Antimicrobial Resistance of Staphylococcus borealis Isolated from Pig Farms: High Prevalence of SCCmec Type V and Emergence of cfr-Positive Isolates
Abstract
1. Introduction
2. Results
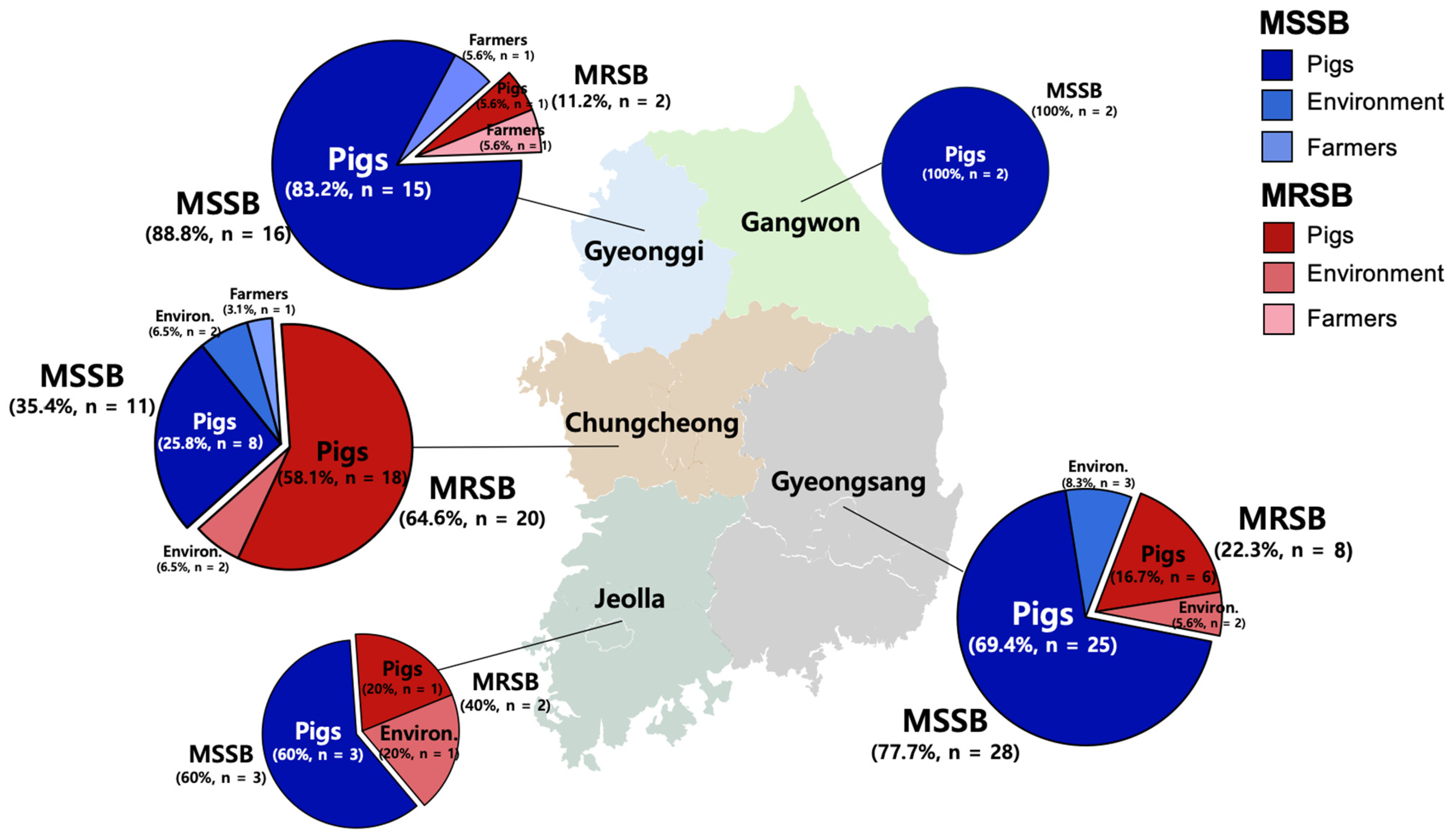
2.1. Prevalence of S. borealis in Pig Farms in Korea
2.2. AMR Profiles of S. borealis Isolates
2.3. High Prevalence of SCCmec V Among MRSB Isolates
2.4. Identification of cfr-Positive Isolates and Comparative Analysis of cfr-Containing Regions
3. Discussion
4. Materials and Methods
4.1. Swab Samples and Isolation of S. borealis
4.2. Antimicrobial Susceptibility Test
4.3. SCCmec Typing and Detection of cfr
4.4. WGS Analysis
4.5. Comparative Analysis of SCCmec V and cfr
4.6. Nucleotide Sequence Accession Number
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fluit, A. Livestock-associated Staphylococcus aureus. Clin. Microbiol. Infect. 2012, 18, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.R. Livestock-associated Staphylococcus aureus: Origin, evolution and public health threat. Trends Microbiol. 2012, 20, 192–198. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.C.; Rodrigues, M.X.; Silva, N.C.C. Methicillin-resistant Staphylococcus aureus in food and the prevalence in Brazil: A review. Braz. J. Microbiol. 2020, 51, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.A.; Vanderhaeghen, W.; Butaye, P. Antimicrobial resistance and population structure of Staphylococcus epidermidis recovered from pig farms in Belgium. Vet. J. 2015, 203, 302–308. [Google Scholar] [CrossRef]
- Vanderhaeghen, W.; Vandendriessche, S.; Crombé, F.; Dispas, M.; Denis, O.; Hermans, K.; Haesebrouck, F.; Butaye, P. Species and staphylococcal cassette chromosome mec (SCCmec) diversity among methicillin-resistant non-Staphylococcus aureus staphylococci isolated from pigs. Vet. Microbiol. 2012, 158, 123–128. [Google Scholar] [CrossRef]
- Osman, K.; Badr, J.; Al-Maary, K.S.; Mousse, I.M.I.; Hessain, A.M.; Girah, Z.M.S.A.; Abo-shamas, U.H.; Orabi, A.; Saad, A. Prevalence of the antibiotic resistance genes in coagulase-positive-and negative-Staphylococcus in chicken meat retailed to consumers. Front. Microbiol. 2016, 7, 1846. [Google Scholar] [CrossRef]
- Price, L.B.; Stegger, M.; Hasman, H.; Aziz, M.; Larsen, J.; Andersen, P.S.; Pearson, T.; Waters, A.E.; Foster, J.T.; Schupp, J. Staphylococcus aureus CC398: Host adaptation and emergence of methicillin resistance in livestock. mBio 2012, 3, 00305–00311. [Google Scholar] [CrossRef]
- Pirolo, M.; Gioffrè, A.; Visaggio, D.; Gherardi, M.; Pavia, G.; Samele, P.; Ciambrone, L.; Di Natale, R.; Spatari, G.; Casalinuovo, F. Prevalence, molecular epidemiology, and antimicrobial resistance of methicillin-resistant Staphylococcus aureus from swine in southern Italy. BMC Microbiol. 2019, 19, 51. [Google Scholar] [CrossRef]
- Venugopal, N.; Mitra, S.; Tewari, R.; Ganaie, F.; Shome, R.; Rahman, H.; Shome, B.R. Molecular detection and typing of methicillin-resistant Staphylococcus aureus and methicillin-resistant coagulase-negative staphylococci isolated from cattle, animal handlers, and their environment from Karnataka, Southern Province of India. Vet. World 2019, 12, 1760. [Google Scholar] [CrossRef]
- Feßler, A.T.; Kadlec, K.; Hassel, M.; Hauschild, T.; Eidam, C.; Ehricht, R.; Monecke, S.; Schwarz, S. Characterization of methicillin-resistant Staphylococcus aureus isolates from food and food products of poultry origin in Germany. Appl. Environ. Microbiol. 2011, 77, 7151–7157. [Google Scholar] [CrossRef]
- Tulinski, P.; Fluit, A.C.; Wagenaar, J.A.; Mevius, D.; van de Vijver, L.; Duim, B. Methicillin-resistant coagulase-negative staphylococci on pig farms as a reservoir of heterogeneous staphylococcal cassette chromosome mec elements. Appl. Environ. Microbiol. 2012, 78, 299–304. [Google Scholar] [CrossRef]
- Takahashi, T.; Kim, H.; Kim, H.S.; Kim, H.S.; Song, W.; Kim, J.S. Comparative genomic analysis of staphylococcal cassette chromosome mec type V Staphylococcus aureus strains and estimation of the emergence of SCCmec V clinical isolates in Korea. Ann. Lab. Med. 2024, 44, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Wolska-Gębarzewska, M.; Międzobrodzki, J.; Kosecka-Strojek, M. Current types of staphylococcal cassette chromosome mec (SCC mec) in clinically relevant coagulase-negative staphylococcal (CoNS) species. Crit. Rev. Microbiol. 2024, 50, 1020–1036. [Google Scholar] [CrossRef] [PubMed]
- Saber, H.; Jasni, A.S.; Jamaluddin, T.Z.M.T.; Ibrahim, R. A review of staphylococcal cassette chromosome mec (SCCmec) types in coagulase-negative staphylococci (CoNS) species. Malays. J. Med. Sci. 2017, 24, 7. [Google Scholar] [CrossRef] [PubMed]
- Srednik, M.E.; Tremblay, Y.D.; Labrie, J.; Archambault, M.; Jacques, M.; Fernández Cirelli, A.; Gentilini, E.R. Biofilm formation and antimicrobial resistance genes of coagulase-negative staphylococci isolated from cows with mastitis in Argentina. FEMS Microbiol. Lett. 2017, 364, fnx001. [Google Scholar] [CrossRef]
- Rossi, C.C.; Pereira, M.F.; Giambiagi-deMarval, M. Underrated Staphylococcus species and their role in antimicrobial resistance spreading. Genet. Mol. Biol. 2020, 43, e20190065. [Google Scholar] [CrossRef]
- Cuny, C.; Arnold, P.; Hermes, J.; Eckmanns, T.; Mehraj, J.; Schoenfelder, S.; Ziebuhr, W.; Zhao, Q.; Wang, Y.; Feßler, A.T. Occurrence of cfr-mediated multiresistance in staphylococci from veal calves and pigs, from humans at the corresponding farms, and from veterinarians and their family members. Vet. Microbiol. 2017, 200, 88–94. [Google Scholar] [CrossRef]
- Ruiz-Ripa, L.; Feßler, A.T.; Hanke, D.; Sanz, S.; Olarte, C.; Mama, O.M.; Eichhorn, I.; Schwarz, S.; Torres, C. Coagulase-negative staphylococci carrying cfr and PVL genes, and MRSA/MSSA-CC398 in the swine farm environment. Vet. Microbiol. 2020, 243, 108631. [Google Scholar] [CrossRef]
- Chen, H.; Du, Y.; Xia, Q.; Li, Y.; Song, S.; Huang, X. Role of linezolid combination therapy for serious infections: Review of the current evidence. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1043–1052. [Google Scholar] [CrossRef]
- Schwarz, S.; Werckenthin, C.; Kehrenberg, C. Identification of a plasmid-borne chloramphenicol-florfenicol resistance gene in Staphylococcus sciuri. Antimicrob. Agents Chemother. 2000, 44, 2530–2533. [Google Scholar] [CrossRef]
- Pain, M.; Wolden, R.; Jaén-Luchoro, D.; Salvà-Serra, F.; Iglesias, B.P.; Karlsson, R.; Klingenberg, C.; Cavanagh, J.P. Staphylococcus borealis sp. nov., isolated from human skin and blood. Int. J. Syst. Evol. Microbiol. 2020, 70, 6067–6078. [Google Scholar] [CrossRef]
- Cavanagh, J.P.; Klingenberg, C.; Venter, H.J.; Afset, J.E.; Stromme, O.; Lindemann, P.C.; Johansen, T.; Zaragkoulias, K.; Aamot, H.V.; Tofteland, S. Revealing the clinical relevance of Staphylococcus borealis. Microbiol. Spectr. 2025, 13, e01988-24. [Google Scholar] [CrossRef]
- Abdullahi, I.N.; Lozano, C.; Simón, C.; Zarazaga, M.; Torres, C. Within-host diversity of coagulase-negative staphylococci resistome from healthy pigs and pig farmers, with the detection of cfr-carrying strains and MDR-S. borealis. Antibiotics 2023, 12, 1505. [Google Scholar] [CrossRef]
- Lee, G.Y.; Seong, H.J.; Sul, W.J.; Yang, S.J. Genomic information on linezolid-resistant sequence-type 398 livestock-associated methicillin-resistant Staphylococcus aureus isolated from a pig. Foodborne Pathog. Dis. 2021, 18, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Kim, G.B.; Yang, S.J. Co-occurrence of cfr-mediated linezolid-resistance in ST398 LA-MRSA and non-aureus staphylococci isolated from a pig farm. Vet. Microbiol. 2022, 266, 109336. [Google Scholar] [CrossRef] [PubMed]
- Weßels, C.; Strommenger, B.; Klare, I.; Bender, J.; Messler, S.; Mattner, F.; Krakau, M.; Werner, G.; Layer, F. Emergence and control of linezolid-resistant Staphylococcus epidermidis in an ICU of a German hospital. J. Antimicrob. Chemother. 2018, 73, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Król, J.; Wanecka, A.; Twardoń, J.; Florek, M.; Marynowska, M.; Banaszkiewicz, S.; Kaczmarek-Pieńczewska, A.; Pląskowska, E.; Brodala, M.; Chwirot, W. Staphylococcus borealis–A newly identified pathogen of bovine mammary glands. Vet. Microbiol. 2023, 286, 109876. [Google Scholar] [CrossRef]
- Back, S.H.; Eom, H.S.; Lee, H.H.; Lee, G.Y.; Park, K.T.; Yang, S.J. Livestock-associated methicillin-resistant Staphylococcus aureus in Korea: Antimicrobial resistance and molecular characteristics of LA-MRSA strains isolated from pigs, pig farmers, and farm environment. J. Vet. Sci. 2020, 21, e2. [Google Scholar] [CrossRef]
- Lee, G.Y.; Lee, H.H.; Yang, S.J. Antimicrobial resistance profiles and clonal diversity of Staphylococcus epidermidis isolates from pig farms, slaughterhouses, and retail pork. Vet. Microbiol. 2023, 282, 109753. [Google Scholar] [CrossRef]
- Scott, H.M.; Acuff, G.; Bergeron, G.; Bourassa, M.W.; Gill, J.; Graham, D.W.; Kahn, L.H.; Morley, P.S.; Salois, M.J.; Simjee, S.; et al. Critically important antibiotics: Criteria and approaches for measuring and reducing their use in food animal agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 8–16. [Google Scholar] [CrossRef]
- Lim, S.K.; Lee, J.E.; Lee, H.S.; Nam, H.M.; Moon, D.C.; Jang, G.C.; Park, Y.J.; Jung, Y.G.; Jung, S.C.; Wee, S.H. Trends in antimicrobial sales for livestock and fisheries in Korea during 2003–2012. Korean J. Vet. Res. 2014, 54, 81–86. [Google Scholar] [CrossRef]
- Moon, D.C.; Jeong, S.K.; Hyun, B.H.; Lim, S.K. Prevalence and characteristics of methicillin-resistant Staphylococcus aureus isolates in pigs and pig farmers in Korea. Foodborne Pathog. Dis. 2019, 16, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, P.; Wu, J.; Chen, S.; Jin, Y.; Long, J.; Duan, G.; Yang, H. Transmission of livestock-associated methicillin-resistant Staphylococcus aureus between animals, environment, and humans in the farm. Environ. Sci. Pollut. Res. 2023, 30, 86521–86539. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Lee, G.Y.; Park, J.H.; Yang, S.J. High prevalence of clonal complex 398 methicillin-susceptible and-resistant Staphylococcus aureus in pig farms: Clonal lineages, multiple drug resistance, and occurrence of the staphylococcal cassette chromosome mec IX. Foodborne Pathog. Dis. 2023, 20, 100–109. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Z.; Fu, J.; Cai, J.; Ma, T.; Xie, N.; Fan, R.; Zhai, W.; Feßler, A.T.; Sun, C. Spreading of cfr-carrying plasmids among staphylococci from humans and animals. Microbiol. Spectr. 2022, 10, e02461-22. [Google Scholar] [CrossRef]
- Lee, G.Y.; Yang, S.J. Occurrence of cfr-positive linezolid-susceptible Staphylococcus aureus and non-aureus Staphylococcal isolates from pig farms. Antibiotics 2023, 12, 359. [Google Scholar] [CrossRef]
- Lee, J.B.; Lim, J.H.; Park, J.H.; Lee, G.Y.; Park, K.T.; Yang, S.J. Genetic characteristics and antimicrobial resistance of Staphylococcus aureus isolates from pig farms in Korea: Emergence of cfr-positive CC398 lineage. Bmc Vet. Res. 2024, 20, 503. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standard Institute (CLSI). VET01S Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 5th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Geha, D.J.; Uhl, J.R.; Gustaferro, C.A.; Persing, D.H. Multiplex PCR for identification of methicillin-resistant staphylococci in the clinical laboratory. J. Clin. Microbiol. 1994, 32, 1768–1772. [Google Scholar] [CrossRef]
- Kondo, Y.; Ito, T.; Ma, X.X.; Watanabe, S.; Kreiswirth, B.N.; Etienne, J.; Hiramatsu, K. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: Rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob. Agents Chemother. 2007, 51, 264–274. [Google Scholar] [CrossRef]
- Tsai, K.; Stojković, V.; Noda-Garcia, L.; Young, I.D.; Myasnikov, A.G.; Kleinman, J.; Palla, A.; Floor, S.N.; Frost, A.; Fraser, J.S. Directed evolution of the rRNA methylating enzyme Cfr reveals molecular basis of antibiotic resistance. eLife 2022, 11, e70017. [Google Scholar] [CrossRef]
- Naorem, R.S.; Urban, P.; Goswami, G.; Fekete, C. Characterization of methicillin-resistant Staphylococcus aureus through genomics approach. 3 Biotech 2020, 10, 401. [Google Scholar] [CrossRef]


| No. of Isolates/No. of Samples (%) | |||
|---|---|---|---|
| S. borealis (n = 92) | Pig Farms (n = 92/1009, 9.1%) | ||
| Pigs | Farmers | Environ. 1 | |
| MRSB 2 (n = 32/92) | 26/760 (3.4%) | 1/34 (2.9%) | 5/215 (2.3%) |
| MSSB 3 (n = 60/92) | 53/760 (7.0%) | 2/34 (5.9%) | 5/215 (2.3%) |
| Total | 79/760 (10.4%) | 3/34 (8.8%) | 10/215 (4.6%) |
| Number (%) of Isolates Resistant to 1 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP | FOX | CHL | CIP | CLI | ERY | GEN | MUP | RIF | SXT | SYN | TET | LZD | MDR | ||
| MRSB 2 (n = 32) | Pigs (n = 26) | 26 (100) | 26 (100) | 25 (96.2) | 0 | 26 (100) | 0 | 5 (19.2) | 0 | 0 | 20 (76.9) | 2 (7.7) | 25 (96.2) | 0 | 26 (100) |
| Farmers (n = 1) | 1 (100) | 1 (100) | 1 (100) | 0 | 1 (100) | 1 (100) | 1 (100) | 0 | 0 | 0 | 0 | 1 (100) | 0 | 1 (100) | |
| Environ. 4 (n = 5) | 4 (80) | 5 (100) | 4 (80) | 0 | 4 (80) | 0 | 0 | 0 | 0 | 2 (40) | 0 | 4 (80) | 0 | 4 (80) | |
| Total | 31 (96.9) | 32 (100) | 30 (93.8) | 0 | 31 (96.9) | 1 (3.1) | 6 (18.8) | 0 | 0 | 22 (68.8) | 2 (6.3) | 30 (93.8) | 0 | 31 (96.9) | |
| MSSB 3 (n = 60) | Pigs (n = 53) | 2 (3.8) | 0 | 41 (77.4) | 1 (1.9) | 44 (83) | 26 (49.1) | 1 (1.9) | 0 | 0 | 0 | 1 (1.9) | 21 (39.6) | 0 | 27 (50.9) |
| Farmers (n = 2) | 0 | 0 | 2 (100) | 0 | 2 (100) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Environ. (n = 5) | 0 | 0 | 5 (100) | 0 | 5 (100) | 3 (60) | 0 | 0 | 0 | 0 | 0 | 3 (60) | 0 | 3 (60) | |
| Total | 2 (3.3) | 0 | 48 (80) | 1 (1.7) | 51 (85) | 29 (48.3) | 1 (1.7) | 0 | 0 | 0 | 1 (1.7) | 24 (40) | 0 | 30 (50) | |
| Total (n = 92) | 33 (35.9) | 32 (34.8) | 78 (84.8) | 1 (1.1) | 82 (89.1) | 30 (32.6) | 7 (7.6) | 0 | 0 | 22 (23.9) | 3 (3.3) | 54 (58.7) | 0 | 61 (66.3) | |
| No. of S. borealis Isolates | ||||
|---|---|---|---|---|
| Methicillin Resistance | Carriage of cfr | |||
| MR/MS | mecA | SCCmec Type | ||
| MRSB 1 (n = 32) | Pigs (n = 26) | 26/26 (100%) | SCCmec V (26/26, 100%) SCCmec V (1/1, 100%) SCCmec V (5/5, 100%) | 17/26 (65.4%) |
| Farmers (n = 1) | 1/1 (100%) | 1/1 (100%) | ||
| Environ. 3 (n = 5) | 5/5 (100%) | 2/5 (40.0%) | ||
| Total | 32/32 (100%) | 32/32 (100%) | 20/32 (62.5%) | |
| MSSB 2 (n = 60) | Pigs (n = 53) | - | - | 2/53 (3.8%) |
| Farmers (n = 2) | - | - | - | |
| Environ. (n = 5) | - | - | - | |
| Total | - | - | 2/60 (3.3%) | |
| Total (n = 92) | 32/92 (34.8%) | 22/92 (23.9%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.H.; Park, J.H.; Lee, G.Y.; Yang, S.-J. Antimicrobial Resistance of Staphylococcus borealis Isolated from Pig Farms: High Prevalence of SCCmec Type V and Emergence of cfr-Positive Isolates. Antibiotics 2025, 14, 910. https://doi.org/10.3390/antibiotics14090910
Lim JH, Park JH, Lee GY, Yang S-J. Antimicrobial Resistance of Staphylococcus borealis Isolated from Pig Farms: High Prevalence of SCCmec Type V and Emergence of cfr-Positive Isolates. Antibiotics. 2025; 14(9):910. https://doi.org/10.3390/antibiotics14090910
Chicago/Turabian StyleLim, Ji Hyun, Ji Heon Park, Gi Yong Lee, and Soo-Jin Yang. 2025. "Antimicrobial Resistance of Staphylococcus borealis Isolated from Pig Farms: High Prevalence of SCCmec Type V and Emergence of cfr-Positive Isolates" Antibiotics 14, no. 9: 910. https://doi.org/10.3390/antibiotics14090910
APA StyleLim, J. H., Park, J. H., Lee, G. Y., & Yang, S.-J. (2025). Antimicrobial Resistance of Staphylococcus borealis Isolated from Pig Farms: High Prevalence of SCCmec Type V and Emergence of cfr-Positive Isolates. Antibiotics, 14(9), 910. https://doi.org/10.3390/antibiotics14090910






