Synergistic Antimicrobial Activity of Vancomycin, Ceftriaxone, and Gentamicin Against Cutibacterium acnes Strains: An In Vitro Checkerboard Analysis and In Vivo Interaction with Bioactive Glass Using Galleria mellonella
Abstract
1. Introduction
2. Results
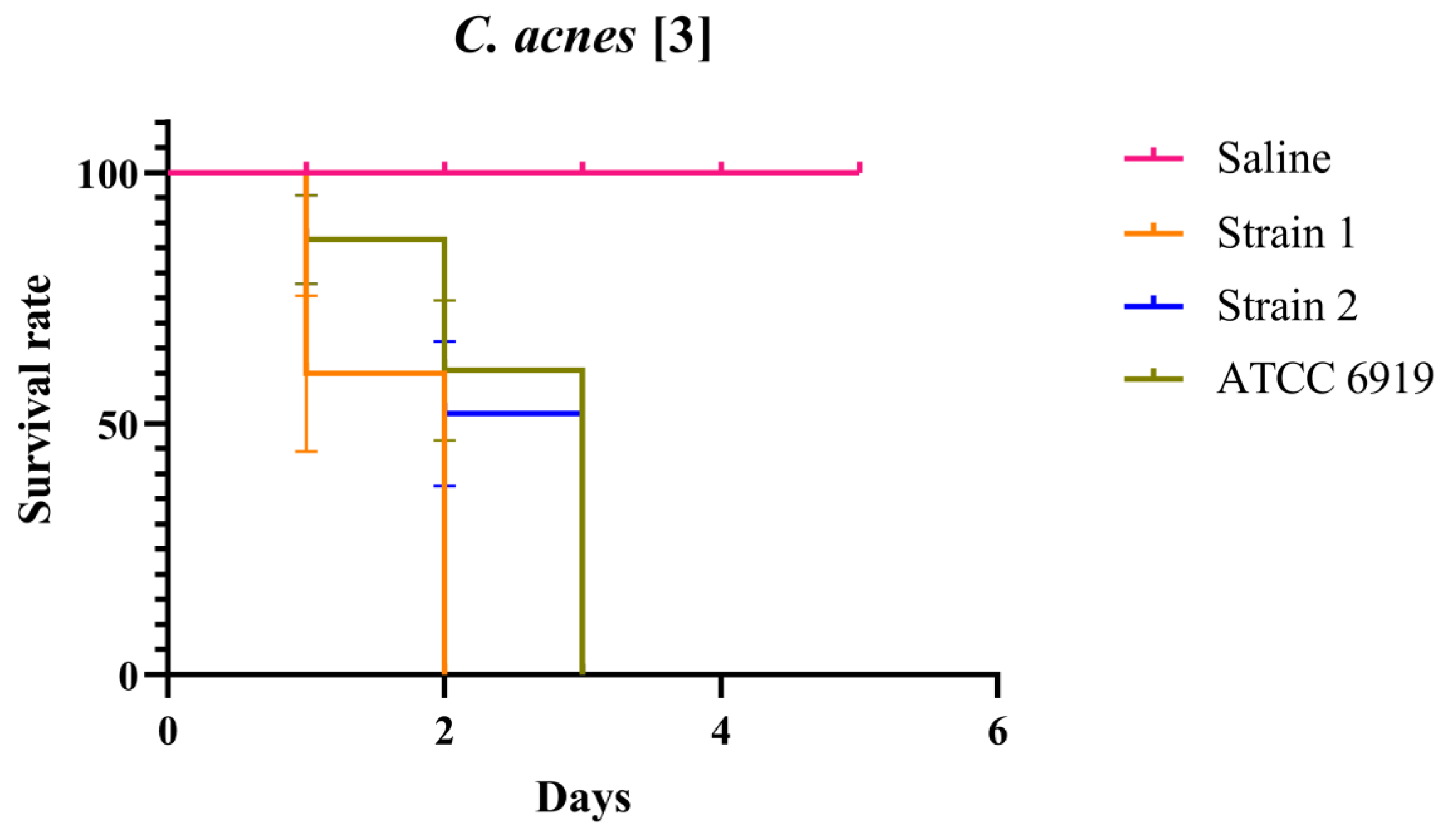
2.1. Optimal Concentration of C. acnes in the Galleria mellonella Infection Assay Using BAG Supernatant and Antimicrobials in a Synergistic Approach
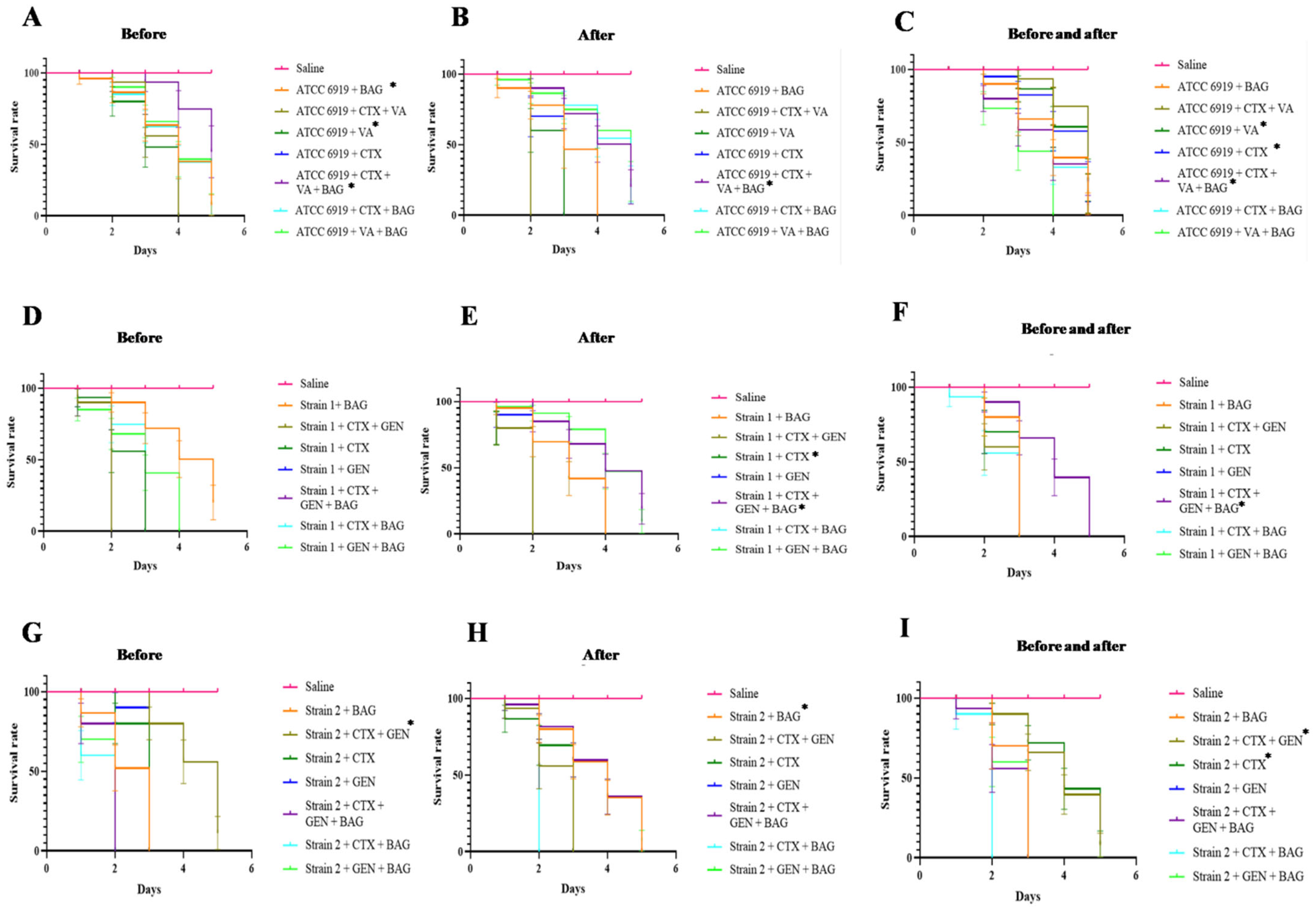
2.2. Efficacy and Synergism of Antibiotics and Interaction with BAG for C. acnes in the G. mellonella Infection Assay
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates
4.2. Determination of Minimum Inhibitory Concentration (MIC) and Checkerboard Assay for Antimicrobial Treatment
- Synergy: ΣFIC ≤ 0.5, indicating a greater effect than that observed with the individual agents;
- Partial synergy: 0.5 < ΣFIC ≤ 1.0, indicating a weakly additive effect;
- Indifference: 1.0 < ΣFIC ≤ 4.0, indicating that the combination has a similar effect to that of the individual agents;
- Antagonism: ΣFIC > 4.0, indicating that the combination is less effective than the individual agents.
4.3. Bioactive Glass S53P4 (BAG) Preparation
4.4. Galleria mellonella Infection Model
4.5. Infection Assay in Galleria mellonella Using BAG Supernatant and Antimicrobials in a Synergistic Approach
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VA | Vancomycin |
| GEN | Gentamicin |
| CTX | Ceftriaxone |
| BAG | Bioactive glass S53P4 |
| MICs | Minimum inhibitory concentrations |
| FICIs | Fractional inhibitory concentration indices |
| TSB | Tryptic soy broth |
| ALBC | Antibiotic-loaded bone cement |
References
- Thoraval, L.; Varin-Simon, J.; Ohl, X.; Velard, F.; Reffuveille, F.; Tang-Fichaux, M. Cutibacterium acnes and its complex host interaction in prosthetic joint infection: Current insights and future directions. Res. Microbiol. 2025, 176, 104265. [Google Scholar] [CrossRef] [PubMed]
- Tafin, U.F.; Trampuz, A.; Corvec, S. In vitro emergence of rifampicin resistance in Propionibacterium acnes and molecular characterization of mutations in the rpoB gene. J. Antimicrob. Chemother. 2013, 68, 523–528. [Google Scholar] [CrossRef]
- Aoki, S.; Nakase, K.; Hayashi, N.; Noguchi, N. Transconjugation of erm(X) conferring high-level resistance of clindamycin for Cutibacterium acnes. J. Med. Microbiol. 2019, 68, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Beig, M.; Shirazi, O.; Ebrahimi, E.; Banadkouki, A.Z.; Golab, N.; Sholeh, M. Prevalence of antibiotic-resistant Cutibacterium acnes (formerly Propionibacterium acnes) isolates, a systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2024, 39, 82–91. [Google Scholar] [CrossRef]
- McDowell, A.; Nagy, I.; Magyari, M.; Barnard, E.; Patrick, S.; Zhou, D. The opportunistic pathogen Propionibacterium acnes: Insights into typing, human disease, clonal diversification and CAMP factor evolution. PLoS ONE 2013, 8, e70897. [Google Scholar] [CrossRef] [PubMed]
- Spittaels, K.-J.; Ongena, R.; Zouboulis, C.C.; Crabbé, A.; Coenye, T. Cutibacterium acnes Phylotype I and II Strains Interact Differently with Human Skin Cells. Front. Cell. Infect. Microbiol. 2020, 10, 575164. [Google Scholar] [CrossRef]
- Salar-Vidal, L.; Achermann, Y.; Aguilera-Correa, J.-J.; Poehlein, A.; Esteban, J.; Brüggemann, H.; on behalf of the ESCMID Study Group for Implant-Associated Infections (ESGIAI). Genomic Analysis of Cutibacterium acnes Strains Isolated from Prosthetic Joint Infections. Microorganisms 2021, 9, 1500. [Google Scholar] [CrossRef]
- Cavallo, I.; Sivori, F.; Truglio, M.; De Maio, F.; Lucantoni, F.; Cardinali, G.; Pontone, M.; Bernardi, T.; Sanguinetti, M.; Capitanio, B.; et al. Skin dysbiosis and Cutibacterium acnes biofilm in inflammatory acne lesions of adolescents. Sci Rep. 2022, 12, 21104. [Google Scholar] [CrossRef]
- van Vugt, T.A.G.; Arts, J.J.; Geurts, J.A.P. Antibiotic-Loaded Polymethylmethacrylate Beads and Spacers in Treatment of Orthopedic Infections and the Role of Biofilm Formation. Front. Microbiol. 2019, 10, 1626. [Google Scholar] [CrossRef]
- Hoch, A.; Fritz, Y.; Dimitriou, D.; Bossard, D.A.; Fucentese, S.F.; Wieser, K.; Achermann, Y.; Zingg, P.O. Treatment outcomes of patients with Cutibacterium acnes-positive cultures during total joint replacement revision surgery: A minimum 2-year follow-up. Arch. Orthop. Trauma Surg. 2023, 143, 2951–2958. [Google Scholar] [CrossRef]
- Kurihara, M.N.L.; Santos, I.N.M.; Eisen, A.K.A.; Caleiro, G.S.; de Araújo, J.; de Sales, R.O.; Pignatari, A.C.; Salles, M.J. Phenotypic and Genotypic Characterization of Cutibacterium acnes Isolated from Shoulder Surgery Reveals Insights into Genetic Diversity. Microorganisms 2023, 11, 2594. [Google Scholar] [CrossRef]
- Kusejko, K.; Auñón, Á.; Jost, B.; Natividad, B.; Strahm, C.; Thurnheer, C.; Pablo-Marcos, D.; Slama, D.; Scanferla, G.; Uckay, I.; et al. The Impact of Surgical Strategy and Rifampin on Treatment Outcome in Cutibacterium Periprosthetic Joint Infections. Clin. Infect. Dis. 2021, 72, e1064–e1073. [Google Scholar] [CrossRef]
- Saltiel, G.; Meyssonnier, V.; Kerroumi, Y.; Heym, B.; Lidove, O.; Marmor, S.; Zeller, V. Cutibacterium acnes Prosthetic Joint Infections: Is Rifampicin-Combination Therapy Beneficial? Antibiotics 2022, 11, 1801. [Google Scholar] [CrossRef]
- Tanwar, Y.S.; Ferreira, N. The role of bioactive glass in the management of chronic osteomyelitis: A systematic review of literature and current evidence. Infect. Dis. 2020, 52, 219–226. [Google Scholar] [CrossRef]
- Mannala, G.K.; Rupp, M.; Alagboso, F.; Kerschbaum, M.; Pfeifer, C.; Sommer, U.; Kampschulte, M.; Domann, E.; Alt, V. Galleria mellonella as an alternative in vivo model to study bacterial biofilms on stainless steel and titanium implants. ALTEX 2021, 38, 245–252. [Google Scholar] [CrossRef]
- Tiltnes, T.S.; Kehrer, M.; Hughes, H.; Morris, T.E.; Justesen, U.S. Ceftriaxone treatment of spondylodiscitis and other serious infections with Cutibacterium acnes. J. Antimicrob. Chemother. 2020, 75, 3046–3048. [Google Scholar] [CrossRef] [PubMed]
- Charles, P.; Hot, A.; Ou, P.; Carbonnelle, E.; Sidi, D.; Nassif, X.; Lortholary, O. Propionibacterium acnes endocarditis in an adolescent boy suffering from a congenital cardiopathy. Pediatr. Infect. Dis. J. 2007, 26, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Kurz, M.; Kaufmann, B.A.; Baddour, L.M.; Widmer, A.F. Propionibacterium acnes prosthetic valve endocarditis with abscess formation: A case report. BMC Infect. Dis. 2014, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Vinod, A.; Listopadzki, T.; Kohut, K.; Pavlesen, S.; Crane, J.; Feng, L.; Duquin, T.; DiPaola, M. An in vitro analysis of various antibiotic cement combinations against Cutibacterium acnes. Semin. Arthroplast. JSES 2023, 33, 707–714. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Lee, J.-H.; Kim, S.; Park, S.; Kim, Y.-J.; Ryu, C.-M.; Seo, H.W.; Lee, J. Inhibition of Biofilm Formation in Cutibacterium acnes, Staphylococcus aureus, and Candida albicans by the Phytopigment Shikonin. Int. J. Mol. Sci. 2024, 25, 2426. [Google Scholar] [CrossRef]
- Usman, M.; Markus, A.; Fatima, A.; Aslam, B.; Zaid, M.; Khattak, M.; Bashir, S.; Masood, S.; Rafaque, Z.; Dasti, J.I. Synergistic Effects of Gentamicin, Cefepime, and Ciprofloxacin on Biofilm of Pseudomonas aeruginosa. Infect. Drug Resist. 2023, 16, 5887–5898. [Google Scholar] [CrossRef] [PubMed]
- Hawas, S.; Verderosa, A.D.; Totsika, M. Combination Therapies for Biofilm Inhibition and Eradication: A Comparative Review of Laboratory and Preclinical Studies. Front. Cell. Infect. Microbiol. 2022, 12, 850030. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Tateyama, Y.; Sugita, T. Evaluation of Antibacterial Drugs Using Silkworms Infected by Cutibacterium acnes. Insects 2021, 12, 619. [Google Scholar] [CrossRef]
- Zhao, Y.; Mannala, G.K.; Youf, R.; Rupp, M.; Alt, V.; Riool, M. Development of a Galleria mellonella Infection Model to Evaluate the Efficacy of Antibiotic-Loaded Polymethyl Methacrylate (PMMA) Bone Cement. Antibiotics 2024, 13, 692. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Zhang, D.; Leppäranta, O.; Munukka, E.; Ylänen, H.; Viljanen, M.K.; Eerola, E.; Hupa, M.; Hupa, L. Antibacterial effects and dissolution behavior of six bioactive glasses. J. Biomed. Mater. Res. Part A 2009, 93, 475–483. [Google Scholar] [CrossRef]
- Gerhardt, L.C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef]
- Brazilian Committee on Antimicrobial Susceptibility Testing—BrCAST. Tabelas de Pontos de Corte Para Interpretação de CIMs e Diâmetros de Halos; Brazilian Committee on Antimicrobial Susceptibility Testing: Rio de Janeiro, Brazil, 2025. [Google Scholar]
- Leber, A.L. Clinical Microbiology Procedures Handbook, 4th ed.; ASM Press: Washington, DC, USA, 2016. [Google Scholar]
| Interpretation | ΣFIC (FIC A + B) | Gentamicin | Ceftriaxone | Plate Well | ||
|---|---|---|---|---|---|---|
| Gentamicin FIC | Concentration (μg/mL) | Ceftriaxone FIC | Concentration (μg/mL) | |||
| Gentamicin MIC | Gentamicin MIC | Gentamicin MIC | 0 | Gentamicin MIC | 0.125 | A4 |
| Partial synergy | 0.625 | 0.5 | 1 | 1 | 0.125 | B4 |
| Partial synergy | 0.625 | 0.5 | 1 | 1 | 0.125 | C4 |
| Partial synergy | 0.625 | 0.5 | 1 | 1 | 0.125 | D4 |
| Partial synergy | 0.625 | 0.5 | 1 | 1 | 0.125 | E4 |
| Partial synergy | 0.625 | 0.5 | 1 | 1 | 0.125 | F4 |
| Partial synergy | 0.56 | 0.5 | 1 | 0.48 | 0.06 | F3 |
| Indifferent | 1.06 | 1 | 2 | 0.48 | 0.06 | G3 |
| Indifferent | 1.03 | 1 | 2 | 0.24 | 0.03 | G2 |
| Ceftriaxone MIC | Ceftriaxone MIC | Ceftriaxone MIC | 2 | Ceftriaxone MIC | Ceftriaxone MIC | G1 |
| Interpretation | ΣFIC (FIC A + B) | Gentamicin | Ceftriaxone | Plate Well | ||
|---|---|---|---|---|---|---|
| Gentamicin FIC | Concentration (μg/mL) | Ceftriaxone FIC | Concentration (μg/mL) | |||
| Gentamicin MIC | Gentamicin MIC | Gentamicin MIC | 0 | Gentamicin MIC | 0.25 | A5 |
| Synergy | 0.265 | 0.015 | 0.03 | 1 | 0.25 | B5 |
| Synergy | 0.28 | 0.03 | 0.06 | 1 | 0.25 | C5 |
| Synergy | 0.31 | 0.06 | 0.125 | 1 | 0.25 | D5 |
| Synergy | 0.25 | 0.125 | 0.25 | 0.5 | 0.125 | D4 |
| Synergy | 0.375 | 0.25 | 0.5 | 0.5 | 0.125 | E4 |
| Synergy | 0.31 | 0.25 | 0.5 | 0.24 | 0.06 | E3 |
| Indifferent | 1.06 | 1 | 2 | 0.24 | 0.06 | F3 |
| Indifferent | 1.06 | 1 | 2 | 0.24 | 0.06 | G3 |
| Indifferent | 1.03 | 1 | 2 | 0.12 | 0.03 | G2 |
| Ceftriaxone MIC | Ceftriaxone MIC | Ceftriaxone MIC | 2 | Ceftriaxone MIC | Ceftriaxone MIC | G1 |
| Interpretation | ΣFIC (FICA + B) | Ceftriaxone | Vancomycin | Plate Well | ||
|---|---|---|---|---|---|---|
| Ceftriaxone FIC | Concentration (μg/mL) | Vancomycin FIC | Concentration (μg/mL) | |||
| Vancomycin MIC | Vancomycin MIC | Vancomycin MIC | 0 | Vancomycin MIC | 0.5 | A11 |
| Indifferent | 1.1 | 0.1 | 0.006 | 1 | 0.5 | B11 |
| Indifferent | 1.01 | 0.01 | 0.001 | 1 | 0.5 | C11 |
| Indifferent | 1.04 | 0.04 | 0.0025 | 1 | 0.5 | D11 |
| Indifferent | 1.08 | 0.08 | 0.005 | 1 | 0.5 | E11 |
| Indifferent | 1.16 | 0.16 | 0.01 | 1 | 0.5 | F11 |
| Partial synergy | 0.66 | 0.16 | 0.01 | 0.5 | 0.25 | F10 |
| Indifferent | 1.5 | 1 | 0.06 | 0.5 | 0.25 | G10 |
| Indifferent | 1.25 | 1 | 0.06 | 0.25 | 0.125 | G9 |
| Indifferent | 1.12 | 1 | 0.06 | 0.12 | 0.06 | G8 |
| Indifferent | 1.06 | 1 | 0.06 | 0.06 | 0.03 | G7 |
| Indifferent | 1.03 | 1 | 0.06 | 0.03 | 0.01 | G6 |
| Indifferent | 1.01 | 1 | 0.06 | 0.01 | 0.005 | G5 |
| Indifferent | 1.005 | 1 | 0.06 | 0.005 | 0.0025 | G4 |
| Indifferent | 1.002 | 1 | 0.06 | 0.002 | 0.001 | G3 |
| Indifferent | 1.012 | 1 | 0.06 | 0.012 | 0.006 | G2 |
| Ceftriaxone MIC | Ceftriaxone MIC | Ceftriaxone MIC | 0.06 | Ceftriaxone MIC | Ceftriaxone MIC | G1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurihara, M.N.L.; Brasil, I.F.; Silva, M.M.d.A.; Salles, M.J. Synergistic Antimicrobial Activity of Vancomycin, Ceftriaxone, and Gentamicin Against Cutibacterium acnes Strains: An In Vitro Checkerboard Analysis and In Vivo Interaction with Bioactive Glass Using Galleria mellonella. Antibiotics 2025, 14, 923. https://doi.org/10.3390/antibiotics14090923
Kurihara MNL, Brasil IF, Silva MMdA, Salles MJ. Synergistic Antimicrobial Activity of Vancomycin, Ceftriaxone, and Gentamicin Against Cutibacterium acnes Strains: An In Vitro Checkerboard Analysis and In Vivo Interaction with Bioactive Glass Using Galleria mellonella. Antibiotics. 2025; 14(9):923. https://doi.org/10.3390/antibiotics14090923
Chicago/Turabian StyleKurihara, Mariana Neri Lucas, Isabelle Frois Brasil, Mayara Muniz de Andrade Silva, and Mauro Jose Salles. 2025. "Synergistic Antimicrobial Activity of Vancomycin, Ceftriaxone, and Gentamicin Against Cutibacterium acnes Strains: An In Vitro Checkerboard Analysis and In Vivo Interaction with Bioactive Glass Using Galleria mellonella" Antibiotics 14, no. 9: 923. https://doi.org/10.3390/antibiotics14090923
APA StyleKurihara, M. N. L., Brasil, I. F., Silva, M. M. d. A., & Salles, M. J. (2025). Synergistic Antimicrobial Activity of Vancomycin, Ceftriaxone, and Gentamicin Against Cutibacterium acnes Strains: An In Vitro Checkerboard Analysis and In Vivo Interaction with Bioactive Glass Using Galleria mellonella. Antibiotics, 14(9), 923. https://doi.org/10.3390/antibiotics14090923








