Development and Validation of a Rapid LC-MS/MS Method for Quantifying Eravacycline in Epithelial Lining Fluid: Application to a Prospective Pulmonary Distribution Study in HAP/VAP Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. LC-MS/MS Method
2.3. The Preparation of Calibration Curve, Quality Control and Internal Standard
2.4. Sample Preparations
2.5. Method Validation
2.6. Calculation of Eravacycline Concentration in Lung Epithelial Linings Fluid
2.7. Clinical Applications
3. Result
3.1. Result of Method Validation
3.1.1. Assay Specificity and Linearity
3.1.2. Accuracy and Precision
3.1.3. Matrix Effect and Extraction Recovery
3.1.4. Stability
3.2. Clinical Application
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HAP | Hospital-acquired pneumonia |
| VAP | Ventilator-associated pneumonia |
| ELF | Pulmonary Epithelial lining fluid |
| PK | Pharmacokinetic |
| PK/PD | pharmacokinetic/pharmacodynamic |
| BALF | Bronchoalveolar lavage fluid |
| ICU | Intensive care units |
| MDR | Multidrug-resistant |
| CRAB | Carbapenem-resistant Acinetobacter baumannii |
| CRKP | Carbapenem-resistant Klebsiella pneumoniae |
| XDR | Extensively drug-resistant |
| CRE | Carbapenem-resistant Enterobacteriaceae |
| cIAI | Complicated intra-abdominal infections |
| TFA | Trifluoroacetic acid |
| MRM | Multiple reaction monitoring |
| NS | Normal saline |
| LLOQ | Lower limit of quantification |
| MF | Matrix factor |
| DF | Dilution factor |
| ESI | Electrospray ionization |
| BAL | Bronchoalveolar lavage |
| TDM | therapeutic drug monitoring |
| fAUC/MIC | free area under the concentration–time curve to the minimum inhibitory concentration |
| PTA | probability of target attainment |
| PPK | population pharmacokinetic |
References
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Li Bassi, G.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar] [CrossRef]
- Miron, M.; Blaj, M.; Ristescu, A.I.; Iosep, G.; Avădanei, A.N.; Iosep, D.G.; Crișan-Dabija, R.; Ciocan, A.; Perțea, M.; Manciuc, C.D.; et al. Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia: A Literature Review. Microorganisms 2024, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.R.; Kovacs, C.S. Hospital-acquired and ventilator-associated pneumonia: Diagnosis, management, and prevention. Cleve Clin. J. Med. 2020, 87, 633–639. [Google Scholar] [CrossRef]
- Zaragoza, R.; Vidal-Cortés, P.; Aguilar, G.; Borges, M.; Diaz, E.; Ferrer, R.; Maseda, E.; Nieto, M.; Nuvials, F.X.; Ramirez, P.; et al. Update of the treatment of nosocomial pneumonia in the ICU. Crit. Care 2020, 24, 383. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Nathanson, B.H.; Puzniak, L.A.; Shorr, A.F. Descriptive Epidemiology and Outcomes of Nonventilated Hospital-Acquired, Ventilated Hospital-Acquired, and Ventilator-Associated Bacterial Pneumonia in the United States, 2012–2019. Crit. Care Med. 2022, 50, 460–468. [Google Scholar] [CrossRef]
- Yin, Y.; Zhao, C.; Li, H.; Jin, L.; Wang, Q.; Wang, R.; Zhang, Y.; Zhang, J.; Wang, H. Clinical and microbiological characteristics of adults with hospital-acquired pneumonia: A 10-year prospective observational study in China. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 683–690. [Google Scholar] [CrossRef]
- Cillóniz, C.; Dominedò, C.; Torres, A. An overview of guidelines for the management of hospital-acquired and ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria. Curr. Opin. Infect. Dis. 2019, 32, 656–662. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Nathanson, B.H.; Sulham, K.; Fan, W.; Shorr, A.F. A Novel Algorithm to Analyze Epidemiology and Outcomes of Carbapenem Resistance Among Patients With Hospital-Acquired and Ventilator-Associated Pneumonia: A Retrospective Cohort Study. Chest 2019, 155, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Van Driel, M.L.; McGuire, T.M.; Zhang, T.; Dong, Y.; Liu, Y.; Liu, L.; Hao, R.; Cao, L.; et al. Network meta-analysis and pharmacoeconomic evaluation of antibiotics for the treatment of patients infected with complicated skin and soft structure infection and hospital-acquired or ventilator-associated penumonia. Antimicrob. Resist. Infect. Control 2019, 8, 72. [Google Scholar] [CrossRef]
- Ataman, M.; Çelik, B. Investigation of the in vitro antimicrobial activity of eravacycline alone and in combination with various antibiotics against MDR Acinetobacter baumanni strains. BMC Microbiol. 2025, 25, 167. [Google Scholar] [CrossRef] [PubMed]
- Van Hise, N.; Petrak, R.M.; Skorodin, N.C.; Fliegelman, R.M.; Anderson, M.; Didwania, V.; Han, A.; Shah, K.; Chundi, V.; Hines, D.; et al. A Real-World Assessment of Clinical Outcomes and Safety of Eravacycline: A Novel Fluorocycline. Infect. Dis. Ther. 2020, 9, 1017–1028. [Google Scholar] [CrossRef]
- Cruz-López, F.; Martínez-Meléndez, A.; Morfin-Otero, R.; Rodriguez-Noriega, E.; Maldonado-Garza, H.J.; Garza-González, E. Efficacy and In Vitro Activity of Novel Antibiotics for Infections With Carbapenem-Resistant Gram-Negative Pathogens. Front. Cell Infect. Microbiol. 2022, 12, 884365. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Cheung, D.; Adam, H.; Zelenitsky, S.; Golden, A.; Schweizer, F.; Gorityala, B.; Lagacé-Wiens, P.R.; Walkty, A.; Gin, A.S.; et al. Review of Eravacycline, a Novel Fluorocycline Antibacterial Agent. Drugs 2016, 76, 567–588. [Google Scholar] [CrossRef]
- Kanj, S.S.; Bassetti, M.; Kiratisin, P.; Rodrigues, C.; Villegas, M.V.; Yu, Y.; van Duin, D. Clinical data from studies involving novel antibiotics to treat multidrug-resistant Gram-negative bacterial infections. Int. J. Antimicrob. Agents 2022, 60, 106633. [Google Scholar] [CrossRef]
- Montravers, P.; Zappella, N.; Tran-Dinh, A. Eravacycline for the treatment of complicated intra-abdominal infections. Expert. Rev. Anti Infect. Ther. 2019, 17, 851–863. [Google Scholar] [CrossRef]
- Connors, K.P.; Housman, S.T.; Pope, J.S.; Russomanno, J.; Salerno, E.; Shore, E.; Redican, S.; Nicolau, D.P. Phase I, open-label, safety and pharmacokinetic study to assess bronchopulmonary disposition of intravenous eravacycline in healthy men and women. Antimicrob. Agents Chemother. 2014, 58, 2113–2118. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef]
- Drwiega, E.N.; Rodvold, K.A. Penetration of Antibacterial Agents into Pulmonary Epithelial Lining Fluid: An Update. Clin. Pharmacokinet. 2022, 61, 17–46. [Google Scholar] [CrossRef]
- Rodvold, K.A.; George, J.M.; Yoo, L. Penetration of anti-infective agents into pulmonary epithelial lining fluid: Focus on antibacterial agents. Clin. Pharmacokinet. 2011, 50, 637–664. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Davidson, K.R.; Ha, D.M.; Schwarz, M.I.; Chan, E.D. Bronchoalveolar lavage as a diagnostic procedure: A review of known cellular and molecular findings in various lung diseases. J. Thorac. Dis. 2020, 12, 4991–5019. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Dorshorst, D.W.; Cai, F.; Bremer, M.; Milanowski, D.; Staton, T.L.; Cape, S.S.; Dean, B.; Ding, X. Development of a multi-matrix LC-MS/MS method for urea quantitation and its application in human respiratory disease studies. J. Pharm. Biomed. Anal. 2017, 133, 96–104. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Chen, Y.; Guo, B.; Liu, J.; Zhang, J. Rapid quantification of polymyxin B in human pulmonary epithelial lining fluid by LC-MS/MS and its clinical application. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2024, 1247, 124332. [Google Scholar] [CrossRef]
- McCarthy, M.W. Clinical Pharmacokinetics and Pharmacodynamics of Eravacycline. Clin. Pharmacokinet. 2019, 58, 1149–1153. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Li, S.; Yu, J.; Jin, Y.; Wu, J.; Yu, S.; Shen, J.; Tang, J.; Fu, Y.; et al. Population pharmacokinetics and pharmacodynamics of eravacycline in patients with complicated intra-abdominal infections and community acquired bacterial pneumonia: Support for a fixed-dose treatment regimen. Int. J. Antimicrob. Agents 2025, 107603. [Google Scholar] [CrossRef]
- Morrissey, I.; Olesky, M.; Hawser, S.; Lob, S.H.; Karlowsky, J.A.; Corey, G.R.; Bassetti, M.; Fyfe, C. In Vitro Activity of Eravacycline against Gram-Negative Bacilli Isolated in Clinical Laboratories Worldwide from 2013 to 2017. Antimicrob. Agents Chemother. 2020, 64, e01699-19. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.Y.; Hsu, C.K.; Tang, H.J.; Lai, C.C. Eravacycline: A comprehensive review of in vitro activity, clinical efficacy, and real-world applications. Expert. Rev. Anti Infect. Ther. 2024, 22, 387–398. [Google Scholar] [CrossRef]
- Jemal, M. High-throughput quantitative bioanalysis by LC/MS/MS. Biomed. Chromatogr. 2000, 14, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.E.; Cramer, J.A.; Tse, F.L. Comparison of manual protein precipitation (PPT) versus a new small volume PPT 96-well filter plate to decrease sample preparation time. J. Pharm. Biomed. Anal. 2001, 25, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Khalikova, M.A.; Skarbalius, L.; Naplekov, D.K.; Jadeja, S.; Švec, F.; Lenčo, J. Evaluation of strategies for overcoming trifluoroacetic acid ionization suppression resulted in single-column intact level, middle-up, and bottom-up reversed-phase LC-MS analyses of antibody biopharmaceuticals. Talanta 2021, 233, 122512. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, D.; Bankir, L.; Ponte, B.; Pruijm, M.; Corre, T.; Ghobril, J.P.; Bouatou, Y.; Ackermann, D.; Vogt, B.; Bochud, M. The urine-to-plasma urea concentration ratio is associated with eGFR and eGFR decline over time in a population cohort. Nephrol. Dial. Transplant. 2023, 39, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Haeger, S.; Moore, C.M.; McManus, S.A.; Moore, P.K.; Janssen, W.J.; Mould, K.J. The bronchoalveolar lavage dilution conundrum: An updated view on a long-standing problem. Am. J. Physiol. Lung Cell Mol. Physiol. 2024, 327, L807–L813. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, C.; Zhang, Z.; Shen, H.; Li, Y.; Lu, L.; Gao, Y. Bronchoalveolar lavage fluid dilution in ICU patients: What we should know and what we should do. Crit. Care 2019, 23, 23. [Google Scholar] [CrossRef]
- Rennard, S.I.; Basset, G.; Lecossier, D.; O’Donnell, K.M.; Pinkston, P.; Martin, P.G.; Crystal, R.G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. (1985) 1986, 60, 532–538. [Google Scholar] [CrossRef]
- Marcy, T.W.; Merrill, W.W.; Rankin, J.A.; Reynolds, H.Y. Limitations of using urea to quantify epithelial lining fluid recovered by bronchoalveolar lavage. Am. Rev. Respir. Dis. 1987, 135, 1276–1280. [Google Scholar] [CrossRef]
- Galvidis, I.A.; Moshcheva, A.G.; Surovoy, Y.A.; Sobolev, P.D.; Sharipov, V.R.; Sidorov, N.G.; Tsarenko, S.V.; Burkin, M.A. Production of antibody and development of enzyme-linked immunosorbent assay for therapeutic drug monitoring of eravacycline. J. Pharm. Biomed. Anal. 2024, 242, 116033. [Google Scholar] [CrossRef]
- Onufrak, N.J.; Forrest, A.; Gonzalez, D. Pharmacokinetic and Pharmacodynamic Principles of Anti-infective Dosing. Clin. Ther. 2016, 38, 1930–1947. [Google Scholar] [CrossRef]
- Bavaro, D.F.; Belati, A.; Diella, L.; Frallonardo, L.; Guido, G.; Papagni, R.; Pellegrino, C.; Brindicci, G.; De Gennaro, N.; Di Gennaro, F.; et al. Loading dose plus continuous/extended infusion versus intermittent bolus of β-lactams for the treatment of Gram-negative bacteria bloodstream infections: A propensity score-adjusted retrospective cohort study. J. Antimicrob. Chemother. 2023, 78, 2175–2184. [Google Scholar] [CrossRef]
- Rubinstein, E.; Vaughan, D. Tigecycline: A novel glycylcycline. Drugs 2005, 65, 1317–1336. [Google Scholar] [CrossRef]
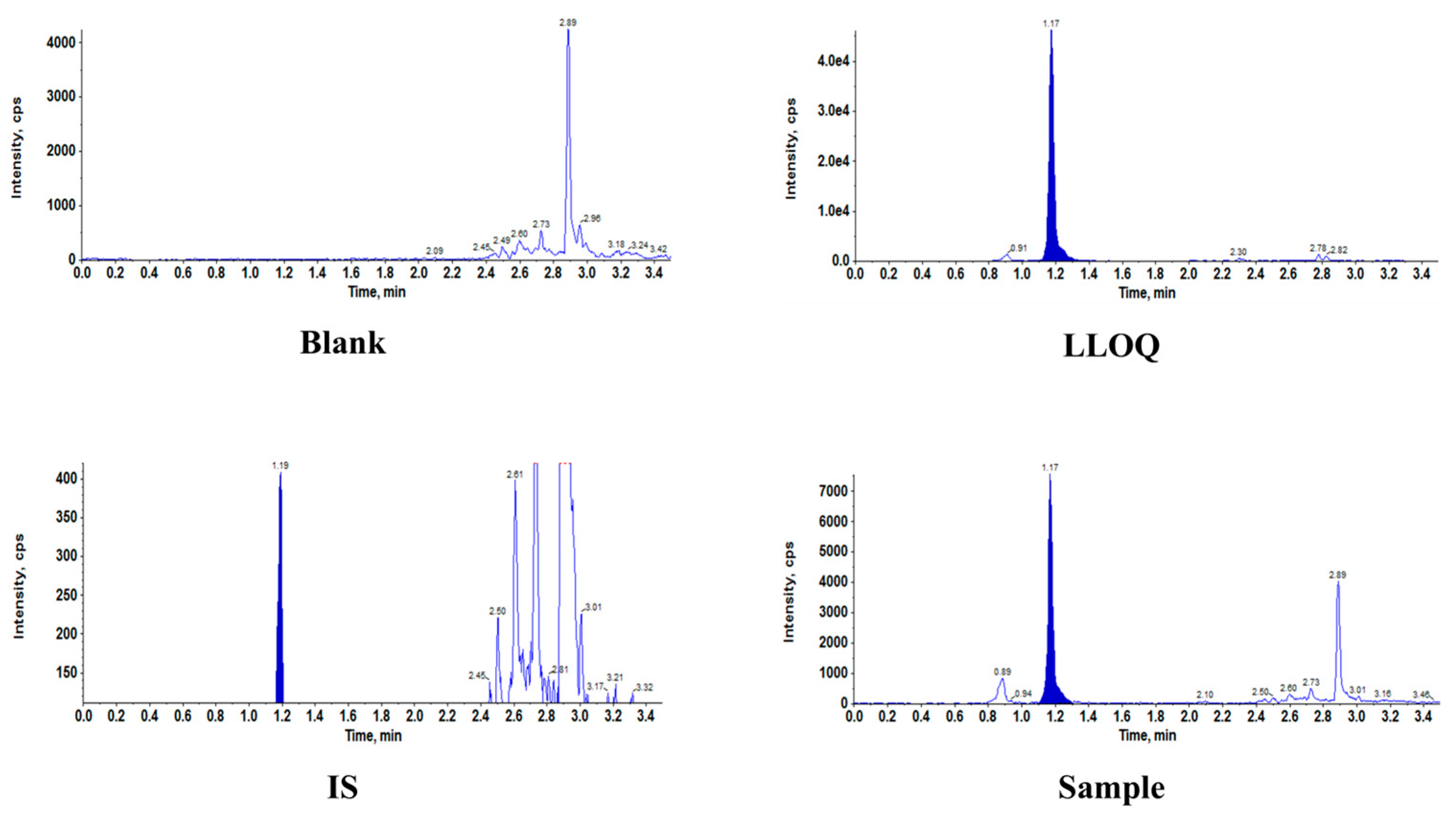
| Analyte | Batch | Slope | Intercept | R2 | Fitted Equation |
|---|---|---|---|---|---|
| Eravacycline in BALF | 1 | 0.00421 | 0.00156 | 0.99870 | y = 0.00421x + 0.00156 |
| 2 | 0.00550 | 0.00172 | 0.99900 | y = 0.0055x + 0.00172 | |
| 3 | 0.00353 | 0.00106 | 0.99850 | y = 0.00353x + 0.00106 | |
| Average | 0.0044 | 0.0014 | 0.9987 |
| Samples | Nominal Conc.† (ng/mL) | Average Measured Conc.† (ng/mL) | Precision (%) | Accuracy (%) |
|---|---|---|---|---|
| Intra-batch (n = 5) | ||||
| LLOQ | 1 | 1.015 | 1.6 | 101.5 |
| QCL | 3 | 2.970 | 6.0 | 99.0 |
| QCM | 60 | 59.460 | 2.4 | 99.1 |
| QCH | 150 | 147.000 | 1.4 | 98.0 |
| Inter-batch (n = 15) | ||||
| LLOQ | 1 | 1.024 | 9.9 | 102.4 |
| QCL | 3 | 3.018 | 4.4 | 100.6 |
| QCM | 60 | 61.380 | 4.0 | 102.3 |
| QCH | 150 | 149.200 | 1.6 | 99.5 |
| QC Samples | BALF (n = 6) | ||
|---|---|---|---|
| Average (%) | SD | CV (%) | |
| Matrix effect (%) | |||
| QCL | 97.4 | 6.6 | 6.8 |
| QCH | 107.6 | 2.7 | 2.5 |
| Extraction recovery (%) | |||
| QCL | 103.5 | / | 6.1 |
| QCM | 103.6 | / | 13.0 |
| QCH | 107.2 | / | 1.4 |
| Condition | QCL | QCH | ||
|---|---|---|---|---|
| Recovery (%) | CV (%) | Recovery (%) | CV (%) | |
| Room temperature 6 h | 112.1 | 4.8 | 107.6 | 0.9 |
| Post-preparation (autosampler, 4 °C) 4 h | 91.8 | 1.8 | 96.3 | 4.4 |
| Three freeze–thaw cycles (−70 °C) | 107.8 | 7.8 | 98.4 | 2.1 |
| Long-term 44 days (−20 °C) | 107.1 | 3.2 | 105.3 | 3.0 |
| Long-term 44 days (−70 °C) | 110.4 | 3.3 | 107.5 | 2.1 |
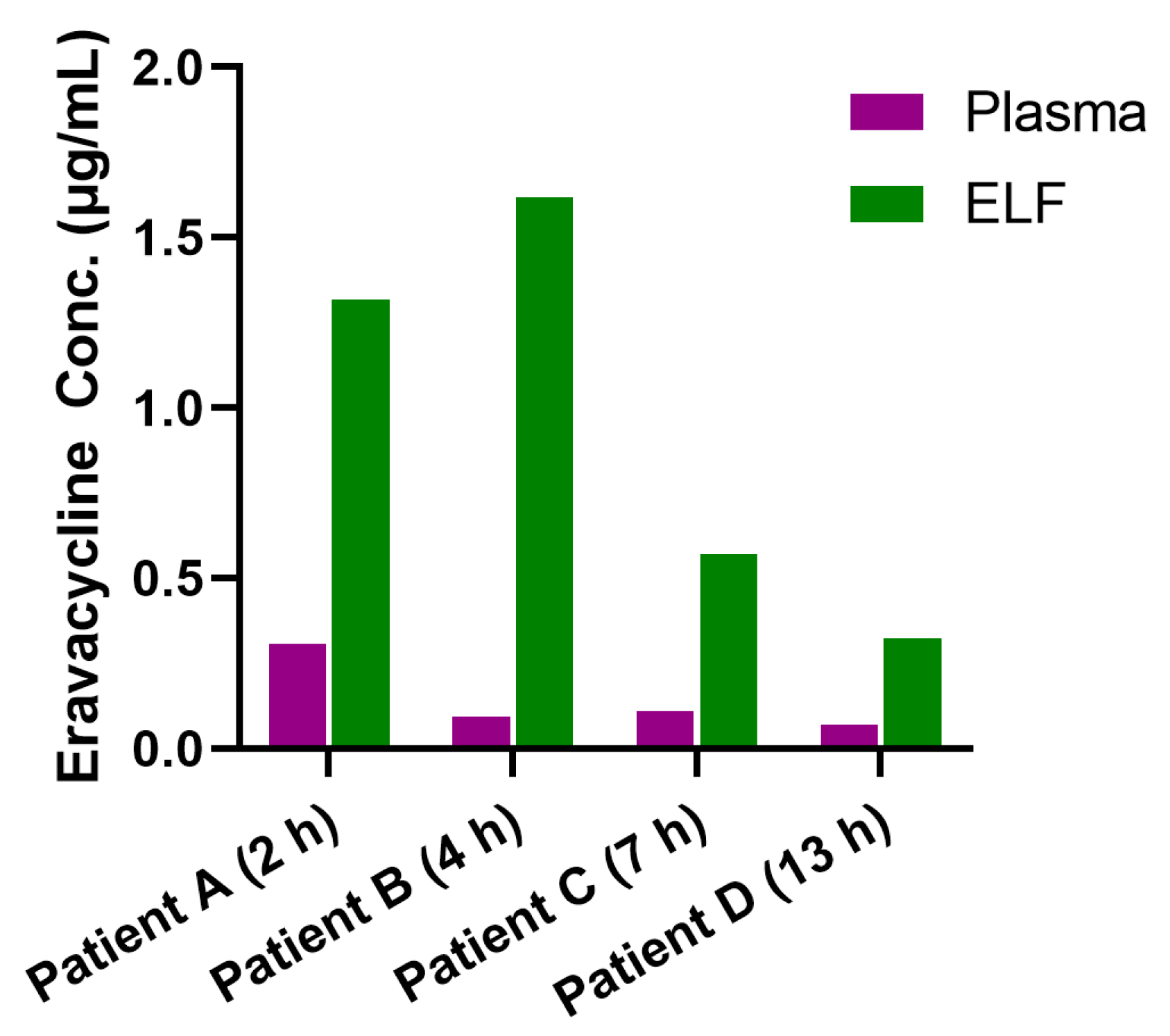
| Patient ID | Time (h) | UreaBlood/UreaBALF | Eravacycline Conc.† (μg/mL) | Pulmonary Penetration Ratios | ||
|---|---|---|---|---|---|---|
| Plasma | BALF | ELF | ||||
| A | 2 h | 30.27 | 0.307 | 0.0436 | 1.318 | 4.29 |
| B | 4 h | 69.10 | 0.0929 | 0.0234 | 1.617 | 17.40 |
| C | 7 h | 16.98 | 0.109 | 0.0336 | 0.570 | 5.22 |
| D | 13 h | 15.23 | 0.0687 | 0.0212 | 0.323 | 4.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Lin, J.; Li, X.; Li, N.; Su, J.; Wu, J.; Hu, J.; Zhang, J.; Liu, X. Development and Validation of a Rapid LC-MS/MS Method for Quantifying Eravacycline in Epithelial Lining Fluid: Application to a Prospective Pulmonary Distribution Study in HAP/VAP Patients. Antibiotics 2025, 14, 957. https://doi.org/10.3390/antibiotics14090957
He J, Lin J, Li X, Li N, Su J, Wu J, Hu J, Zhang J, Liu X. Development and Validation of a Rapid LC-MS/MS Method for Quantifying Eravacycline in Epithelial Lining Fluid: Application to a Prospective Pulmonary Distribution Study in HAP/VAP Patients. Antibiotics. 2025; 14(9):957. https://doi.org/10.3390/antibiotics14090957
Chicago/Turabian StyleHe, Jingjing, Jingjing Lin, Xin Li, Nanyang Li, Jianguang Su, Jufang Wu, Jin Hu, Jing Zhang, and Xiaofen Liu. 2025. "Development and Validation of a Rapid LC-MS/MS Method for Quantifying Eravacycline in Epithelial Lining Fluid: Application to a Prospective Pulmonary Distribution Study in HAP/VAP Patients" Antibiotics 14, no. 9: 957. https://doi.org/10.3390/antibiotics14090957
APA StyleHe, J., Lin, J., Li, X., Li, N., Su, J., Wu, J., Hu, J., Zhang, J., & Liu, X. (2025). Development and Validation of a Rapid LC-MS/MS Method for Quantifying Eravacycline in Epithelial Lining Fluid: Application to a Prospective Pulmonary Distribution Study in HAP/VAP Patients. Antibiotics, 14(9), 957. https://doi.org/10.3390/antibiotics14090957








