interactions. Crystallogr. Rev. 2006, 12, 301–333. [Google Scholar] [CrossRef]
- Hyde, J.E. Targeting purine and pyrimidine metabolism in human apicomplexan parasites. Curr. Drug Targets 2007, 8, 31–47. [Google Scholar] [CrossRef]
- Jabrin, S.; Ravanel, S.; Gambonnet, B.; Douce, R.; Rebeille, F. One-carbon metabolism in plants. Regulation of tetrahydrofolate synthesis during germination and seedling development. Plant. Physiol. 2003, 131, 1431–1439. [Google Scholar] [CrossRef]
- Nzila, A. Inhibitors of de novo folate enzymes in Plasmodium falciparum. Drug Discov. Today 2006, 11, 939–944. [Google Scholar] [CrossRef]
- Van Hoek, A.H.; Mevius, D.; Guerra, B.; Mullany, P.; Roberts, A.P.; Aarts, H.J. Acquired antibiotic resistance genes: An overview. Front. Microbiol. 2011, 2, e203. [Google Scholar]
- Volpato, J.P.; Pelletier, J.N. Mutational “hot-spots’” in mammalian, bacterial and protozoal dihydrofolate reductases associated with antifolate resistance: Sequence and structural comparison. Drug Resist. Updat. 2009, 12, 28–41. [Google Scholar] [CrossRef]
- Wright, D.L.; Anderson, A.C. Antifolate agents: A patent review (2006–2010). Expert Opin. Ther. Pat. 2011, 21, 1293–1308. [Google Scholar] [CrossRef]
- Keiser, M.J.; Roth, B.L.; Armbruster, B.N.; Ernsberger, P.; Irwin, J.J.; Shoichet, B.K. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 2007, 25, 197–206. [Google Scholar] [CrossRef]
- Brown, J.B.; Okuno, Y. Systems biology and systems chemistry: New directions for drug discovery. Chem. Biol. 2012, 19, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Paolini, G.V.; Shapland, R.H.; van Hoorn, W.P.; Mason, J.S.; Hopkins, A.L. Global mapping of pharmacological space. Nat. Biotechnol. 2006, 24, 805–815. [Google Scholar] [CrossRef]
- Schreiber, S.L. Small molecules: The missing link in the central dogma. Nat. Chem. Biol. 2005, 1, 64–66. [Google Scholar] [CrossRef]
- Li, C.; Xu, L.; Wolan, D.W.; Wilson, I.A.; Olson, A.J. Virtual screening of human 5-aminoimidazole-4-carboxamide ribonucleotide transformylase against the NCI diversity set by use of AutoDock to identify novel nonfolate inhibitors. J. Med. Chem. 2004, 47, 6681–6690. [Google Scholar] [CrossRef]
- Baggott, J.E.; Morgan, S.L.; Ha, T.; Vaughn, W.H.; Hine, R.J. Inhibition of folate-dependent enzymes by non-steroidal anti-inflammatory drugs. Biochem. J. 1992, 282, 197–202. [Google Scholar]
- Cheong, C.G.; Wolan, D.W.; Greasley, S.E.; Horton, P.A.; Beardsley, G.P.; Wilson, I.A. Crystal structures of human bifunctional enzyme aminoimidazole-4-carboxamide ribonucleotide transformylase/IMP cyclohydrolase in complex with potent sulfonyl-containing antifolates. J. Biol. Chem. 2004, 279, 18034–18045. [Google Scholar]
- Babaoglu, K.; Qi, J.; Lee, R.E.; White, S.W. Crystal structure of 7,8-dihydropteroate synthase from Bacillus anthracis; Mechanism and novel inhibitor design. Structure 2004, 12, 1705–1717. [Google Scholar] [CrossRef]
- Shi, G.; Blaszczyk, J.; Ji, X.; Yan, H. Bisubstrate analogue inhibitors of 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase: Synthesis and biochemical and crystallographic studies. J. Med. Chem. 2001, 44, 1364–1371. [Google Scholar] [CrossRef]
- Shi, G.; Shaw, G.; Li, Y.; Wu, Y.; Yan, H.; Ji, X. Bisubstrate analog inhibitors of 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase: New lead exhibits a distinct binding mode. Bioorg. Med. Chem. 2012, 20, 4303–4309. [Google Scholar] [CrossRef]
- Bennett, B.C.; Wan, Q.; Ahmad, M.F.; Langan, P.; Dealwis, C.G. X-ray structure of the ternary MTX-NADPH complex of the anthrax dihydrofolate reductase: A pharmacophore for dual-site inhibitor design. J. Struct. Biol. 2009, 166, 162–171. [Google Scholar] [CrossRef]
- Pemble, C.W.T.; Mehta, P.K.; Mehra, S.; Li, Z.; Nourse, A.; Lee, R.E.; White, S.W. Crystal structure of the 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase*dihydropteroate synthase bifunctional enzyme from Francisella tularensis. PLoS One 2010, 5, e14165. [Google Scholar]
- Chan, D.C.; Anderson, A.C. Towards species-specific antifolates. Curr. Med. Chem. 2006, 13, 377–398. [Google Scholar] [CrossRef]
- De Crecy-Lagard, V.; El Yacoubi, B.; de la Garza, R.D.; Noiriel, A.; Hanson, A.D. Comparative genomics of bacterial and plant folate synthesis and salvage: Predictions and validations. BMC Genomics 2007, 8, e245. [Google Scholar] [CrossRef]
- Levin, I.; Giladi, M.; Altman-Price, N.; Ortenberg, R.; Mevarech, M. An alternative pathway for reduced folate biosynthesis in bacteria and halophilic archaea. Mol. Microbiol. 2004, 54, 1307–1318. [Google Scholar] [CrossRef]
- Schwalbe, C.H.; Cody, V. Structural characteristics of small-molecule antifolate compounds. Crystallogr. Rev. 2006, 12, 267–300. [Google Scholar] [CrossRef]
- Swarbrick, J.; Iliades, P.; Simpson, J.S.; Macreadie, I. Folate biosynthesis-Reappraisal of old and novel targets in the search for new antimicrobials. Open Enzyme Inhib. J. 2008, 1, 12–33. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Young, P.G.; Smith, C.A.; Metcalf, P.; Baker, E.N. Structures of Mycobacterium tuberculosis folylpolyglutamate synthase complexed with ADP and AMPPCP. Acta Crystallogr. D Biol. Crystallogr. 2008, D64, 745–753. [Google Scholar]
- Haruki, H.; Pedersen, M.G.; Gorska, K.I.; Pojer, F.; Johnsson, K. Tetrahydrobiopterin biosynthesis as an off-target of sulfa drugs. Science 2013, 340, 987–991. [Google Scholar] [CrossRef]
- Ichinose, H.; Ohye, T.; Matsuda, Y.; Hori, T.; Blau, N.; Burlina, A.; Rouse, B.; Matalon, R.; Fujita, K.; Nagatsu, T. Characterization of mouse and human GTP cyclohydrolase I genes. Mutations in patients with GTP cyclohydrolase I deficiency. J. Biol. Chem. 1995, 270, 10062–10071. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakagawa, N.; Kuramitsu, S.; Yokoyama, S.; Masui, R. Novel reaction mechanism of GTP cyclohydrolase I. High-resolution X-ray crystallography of Thermus thermophilus HB8 enzyme complexed with a transition state analogue, the 8-oxoguanine derivative. J. Biochem. 2005, 138, 263–275. [Google Scholar] [CrossRef]
- Cheng, W.C.; Chen, Y.F.; Wang, H.J.; Hsu, K.C.; Lin, S.C.; Chen, T.J.; Yang, J.M.; Wang, W.C. Structures of Helicobacter pylori shikimate kinase reveal a selective inhibitor-induced-fit mechanism. PLoS One 2012, 7, e33481. [Google Scholar]
- Ducati, R.G.; Basso, L.A.; Santos, D.S. Mycobacterial shikimate pathway enzymes as targets for drug design. Curr. Drug Targets 2007, 8, 423–435. [Google Scholar] [CrossRef]
- Funke, T.; Han, H.; Healy-Fried, M.L.; Fischer, M.; Schonbrunn, E. Molecular basis for the herbicide resistance of Roundup Ready crops. Proc. Natl. Acad. Sci. USA 2006, 103, 13010–13015. [Google Scholar]
- Pitchandi, P.; Hopper, W.; Rao, R. Comprehensive database of Chorismate synthase enzyme from shikimate pathway in pathogenic bacteria. BMC Pharmacol. Toxicol. 2013, 14, e29. [Google Scholar] [CrossRef]
- Tapas, S.; Kumar, A.; Dhindwal, S.; Preeti; Kumar, P. Structural analysis of chorismate synthase from Plasmodium falciparum: A novel target for antimalaria drug discovery. Int. J. Biol. Macromol. 2011, 49, 767–777. [Google Scholar] [CrossRef]
- Christensen, K.E.; MacKenzie, R.E. Mitochondrial one-carbon metabolism is adapted to the specific needs of yeast, plants and mammals. Bioessays 2006, 28, 595–605. [Google Scholar] [CrossRef]
- Rebelo, J.; Auerbach, G.; Bader, G.; Bracher, A.; Nar, H.; Hosl, C.; Schramek, N.; Kaiser, J.; Bacher, A.; Huber, R.; et al. Biosynthesis of pteridines. Reaction mechanism of GTP cyclohydrolase I. J. Mol. Biol. 2003, 326, 503–516. [Google Scholar] [CrossRef]
- Nar, H.; Huber, R.; Meining, W.; Schmid, C.; Weinkauf, S.; Bacher, A. Atomic structure of GTP cyclohydrolase I. Structure 1995, 3, 459–466. [Google Scholar] [CrossRef]
- Maita, N.; Okada, K.; Hatakeyama, K.; Hakoshima, T. Crystal structure of the stimulatory complex of GTP cyclohydrolase I and its feedback regulatory protein GFRP. Proc. Natl. Acad. Sci. USA 2002, 99, 1212–1217. [Google Scholar] [CrossRef]
- Auerbach, G.; Herrmann, A.; Bracher, A.; Bader, G.; Gutlich, M.; Fischer, M.; Neukamm, M.; Garrido-Franco, M.; Richardson, J.; Nar, H.; et al. Zinc plays a key role in human and bacterial GTP cyclohydrolase I. Proc. Natl. Acad. Sci. USA 2000, 97, 13567–13572. [Google Scholar] [CrossRef]
- El Yacoubi, B.; Bonnett, S.; Anderson, J.N.; Swairjo, M.A.; Iwata-Reuyl, D.; de Crecy-Lagard, V. Discovery of a new prokaryotic type I GTP cyclohydrolase family. J. Biol. Chem. 2006, 281, 37586–37593. [Google Scholar] [CrossRef]
- Sankaran, B.; Bonnett, S.A.; Shah, K.; Gabriel, S.; Reddy, R.; Schimmel, P.; Rodionov, D.A.; de Crecy-Lagard, V.; Helmann, J.D.; Iwata-Reuyl, D.; et al. Zinc-independent folate biosynthesis: Genetic, biochemical, and structural investigations reveal new metal dependence for GTP cyclohydrolase IB. J. Bacteriol. 2009, 191, 6936–6949. [Google Scholar] [CrossRef]
- Maltseva, N.; Kim, Y.; Kwon, K.; Anderson, W.F.; Joachimiak, A. Crystal structure of GTP cyclohydrolase I from Yersinia pestis complexed with GTP. Available online: http://rcsb.org/pdb/explore/explore.do?structureId=4DU6/ (accessed on 26 October 2013).
- Gabelli, S.B.; Bianchet, M.A.; Xu, W.; Dunn, C.A.; Niu, Z.D.; Amzel, L.M.; Bessman, M.J. Structure and function of the E. coli dihydroneopterin triphosphate pyrophosphatase: A Nudix enzyme involved in folate biosynthesis. Structure 2007, 15, 1014–1022. [Google Scholar] [CrossRef]
- Blaszczyk, J.; Li, Y.; Gan, J.; Yan, H.; Ji, X. Structural basis for the aldolase and epimerase activities of Staphylococcus aureus dihydroneopterin aldolase. J. Mol. Biol. 2007, 368, 161–169. [Google Scholar] [CrossRef]
- Goulding, C.W.; Apostol, M.I.; Sawaya, M.R.; Phillips, M.; Parseghian, A.; Eisenberg, D. Regulation by oligomerization in a mycobacterial folate biosynthetic enzyme. J. Mol. Biol. 2005, 349, 61–72. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Yan, H. Mechanism of dihydroneopterin aldolase: Functional roles of the conserved active site glutamate and lysine residues. Biochemistry 2006, 45, 15232–15239. [Google Scholar] [CrossRef]
- Garcon, A.; Levy, C.; Derrick, J.P. Crystal structure of the bifunctional dihydroneopterin aldolase/6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase from Streptococcus pneumoniae. J. Mol. Biol. 2006, 360, 644–653. [Google Scholar] [CrossRef]
- Lopez, P.; Lacks, S.A. A bifunctional protein in the folate biosynthetic pathway of Streptococcus pneumoniae with dihydroneopterin aldolase and hydroxymethyldihydropterin pyrophosphokinase activities. J. Bacteriol. 1993, 175, 2214–2220. [Google Scholar]
- Volpe, F.; Ballantine, S.P.; Delves, C.J. Two domains with amino-acid sequence similarity are required for dihydroneopterin aldolase function in the multifunctional folic acid synthesis Fas protein of Pneumocystis carinii. Gene 1995, 160, 41–46. [Google Scholar] [CrossRef]
- Sanders, W.J.; Nienaber, V.L.; Lerner, C.G.; McCall, J.O.; Merrick, S.M.; Swanson, S.J.; Harlan, J.E.; Stoll, V.S.; Stamper, G.F.; Betz, S.F.; et al. Discovery of potent inhibitors of dihydroneopterin aldolase using CrystaLEAD high-throughput X-ray crystallographic screening and structure-directed lead optimization. J. Med. Chem. 2004, 47, 1709–1718. [Google Scholar] [CrossRef]
- Bauer, S.; Schott, A.K.; Illarionova, V.; Bacher, A.; Huber, R.; Fischer, M. Biosynthesis of tetrahydrofolate in plants: Crystal structure of 7,8-dihydroneopterin aldolase from Arabidopsis thaliana reveals a novel adolase class. J. Mol. Biol. 2004, 339, 967–979. [Google Scholar] [CrossRef]
- Pribat, A.; Jeanguenin, L.; Lara-Nunez, A.; Ziemak, M.J.; Hyde, J.E.; de Crecy-Lagard, V.; Hanson, A.D. 6-pyruvoyltetrahydropterin synthase paralogs replace the folate synthesis enzyme dihydroneopterin aldolase in diverse bacteria. J. Bacteriol. 2009, 191, 4158–4165. [Google Scholar] [CrossRef]
- Dittrich, S.; Mitchell, S.L.; Blagborough, A.M.; Wang, Q.; Wang, P.; Sims, P.F.; Hyde, J.E. An atypical orthologue of 6-pyruvoyltetrahydropterin synthase can provide the missing link in the folate biosynthesis pathway of malaria parasites. Mol. Microbiol. 2008, 67, 609–618. [Google Scholar] [CrossRef]
- Hyde, J.E.; Dittrich, S.; Wang, P.; Sims, P.F.; de Crecy-Lagard, V.; Hanson, A.D. Plasmodium falciparum: A paradigm for alternative folate biosynthesis in diverse microorganisms? Trends Parasitol. 2008, 24, 502–508. [Google Scholar] [CrossRef]
- Cicmil, N. Crystallization and preliminary X-ray crystallographic characterization of TrmFO, a folate-dependent tRNA methyltransferase from Thermotoga maritima. Acta Crystallogr. D Biol. Crystallogr. 2008, 64, 193–195. [Google Scholar]
- Derrick, J.P. The structure and mechanism of 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase. Vitam. Horm. 2008, 79, 411–433. [Google Scholar]
- Shi, G.; Shaw, G.; Liang, Y.H.; Subburaman, P.; Li, Y.; Wu, Y.; Yan, H.; Ji, X. Bisubstrate analogue inhibitors of 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase: New design with improved properties. Bioorg. Med. Chem. 2012, 20, 47–57. [Google Scholar] [CrossRef]
- Stammers, D.K.; Achari, A.; Somers, D.O.; Bryant, P.K.; Rosemond, J.; Scott, D.L.; Champness, J.N. A X-ray structure of the ternary complex of 7,8-dihydro-6-hydroxymethylpterinpyrophosphokinase from Escherichia coli with ATP and a substrate analogue. FEBS Lett. 1999, 456, 49–53. [Google Scholar] [CrossRef]
- Chhabra, S.; Barlow, N.; Dolezal, O.; Hattarki, M.K.; Newman, J.; Peat, T.S.; Graham, B.; Swarbrick, J.D. Exploring the chemical space around 8-mercaptoguanine as a route to new inhibitors of the folate biosynthesis enzyme HPPK. PLoS One 2013, 8, e59535. [Google Scholar]
- Chhabra, S.; Dolezal, O.; Collins, B.M.; Newman, J.; Simpson, J.S.; Macreadie, I.G.; Fernley, R.; Peat, T.S.; Swarbrick, J.D. Structure of S. aureus HPPK and the discovery of a new substrate site inhibitor. PLoS One 2012, 7, e29444. [Google Scholar]
- Garcon, A.; Bermingham, A.; Lian, L.Y.; Derrick, J.P. Kinetic and structural characterization of a product complex of 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase from Escherichia coli. Biochem. J. 2004, 380, 867–873. [Google Scholar] [CrossRef]
- Xiao, B.; Shi, G.; Gao, J.; Blaszczyk, J.; Liu, Q.; Ji, X.; Yan, H. Unusual conformational changes in 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase as revealed by X-ray crystallography and NMR. J. Biol. Chem. 2001, 276, 40274–40281. [Google Scholar]
- Xiao, B.; Shi, G.; Chen, X.; Yan, H.; Ji, X. Crystal structure of 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase, a potential target for the development of novel antimicrobial agents. Structure 1999, 7, 489–496. [Google Scholar] [CrossRef]
- Blaszczyk, J.; Li, Y.; Cherry, S.; Alexandratos, J.; Wu, Y.; Shaw, G.; Tropea, J.E.; Waugh, D.S.; Yan, H.; Ji, X. Structure and activity of Yersinia pestis 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase as a novel target for the development of antiplague therapeutics. Acta Crystallogr. D Biol. Crystallogr. 2007, 63, 1169–1177. [Google Scholar] [CrossRef]
- Blaszczyk, J.; Shi, G.; Li, Y.; Yan, H.; Ji, X. Reaction trajectory of pyrophosphoryl transfer catalyzed by 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase. Structure 2004, 12, 467–475. [Google Scholar] [CrossRef]
- Blaszczyk, J.; Li, Y.; Wu, Y.; Shi, G.; Ji, X.; Yan, H. Essential roles of a dynamic loop in the catalysis of 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase. Biochemistry 2004, 43, 1469–1477. [Google Scholar] [CrossRef]
- Blaszczyk, J.; Shi, G.; Yan, H.; Ji, X. Catalytic center assembly of HPPK as revealed by the crystal structure of a ternary complex at 1.25 A resolution. Structure 2000, 8, 1049–1058. [Google Scholar] [CrossRef]
- Chhabra, S.; Peat, T.S.; Swarbrick, J. Synthesis and SAR study of guanine based analogues for HPPK inhibitors. Available online: http://www.rcsb.org/pdb/explore/explore.do?pdbId=4ad6/ (accessed on 27 October 2013).
- Hevener, K.E.; Yun, M.K.; Qi, J.; Kerr, I.D.; Babaoglu, K.; Hurdle, J.G.; Balakrishna, K.; White, S.W.; Lee, R.E. Structural studies of pterin-based inhibitors of dihydropteroate synthase. J. Med. Chem. 2010, 53, 166–177. [Google Scholar] [CrossRef]
- Lawrence, M.C.; Iliades, P.; Fernley, R.T.; Berglez, J.; Pilling, P.A.; Macreadie, I.G. The three-dimensional structure of the bifunctional 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase/dihydropteroate synthase of Saccharomyces cerevisiae. J. Mol. Biol. 2005, 348, 655–670. [Google Scholar] [CrossRef]
- Morgan, R.E.; Batot, G.O.; Dement, J.M.; Rao, V.A.; Eadsforth, T.C.; Hunter, W.N. Crystal structures of Burkholderia cenocepacia dihydropteroate synthase in the apo-form and complexed with the product 7,8-dihydropteroate. BMC Struct. Biol. 2011, 11, e21. [Google Scholar] [CrossRef]
- Levy, C.; Minnis, D.; Derrick, J.P. Dihydropteroate synthase from Streptococcus pneumoniae: Structure, ligand recognition and mechanism of sulfonamide resistance. Biochem. J. 2008, 412, 379–388. [Google Scholar] [CrossRef]
- Hampele, I.C.; D’Arcy, A.; Dale, G.E.; Kostrewa, D.; Nielsen, J.; Oefner, C.; Page, M.G.; Schonfeld, H.J.; Stuber, D.; Then, R.L. Structure and function of the dihydropteroate synthase from Staphylococcus aureus. J. Mol. Biol. 1997, 268, 21–30. [Google Scholar] [CrossRef]
- Yun, M.K.; Wu, Y.; Li, Z.; Zhao, Y.; Waddell, M.B.; Ferreira, A.M.; Lee, R.E.; Bashford, D.; White, S.W. Catalysis and sulfa drug resistance in dihydropteroate synthase. Science 2012, 335, 1110–1114. [Google Scholar] [CrossRef]
- Roland, S.; Ferone, R.; Harvey, R.J.; Styles, V.L.; Morrison, R.W. The characteristics and significance of sulfonamides as substrates for Escherichia coli dihydropteroate synthase. J. Biol. Chem. 1979, 254, 10337–10345. [Google Scholar]
- Lever, O.W.; Hyman, C.; Ray, P.H.; Ferone, R.; Kelsey, J.E. A galactoside derivative of a nitrosoisocytosine inhibitor of dihydropteroate synthase: Synthesis and biological evaluation. J. Heterocycl. Chem. 1986, 23, 629–631. [Google Scholar] [CrossRef]
- Valderas, M.W.; Bourne, P.C.; Barrow, W.W. Genetic basis for sulfonamide resistance in Bacillus anthracis. Microb. Drug Resist. 2007, 13, 12–21. [Google Scholar]
- Chakraborty, S.; Gruber, T.; Barry, C.E., 3rd; Boshoff, H.I.; Rhee, K.Y. Para-aminosalicylic acid acts as an alternative substrate of folate metabolism in Mycobacterium tuberculosis. Science 2013, 339, 88–91. [Google Scholar] [CrossRef]
- De Beer, T.A.; Louw, A.I.; Joubert, F. Elucidation of sulfadoxine resistance with structural models of the bifunctional Plasmodium falciparum dihydropterin pyrophosphokinase-dihydropteroate synthase. Bioorg. Med. Chem. 2006, 14, 4433–4443. [Google Scholar] [CrossRef]
- Baca, A.M.; Sirawaraporn, R.; Turley, S.; Sirawaraporn, W.; Hol, W.G. Crystal structure of Mycobacterium tuberculosis 7,8-dihydropteroate synthase in complex with pterin monophosphate: New insight into the enzymatic mechanism and sulfa-drug action. J. Mol. Biol. 2000, 302, 1193–1212. [Google Scholar] [CrossRef]
- Mathieu, M.; Debousker, G.; Vincent, S.; Viviani, F.; Bamas-Jacques, N.; Mikol, V. Escherichia coli FolC structure reveals an unexpected dihydrofolate binding site providing an attractive target for anti-microbial therapy. J. Biol. Chem. 2005, 280, 18916–18922. [Google Scholar] [CrossRef]
- McDonald, D.; Atkinson, I.J.; Cossins, E.A.; Shane, B. Isolation of dihydrofolate and folylpolyglutamate synthetase activities from Neurospora. Phytochemistry 1995, 38, 327–333. [Google Scholar] [CrossRef]
- Shane, B. Pteroylpoly(gamma-glutamate) synthesis by Corynebacterium species. Studies on the mechanism of folypoly(gamma-glutamate) synthetase. J. Biol. Chem. 198, 255, 5663–5667. [Google Scholar]
- Fussenegger, M.; Meyer, T.F. Cloning and characterization of the Neisseria gonorrhoeae MS11 folC gene. Mol. Gen. Genet. 1996, 250, 277–285. [Google Scholar]
- Bognar, A.L.; Osborne, C.; Shane, B.; Singer, S.C.; Ferone, R. Folylpoly-gamma-glutamate synthetase-dihydrofolate synthetase. Cloning and high expression of the Escherichia coli folC gene and purification and properties of the gene product. J. Biol. Chem. 1985, 260, 5625–5630. [Google Scholar]
- Sun, X.; Cross, J.A.; Bognar, A.L.; Baker, E.N.; Smith, C.A. Folate-binding triggers the activation of folylpolyglutamate synthetase. J. Mol. Biol. 2001, 310, 1067–1078. [Google Scholar] [CrossRef]
- Smith, C.A.; Cross, J.A.; Bognar, A.L.; Sun, X. Mutation of Gly51 to serine in the P-loop of Lactobacillus casei folylpolyglutamate synthetase abolishes activity by altering the conformation of two adjacent loops. Acta Crystallogr. D Biol. Crystallogr. 2006, 62, 548–558. [Google Scholar]
- Sheng, Y.; Khanam, N.; Tsaksis, Y.; Shi, X.M.; Lu, Q.S.; Bognar, A.L. Mutagenesis of folylpolyglutamate synthetase indicates that dihydropteroate and tetrahydrofolate bind to the same site. Biochemistry 2008, 47, 2388–2396. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Q.; Yang, Y.; Coward, J.K.; Nzila, A.; Sims, P.F.; Hyde, J.E. Characterisation of the bifunctional dihydrofolate synthase-folylpolyglutamate synthase from Plasmodium falciparum; a potential novel target for antimalarial antifolate inhibition. Mol. Biochem. Parasitol. 2010, 172, 41–51. [Google Scholar] [CrossRef]
- Kwon, Y.K.; Lu, W.; Melamud, E.; Khanam, N.; Bognar, A.; Rabinowitz, J.D. A domino effect in antifolate drug action in Escherichia coli. Nat. Chem. Biol. 2008, 4, 602–608. [Google Scholar] [CrossRef]
- Banerjee, R.V.; Shane, B.; McGuire, J.J.; Coward, J.K. Dihydrofolate synthetase and folylpolyglutamate synthetase: Direct evidence for intervention of acyl phosphate intermediates. Biochemistry 1988, 27, 9062–9070. [Google Scholar] [CrossRef]
- Bystroff, C.; Oatley, S.J.; Kraut, J. Crystal structures of Escherichia coli dihydrofolate reductase: The NADP+ holoenzyme and the folate .cntdot. NADP+ ternary complex. Substrate binding and a model for the transition state. Biochemistry 1990, 29, 3263–3277. [Google Scholar] [CrossRef]
- Sawaya, M.R.; Kraut, J. Loop and subdomain movements in the mechanism of Escherichia coli dihydrofolate reductase: Crystallographic evidence. Biochemistry 1997, 36, 586–603. [Google Scholar]
- Cody, V.; Chan, D.; Galitsky, N.; Rak, D.; Luft, J.R.; Pangborn, W.; Queener, S.F.; Laughton, C.A.; Stevens, M.F. Structural studies on bioactive compounds. 30. Crystal structure and molecular modeling studies on the Pneumocystis carinii dihydrofolate reductase cofactor complex with TAB, a highly selective antifolate. Biochemistry 2000, 39, 3556–3564. [Google Scholar] [CrossRef]
- Liu, C.T.; Hanoian, P.; French, J.B.; Pringle, T.H.; Hammes-Schiffer, S.; Benkovic, S.J. Functional significance of evolving protein sequence in dihydrofolate reductase from bacteria to humans. Proc. Natl. Acad. Sci. USA 2013, 110, 10159–10164. [Google Scholar]
- Matherly, L.H.; Barlowe, C.K.; Phillips, V.M.; Goldman, I.D. The effects on 4-aminoantifolates on 5-formyltetrahydrofolate metabolism in L1210 cells. A biochemical basis of the selectivity of leucovorin rescue. J. Biol. Chem. 1987, 262, 710–717. [Google Scholar]
- Zervos, M.J.; Schaberg, D.R. Reversal of the in vitro susceptibility of enterococci to trimethoprim-sulfamethoxazole by folinic acid. Antimicrob. Agents Chemother. 1985, 28, 446–448. [Google Scholar] [CrossRef]
- Lewis, W.S.; Cody, V.; Galitsky, N.; Luft, J.R.; Pangborn, W.; Chunduru, S.K.; Spencer, H.T.; Appleman, J.R.; Blakley, R.L. Methotrexate-resistant variants of human dihydrofolate reductase with substitutions of leucine 22. Kinetics, crystallography, and potential as selectable markers. J. Biol. Chem. 1995, 270, 5057–5064. [Google Scholar] [CrossRef]
- Buffet-Bataillon, S.; le Jeune, A.; le Gall-David, S.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Molecular mechanisms of higher MICs of antibiotics and quaternary ammonium compounds for Escherichia coli isolated from bacteraemia. J. Antimicrob. Chemother. 2012, 67, 2837–2842. [Google Scholar] [CrossRef]
- Floyd, J.L.; Smith, K.P.; Kumar, S.H.; Floyd, J.T.; Varela, M.F. LmrS is a multidrug efflux pump of the major facilitator superfamily from Staphylococcus aureus. Antimicrob. Agents Chemother. 2010, 54, 5406–5412. [Google Scholar] [CrossRef]
- Kohler, T.; Kok, M.; Michea-Hamzehpour, M.; Plesiat, P.; Gotoh, N.; Nishino, T.; Curty, L.K.; Pechere, J.C. Multidrug efflux in intrinsic resistance to trimethoprim and sulfamethoxazole in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1996, 40, 2288–2290. [Google Scholar]
- Piddock, L.J.; Garvey, M.I.; Rahman, M.M.; Gibbons, S. Natural and synthetic compounds such as trimethoprim behave as inhibitors of efflux in Gram-negative bacteria. J. Antimicrob. Chemother. 2010, 65, 1215–1223. [Google Scholar] [CrossRef]
- Cody, V.; Galitsky, N.; Rak, D.; Luft, J.R.; Pangborn, W.; Queener, S.F. Ligand-induced conformational changes in the crystal structures of Pneumocystis carinii dihydrofolate reductase complexes with folate and NADP+. Biochemistry 1999, 38, 4303–4312. [Google Scholar] [CrossRef]
- Cody, V.; Pace, J. Structural analysis of Pneumocystis carinii and human DHFR complexes with NADPH and a series of five potent 6-[5'-(omega-carboxyalkoxy)benzyl]pyrido[2,3-d]pyrimidine derivatives. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 1–7. [Google Scholar] [CrossRef]
- Cody, V.; Pace, J.; Queener, S.F.; Adair, O.O.; Gangjee, A. Kinetic and structural analysis for potent antifolate inhibition of Pneumocystis jirovecii, Pneumocystis carinii, and human dihydrofolate reductases and their active-site variants. Antimicrob. Agents Chemother. 2013, 57, 2669–2677. [Google Scholar] [CrossRef]
- Gangjee, A.; Adair, O.; Queener, S.F. Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase inhibitors and antitumor agents: Synthesis and biological activities of 2,4-diamino-5-methyl-6-[(monosubstituted anilino) methyl]-pyrido [2,3-d] pyrimidines. J. Med. Chem. 1999, 42, 2447–2455. [Google Scholar] [CrossRef]
- Rosowsky, A.; Forsch, R.A.; Queener, S.F. Inhibition of Pneumocystis carinii, Toxoplasma gondii, and Mycobacterium avium dihydrofolate reductases by 2,4-diamino-5-[2-methoxy-5-(omega-carboxyalkyloxy)benzyl]pyrimidines: Marked improvement in potency relative to trimethoprim and species selectivity relative to piritrexim. J. Med. Chem. 2002, 45, 233–241. [Google Scholar] [CrossRef]
- Darrell, J.H.; Garrod, L.P.; Waterworth, P.M. Trimethoprim: Laboratory and clinical studies. J. Clin. Pathol. 1968, 21, 202–209. [Google Scholar] [CrossRef]
- Morgan, A.; Cofer, C.; Stevens, D.L. Iclaprim: A novel dihydrofolate reductase inhibitor for skin and soft tissue infections. Future Microbiol. 2009, 4, 131–144. [Google Scholar] [CrossRef]
- Schneider, P.; Hawser, S.; Islam, K. Iclaprim, a novel diaminopyrimidine with potent activity on trimethoprim sensitive and resistant bacteria. Bioorg. Med. Chem. Lett. 2003, 13, 4217–4221. [Google Scholar] [CrossRef]
- Oefner, C.; Bandera, M.; Haldimann, A.; Laue, H.; Schulz, H.; Mukhija, S.; Parisi, S.; Weiss, L.; Lociuro, S.; Dale, G.E. Increased hydrophobic interactions of iclaprim with Staphylococcus aureus dihydrofolate reductase are responsible for the increase in affinity and antibacterial activity. J. Antimicrob. Chemother. 2009, 63, 687–698. [Google Scholar] [CrossRef]
- Polshakov, V.I.; Birdsall, B.; Feeney, J. Characterization of rates of ring-flipping in trimethoprim in its ternary complexes with Lactobacillus casei dihydrofolate reductase and coenzyme analogues. Biochemistry 1999, 38, 15962–15969. [Google Scholar] [CrossRef]
- Maskell, J.P.; Sefton, A.M.; Hall, L.M. Multiple mutations modulate the function of dihydrofolate reductase in trimethoprim-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 1104–1108. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Perdikouli, M.; Beeck, A.; Verhoef, J.; Fluit, A.C. Resistance to trimethoprim-sulfamethoxazole and modifications in genes coding for dihydrofolate reductase and dihydropteroate synthase in European Streptococcus pneumoniae isolates. J. Antimicrob. Chemother. 2001, 48, 935–936. [Google Scholar] [CrossRef]
- Skold, O. Resistance to trimethoprim and sulfonamides. Vet. Res. 2001, 32, 261–273. [Google Scholar] [CrossRef]
- Barrow, E.W.; Bourne, P.C.; Barrow, W.W. Functional cloning of Bacillus anthracis DHFR and confirmation of natural resistance to trimethoprim. Antimicrob. Agents Chemother. 2004, 48, 4643–4649. [Google Scholar] [CrossRef]
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Med. 2006, 119, S3–S10. [Google Scholar] [CrossRef]
- Beierlein, J.M.; Karri, N.G.; Anderson, A.C. Targeted mutations of Bacillus anthracis dihydrofolate reductase condense complex structure-activity relationships. J. Med. Chem. 2010, 53, 7327–7336. [Google Scholar] [CrossRef]
- Bourne, C.R.; Barrow, E.W.; Bunce, R.A.; Bourne, P.C.; Berlin, K.D.; Barrow, W.W. Inhibition of antibiotic-resistant Staphylococcus aureus by the broad-spectrum dihydrofolate reductase inhibitor RAB1. Antimicrob. Agents Chemother. 2010, 54, 3825–3833. [Google Scholar] [CrossRef]
- Frey, K.M.; Lombardo, M.N.; Wright, D.L.; Anderson, A.C. Towards the understanding of resistance mechanisms in clinically isolated trimethoprim-resistant, methicillin-resistant Staphylococcus aureus dihydrofolate reductase. J. Struct. Biol. 2010, 170, 93–97. [Google Scholar] [CrossRef]
- Bourne, C.R.; Wakeham, N.; Nammalwar, B.; Tseitin, V.; Bourne, P.C.; Barrow, E.W.; Mylvaganam, S.; Ramnarayan, K.; Bunce, R.A.; Berlin, K.D.; et al. Structure-activity relationship for enantiomers of potent inhibitors of B. anthracis dihydrofolate reductase. Biochim. Biophys. Acta 2013, 1834, 46–52. [Google Scholar] [CrossRef]
- Bowden, K.; Harris, N.V.; Watson, C.A. Structure-activity relationships of dihydrofolate reductase inhibitors. J. Chemother. 1993, 5, 377–388. [Google Scholar]
- Frey, K.M.; Liu, J.; Lombardo, M.N.; Bolstad, D.B.; Wright, D.L.; Anderson, A.C. Crystal structures of wild-type and mutant methicillin-resistant Staphylococcus aureus dihydrofolate reductase reveal an alternate conformation of NADPH that may be linked to trimethoprim resistance. J. Mol. Biol. 2009, 387, 1298–1308. [Google Scholar] [CrossRef]
- Popov, V.M.; Chan, D.C.; Fillingham, Y.A.; Yee, W.A.; Wright, D.L.; Anderson, A.C. Analysis of compleses of inhibitors with Cryptosporidium hominis DHFR leads to a new trimethoprim derivative. Bioorg. Med. Chem. Lett. 2006, 16, 4366–4370. [Google Scholar] [CrossRef]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef]
- Bhullar, K.; Waglechner, N.; Pawlowski, A.; Koteva, K.; Banks, E.D.; Johnston, M.D.; Barton, H.A.; Wright, G.D. Antibiotic resistance is prevalent in an isolated cave microbiome. PLoS One 2012, 7, e34953. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bourne, C.R. Utility of the Biosynthetic Folate Pathway for Targets in Antimicrobial Discovery. Antibiotics 2014, 3, 1-28. https://doi.org/10.3390/antibiotics3010001
Bourne CR. Utility of the Biosynthetic Folate Pathway for Targets in Antimicrobial Discovery. Antibiotics. 2014; 3(1):1-28. https://doi.org/10.3390/antibiotics3010001
Chicago/Turabian StyleBourne, Christina R. 2014. "Utility of the Biosynthetic Folate Pathway for Targets in Antimicrobial Discovery" Antibiotics 3, no. 1: 1-28. https://doi.org/10.3390/antibiotics3010001
APA StyleBourne, C. R. (2014). Utility of the Biosynthetic Folate Pathway for Targets in Antimicrobial Discovery. Antibiotics, 3(1), 1-28. https://doi.org/10.3390/antibiotics3010001