Antimicrobial Resistance of Clostridioides (Clostridium) difficile in Cambodia
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Resistance
2.2. Antimicrobial Resistance by Condition
3. Discussion
4. Materials and Methods
4.1. C. difficile Isolates
4.2. MIC Determination
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| CDI | Clostridioides difficile infection |
| MLSB | Macrolide lincosamide streptogramin B |
| MGEs | Mobile genetic elements |
| MIC | Minimal inhibitory concentration |
| BA | Blood agar |
| RT | Ribotype |
| MDR | Multidrug resistance |
| OPD | Outpatient department |
| S | Susceptible |
| I | Intermediate |
| R | Resistant |
| TT | Total toxigenic strains |
| OT | Other toxigenic strains |
| TNT | Total non-toxigenic strains |
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013.
- Cetin, S.; Ugur, M. Clostridioides difficile infections and factors associated with recurrence. Infect. Dis. Clin. Microbiol. 2024, 6, 264–275. [Google Scholar] [CrossRef]
- McFarland, L.V.; Elmer, G.W.; Surawicz, C.M. Breaking the cycle: Treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am. J. Gastroenterol. 2002, 97, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.D.; Seyboldt, C.; Rupnik, M. Non-human C. difficile reservoirs and sources: Animals, food, environment. In Updates on Clostridium Difficile in Europe: Advances in Microbiology, Infectious Diseases and Public Health; Mastrantonio, P., Rupnik, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 8, pp. 227–243. [Google Scholar]
- Cromwell, G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002, 13, 7–27. [Google Scholar] [CrossRef]
- Longhitano, A.; Roder, C.; Blackmore, T.; Campbell, A.; May, M.; Athan, E. Australasian Society of Infectious Diseases updated guidelines for the management of Clostridioides difficile infection in adults and children in Australia and New Zealand. Intern. Med. J. 2025, 55, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin. Infect. Dis. 2021, 73, e1029–e1044. [Google Scholar] [CrossRef] [PubMed]
- Imwattana, K.; Putsathit, P.; Leepattarakit, T.; Kiratisin, P.; Riley, T.V. Mild or malign: Clinical characteristics and outcomes of Clostridium difficile infection in Thailand. J. Clin. Microbiol. 2020, 58, e01217-20. [Google Scholar] [CrossRef]
- Collins, D.A.; Sohn, K.M.; Wu, Y.; Ouchi, K.; Ishii, Y.; Elliott, B.; Riley, T.V.; Tateda, K. Clostridioides difficile infection in the Asia-Pacific region. Emerg. Microbes Infect. 2020, 9, 42–52. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, C.; Zhang, H.; Zhao, C.; Guo, R.; Liu, J.; Wang, J.; Yuan, J.; Jia, K.; Wu, A.; et al. Toxin genotypes, antibiotic resistance and their correlations in Clostridioides difficile isolated from hospitals in Xi’an, China. BMC Microbiol. 2024, 24, 177. [Google Scholar] [CrossRef]
- Lew, T.; Putsathit, P.; Sohn, K.M.; Wu, Y.; Ouchi, K.; Ishii, Y.; Tateda, K.; Riley, T.V.; Collins, D.A. Antimicrobial Susceptibilities of Clostridium difficile Isolates from 12 Asia-Pacific Countries in 2014 and 2015. Antimicrob. Agents Chemother. 2020, 64, e00296-20. [Google Scholar] [CrossRef]
- Wieczorkiewicz, J.T.; Skinner, A.M.; Cheknis, A.; Petrella, L.A.; Stevens, V.W.; Wright, L.M.; Gerding, D.N.; Johnson, S. Epidemiology of Clostridioides difficile infection at one hospital 10 years after an outbreak of the epidemic C. difficile strain BI/027: Changing strain prevalence, antimicrobial susceptibilities, and patient antibiotic exposures. Antimicrob. Agents Chemother. 2024, T, e00698-24. [Google Scholar] [CrossRef] [PubMed]
- Tickler, I.A.; Obradovich, A.E.; Goering, R.V.; Fang, F.C.; Tenover, F.C.; Consortium, H.A.I. Changes in molecular epidemiology and antimicrobial resistance profiles of Clostridioides (Clostridium) difficile strains in the United States between 2011 and 2017. Anaerobe 2019, 60, 102050. [Google Scholar] [CrossRef] [PubMed]
- Putsathit, P.; Hong, S.; George, N.; Hemphill, C.; Huntington, P.G.; Korman, T.M.; Kotsanas, D.; Lahra, M.; McDougall, R.; McGlinchey, A.; et al. Antimicrobial resistance surveillance of Clostridioides difficile in Australia, 2015–18. J. Antimicrob. Chemother. 2021, 76, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Abdrabou, A.M.M.; Ul Habib Bajwa, Z.; Halfmann, A.; Mellmann, A.; Nimmesgern, A.; Margardt, L.; Bischoff, M.; von Muller, L.; Gartner, B.; Berger, F.K. Molecular epidemiology and antimicrobial resistance of Clostridioides difficile in Germany, 2014-2019. Int. J. Med. Microbiol. 2021, 311, 151507. [Google Scholar] [CrossRef]
- Imwattana, K.; Putsathit, P.; Knight, D.R.; Kiratisin, P.; Riley, T.V. Molecular characterization of, and antimicrobial resistance in Clostridioides difficile from Thailand, 2017–2018. Microb. Drug Resist. 2021, 27, 1505–1512. [Google Scholar] [CrossRef]
- Khun, P.A.; Phi, L.D.; Bui, H.T.T.; Collins, D.A.; Riley, T.V. Clostridioides (Clostridium) difficile in adults with diarrhoea in Vietnam. Anaerobe 2023, 81, 102741. [Google Scholar] [CrossRef]
- Khun, P.A.; Phi, L.D.; Pham, P.T.; Thu Nguyen, H.T.; Huyen Vu, Q.T.; Collins, D.A.; Riley, T.V. Clostridioides (Clostridium) difficile in children with diarrhoea in Vietnam. Anaerobe 2022, 74, 102550. [Google Scholar] [CrossRef]
- Wolfe, C.; Pagano, P.; Pillar, C.M.; Shinabarger, D.L.; Boulos, R.A. Comparison of the in vitro antibacterial activity of Ramizol, fidaxomicin, vancomycin, and metronidazole against 100 clinical isolates of Clostridium difficile by broth microdilution. Diagn. Microbiol. Infect. Dis. 2018, 92, 250–252. [Google Scholar] [CrossRef]
- Freeman, J.; Vernon, J.; Pilling, S.; Morris, K.; Nicolson, S.; Shearman, S.; Clark, E.; Palacios-Fabrega, J.A.; Wilcox, M.; Pan-European Longitudinal Surveillance of Antibiotic Resistance among Prevalent Clostridium difficile Ribotypes’ Study, G. Five-year Pan-European, longitudinal surveillance of Clostridium difficile ribotype prevalence and antimicrobial resistance: The extended ClosER study. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 169–177. [Google Scholar] [CrossRef]
- Imwattana, K.; Rodriguez, C.; Riley, T.V.; Knight, D.R. A species-wide genetic atlas of antimicrobial resistance in Clostridioides difficile. Microb. Genom. 2021, 7, 000696. [Google Scholar] [CrossRef]
- Knight, D.R.; Elliott, B.; Chang, B.J.; Perkins, T.T.; Riley, T.V. Diversity and evolution in the genome of Clostridium difficile. Clin. Microbiol. Rev. 2015, 28, 721–741. [Google Scholar] [CrossRef] [PubMed]
- Imwattana, K.; Kiratisin, P.; Riley, T.V.; Knight, D.R. Genomic basis of antimicrobial resistance in non-toxigenic Clostridium difficile in Southeast Asia. Anaerobe 2020, 66, 102290. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Putsathit, P.; Maneerattanaporn, M.; Piewngam, P.; Knight, D.R.; Kiratisin, P.; Riley, T.V. Antimicrobial susceptibility of Clostridium difficile isolated in Thailand. Antimicrob. Resist. Infect. Control 2017, 6, 58. [Google Scholar] [CrossRef][Green Version]
- O’Connor, J.R.; Galang, M.A.; Sambol, S.P.; Hecht, D.W.; Vedantam, G.; Gerding, D.N.; Johnson, S. Rifampin and rifaximin resistance in clinical isolates of Clostridium difficile. Antimicrob. Agents Chemother. 2008, 52, 2813–2817. [Google Scholar] [CrossRef]
- Jon, J.V.; Mark, H.W.; Jane, F. Antimicrobial resistance progression in the United Kingdom: A temporal comparison of Clostridioides difficile antimicrobial susceptibilities. Anaerobe 2021, 70, 102385. [Google Scholar] [CrossRef]
- CENAT. Tuberculosis Report 2023; National Center for Tuberculosis and Leprosy Control: Phnom Penh, Cambodia, 2023. [Google Scholar]
- Suh, J.W.; Jeong, Y.J.; Ahn, H.G.; Kim, J.Y.; Sohn, J.W.; Yoon, Y.K. Epidemiologic characteristics and risk factors of Clostridioides difficile infection in patients with active tuberculosis in the Republic of Korea: A nationwide population-based study. J. Hosp. Infect. 2024, 154, 1–8. [Google Scholar] [CrossRef]
- Lee, Y.M.; Huh, K.C.; Yoon, S.M.; Jang, B.I.; Shin, J.E.; Koo, H.S.; Jung, Y.; Kim, S.H.; Moon, H.S.; Lee, S.W. Incidence and clinical outcomes of Clostridium difficile infection after treatment with tuberculosis medication. Gut Liver 2016, 10, 250–254. [Google Scholar] [CrossRef]
- Dingle, K.E.; Didelot, X.; Quan, T.P.; Eyre, D.W.; Stoesser, N.; Marwick, C.A.; Coia, J.; Brown, D.; Buchanan, S.; Ijaz, U.Z.; et al. A role for tetracycline selection in recent evolution of agriculture-associated Clostridium difficile PCR rbotype 078. mBio 2019, 10, e02790-18. [Google Scholar] [CrossRef]
- Eng, L.; Collins, D.A.; Alene, K.A.; Bory, S.; Theng, Y.; Vann, P.; Meng, S.; Limsreng, S.; Clements, A.C.; Riley, T.V. Clostridioides (Clostridium) difficile infection in hospitalised adult patients in Cambodia. Microbiol. Spectr. 2025, 13, e02747-24. [Google Scholar] [CrossRef]
- Chea, B.; Kong, S.; Thim, S.; Ban, N.; Seng, S.; Fernandez-Colorado, C.; Kang, K. Knowledge, attitudes, and practices of antimicrobial use and resistance among livestock producers in Cambodia. Open J. Anim. Sci. 2022, 12, 454–466. [Google Scholar] [CrossRef]
- Loo, V.G.; Poirier, L.; Miller, M.A.; Oughton, M.; Libman, M.D.; Michaud, S.; Bourgault, A.M.; Nguyen, T.; Frenette, C.; Kelly, M.; et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 2005, 353, 2442–2449. [Google Scholar] [CrossRef] [PubMed]
- Goorhuis, A.; Van der Kooi, T.; Vaessen, N.; Dekker, F.W.; Van den Berg, R.; Harmanus, C.; van den Hof, S.; Notermans, D.W.; Kuijper, E.J. Spread and epidemiology of Clostridium difficile polymerase chain reaction ribotype 027/toxinotype III in the Netherlands. Clin. Infect. Dis. 2007, 45, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Imwattana, K.; Knight, D.R.; Kullin, B.; Collins, D.A.; Putsathit, P.; Kiratisin, P.; Riley, T.V. Clostridium difficile ribotype 017—Characterization, evolution and epidemiology of the dominant strain in Asia. Emerg. Microbes Infect. 2019, 8, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Riley, T.V.; Collins, D.A.; Karunakaran, R.; Kahar, M.A.; Adnan, A.; Hassan, S.A.; Zainul, N.H.; Rustam, F.R.M.; Wahab, Z.A.; Ramli, R.; et al. High prevalence of toxigenic and nontoxigenic Clostridium difficile strains in Malaysia. J. Clin. Microbiol. 2018, 56, e00170-18. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mith, H.; Taminiau, B.; Bouchafa, L.; Van Broeck, J.; Soumillion, K.; Ngyuvula, E.; García-Fuentes, E.; Korsak, N.; Delmée, M.; et al. First isolation of Clostridioides difficile from smoked and dried freshwater fish in Cambodia. Food Control 2021, 124, 107895. [Google Scholar] [CrossRef]
- Eng, L.; Alene, K.A.; Collins, D.A.; Lim, S.C.; Srey, V.; Chea, C.; Yohn, S.; Leng, S.; Clements, A.C.A.; Riley, T.V. Clostridioides (Clostridium) difficile in hospitalised children in Cambodia. Anaerobe 2025, 93, 102959. [Google Scholar] [CrossRef]
- Eng, L.; Turner, P.; Alene, K.A.; Collins, D.A.; Lim, S.-C.; Tan, P.; Soeng, S.; Hun, D.; Yohn, S.; Vong, S.; et al. Clostridioides (Clostridium) difficile in children and adolescents in the community in Cambodia. Anaerobe 2025, 94, 102982. [Google Scholar] [CrossRef]
- Khun, P.A.; Phi, L.D.; Bui, H.T.T.; Bui, N.T.; Vu, Q.T.H.; Trinh, L.D.; Collins, D.A.; Riley, T.V. Environmental contamination with Clostridioides (Clostridium) difficile in Vietnam. J. Appl. Microbiol. 2023, 134, lxad118. [Google Scholar] [CrossRef]
- CLSI M11; Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, M11-A7, 7th Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2007.
- EUCAST; The European Society of Clinical Microbiology and Infectious Diseases. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 14.0; EUCAST: Växjö, Sweden, 2024. [Google Scholar]
- CLSI M100-ED34; 2024 Performance Standards for Antimicrobial Susceptibility Testing, 34th Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024.

| Antimicrobial | Interpretive Categories and MIC Breakpoints (mg/L) | Result Ranges (mg/L) | MIC50/MIC90 (mg/L) | Susceptibility % (N = 192) | ||||
|---|---|---|---|---|---|---|---|---|
| S | I | R | S | I | R | |||
| Vancomycin | ≤2 | >2 | 0.5–2 | 0.5/1 | 100% | 0 | 0 | |
| Metronidazole | ≤2 | >2 | 0.12–0.5 | 0.25/0.25 | 100% | 0 | 0 | |
| Fidaxomicin | ≤0.5 | - | >0.5 | 0.015–0.25 | 0.06/0.12 | 100% | 0 | 0 |
| Rifaximin | <32 | ≥32 | 0.0008–>64 | 0.015/0.015 | 92% (177) | 0 | 8% (15) | |
| Meropenem | ≤4 | 8 | ≥16 | 2 to 8 | 4/8. | 86% (166) | 14% (26) | 0 |
| Amoxicillin/clavulanic acid | ≤4 | 8 | ≥16 | 0.25–2 | 0.5/1 | 100% | 0 | 0 |
| Clindamycin | ≤2 | 4 | ≥8 | 2–>64 | 16/>64 | 1% (1) | 11% (21) | 88% (169) |
| Erythromycin | - | - | 0.25–>512 | >512/>512 | - | - | - | |
| Tetracycline | ≤4 | 8 | ≥16 | 0.12–64 | 8/32 | 48% | 2% (3) | 50% (96) |
| Moxifloxacin | ≤2 | 4 | ≥8 | 2–>32 | 2/32 | 80% | 0 | 20% (38) |
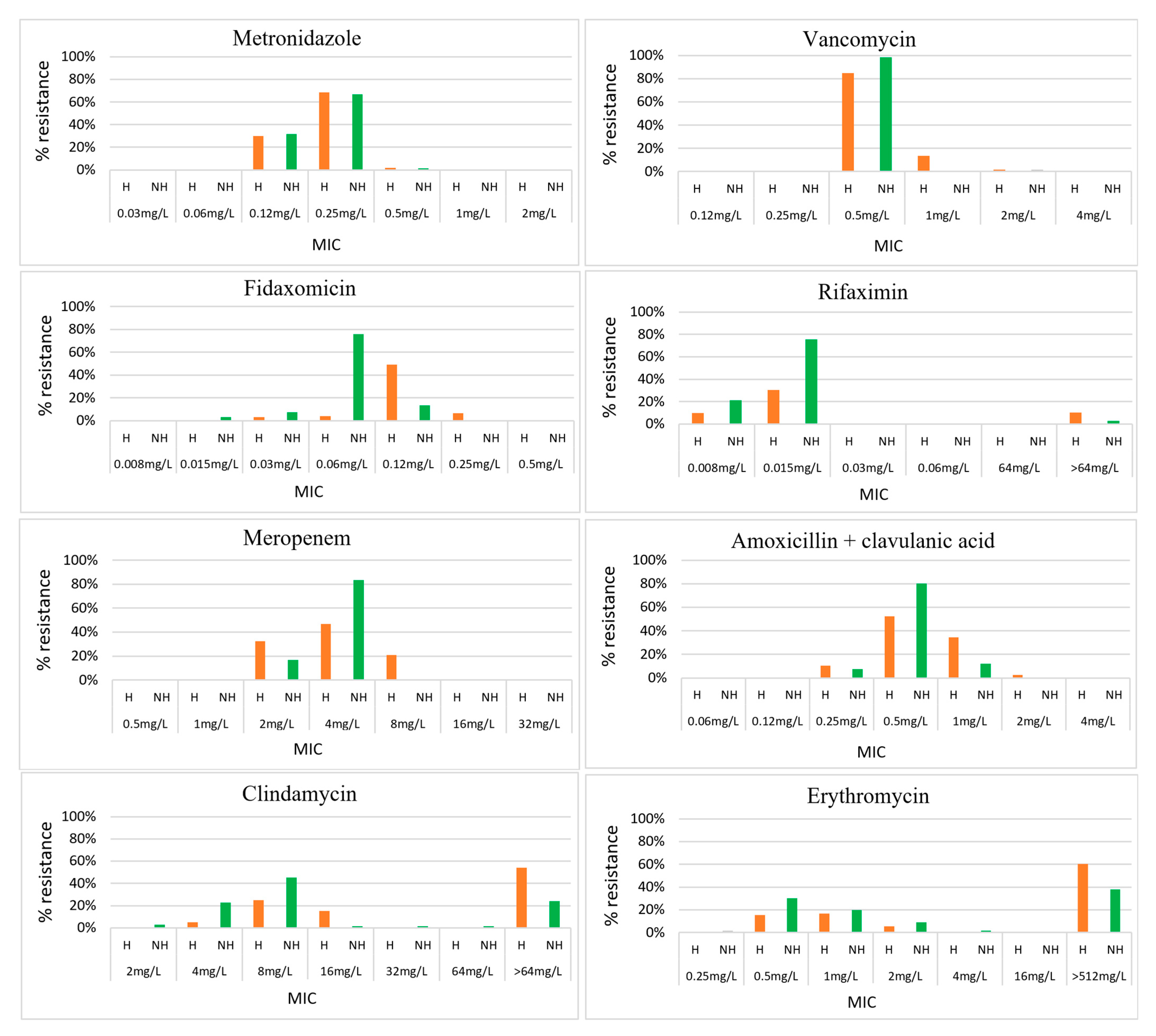
| Antimicrobials | Interpretive Categories and MIC Breakpoints (mg/L) | C. difficile in Hospitalised Patients (n = 124) | C. difficile in Non-Hospitalised Individuals (n = 66) | p-Value | C. difficile in Those Living in the Capital City (n = 37) | C. difficile in Those Living Outside the Capital City (n = 145) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| S | I | R | Resistant (%) | Resistant (%) | Resistant (%) | Resistant (%) | |||
| Vancomycin | ≤2 | >2 | 0% | 0% | 0% | 0% | |||
| Metronidazole | ≤2 | >2 | 0% | 0% | 0% | 0% | |||
| Fidaxomicin | ≤0.5 | - | >0.5 | 0% | 0% | 0% | 0% | ||
| Rifaximin | <32 | ≥32 | 10% | 3% | 0.07 | 0% | 10% | ||
| Meropenem | ≤4 | 8 | ≥16 | 0% | 0% | 0% | 0% | ||
| Amoxicillin/clavulanic acid | ≤4 | 8 | ≥16 | 0% | 0% | 0% | 0% | ||
| Clindamycin | ≤2 | 4 | ≥8 | 95% | 74% | <0.0001 | 92% | 88% | 0.464 |
| Erythromycin | - | - | - | - | - | - | |||
| Tetracycline | ≤4 | 8 | ≥16 | 55% | 41% | 0.067 | 68% | 46% | 0.020 |
| Moxifloxacin | ≤2 | 4 | ≥8 | 29% | 3% | <0.0001 | 24% | 18% | 0.378 |
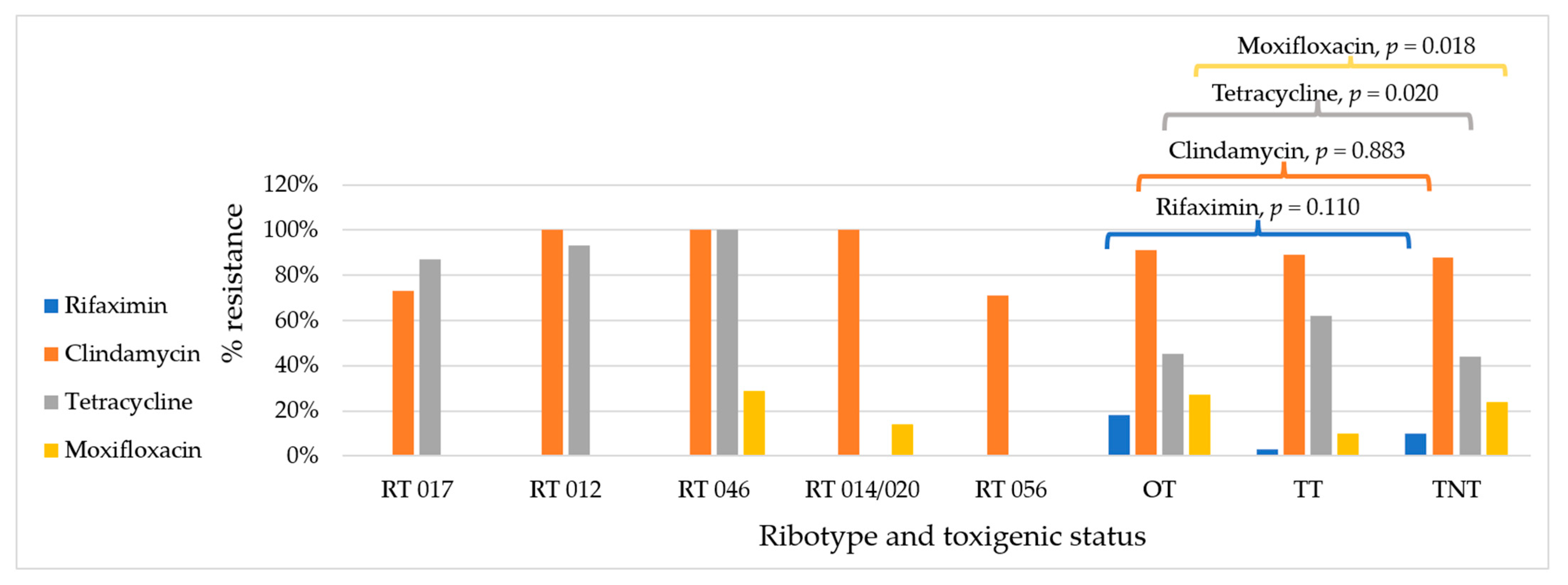
| Antimicrobials | Interpretive Categories and MIC Breakpoints (mg/L) | Hospitalised Patients | Non-Hospitalised Individuals | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Toxigenic Strains (n = 36) | Non-Toxigenic Strains (n = 88) | p-Value | Toxigenic Strains (n = 24) | Non-Toxigenic Strains (n = 42) | p-Value | ||||
| S | I | R | Resistant (%) | Resistant (%) | Resistant (%) | Resistant (%) | |||
| Vancomycin | ≤2 | >2 | 0% | 0% | 0% | 0% | |||
| Metronidazole | ≤2 | >2 | 0% | 0% | 0% | 0% | |||
| Fidaxomicin | ≤0.5 | - | >0.5 | 0% | 0% | 0% | 0% | ||
| Rifaximin | <32 | ≥32 | 3% | 14% | 0.073 | 4% | 2% | 0.684 | |
| Meropenem | ≤4 | 8 | ≥16 | 0% | 0% | 0% | 0% | ||
| Amoxicillin/clavulanic acid | ≤4 | 8 | ≥16 | 0% | 0% | 0% | 0% | ||
| Clindamycin | ≤2 | 4 | ≥8 | 94% | 95% | 0.812 | 79% | 71% | 0.489 |
| Erythromycin | - | - | - | - | - | - | |||
| Tetracycline | ≤4 | 8 | ≥16 | 58% | 53% | 0.617 | 67% | 26% | 0.001 |
| Moxifloxacin | ≤2 | 4 | ≥8 | 14% | 35% | 0.017 | 4% | 2% | 0.684 |
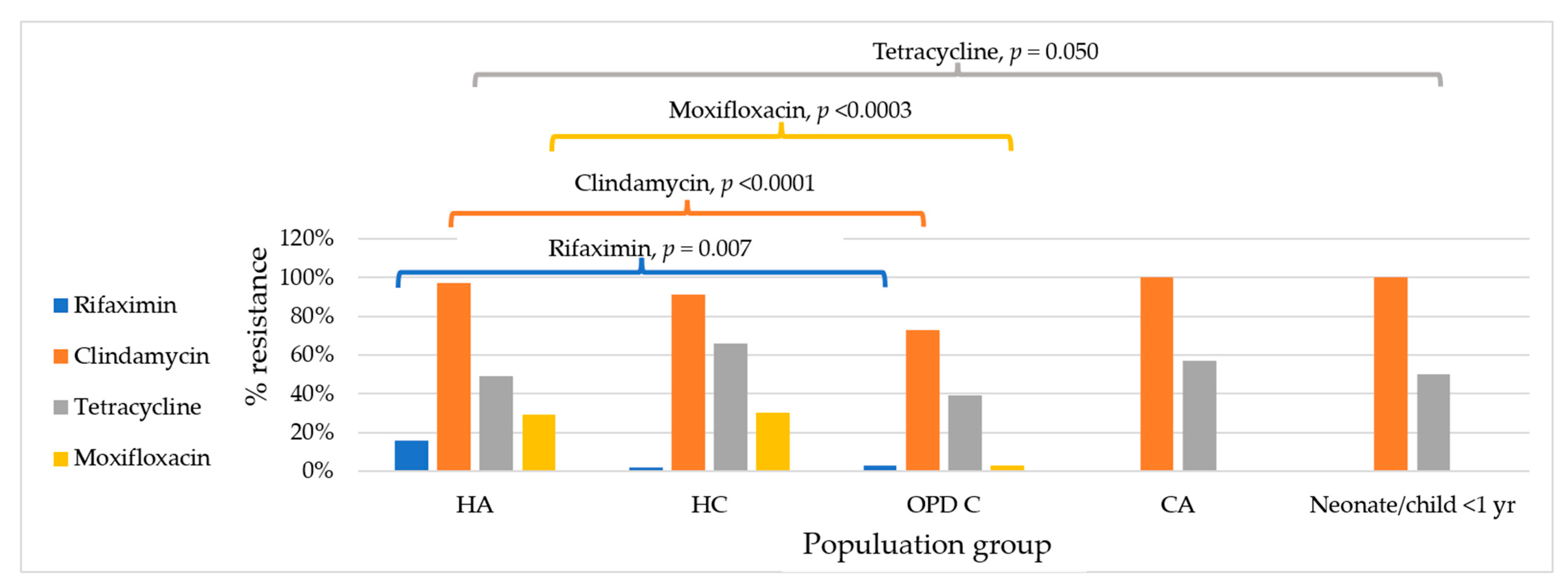
| Ribotype | Hospitalised Adults | Hospitalised Children | OPD Children | Adolescents | Neonate and Children < 1 yr | Total |
|---|---|---|---|---|---|---|
| 017 | 5 | 1 | 7 | 1 | 1 | 15 |
| 012 | 2 | 7 | 2 | 3 | 0 | 14 |
| 046 | 5 | 0 | 2 | 0 | 0 | 7 |
| 014/020 | 3 | 3 | 1 | 0 | 0 | 7 |
| 056 | 3 | 0 | 3 | 1 | 0 | 7 |
| Other toxigenic strains | 6 | 1 | 2 | 2 | 0 | 11 |
| Non-toxigenic strains | 53 | 35 | 42 | 0 | 1 | 131 |
| Total | 77 | 47 | 59 | 7 | 2 | 192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eng, L.; Putsathit, P.; Lim, S.-C.; Chisholm, J.M.; Collins, D.A.; Clements, A.C.A.; Alene, K.A.; Riley, T.V. Antimicrobial Resistance of Clostridioides (Clostridium) difficile in Cambodia. Antibiotics 2025, 14, 950. https://doi.org/10.3390/antibiotics14090950
Eng L, Putsathit P, Lim S-C, Chisholm JM, Collins DA, Clements ACA, Alene KA, Riley TV. Antimicrobial Resistance of Clostridioides (Clostridium) difficile in Cambodia. Antibiotics. 2025; 14(9):950. https://doi.org/10.3390/antibiotics14090950
Chicago/Turabian StyleEng, Lengsea, Papanin Putsathit, Su-Chen Lim, Jessica M. Chisholm, Deirdre A. Collins, Archie C. A. Clements, Kefyalew Addis Alene, and Thomas V. Riley. 2025. "Antimicrobial Resistance of Clostridioides (Clostridium) difficile in Cambodia" Antibiotics 14, no. 9: 950. https://doi.org/10.3390/antibiotics14090950
APA StyleEng, L., Putsathit, P., Lim, S.-C., Chisholm, J. M., Collins, D. A., Clements, A. C. A., Alene, K. A., & Riley, T. V. (2025). Antimicrobial Resistance of Clostridioides (Clostridium) difficile in Cambodia. Antibiotics, 14(9), 950. https://doi.org/10.3390/antibiotics14090950







