1. Introduction
Chagas disease, caused by the hemoflagellate protozoan
Trypanosoma cruzi, remains one of the most neglected tropical diseases in the world, predominantly affecting low-income populations [
1,
2]. It is endemic in 21 countries across the Americas, with the highest prevalence in rural areas of Bolivia, Argentina, Brazil, Colombia, and Mexico [
3]. However, due to human migration, cases have been reported in non-endemic countries such as the United States, Spain, and Australia, where transmission occurs mainly through congenital or transfusional routes [
4]. As a matter of fact, this disease exhibits complex epidemiology, with multiple transmission routes that include not only the traditional triatomine vector but also congenital transmission, blood transfusion, and oral transmission, significantly complicating its control [
4,
5,
6]. Despite global efforts in disease control and improved diagnostics,
T. cruzi poses a serious public health challenge, with millions of people infected and at risk of developing chronic complications [
7]. The World Health Organization (WHO) estimates that approximately 6 to 7 million individuals are infected, with around 70 million living in at-risk areas [
8].
The disease progresses through two distinct phases: acute and chronic. The acute phase is often asymptomatic or presents with fever, lymphadenopathy, and, in severe cases, myocarditis or meningoencephalitis [
9]. The chronic phase may remain indeterminate or progress to Chagas cardiomyopathy (30–40% of cases) [
10] and megacolon/megaesophagus (10%) [
11]. Current treatment relies on two main drugs, benznidazole and nifurtimox, which exhibit variable efficacy (60–80% in the acute phase, <20% in the chronic phase) and severe adverse effects such as allergies, neuropathies, and hepatotoxicity, often leading to treatment discontinuation [
12]. Additionally, resistance of
T. cruzi to these compounds has been reported, underscoring the urgent need for new therapeutic alternatives [
13].
In this context, antimicrobial peptides (AMPs) emerge as promising therapeutic candidates due to their broad-spectrum activity against many pathogens, including bacteria, fungi, viruses, and protozoa [
14,
15]. These molecules, essential components of innate immunity, act through multifactorial mechanisms, reducing the likelihood of resistance development [
16,
17,
18]. Recent studies have demonstrated the efficacy of AMPs against trypanosomatids, including
T. cruzi, highlighting their potential as a therapeutic alternative [
19,
20,
21]. AMPs combat
T. cruzi through multifaceted mechanisms, where their positive charge allows binding to anionic phospholipids in the parasite’s membrane, inducing pore formation and cell lysis [
22]. Additionally, they modulate the host immune response stimulating the production of pro-inflammatory cytokines (such as IL-12 and TNF-α), which enhance parasite clearance [
23]. Several families of AMPs have demonstrated trypanocidal activity, including defensins, cathelicidins, cecropins, and temporins, each with specific mechanisms of action against
T. cruzi [
22]. Defensins, particularly human β-defensin 1 (HBD-1) and α-defensin 1 (HNP-1), have shown the ability to significantly reduce the infectivity of the parasite’s trypomastigote forms [
24]. Cathelicidins, such as human-derived LL-37, act by disrupting the parasite’s mitochondrial membrane, leading to its death [
22]. Cecropins, originally isolated from insects, exhibit potent trypanocidal activity at micromolar concentrations, with cecropin A showing particular efficacy against intracellular forms of the parasite [
22].
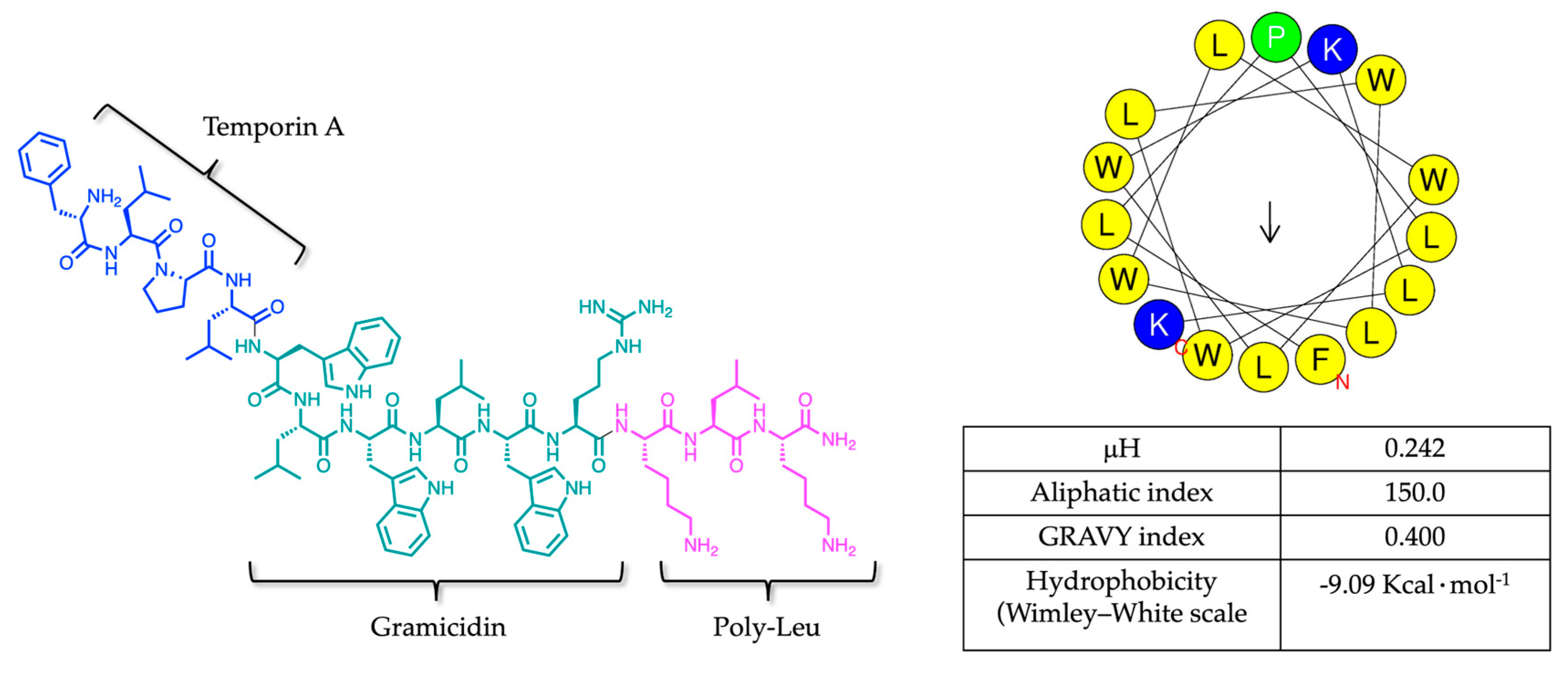
Among AMPs, two temporin-derived molecules, Temporizin (FLPLWLWLWLWLWKLK) and its synthetic analog Temporizin-1 (FLPLWLWLWRKLK), deserve particular attention for their trypanocidal potential. Temporizin is a synthetic hybrid peptide composed of three distinct regions: the N-terminal segment of Temporin A, from the skin of the amphibian
Rana temporaria [
25]; the pore-forming domain of Gramicidin; and a C-terminal tail featuring alternating leucine and lysine residues. Although AMPs generally offer significant therapeutic potential, some can also penetrate mammalian plasma membranes, leading to undesirable cytotoxic effects. Temporizin demonstrated an EC
50 of 116.9 μg/mL against mammalian cells, while exhibiting potent activity against
Trypanosoma spp. at concentrations approximately 100-fold lower, indicating a favorable therapeutic index and selective toxicity toward the parasite [
25]. To further improve its safety profile, Temporizin-1, characterized by a truncated pore-forming domain, was engineered to reduce its interaction with lipid bilayers and consequently cytotoxicity against mammalian cells while maintaining antiparasitic efficacy [
25].
To better understand the enhanced activity of Temporizin-1 against Trypanosoma spp. alongside its reduced cytotoxicity toward mammalian cells, it is essential to investigate its mechanism of action using membrane-mimicking systems. Model membranes that replicate the distinct lipid compositions of protozoan and mammalian cells provide a valuable platform for elucidating these interactions at the molecular level and further optimizing the AMP’s structure for therapeutic applications, enhancing selectivity, minimizing off-target effects, and informing the rational design of next-generation therapeutics. Moreover, the significant antiparasitic activity of Temporizin-1 was also confirmed against the trypomastigote and intracellular amastigote forms, and its additive effect was observed when administered in combination with the traditional benznidazole.
3. Discussion
AMPs represent a promising strategy to counteract antibiotic resistance, as their primary mechanism of action involves direct interaction with and subsequent disruption of microbial membranes. The physicochemical properties including hydrophobicity, amphipathicity, and net positive charge, control the AMP’s behavior towards the membrane [
43]. The net positive charge is implicated in the membrane anchoring by establishing electrostatic interactions, while the hydrophobicity plays a key role in the insertion and incorporation of AMPs inside the lipid core [
44].
Herein, we explored the potential membranolytic activity of Temporizin-1, a synthetic hybrid peptide derived from the Temporin A, Gramidicin, and poly-leu peptides, which displayed strong activity on
T. cruzi and moderate cytotoxicity against mammalian cells [
25].
Firstly, we determined the physicochemical properties of Temporizin-1, including amphipathicity and hydrophobicity, which is featured by a net positive charge of +4 essential for electrostatic interactions with negatively charged phospholipids, and a repeated motif WL conferring hydrophobicity and the ability to incorporate inside the lipid core. The intrinsic peptide hydrophobicity is evidenced by its hydrophobic moment (0.242), aliphatic index (150.0), and GRAVY score (0.400), parameters that highlight its amphipathic nature and potential membrane activity. All these theorical parameters were obtained by HeliQuest and ExPASy, while the Wimley–White hydrophobicity scale was calculated from the MPEx software obtaining the negative Gibbs energy of −9.09 Kcal·mol
−1 and suggesting a membrane insertion of Temporizin-1 [
45].
Moreover, these physicochemical properties endow Temporizin-1 with the capacity to interact with and insert into lipid membranes, leading to membrane disruption. To investigate its mechanism of action, herein, we employed liposomes designed to mimic both the lipid composition of protozoan and eukaryotic membranes and conducted a series of biophysical assays. These two-membrane model systems enable a comparative analysis of membrane interactions, providing insights into potential selective targeting based on lipid composition differences.
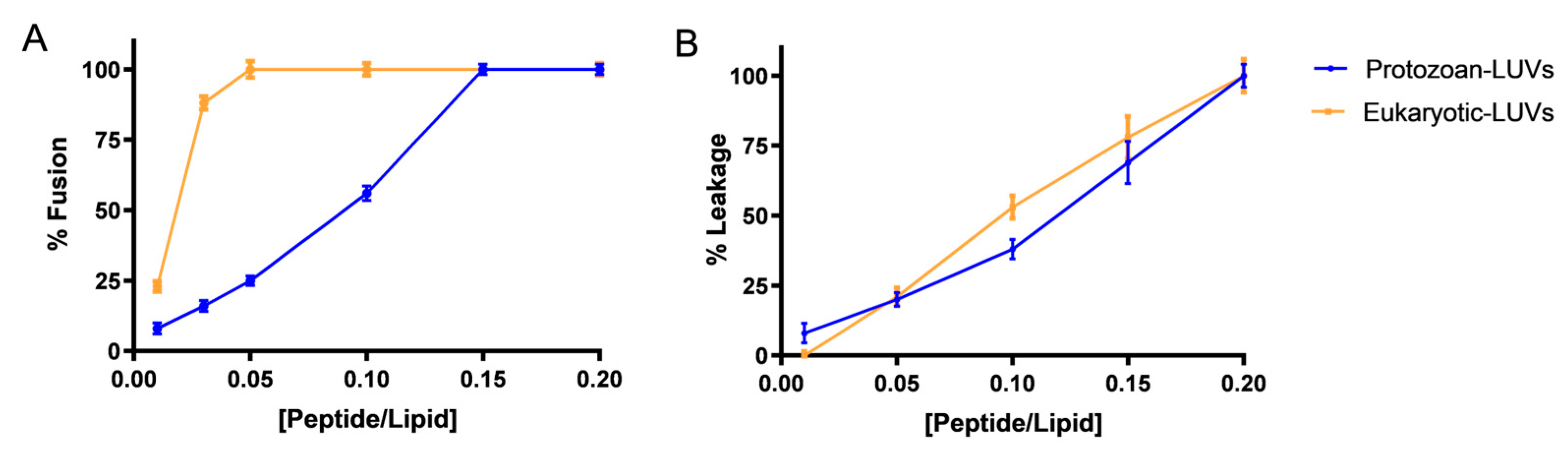
By performing the lipid mixing and leakage assays, we observed strong membranolytic activity of Temporizin-1 both in the presence of protozoan and eukaryotic LUVs. In both conditions, the peptide induced a complete membrane fusion, but at different peptide/lipid ratios. Specifically, Temporizin-1 induced the complete fusion of eukaryotic LUVs already at the low P/L ratio of 0.05; in contrast, in protozoan-mimetic LUVs, Temporizin-1 showed a gradual increase in lipid mixing, reaching the full membrane fusion at a higher P/L of 0.15.
Additionally, Temporizin-1 exhibited pronounced membranolytic activity as evidenced by its ability to induce leakage both of protozoan and eukaryotic LUVs. When we performed the LUVs’ titration with Temporizin-1, we observed a complete protozoan LUVs’ disruption at a P/L ratio of 0.2, measuring a significant calcein release from the LUVs. These findings are consistent with previous studies reporting that Temporizin-1 can induce pore formation in the
Trypanosoma membrane, likely due to the presence of the gramicidin-derived sequence. Furthermore, the significant leakage observed in eukaryotic LUVs at all P/L ratios highlights the cytotoxic potential of Temporizin-1 previously reported [
25], where the pore formation was observed in mammalian cells but at lower concentrations compared to the native peptide Temporizin.
Once the membrane action of Temporizin-1 was ascertained, we accomplished a systematic study to understand how the physicochemical properties can influence the peptide affinity and interaction with membranes featured by different compositions. Firstly, the presence of repeated WL motifs can favor the insertion of Temporizin-1 into the hydrophobic core of lipid bilayers. Tryptophan residues are particularly important in this process, as they show a strong preference for the interfacial regions of lipid membranes and thus facilitate AMP insertion [
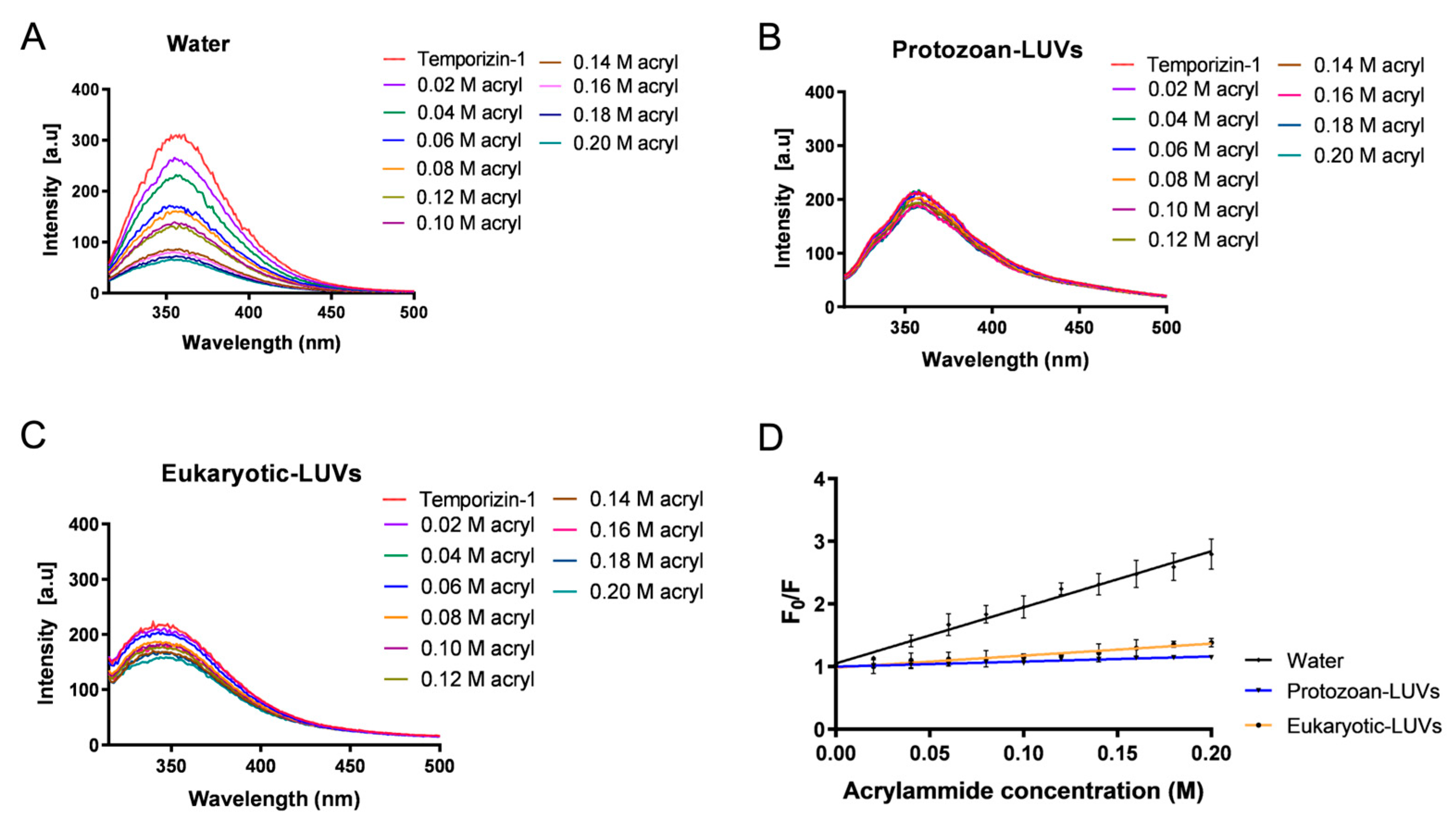
46]. In our study, we confirmed Trp insertion by performing fluorescence quenching experiments with acrylamide, a quencher unable to interact with lipids and which prefers the aqueous solution. While the acrylamide is able to strongly quench Temporizin-1 in water, in protozoan and eukaryotic LUVs, the Trp residues are less accessible because they are buried inside the lipid core. Interestingly, these results were further supported by calculating the Stern–Volmer constants (K
sv). The high Ksv value (8.9 ± 0.3) of Temporizin-1 in water confirmed the Trp accessibility to acrylamide in this condition, whereas the low K
sv values, 0.81 ± 0.05 and 1.9 ± 0.1 for protozoan and eukaryotic LUVs, demonstrated the reduced Trp accessibility. This slight difference between K
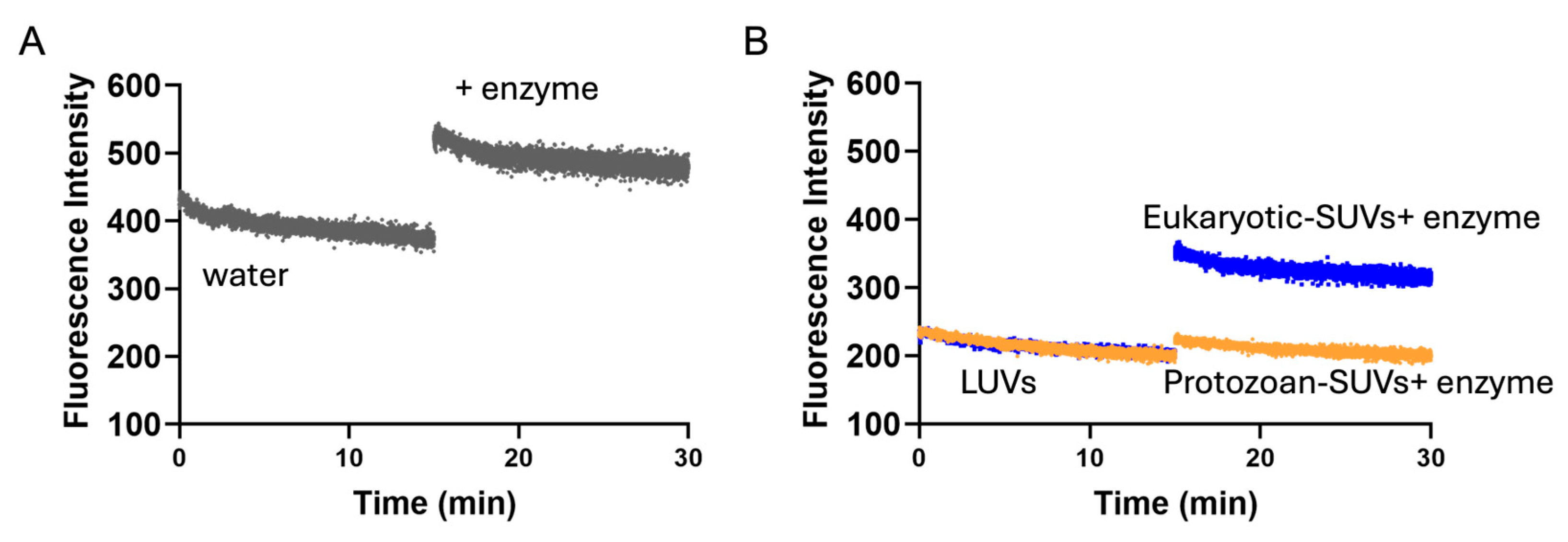
sv values obtained in LUVs highlighted a potential different penetration of Temporizin-1 in the membrane. To investigate this aspect, we performed proteolytic cut with chymotrypsin following peptide incubation with SUVs. If the Trp residues are at the interface, the enzyme recognizes and cuts them, resulting in the enhancement of Trp fluorescence; conversely, when Trp residues are located inside the core, the enzyme is not able to cut, and an increase in fluorescence is not recorded. Interestingly, we observed that Temporizin-1 is completely incorporated inside the protozoan SUVs, while in eukaryotic SUVs, the peptide is probably partially inserted inside the lipid bilayer and located at the membrane interface since the Trp fluorescence emission increased significantly after the proteolytic cut. This different behavior could be attributed to the different lipid composition of membranes. Indeed, eukaryotic membranes, rich in zwitterionic lipids, could favor a more interfacial peptide localization, whereas protozoan membranes, enriched in anionic lipids, promote stronger electrostatic interactions with Temporizin-1, driving Trp residues deeper into the bilayer and reducing their enzymatic accessibility.
Moreover, this different membrane interaction was also further investigated by BLI experiment using APS biosensors to immobilize protozoan and eukaryotic LUVs. Temporizin-1 showed a strong interaction and high affinity with the protozoan-like membrane, while its interaction with eukaryotic LUVs was comparatively weaker. This result was also supported by KD values calculated using the Langmuir binding model, presenting values of 1.7 ± 0.9 µM for protozoan LUVs and 62.1 ± 8.2 µM for eukaryotic LUVs, thereby confirming the weaker affinity of Temporizin-1 for eukaryotic LUVs.
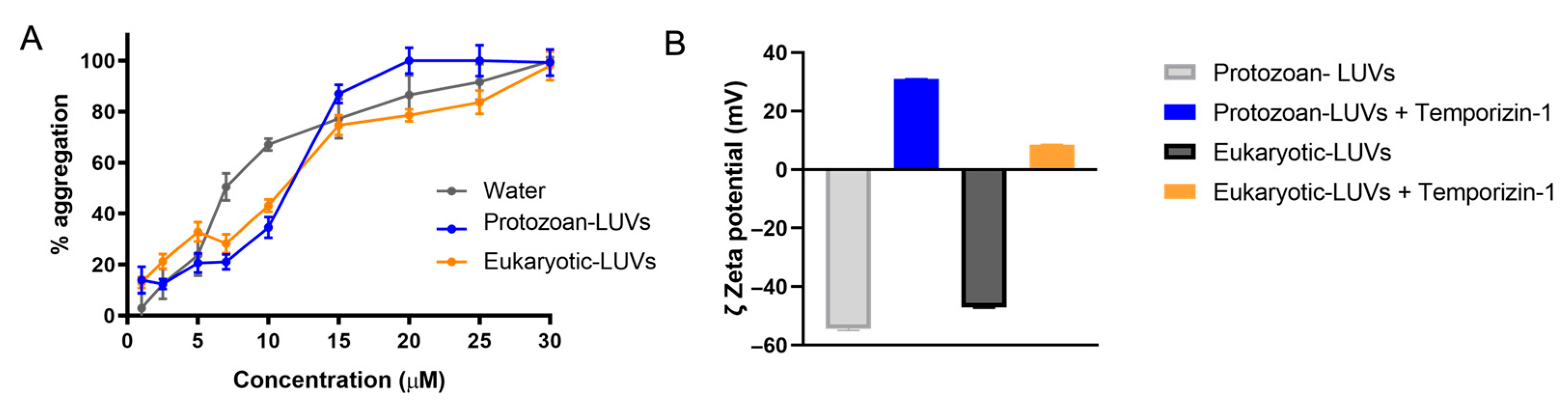
Additionally, the effect on the membrane due to peptide interaction and insertion was investigated in terms of zeta potential, aggregation, and membrane fluidity.
Through the DLS analysis, we observed a strong binding between positively charged Temporizin-1 and protozoan LUVs, where the zeta potential changed from −54 mV to +31 mV. Similarly, this variation was also measured in the presence of eukaryotic LUVs. These significant changes indicate a strong electrostatic interaction between Temporizin-1 and the surface of both types of LUVs driven by the peptide’s net positive charge of +4. Additionally, upon the LUVs’ interaction, the peptide showed a strong propensity to aggregate and change the membrane fluidity both in the presence of protozoan and eukaryotic LUVs. ThT fluorescence measurements revealed a concentration-dependent aggregation, with progressive oligomerization occurring between 2.5 and 50 µM and complete oligomer formation observed at the highest concentrations. Notably, the peptide already displayed partial aggregation (~65%) in aqueous solution at 10 µM, indicating an intrinsic propensity to oligomerize. In the presence of protozoan LUVs, complete aggregation was achieved at 20 µM, whereas in eukaryotic LUVs, full oligomerization was only observed at 30 µM.
In these same conditions, we also observed an increase in the GP value by recording the Laurdan probe emission in both liposomal compositions, indicating a partial modification of the membrane order and a resulting decrease in membrane fluidity, likely due to extensive aggregation on the membrane surface.
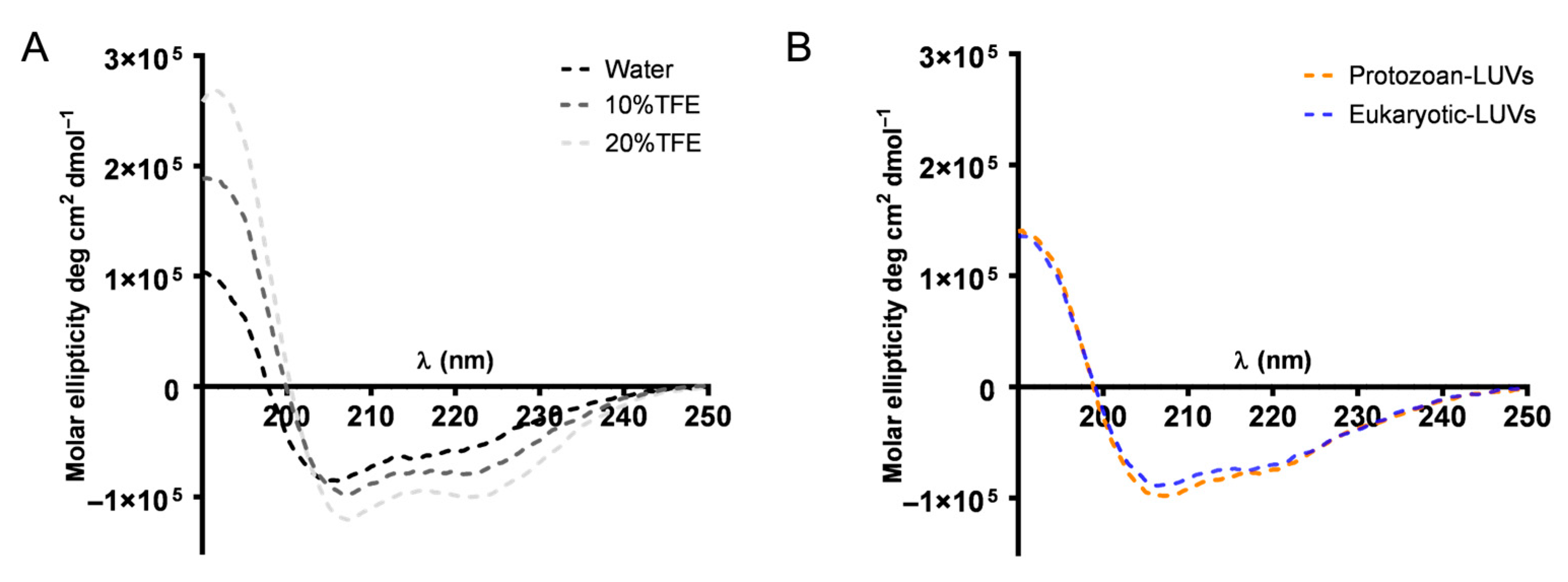
In addition, we also studied the conformational change occurring when AMPs meet the membrane. Generally, AMPs are in a disordered or partially structured state in water, and upon the membrane binding, electrostatic attraction to negatively charged lipid headgroups and hydrophobic interactions with the lipid core can induce folding into structured conformations, typically α-helices or β-sheets [
47]. Interestingly, Temporizin-1 adopts a structured conformation even in aqueous solution. The presence of an α-helical structure plays a crucial role in promoting peptide interactions with target membranes and enabling membrane disruption [
48]. In this amphiphilic arrangement, the cationic and hydrophobic residues are segregated on opposite faces of the helix, thereby facilitating the interaction of antimicrobial peptides (AMPs) with membranes [
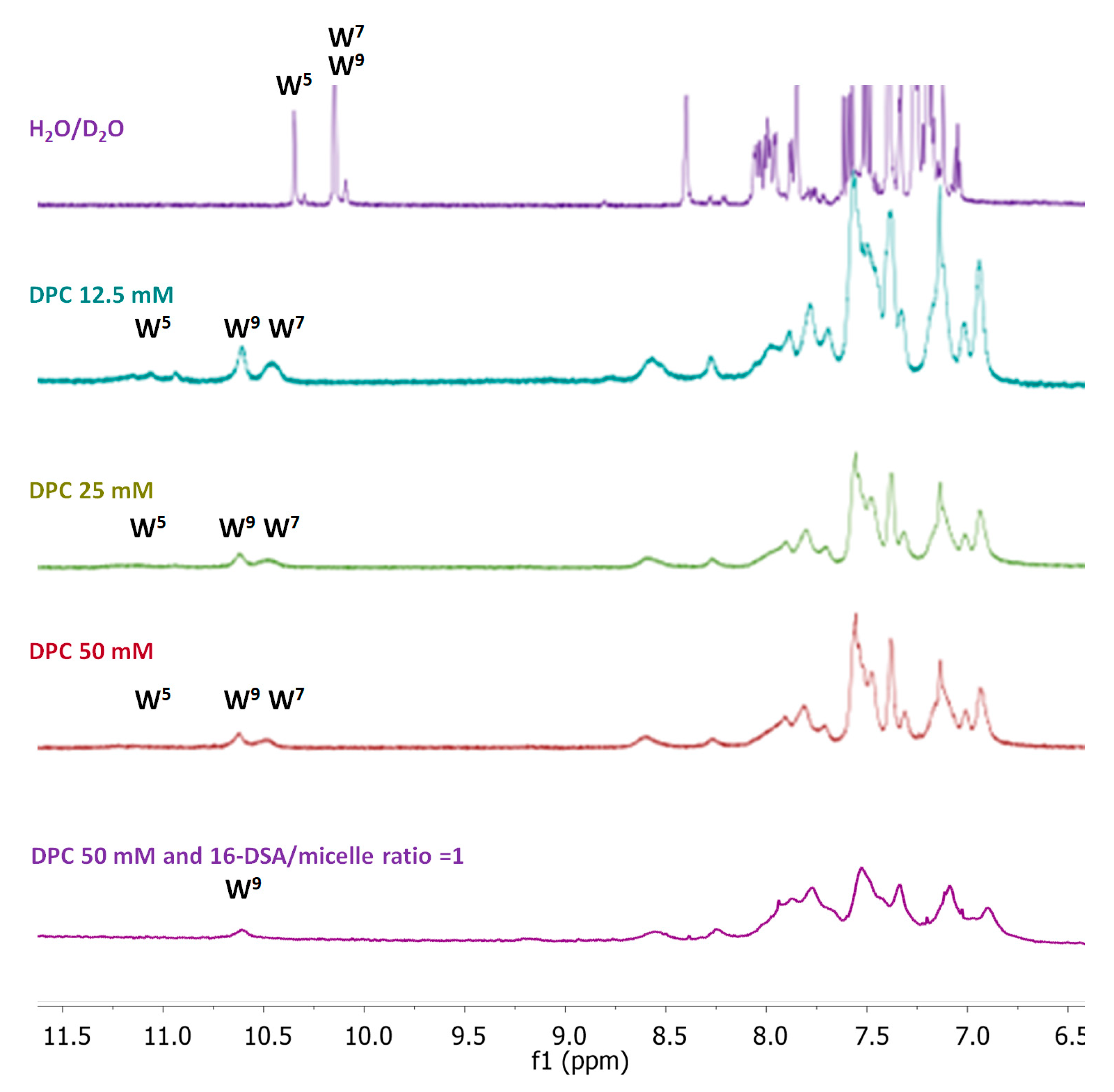
49]. The CD spectrum evidenced the presence of a stable helical structure that is kept after the membrane interaction, as shown by CD spectra recorded in the presence of LUVs. The CD data were further confirmed by NMR analysis. The NMR provided the experimental structure of free Temporizin-1 in water, confirming the molecule’s ability to interact with mimetic micelles, and determined the way the peptide interacts with the micelle. The free state sequence adopts a helical conformation in the L4-L12 segment. Temporizin-1’s ability to interact with micelle systems was analyzed using DPC, a monomer whose aggregation produces micelles of moderate size and is therefore compatible with the observation technique. The Temporizin-1/DPC micelles interaction is confirmed by both the change in chemical shifts and the broadening of the peptide’s spectral signals. This last observation is due to the fact that in its micelle-bound state, the peptide adopts longer rotational correlation times than in its free state, resulting in increased nuclear relaxation rates and signal broadening. This phenomenon also allowed us to deduce the geometry of the peptide–micelle interaction. We can observe how the indole NH proton signals of W5, W7, and W9 shift from their position in the free peptide and that they all broaden, albeit to varying degrees. While the W5 signal broadens so much that it almost disappears, the corresponding signal of W7 broadens less, and that of W9 even less. This ordered pattern of signal widening that sequentially decreases in the C-terminal direction indicates that Temporizin-1 dives into the core of the micelle with its hydrophobic N-terminal portion. This observation is consistent with the effects produced by the addition of 16-DSA to the Temporizin-1 micelle solution, a radical species that penetrates the micelle and “turns off” the resonances of the most internalized portions of the peptide.
Overall, our biophysical results were supported by biological studies demonstrating that Temporizin-1 exerts significant activity against the infective forms of
T. cruzi, in addition to previously reported effects on non-infective epimastigotes [
25]. Notably, Temporizin-1 displayed high selectivity against intracellular amastigotes, with a selectivity index of 15.5. This result is consistent with the strong interaction with the eukaryotic membrane, where its effect consists of the membrane disruption with the ability to reach and kill intracellular amastigotes.
However, biophysical and biological studies on the mode of action demonstrate that Temporizin-1 induces membrane permeabilization, allowing PI entry into intracellular compartments; however, the selective action of the peptide against intracellular forms suggests additional mechanisms, including induction of apoptosis, mitochondrial damage, DNA fragmentation, and immunomodulatory activity [
50].
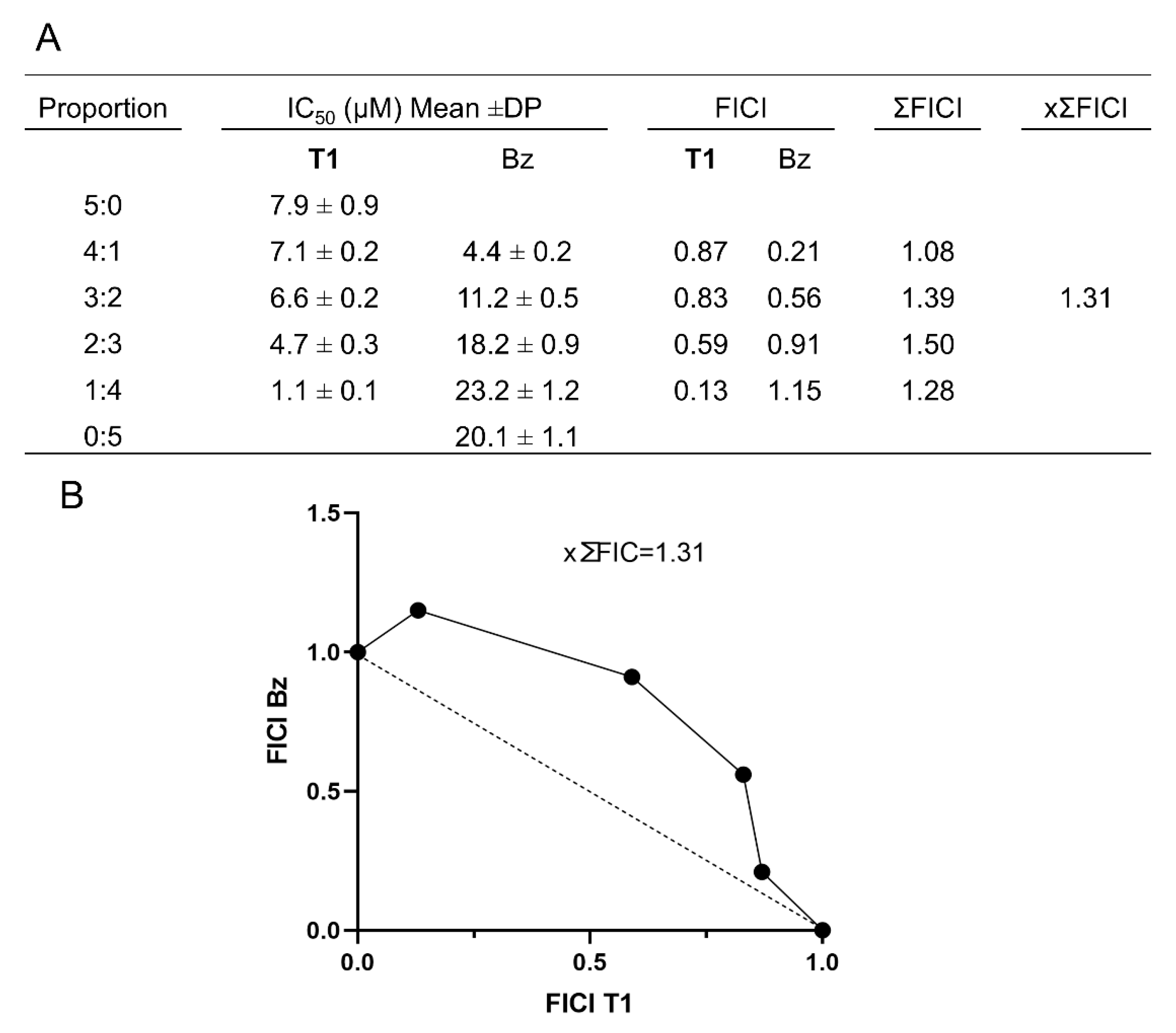
Moreover, the combination of Temporizin-1 with the traditional drug Bz has determined an additive effect (as shown by ×∑FICI ≤ 0.5) that could enhance treatment efficacy by reducing drug dosages, shortening overall treatment duration, and mitigating adverse effects [
51]. This observation aligns with our biophysical findings, suggesting that Temporizin-1 may facilitate drug uptake through perturbation of the protozoan membrane, thereby contributing to the observed synergistic effect.
Based on our biophysical and biological studies, we demonstrated membranolytic activity of Temporizin-1, supporting a potential toroidal or barrel stave mechanism both on protozoan and eukaryotic LUVs. Nevertheless, additional intracellular mechanisms contributing to the peptide’s potential activity within the cell cannot be ruled out. Moreover, the combination of Temporizin-1 with conventional drugs may represent a promising strategy to restore the efficacy of agents that have lost clinical utility due to the development of pathogen resistance.
4. Materials and Methods
4.1. Materials
All conventional Nα-Fmoc-amino acids were acquired from GL Biochem Ltd. (Shanghai, China). Fmoc-Rink amide resin, N,N-diisopropylethylamine (DIEA), piperidine, ergosterol (ERG), and trifluoroacetic acid (TFA) were purchased from Iris-Biotech GMBH (Marktredwitz, Germany). Oxyma pure, N,N′-Diisopropylcarbodiimide (DIC), 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo [4,5-b]pyridinium 3-oxid hexafluorophosphate (HATU), triisopropylsilane (TIS), calcein, Thioflavin T, Laurdan, acrylamide, α-chymotrypsin from bovine pancreas, Deuterium oxide (D2O) (99.9% isotopic purity), dimethyl sulfoxide (DMSO-d6) (99.9% isotopic purity), Dodecylphosphorylcholine-d38 (DPC-d38) (98% isotopic purity), and 16-doxylstearic acid were purchased from Merck (Milan, Italy). Phospholipids: phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), sphingomyelin (SM), phosphatidylserine (PS), Rhodamine and 12-(N-methyl-N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)) (NBD)–phosphatidylethanolamine (Rho-PE and NBD-PE, respectively), and cholesterol (Chol) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Sodium-3-(trimethylsilyl) propionate 2,2,3,3-d4 (TSP) was from Cambridge Isotope Laboratories (CIL), Inc. (Andover, MA, USA).
4.2. Peptide Synthesis
Temporizin-1 was synthesized using the Fmoc/
tBu approach via solid-phase peptide synthesis (SPPS) [
52]. The synthesis was performed using an Fmoc-Rink amide resin (0.67 mmol/g). During the synthesis, the Fmoc removal was performed using two repeated 10 min treatments with 20% piperidine in DMF under shaking at room temperature (rt). The coupling reaction was carried out in two different steps: (i) Fmoc-AA (2 equiv), DIC (2 equiv), and Oxyma Pure (2 quiv), in DMF as solvent for 25 min at rt; (ii) Fmoc-AA (2 equiv), HATU (2 equiv), DIPEA (4 equiv), in DMF for 25 min at rt. Once the final peptide-chain was assembled, Temporizin-1 was cleaved from the resin along with the side-chain protecting groups by treating the resin with a cleavage mixture of TFA:TIS:H
2O (95:2.5:2.5,
v/
v/
v) for 3 h at rt. Then, the peptide was purified by preparative reverse-phase high-performance liquid chromatography (RP-HPLC) using a Phenomenex Kinetex C18 column and a linear gradient of MeCN (0.1% TFA) in water (0.1% TFA) from 10% to 70% over 35 min. Peptide identity was determined by electrospray ionization mass spectrometry (ESI-MS), and purity was assessed by analytical HPLC (Jasco LC-4000, Cremella, Italy) (
Figures S2 and S3).
4.3. Liposome Preparation
Large unilamellar vesicles (LUVs) and small unilamellar vesicles (SUVs), consisting of PC:PE:PI:SM:PS:ERG (35:30:15:5:10:5, ratio in moles) and PC:Chol (70:30, ratio in moles), mimic the protozoan and eukaryotic membranes, respectively. LUVs were prepared using the extrusion method, while SUVs were obtained through ultrasonication. Firstly, we prepared the lipid film by dissolving each phospholipid in chloroform and mixing them with each other at a specific ratio as reported above. Then, the chloroform was removed under nitrogen, and the lipid film was lyophilized overnight. For the LUVs’ preparation, the lipid film was hydrated with water or buffer for 1h and was then freeze–thawed 6 times and extruded 10 times through polycarbonate membranes with 0.1 mm diameter pores [
53]. In all experiments, LUVs were characterized for their average size and polydispersity index (PDI) measured by the Dynamic Light Scattering (DLS) analysis. Specifically, the average size of protozoan and eukaryotic LUVs was 117.4 ± 0.8 nm (PDI = 0.14 ± 0.01) and 124.9 ± 0.3 nm (PDI = 0.15 ± 0.02), respectively. Instead, for the SUVs preparation, the suspension was sonicated for 30 min after the lipid hydration. The average size of protozoan and eukaryotic SUVs was 80.5 ± 0.8 nm (PDI = 0.21 ± 0.01) and 82.5 ± 0.5 nm (PDI = 0.17 ± 0.02), respectively.
4.4. Lipid Mixing Assay
The fusogenic activity of Temporizin-1 was evaluated via fluorescence resonance energy transfer (FRET), using two distinct sets of liposomes, including unlabeled and labeled LUVs [
54]. The liposomes were labeled using NBD as donor and Rho as acceptor probe. We prepared unlabeled LUVs mimicking protozoan membranes by mixing phospholipids PC:PE:PI:SM:PS:ERG (35:30:15:5:10:5, ratio in moles), at a final lipid concentration of 0.16 mM, while labeled LUVs PC:PE:PI:SM:PS:ERG were prepared with 0.6 mol% NBD-PE and 0.6 mol% Rho-PE, at a final lipid concentration of 0.04 mM [
55]. To evaluate membrane mixing, unlabeled and labeled LUVs were mixed at a ratio of 4:1 to obtain a final lipid concentration of 0.1 mM, while Temporizin-1 was added to LUVs at increasing concentrations at lipid/peptide ratios of 0.01, 0.03, 0.05, 0.1, 0.15, and 0.20. The lipid mixing was assessed measuring the NBD emission at 530 nm and Rho emission at 590 nm with an NBD excitation at 465 nm [
56]. To quantify membrane fusion, the percentage of fusion was calculated after each peptide addition. This was normalized against a reference value of 100%, which corresponded to maximal lipid mixing upon the addition of Triton X-100 (0.05%
v/
v). The equation for calculating the percentage of fusion is as follows:
where F530 and F590 represent the intensity at 530 nm and 590 nm, respectively, which we calculated in the presence and absence of the peptide and triton.
4.5. Trp Quenching by Acrylamide
The incorporation of tryptophan residues inside the lipid bilayer was investigated by performing their quenching with acrylamide. Acrylamide is a water-soluble quencher unable to enter lipid bilayers, and this makes it well-suited to detect Trp residues exposed to an aqueous environment [
57]. The experiment was performed in buffer solution and in LUVs mimicking protozoan and eukaryotic membranes (lipid final concentration = 250 µM). Temporizin-1 was dissolved at 10 µM in water or incubated with LUVs and then the quenching was performed at increasing acrylamide concentrations from 0.02 M to 0.22 M [
58]. The Trp fluorescence spectrum was obtained exciting at 295 nm. The data were analyzed using the Stern–Volmer equation: F
0/F = 1 + K
sv[Q], where F
0 and F correspond to the fluorescence intensities before and after the addition of the quencher (Q), respectively, and K
sv is the Stern–Volmer quenching constant, which correlates to the accessibility of the Trp residue to acrylamide.
4.6. Chymotrypsin Proteolysis Monitored by Fluorescence
The insertion of the Trp residue was assessed in the presence of α-chymotrypsin, both in buffer and small unilamellar vesicles (SUVs) mimicking protozoan and eukaryotic membranes. Chymotrypsin has a strong affinity towards aromatic amino acids and cuts peptide bonds involving Trp residues, resulting in an increase in Trp fluorescence [
59]. In the buffer condition, the peptide was dissolved at 10 μM in 100 mM Tris-HCl buffer (pH 7.3) and was then incubated with 3 μM chymotrypsin [
60]. Fluorescence emission was monitored for 15 min using excitation and emission wavelengths of 295 and 355 nm, respectively. Spectra were collected before and after enzyme treatment. The same procedure was performed in the presence of SUVs (lipid final concentration = 250 uM). The peptide at 10 μM was incubated with SUVs for 10 min, and then the enzyme was added. The fluorescence emission of Trp was recorded under the same conditions over a 15 min period.
4.7. Leakage Assay
The ability of Temporizin-1 to induce the pore formation was evaluated by a calcein leakage assay. We prepared LUVs mimicking protozoan and eukaryote membranes loaded with calcein. Once the lipid film was prepared, it was hydrated with a solution of 10 mM Tris, 150 mM NaCl (pH 7.4), and 60 mM calcein for 1 h under stirring [
33]. The liposome suspension was treated for obtaining LUVs, and then free calcein was removed by gel filtration using a Sephadex G-25 column (1.5 × 10 cm) at rt. Calcein release induced by the peptide treatment was monitored by measuring the fluorescence increase setting an excitation fluorescence at 485 nm and an emission at 535 nm. The data were normalized relative to the complete calcein release obtained by adding 0.1% Triton X to LUVs, which causes total liposome disruption. The percentage of leakage at different peptide concentrations was calculated using the formula % leakage = (F
i − F
0)/(F
t − F
0), where F
0 represents the fluorescence of intact LUVs before the addition of the peptide, while F
i and F
t indicate the fluorescence intensity before and after the addition of the peptide and Triton X, respectively.
4.8. Bio-Layer Interferometry (BLI) Experiment
A Bio-Layer Interferometry (BLI) interaction assay was performed by employing the Sartorius Octet
® N1 System and APS Biosensors coated with aminopropylsilane (Sartorius Italy S.r.l., Grassina-Bagno a Ripoli (FI), Italy). Firstly, APS tips were hydrated in 1× PBS buffer for 600 s followed by equilibration for 300 s with PBS. Then, to immobilize the ligand, the tips were dipped, for 1800 s with 1000 rpm, into 200 µL liposome solution (5 mM) followed by wash step with PBS for 300 s to remove unbound vesicles. Afterwards, a blocking step with BSA (1 mg mL
−1) was performed followed by another washing step with PBS for 300 s. Association of Temporizin-1 in a concentration range of 1–20 μM for protozoan LUVs and 1–70 μM for eukaryotic LUVs was performed for 300 s, while the dissociation was monitored for 1800 s with PBS [
61]. All data analysis were processed using the Octet Analysis Studio 12.2 software (Sartorius, Göttingen, Germany). The data were fitted with a 1:1 Langmuir binding model, and the KDs were determined through the global analysis mode.
4.9. Peptide Oligomerization by Thioflavin T Assay
To detect peptide oligomerization in an aqueous solution and in LUVs mimicking protozoan and eukaryotic membranes, we used the fluorescent dye Thioflavin T (ThT) [
62]. ThT strongly binds to aggregated structures, producing an increase in fluorescence emission at 482 nm and excited at 450 nm [
63]. LUVs were prepared at 100 µM and hydrated with the buffer solution 100 mM NaCl, 10 mM Tris-HCl, 25 µM ThT, pH 7.4, for 1 h. The oligomerization was monitored by titrating LUVs with Temporizion-1 concentrations of 5, 2.5, 5, 7, 10, 15, 20, 25, 30, 40 and 50 µM. ThT fluorescence was recorded before and after the addition of the peptides using a Varian Cary Eclipse fluorescence spectrophotometer (Milan, Italy). The emission spectrum was measured at 482 nm upon excitation at 450 nm. Peptide aggregation was expressed as the percentage increase in ThT fluorescence according to the following equation:
where F
f indicates the fluorescence after peptide addition, F
0 the initial fluorescence in the absence of peptides, and F
max is the fluorescence maximum obtained immediately after peptide addition.
4.10. Zeta Potential Measurement
Zeta potential measurements were carried out in the presence of protozoan and eukaryotic LUVs (final lipid concentration = 100 µM), and at peptide concentrations of 20, 30, and 50 µM. These measurements were conducted using a Zetasizer Nano-ZS (Malvern Instruments Ltd., Worcestershire, UK) equipped with a 4 mW He–Ne laser operating at 633 nm. Measurements were taken at a fixed backscattering angle of 173° and at a temperature of 25 °C. The results were performed in triplicate.
4.11. Laurdan-Based Assessment of Membrane Fluidity
To evaluate the effect of Temporizin-1 on membrane fluidity, we used the fluorescent probe Laurdan to observe phase transitions and measure variations in lipid packing, calculating the generalized polarization (GP) parameter [
64]. Laurdan inserts into lipid bilayers and indicates the physical membrane state by altering its emission spectrum. The maximum emission wavelength at 490 nm indicated the liquid-disordered membrane phase, while the emission at 440 nm indicated the more liquid-ordered membrane phase. Lipid films mimicking both membranes were prepared at 100 uM and Laurdan was added to a final concentration of 5 µM. The lipid mixture was lyophilized and then hydrated with PBS 1× (pH 7.4) for 1 h under shaking. The LUVs were obtained by extrusion method as described above. Membrane fluidity was evaluated by incubating the LUVs with the peptide at concentrations from 5 µM to 50 µM. Laurdan emission spectra were recorded from 400 nm to 550 nm with excitation at 365 nm. Peptide-induced changes in membrane fluidity were quantified using the GP value, which is calculated as follows:
where I
440 and I
490 indicate the fluorescence intensities at the maximum emission wavelength in the ordered and disordered phases, respectively.
4.12. Circular Dichroism Studies
The secondary structure of Temporizin-1 was studied by Circular Dichroism (CD) spectroscopy both in solution and in SUVs mimicking protozoan and eukaryotic membranes. CD spectra in water and 2,2,2-Trifluoroethanol (TFE) at 10% and 20% were recorded at 100 μM from 190 to 260 nm using a Jasco J-1500 CD Spectrometer (Cremella, Italy) with a quartz cell (0.1 cm) [
65]. In membrane environments, SUVs (100 μM) were prepared and Temporizin-1 was added at the peptide/lipid ratio of 1. Each CD spectrum was recorded from 190 to 260 nm using a 0.1 cm quartz cell and converting the signal to molar ellipticity.
4.13. Nuclear Magnetic Resonance (NMR) Spectroscopy
Temporizin-1 was dissolved in 0.600 mL H
2O/D
2O (90/10,
v/
v) to achieve a peptide concentration of 0.5–0.6 mM for the NMR analysis. Incremental volumes (μL) of a DPC-d
38 stock solution (1.0 M in H
2O/D
2O, 90/10
v/
v, pH 6) were then added until no further changes were observed in the one-dimensional spectra. The NMR measurements in micellar environments were performed at DPC concentrations of 50 mM and peptide concentration of 0.55 mM for a DPC/peptide molar ratio R of 94. Assuming a micelle aggregation number of ∼30, the micelle/peptide molar ratio R is ∼ 3. NMR spectra were recorded on a Bruker 600 MHz Spectrometer (Department of Pharmacy—University “Federico II” of Naples) and equipped with a z-gradient 5 mm triple-resonance cryoprobe. One-dimensional proton (1D) and two-dimensional homonuclear (2D) NMR spectra were acquired at 298 K. Proton chemical shifts were referred to TSP (0.00 ppm). Two-dimensional TOCSY and NOESY were acquired with typical mixing times of 70 and 300 ms, respectively. Water resonance suppression was achieved using gradient methods. NMR spectra were analyzed by MESTRENOVA 6.0 software (Mestrelab Research, S.L, Santiago de Compostela, Spain) and two-dimensional NMR spectra by CARA program (Version 1.8.4) freeware at (
http://cara.nmr.ch/doku.php/home, accessed on 5 May 2025). Proton resonances were sequentially assigned according to the Wuthrich standard method [
31] and are reported in
Table S1 of the Supplementary Materials. NMR data are accessible by BMRB entry 35009. For the spin-label experiments, a few microliters of a stock solution of 16-doxylstearic acid (16-DSA) in DMSO-d
6 was added to the DPC peptide samples to achieve a 1:1 ratio spin-label/micelle.
4.14. Structure Determinations
The NOE-based distance restraints were derived from NOESY spectra recorded with a mixing time of 300 ms. The NOE cross-peaks were integrated with CARA and converted into upper distance bounds using the CALIBA program [
66] incorporated into CYANA [
40]. Geminal protons were chosen as distance reference with 2.0 Å. Inter-proton NOE-derived distances were applied as upper bounds in the structure calculation performed by the CYANA simulated annealing protocol [
40]. Starting from 100 randomized conformers, structures were generated using the standard CYANA simulated annealing protocol with 20,000 torsion angle dynamics steps per conformer. The statistical treatment of the structures obtained is reported in
Table S1. MolMol [
67] software (Version 6.0.2-5475) was used to analyze the structures and Chimera-UCSF [
40] to cluster them by similarity. Chosen three-dimensional models were visualized by PyMOL software (Version 3.0) (DeLano, CA, USA (2002)).
4.15. Cell Culture
Vero cells (code BCRT 0245), supplied by the Rio de Janeiro Cell Bank (Rio de Janiero, Brazil), were cultured and subcultured weekly using 0.25% trypsin-EDTA solution once they reached 80–100% confluence. After dissociation, the cells were washed and resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). These cultures were then maintained at 37 °C in a humidified incubator with 5% CO2. The cultures were used for both screening assays and propagation of cultured T. cruzi trypomastigotes.
4.16. Trypanosoma cruzi
T. cruzi clone Dm28c (TcI), genetically modified to express luciferase (Dm28c-Luc), was kindly provided by Dr. Cristina Henriques (Oswaldo Cruz Institute—Fiocruz) [
68]. The parasites were cultured in Vero cells maintained in RPMI 1640 medium supplemented with 10% FBS. At four days post-infection (dpi), the supernatant containing trypomastigotes was harvested, and the parasites were centrifuged at 800×
g for 20 min at 4 °C. The resulting parasites were counted using a Neubauer chamber and subsequently used for phenotypic screening and studies of therapeutic combinations.
4.17. Cytotoxicity Assay
Vero cells were seeded at a density of 1.5 × 104 cells/well in white 96-well plates with clear bottoms. After 24 h of culture, the cells were treated with increasing concentrations of Temporizin-1 or benznidazole (Bz), ranging from 3.12 to 100 µM for Temporizin-1 (3.12, 6.25, 12.5, 25, 50, and 100 µM) and 15.62 to 500 µM (15.62, 31.25, 62.5, 125, and 500 µM) for benznidazole. After an incubation period of 72 h, cell viability was assessed using CellTiter Glo reagent (Promega, Milan, Italy), which induces cell lysis and generates luminescence proportional to the intracellular ATP level. Luminescence was measured using a Glomax reader (Promega), and CC50 values were calculated by linear regression analysis using GraphPad Prism software (version 8.2.1). A low concentration of dimethyl sulfoxide (DMSO) (<1%) was employed as a negative control. Results are expressed as the means ± standard deviations of at least three independent experiments, each performed in duplicate.
4.18. Anti-T. cruzi In Vitro Assay
The susceptibility of
T. cruzi (Dm28c-Luc) to treatment with Temporizin-1 was evaluated in both trypomastigote and amastigote stages. For the trypomastigote assays, 1 × 10
6 parasites were inoculated into 96-well white plates with clear bottoms. After a 24 h exposure to different concentrations (0.39, 0.78, 1.56, 3.12, 6.25, 12.5, 25, and 50 µM) of the Temporizin-1 or Bz, D-luciferin (300 µg/mL) was added to measure luciferase activity, serving as a viability indicator for the parasites. Luminescence was measured using the Glomax reader (Promega). To assess the effect of Temporizin-1 on intracellular amastigotes, Vero cells (1.5 × 10
4 cells/well) were cultured and then infected with
T. cruzi Dm28c-Luc at a 10:1 parasite-to-host cell ratio for 24 h. Following infection, the cultures were treated with serial dilutions of the compounds (0.39, 0.78, 1.56, 3.12, 6.25, 12.5, 25, and 50 µM) for 72 h at 37 °C. The viability of the parasite was then measured by luminescence after the addition of D-luciferin (300 µg/mL). The IC
50 values, representing the concentrations required to achieve a 50% reduction in viable parasite population, were calculated using linear regression analysis with GraphPad Prism software version 8.2.1. The selectivity index (SI) was derived from the ratio of the CC
50 in Vero cells to the IC
50 against
T. cruzi, providing insight into the therapeutic margin of the tested compounds. DMSO concentrations (<1%) served as a negative control. Each experimental condition was replicated in duplicate in a minimum of three independent trials [
69].
4.19. Combination Assay
To investigate the interaction between Temporizin-1 and Bz, we used the isobologram approach in
T. cruzi trypomastigotes [
70]. We assessed combinations of varying concentrations of Bz and Temporizin-1 at ratios of 5:0, 4:1, 3:2, 2:3, 1:4, and 0:5, by adding these combinations to 96-well plates containing trypomastigotes (1 × 10
6 parasites per well) for a 24 h treatment period. Following exposure, we evaluated parasite viability using luminescence measured by a Glomax reader after the addition of luciferin. For each treatment combination, we calculated the IC
50 values and derived the fractional inhibitory concentration index (FICI) using the following equation: FICI = IC
50 of the combination/IC
50 of the individual compound. The mean FICI (×ΣFICI) represented the average across the combination groups. Interactions classification was based on ×ΣFICI values as follows: synergistic (×ΣFICI ≤ 0.5), additive (0.5 < ×ΣFICI < 4), or antagonistic (×ΣFICI > 4). Isobologram plots were generated using GraphPad Prism version 8.2.1, with mean FICIs derived from three independent experiments.
4.20. Membrane Permeability Assay
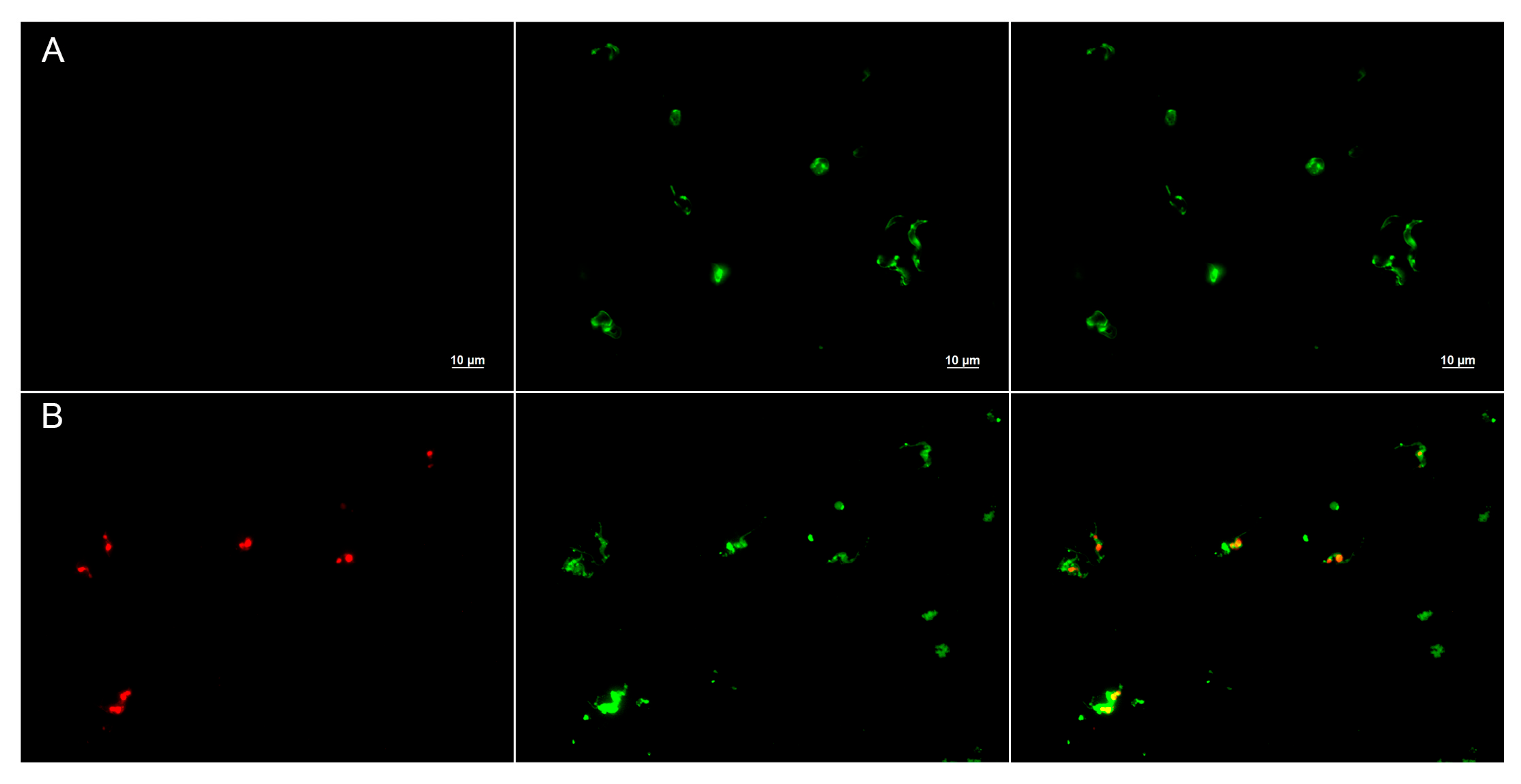
Cytoplasmic membrane damage was determined using steady-state fluorescence, as described before [
71], with some modifications. Briefly, tissue cultured with trypomastigotes (1 × 10
6) was treated with 10 µM of Temporizin-1 for 24 h. Next, cultures were incubated with 10 µM of propidium iodide (PI) at 37 °C for 15 min in the dark. In addition, parasite membranes were stained with Concanavalin A-FITC (50 µg/mL) to allow visualization of glycoproteins on the parasite membrane. Cells were collected by centrifugation (10,000×
g for 5 min) and washed three times in PBS. A final cell suspension was smeared onto a glass slide for imaging on an Axio Imager M2 microscope (Carl Zeiss, Oberkochen, Germany).