Combined Toxicity of Microplastics and Antimicrobials on Animals: A Review
Abstract
1. Introduction
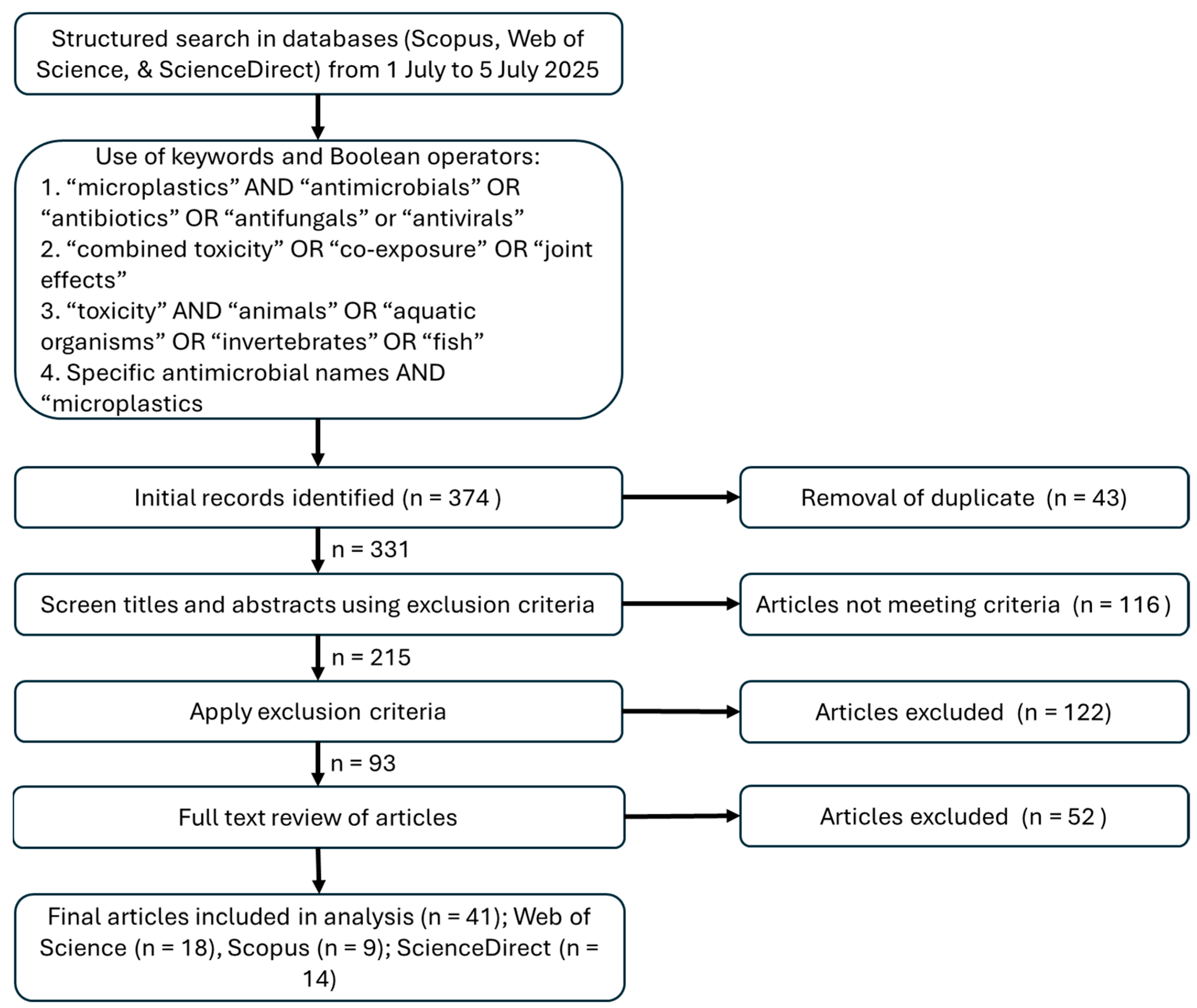
2. Review Methodology
- “microplastics” AND “antimicrobials” OR “antibiotics” OR “antifungals” OR “antivirals”
- “combined toxicity” OR “co-exposure” OR “joint effects”
- “toxicity” AND “animals” OR “aquatic organisms” OR “invertebrates” OR “fish”
- Specific antimicrobial names (e.g., sulfamethoxazole, tetracycline, ciprofloxacin) AND “microplastics”
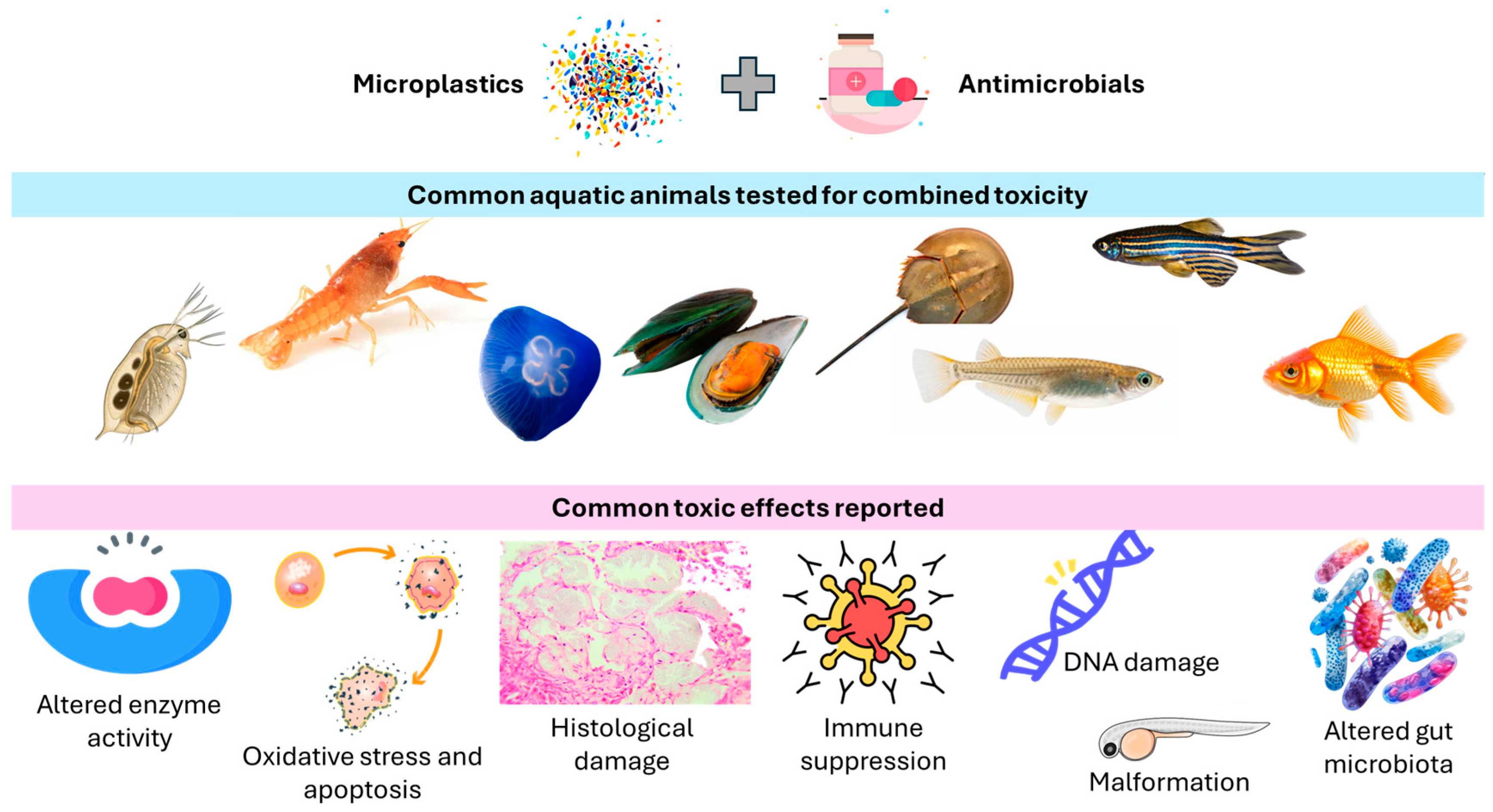
3. Toxicity on Aquatic Animals
3.1. Crustaceans
3.2. Mollusks
3.3. Freshwater Fish Models
3.4. Marine Fish Models
3.5. Other Aquatic Animals
| Organism Type | Species | Microplastics + Antimicrobials | Main Effects | Reference |
|---|---|---|---|---|
| Crustaceans | Daphnia magna | Polystyrene microplastics (1 and 10 μm) + roxithromycin (0.01 mg/L) | Co-exposure reduced glutathione peroxidase activity, lowered malondialdehyde levels (1 μm), decreased glutathione S-transferase activity (10 μm); oxidative stress modulation; 1 μm microplastics mitigated roxithromycin toxicity | [22] |
| Daphnia magna | UV-aged polystyrene microplastics (0.1, 10 µg/L) + roxithromycin | Increased F0 survival (20–40%), mitigated reproductive toxicity in F0; in F1, reproductive toxicity worsened; co-exposure shifted swimming inhibition to stimulation; elevated acetylcholinesterase activity (1.61–3.25×), increased oxidative damage | [41] | |
| Daphnia magna | 5.8 µm polystyrene microplastics (≥2 mg/L) + triclosan, triclocarban, or methyl-triclosan | Chronic exposure delayed first brood, reduced brood frequency and offspring; polystyrene enhanced antimicrobial reproductive toxicity; methyl-triclosan + polystyrene caused greatest population decline | [42] | |
| Ceriodaphnia dubia | 1 µm polystyrene microplastics + acyclovir or imidacloprid | Short-term antagonistic genotoxicity; reproduction decreased by 54.8% at 0.15 µg/L polystyrene + 0.0379 µg/L acyclovir; chronic toxicity increased at low concentration | [43] | |
| Procambarus clarkii | Polystyrene microplastics (100 mg/L) + pyrogallol (10 mg/L) | Altered hemocyte counts; liver enzymes increased; antioxidant enzymes decreased; histological damage | [44] | |
| Mollusks | Mytilus coruscus | Polystyrene microplastics + norfloxacin (≤500 µg/L) | Upregulated antioxidant genes (CYP3A-1, Nrf2); immune genes altered (IRAK-1, IRAK-4, HSP70); microplastics intensified norfloxacin effects on antioxidant and immune responses | [48] |
| Thick-shelled mussels | Polystyrene microplastics (0.26 mg/L, 500 nm) + oxytetracycline (270 ng/L), florfenicol (42 ng/L), or sulfamethoxazole (140 ng/L) | Co-exposure impaired immune function: reduced phagocytic rates (11–34%), total hemocyte counts (37–62%), increased reactive oxygen species, disrupted cytoskeleton, suppressed immune/detoxification gene expression | [45] | |
| Corbicula fluminea | Polystyrene microplastics/nanoplastics + ciprofloxacin | Oxidative stress, neurotoxicity, digestive gland damage; reduced filtration rate; polystyrene lowered ciprofloxacin toxicity in digestive gland but increased siphoning inhibition in nano-polystyrene + antibiotic group | [46] | |
| Tegillarca granosa | Polystyrene microplastics + oxytetracycline, or florfenicol | Reactive oxygen species increased; immune gene suppression; DNA damage; hemocyte viability decreased; apoptosis gene upregulated | [47] | |
| Freshwater fish | Carassius auratus | Polystyrene microplastics + oxytetracycline | Liver and intestinal damage; immune suppression | [49] |
| Danio rerio | Polystyrene microplastics + sulfamethoxazole | Increased mortality (25%), malformation (20–35%), reduced fetal movement (31–37%), swimming activity (27–37%), elevated heart rate (19–21%); endocrine disruption (vitellogenin, 17β-estradiol, testosterone, T3); slight antagonistic effect | [19] | |
| Danio rerio | Aged polystyrene microplastics + penicillin | Pristine and UV-aged polystyrene + penicillin reduced heartbeat, impaired movement; ozonated polystyrene had no effect; co-exposure showed antagonistic effects with aged polystyrene | [50] | |
| Danio rerio | Polystyrene microplastics (10 mg/L) + 3,6-dibromocarbazole (0.5 mg/L) | Embryo deformities increased; reactive oxygen species decreased in co-exposure | [51] | |
| Danio rerio | Polystyrene microplastics + difenoconazole | Reduced difenoconazole accumulation; mitigated liver oxidative stress; moderated gene expression changes | [52] | |
| Danio rerio | Polystyrene microplastics (1 mg/L) + ketoconazole or fluconazole (1 mg/L) | Reduced hatching, survival, and heart rates; increased malformations, catalase activity, bax/bcl2 ratio; polystyrene intensified azole antifungal toxicity via reactive oxygen species and apoptosis | [53] | |
| Danio rerio | Polyethylene microplastics + tetracycline | Reduced heartbeats, heart toxicity; increased mortality; shortened body length, deformities; elevated reactive oxygen species; inflammatory response; altered gene expression | [55] | |
| Danio rerio | Microplastics + sulfamethazine | Larvae: liver damage, macrophage/neutrophil reduction, elevated inflammatory cytokines and antioxidant activity; adults: altered oxidative stress, inflammation, MAPK signaling, liver apoptosis | [56] | |
| Oreochromis sp. | Polystyrene microplastics (100 µg/L) + roxithromycin (50 µg/L) | Increased tissue roxithromycin accumulation; reduced neurotoxicity; altered CYP450 liver enzyme activities; increased superoxide dismutase, decreased malondialdehyde; oxidative stress mitigation | [21] | |
| Carassius auratus | Aged microplastics + roxithromycin | Increased liver/gut antioxidant activity; suppressed brain acetylcholinesterase; smaller microplastics caused more liver/gill/brain damage; larger microplastics caused more intestinal injury; altered gut microbiota/metabolites | [57] | |
| Pelteobagrus fulvidraco | Polystyrene microplastics (100 or 500 µg/L) + oxytetracycline (500 ng/L) | 100 μg/L + oxytetracycline: mild intestinal damage, increased superoxide dismutase and catalase, higher Proteobacteria; 500 μg/L + oxytetracycline: suppressed growth, digestion impairment, oxidative stress, reduced Firmicutes; synergistic toxicity | [58] | |
| Marine fish | Pomatoschistus microps | Polystyrene microplastics + cefalexin (≥1.25 mg/L) | Predation decreased; acetylcholine and lipid peroxidation increased; temperature-dependent | [59] |
| Oryzias melastigma | Polylactic acid microplastics + sulfamethazine | Weight gain (20.9–26.2%); fatty liver symptoms; gut microbiota altered | [60] | |
| Oryzias melastigma | Polystyrene microplastics (0.2% w/w in food) + tetracyclines (50 µg/L) | Weight gain and liver lipid decreased; suppressed body length growth; gut microbiota altered | [61] | |
| Other aquatic taxa | Aurelia aurita | Polystyrene microplastics + tetracycline | Apoptosis increased; oxidative stress; metabolic disruption | [62] |
| Tachypleus tridentatus | Polystyrene nanoplastics (104 particles/L) + norfloxacin (0–5 µg/L) | Oxidative stress; microbiota Bacteroidetes increased | [63] |
3.6. Implications
3.7. Limitations and Future Directions
4. Toxicity on Terrestrial Animals
4.1. Rodent Models
4.2. Amphibians
4.3. Avians
4.4. Earthworm Models
4.5. Enchytraeids
| Organism Type | Species | Microplastics + Antimicrobials | Main Effects | Reference |
|---|---|---|---|---|
| Rodent | Mouse | Polystyrene microplastics + sulfamethoxazole | Increased sulfamethoxazole accumulation in liver (41.7 μg/kg); microplastics retained in kidneys (3.83%); liver tissue damage (amyloidosis, necrocytosis); increased malonaldehyde (174%) and NF-κβ (104%); decreased antioxidant enzymes (22%); disrupted Keap1–Nrf2 signaling; enhanced oxidative stress and inflammation | [76] |
| Juvenile mouse | Polystyrene microplastics + tetracycline | Impaired intestinal barrier; increased inflammation and oxidative stress; decreased probiotics, increased opportunistic pathogens; increased ARGs and virulence genes in microbiota | [79] | |
| Mouse | Polystyrene microplastics + epoxiconazole | Co-exposure (0.120 mg/kg microplastics) caused synergistic toxicity: increased tissue damage, oxidative imbalance, metabolic disruption; mutual enhancement of bioaccumulation; gut barrier disruption, causing increased absorption of microplastics and antifungal pesticide | [80] | |
| Mouse | Polystyrene microplastics + doxycycline | Gut microbiota disruption causing brain lesions, inflammation, decreased learning and memory; intestinal barrier damage by microplastics (not accelerated by doxycycline); fecal microbiota transplant restored cognitive functions | [81] | |
| Amphibian | Rana nigromaculata | Polystyrene microplastics (0.1–10 µm) + levofloxacin | Growth and development inhibition (size-dependent); microplastics crossed blood–brain barrier; thyroid axis disruption stronger with co-exposure; neurotoxicity | [82] |
| Rana nigromaculata (tadpoles) | Polystyrene microplastics (0.1, 1, 10 µm) + levofloxacin | Neurotoxicity and behavioral effects (10 µm > 0.1 µm > 1 µm microplastics); disrupted neural function (cell adhesion molecule pathway); levofloxacin mitigated dysbiosis with 1 µm microplastics | [83] | |
| Avian | Muscovy duck | Polystyrene microplastics + chlortetracycline | Microplastics decreased chlortetracycline accumulation in liver/intestines, increased fecal excretion; microplastics caused oxidative stress, inflammation, gut barrier damage; co-exposure modulated gut microbiome, partially mitigating intestinal damage | [84] |
| Earthworm | Eisenia fetida | Polyethylene microplastics (13 µm, 48 µm, 150 µm) + tebuconazole | Greatest accumulation with 13 µm microplastics; increased oxidative stress markers; DNA damage and high toxicity | [85] |
| Eisenia fetida | Polyethylene nanoplastics + pyraclostrobin | Extended persistence of pyraclostrobin in soil (+13 days); increased accumulation in worms (+8.4%); decreased body weight (–26.8%); altered gut microbiota; increased ARG diversity and plasmid-associated ARGs | [86] | |
| Eisenia fetida | Aged/UV-aged microplastics + azoxystrobin | Aged microplastics had higher adsorption/desorption; UV-aged microplastics + pesticide had lowest LC50 (highest toxicity); caused oxidative stress, skin and intestinal damage, impaired digestion; toxicity linked to pesticide desorption | [87] | |
| Eisenia fetida | Polystyrene microplastics + dufulin | Microplastics enhanced dufulin accumulation; oxidative stress at earlier stage (day 7 vs. day 14 for dufulin alone); altered 21 metabolites, disrupted 3 pathways (vs. 14 metabolites, 2 pathways for dufulin alone) | [88] | |
| Enchytraeus crypticus | Polyamide microplastics/polyvinyl chloride microplastics + tetracycline | Increased tetracycline accumulation; microplastics + tetracycline did not further enhance accumulation compared to tetracycline alone; decreased microbiota diversity; increased ARG diversity | [89] |
4.6. Implications
4.7. Limitations and Future Directions
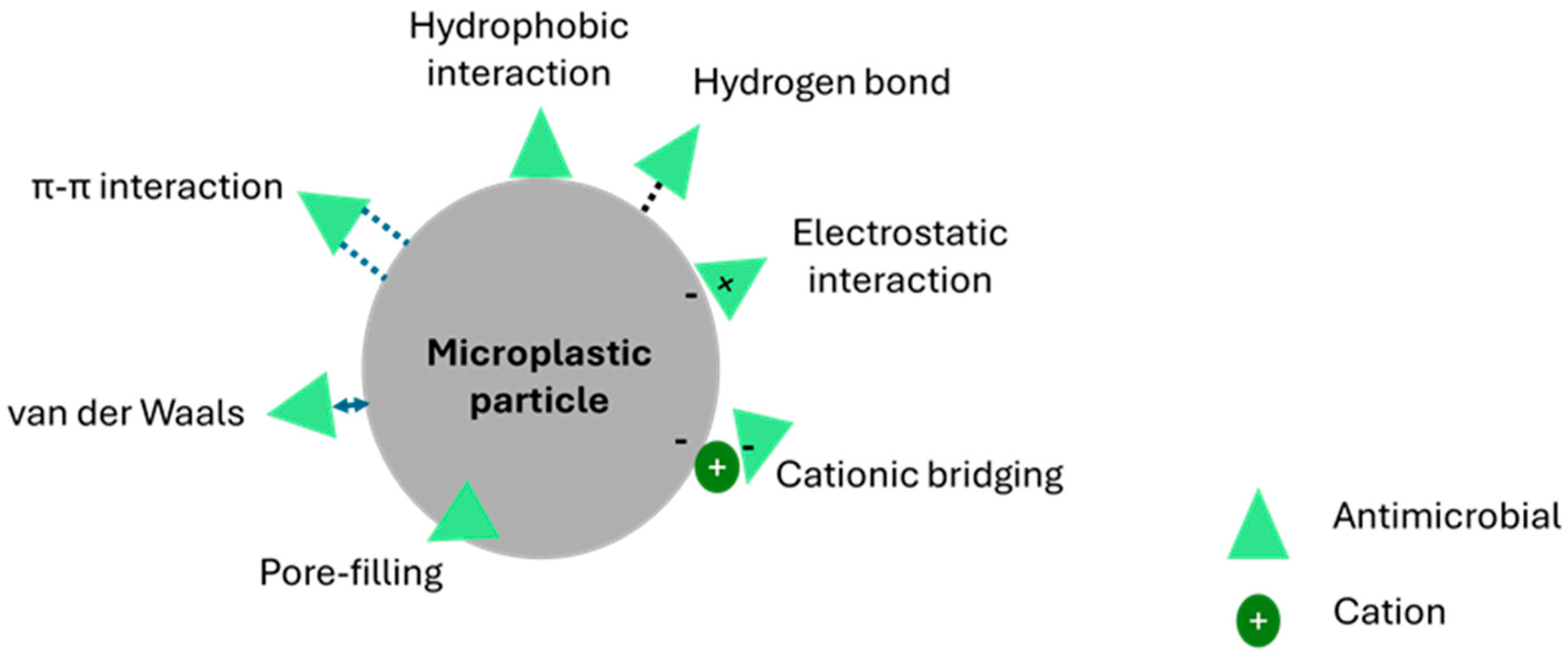
5. Brief Overview of Influences of Microplastic Properties on Combined Toxicity
6. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WasteDirect. Plastic Waste Statistics & Trends. Available online: https://wastedirect.co.uk/blog/plastic-waste-statistics/ (accessed on 22 July 2025).
- Ritchie, H.; Samborska, V.; Roser, M. Plastic Pollution. 2023. Available online: https://ourworldindata.org/plastic-pollution (accessed on 22 July 2025).
- Tang, K.H.D. Microplastics and Antibiotics in Aquatic Environments: A Review of Their Interactions and Ecotoxicological Implications. Trop. Aquat. Soil Pollut. 2024, 4, 60–78. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Li, R. Aged Microplastics and Antibiotic Resistance Genes: A Review of Aging Effects on Their Interactions. Antibiotics 2024, 13, 941. [Google Scholar] [CrossRef]
- Crew, A.; Gregory-Eaves, I.; Ricciardi, A. Distribution, abundance, and diversity of microplastics in the upper St. Lawrence River. Environ. Pollut. 2020, 260, 113994. [Google Scholar] [CrossRef]
- Hermsen, E.; Pompe, R.; Besseling, E.; Koelmans, A.A. Detection of low numbers of microplastics in North Sea fish using strict quality assurance criteria. Mar. Pollut. Bull. 2017, 122, 253–258. [Google Scholar] [CrossRef]
- Au, S.Y.; Lee, C.M.; Weinstein, J.E.; Hurk, P.v.d.; Klaine, S.J. Trophic transfer of microplastics in aquatic ecosystems: Identifying critical research needs. Integr. Environ. Assess. Manag. 2017, 13, 505–509. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Zhou, J. Ecotoxicity of Biodegradable Microplastics and Bio-based Microplastics: A Review of in vitro and in vivo Studies. Environ. Manag. 2024, 75, 663–679. [Google Scholar] [CrossRef]
- Ateia, M.; Zheng, T.; Calace, S.; Tharayil, N.; Pilla, S.; Karanfil, T. Sorption behavior of real microplastics (MPs): Insights for organic micropollutants adsorption on a large set of well-characterized MPs. Sci. Total Environ. 2020, 720, 137634. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Wang, F.; Yang, H.; Liu, L. Adsorption of tetracyclines onto polyethylene microplastics: A combined study of experiment and molecular dynamics simulation. Chemosphere 2021, 265, 129133. [Google Scholar] [CrossRef]
- Wei, X.; Li, M.; Wang, Y.; Jin, L.; Ma, G.; Yu, H. Developing Predictive Models for Carrying Ability of Micro-Plastics towards Organic Pollutants. Molecules 2019, 24, 1784. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, L. Transport of micro- and nanoplastics in the environment: Trojan-Horse effect for organic contaminants. Crit. Rev. Environ. Sci. Technol. 2022, 52, 810–846. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a Vector for Chemicals in the Aquatic Environment: Critical Review and Model-Supported Reinterpretation of Empirical Studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef]
- Tang, K.H.D. Environmental Co-existence of Microplastics and Perfluorochemicals: A Review of Their Interactions. Biointerface Res. Appl. Chem. 2023, 13, 587. [Google Scholar] [CrossRef]
- Yuan, F.; Chen, H.; Ding, Y.; Wang, Y.; Liao, Q.; Wang, T.; Fan, Q.; Feng, Z.; Zhang, C.; Fu, G.; et al. Effects of microplastics on the toxicity of co-existing pollutants to fish: A meta-analysis. Water Res. 2023, 240, 120113. [Google Scholar] [CrossRef]
- Rainieri, S.; Conlledo, N.; Larsen, B.K.; Granby, K.; Barranco, A. Combined effects of microplastics and chemical contaminants on the organ toxicity of zebrafish (Danio rerio). Environ. Res. 2018, 162, 135–143. [Google Scholar] [CrossRef]
- Chen, Q.; Yin, D.; Jia, Y.; Schiwy, S.; Legradi, J.; Yang, S.; Hollert, H. Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Sci. Total Environ. 2017, 609, 1312–1321. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Zhao, H.; Cai, J.; Sultan, Y.; Fang, H.; Zhang, B.; Ma, J. Effects of polyvinyl chloride microplastics on reproduction, oxidative stress and reproduction and detoxification-related genes in Daphnia magna. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 254, 109269. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Gong, L.; Cheng, Y.; Yuan, Q.; He, Y. Combined toxicity of polystyrene microplastics and sulfamethoxazole on zebrafish embryos. Environ. Sci. Pollut. Res. 2022, 29, 19273–19282. [Google Scholar] [CrossRef]
- Li, N.; Zeng, Z.; Zhang, Y.; Zhang, H.; Tang, N.; Guo, Y.; Lu, L.; Li, X.; Zhu, Z.; Gao, X.; et al. Higher toxicity induced by co-exposure of polystyrene microplastics and chloramphenicol to Microcystis aeruginosa: Experimental study and molecular dynamics simulation. Sci. Total Environ. 2023, 866, 161375. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, J.; Razanajatovo, R.M.; Jiang, H.; Zou, H.; Zhu, W. Interactive effects of polystyrene microplastics and roxithromycin on bioaccumulation and biochemical status in the freshwater fish red tilapia (Oreochromis niloticus). Sci. Total Environ. 2019, 648, 1431–1439. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, Z.; Lu, G.; Ji, Y. Single and combined effects of microplastics and roxithromycin on Daphnia magna. Environ. Sci. Pollut. Res. 2019, 26, 17010–17020. [Google Scholar] [CrossRef]
- Tang, K.H.D. Abundance of microplastics in wastewater treatment sludge. J. Hum. Earth Future 2022, 3, 138–146. [Google Scholar] [CrossRef]
- Löffler, P.; Escher, B.I.; Baduel, C.; Virta, M.P.; Lai, F.Y. Antimicrobial Transformation Products in the Aquatic Environment: Global Occurrence, Ecotoxicological Risks, and Potential of Antibiotic Resistance. Environ. Sci. Technol. 2023, 57, 9474–9494. [Google Scholar] [CrossRef]
- Prajapati, A.; Vaidya, A.N.; Kumar, A.R. Microplastic properties and their interaction with hydrophobic organic contaminants: A review. Environ. Sci. Pollut. Res. 2022, 29, 49490–49512. [Google Scholar] [CrossRef]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Tang, K.H.D. Terrestrial and Aquatic Plastisphere: Formation, Characteristics, and Influencing Factors. Sustainability 2024, 16, 2163. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Lai, K.P.; Tsang, C.F.; Li, L.; Yu, R.M.K.; Kong, R.Y.C. Microplastics act as a carrier for wastewater-borne pathogenic bacteria in sewage. Chemosphere 2022, 301, 134692. [Google Scholar] [CrossRef]
- Cholewińska, P.; Moniuszko, H.; Wojnarowski, K.; Pokorny, P.; Szeligowska, N.; Dobicki, W.; Polechoński, R.; Górniak, W. The Occurrence of Microplastics and the Formation of Biofilms by Pathogenic and Opportunistic Bacteria as Threats in Aquaculture. Int. J. Environ. Res. Public Health 2022, 19, 8137. [Google Scholar] [CrossRef]
- Su, H.; Xu, W.; Hu, X.; Xu, Y.; Wen, G.; Cao, Y. The impact of microplastics on antibiotic resistance genes, metal resistance genes, and bacterial community in aquaculture environment. J. Hazard. Mater. 2025, 489, 137704. [Google Scholar] [CrossRef]
- Zhuang, S.; Wang, J. Interaction between antibiotics and microplastics: Recent advances and perspective. Sci. Total Environ. 2023, 897, 165414. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Xu, J.; Li, Z.; Cheng, L.; Fu, J.; Sun, W.; Dang, C. When antibiotics encounter microplastics in aquatic environments: Interaction, combined toxicity, and risk assessments. Sci. Total Environ. 2024, 929, 172455. [Google Scholar] [CrossRef]
- Yu, Z.; An, Q.; Zhou, T.; Zhou, L.; Yan, B. Meta-analysis unravels the complex combined toxicity of microplastics and antibiotics in aquatic ecosystems. Sci. Total Environ. 2024, 929, 172503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Liu, X.; Zhao, J.; Liu, R.; Xing, B. Interaction of Microplastics with Antibiotics in Aquatic Environment: Distribution, Adsorption, and Toxicity. Environ. Sci. Technol. 2021, 55, 15579–15595. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Guo, C.; Li, J.; Wang, K.; Liang, S.; Wang, W.; Wang, J. A critical review of the adsorption-desorption characteristics of antibiotics on microplastics and their combined toxic effects. Environ. Technol. Innov. 2024, 35, 103729. [Google Scholar] [CrossRef]
- Wei, J.; Chen, M.; Wang, J. Insight into combined pollution of antibiotics and microplastics in aquatic and soil environment: Environmental behavior, interaction mechanism and associated impact of resistant genes. TrAC Trends Anal. Chem. 2023, 166, 117214. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Chen, X.; Li, J.; Chen, J.; Wang, H. The fate and risk of microplastic and antibiotic sulfamethoxazole coexisting in the environment. Environ. Geochem. Health 2023, 45, 2905–2915. [Google Scholar] [CrossRef]
- Feng, L.-J.; Zhang, K.-X.; Shi, Z.-L.; Zhu, F.-P.; Yuan, X.-Z.; Zong, W.-S.; Song, C. Aged microplastics enhance their interaction with ciprofloxacin and joint toxicity on Escherichia coli. Ecotoxicol. Environ. Saf. 2022, 247, 114218. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Tong, J.; Xiong, W.; Xiang, Y.; Peng, H.; Wang, W.; Yang, Y.; Ye, Y.; Hu, M.; Yang, Z.; et al. Microplastics influence the fate of antibiotics in freshwater environments: Biofilm formation and its effect on adsorption behavior. J. Hazard. Mater. 2023, 442, 130078. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.; Meng, Q.; Feng, Q.; Yan, Z.; Liu, J.; Liu, Z.; Zhou, Z. Intergenerational and biological effects of roxithromycin and polystyrene microplastics to Daphnia magna. Aquat. Toxicol. 2022, 248, 106192. [Google Scholar] [CrossRef]
- Yin, C.; Yang, X.; Zhao, T.; Watson, P.; Yang, F.; Liu, H. Changes of the acute and chronic toxicity of three antimicrobial agents to Daphnia magna in the presence/absence of micro-polystyrene. Environ. Pollut. 2020, 263, 114551. [Google Scholar] [CrossRef]
- Nugnes, R.; Russo, C.; Lavorgna, M.; Orlo, E.; Kundi, M.; Isidori, M. Polystyrene microplastic particles in combination with pesticides and antiviral drugs: Toxicity and genotoxicity in Ceriodaphnia dubia. Environ. Pollut. 2022, 313, 120088. [Google Scholar] [CrossRef]
- Said, R.E.M.; Hamed, M.; Shaalan, W.M.; Elbaghdady, H.A.M.; Sayed, A.E.-D.H. Exploring the Coexposure Effects of Pyrogallol and Microplastic on the Red Swamp Crayfish Procambarus clarkii. Aquac. Res. 2025, 2025, 6084150. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, W.; Tang, Y.; Shi, W.; Shao, Y.; Ren, P.; Zhang, J.; Xiao, G.; Sun, H.; Liu, G. Microplastics aggravate the bioaccumulation of three veterinary antibiotics in the thick shell mussel Mytilus coruscus and induce synergistic immunotoxic effects. Sci. Total Environ. 2021, 770, 145273. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cai, Y.; Ma, C.; Han, L.; Yang, Z. Combined toxicity of micro/nano scale polystyrene plastics and ciprofloxacin to Corbicula fluminea in freshwater sediments. Sci. Total Environ. 2021, 789, 147887. [Google Scholar] [CrossRef]
- Zhou, W.; Tang, Y.; Du, X.; Han, Y.; Shi, W.; Sun, S.; Zhang, W.; Zheng, H.; Liu, G. Fine polystyrene microplastics render immune responses more vulnerable to two veterinary antibiotics in a bivalve species. Mar. Pollut. Bull. 2021, 164, 111995. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, Y.; Xu, R.; Li, D.; Waiho, K.; Wang, Y.; Hu, M. Combined toxic effects of nanoplastics and norfloxacin on antioxidant and immune genes in mussels. Mar. Environ. Res. 2024, 193, 106277. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, G.; Sun, Y.; Yan, Z.; Zhang, L.; Liu, J. Effect of microplastics on oxytetracycline trophic transfer: Immune, gut microbiota and antibiotic resistance gene responses. J. Hazard. Mater. 2024, 470, 134147. [Google Scholar] [CrossRef]
- Chen, J.; Lei, Y.; Wen, J.; Zheng, Y.; Gan, X.; Liang, Q.; Huang, C.; Song, Y. The neurodevelopmental toxicity induced by combined exposure of nanoplastics and penicillin in embryonic zebrafish: The role of aging processes. Environ. Pollut. 2023, 335, 122281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bai, Y.; Meng, H.; Zhu, Y.; Yue, H.; Li, B.; Wang, J.; Wang, J.; Zhu, L.; Du, Z. Combined toxic effects of polystyrene microplastics and 3,6-dibromocarbazole on zebrafish (Danio rerio) embryos. Sci. Total Environ. 2024, 913, 169787. [Google Scholar] [CrossRef]
- Li, C.; Yuan, S.; Zhou, Y.; Li, X.; Duan, L.; Huang, L.; Zhou, X.; Ma, Y.; Pang, S. Microplastics reduce the bioaccumulation and oxidative stress damage of triazole fungicides in fish. Sci. Total Environ. 2022, 806, 151475. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, J.; Zang, L.; Nakayama, H.; Nishimura, N.; Shimada, Y. Effects of nanoplastic on toxicity of azole fungicides (ketoconazole and fluconazole) in zebrafish embryos. Sci. Total Environ. 2021, 800, 149463. [Google Scholar] [CrossRef]
- Bhagat, J.; Nishimura, N.; Shimada, Y. Toxicological interactions of microplastics/nanoplastics and environmental contaminants: Current knowledge and future perspectives. J. Hazard. Mater. 2021, 405, 123913. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, Z.; Zhong, R.; Fang, X.; Wang, X.; Huang, Y.; Gong, H.; Yan, M. Microplastics Enhance the Toxic Effects of Tetracycline on the Early Development of Zebrafish in a Dose-Dependent Manner. Fishes 2025, 10, 150. [Google Scholar] [CrossRef]
- Xiong, G.; Zhang, H.; Shi, H.; Peng, Y.; Han, M.; Hu, T.; Liao, X.; Liu, Y.; Zhang, J.e.; Xu, G. Enhanced hepatotoxicity in zebrafish due to co-exposure of microplastics and sulfamethoxazole: Insights into ROS-mediated MAPK signaling pathway regulation. Ecotoxicol. Environ. Saf. 2024, 278, 116415. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, G.; Sun, Y.; Zhang, J.; Liu, J.; Yan, Z. Aged microplastics change the toxicological mechanism of roxithromycin on Carassius auratus: Size-dependent interaction and potential long-term effects. Environ. Int. 2022, 169, 107540. [Google Scholar] [CrossRef] [PubMed]
- Juan, K.; Boya, F.; Julin, Y.; Liqin, Y. Combined Effects of Environmental Concentration of Oxytetracycline and Polystyrene Microplastics on Intestinal Tract of Juvenile Yellow Catfish (Pelteobagrus fulvidraco). Asian J. Ecotoxicol. 2023, 18, 426. [Google Scholar]
- Fonte, E.; Ferreira, P.; Guilhermino, L. Temperature rise and microplastics interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): Post-exposure predatory behaviour, acetylcholinesterase activity and lipid peroxidation. Aquat. Toxicol. 2016, 180, 173–185. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Gouveia, A.; Chen, R.; Xing, D.; Wang, J.; Mu, J. Biomicroplastics and Antibiotics: A Toxic Cocktail for Fatty Liver Disease in Marine Medaka. Environ. Sci. Technol. 2025, 59, 12485–12494. [Google Scholar] [CrossRef]
- Zhao, P.; Lu, W.; Avellán-Llaguno, R.D.; Liao, X.; Ye, G.; Pan, Z.; Hu, A.; Huang, Q. Gut microbiota related response of Oryzias melastigma to combined exposure of polystyrene microplastics and tetracycline. Sci. Total Environ. 2023, 905, 167359. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, X.; Liao, H.; Guo, J.; Ma, Z.; Fu, Z. Microplastics and tetracycline affecting apoptosis, enzyme activities and metabolism processes in the Aurelia aurita polyps: Insights into combined pollutant effects. Front. Mar. Sci. 2025, 12, 1545131. [Google Scholar] [CrossRef]
- Huang, M.; Ma, Y.; Qian, J.; Sokolova, I.M.; Zhang, C.; Waiho, K.; Fang, J.K.H.; Ma, X.; Wang, Y.; Hu, M. Combined effects of norfloxacin and polystyrene nanoparticles on the oxidative stress and gut health of the juvenile horseshoe crab Tachypleus tridentatus. J. Hazard. Mater. 2024, 468, 133801. [Google Scholar] [CrossRef]
- Kinigopoulou, V.; Pashalidis, I.; Kalderis, D.; Anastopoulos, I. Microplastics as carriers of inorganic and organic contaminants in the environment: A review of recent progress. J. Mol. Liq. 2022, 350, 118580. [Google Scholar] [CrossRef]
- Dey, S.; Rout, A.K.; Ghosh, K.; Jana, A.K.; Behera, B.K. Microbial Ecology in Microplastics: Impact on Aquatic Ecosystems and Bioremediation. In Current Trends in Fisheries Biotechnology; Behera, B.K., Ed.; Springer Nature: Singapore, 2024; pp. 79–93. [Google Scholar]
- Liu, P.; Dai, J.; Bie, C.; Li, H.; Zhang, Z.; Guo, X.; Zhu, L. Bioaccessibility of Microplastic-Associated Antibiotics in Freshwater Organisms: Highlighting the Impacts of Biofilm Colonization via an In Vitro Protocol. Environ. Sci. Technol. 2022, 56, 12267–12277. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Yang, X.; Osman, R.; Geissen, V. The role of microplastic aging on chlorpyrifos adsorption-desorption and microplastic bioconcentration. Environ. Pollut. 2023, 331, 121910. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, H.; He, C.; Jin, Y.; Fu, Z. Polystyrene nanoparticles trigger the activation of p38 MAPK and apoptosis via inducing oxidative stress in zebrafish and macrophage cells. Environ. Pollut. 2021, 269, 116075. [Google Scholar] [CrossRef]
- Tang, K.H.D. A review of the toxic effects of microplastics based on studies on mammals and mammalian cell lines. Environ. Sci. Adv. 2024, 3, 1669–1678. [Google Scholar] [CrossRef]
- Cui, W.; Hale, R.C.; Huang, Y.; Zhou, F.; Wu, Y.; Liang, X.; Liu, Y.; Tan, H.; Chen, D. Sorption of representative organic contaminants on microplastics: Effects of chemical physicochemical properties, particle size, and biofilm presence. Ecotoxicol. Environ. Saf. 2023, 251, 114533. [Google Scholar] [CrossRef]
- Du, J.; Zhan, L.; Zhang, G.; Zhou, Q.; Wu, W. Antibiotic sorption onto MPs in terrestrial environment: A critical review of the transport, bioaccumulation, ecotoxicological effects and prospects. Drug Chem. Toxicol. 2025, 48, 266–280. [Google Scholar] [CrossRef]
- Ullah, F.; Wang, P.-Y.; Saqib, S.; Zhao, L.; Ashraf, M.; Khan, A.; Khan, W.; Khan, A.; Chen, Y.; Xiong, Y.-C. Toxicological complexity of microplastics in terrestrial ecosystems. iScience 2025, 28, 1118790. [Google Scholar] [CrossRef]
- Tang, K.H.D. Microplastics in Soil: Uncovering Their Hidden Chemical Implications. Trop. Aquat. Soil Pollut. 2025, 5, 88–109. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Kim, S.; Sarkar, B.; Oleszczuk, P.; Sang, M.K.; Haque, M.N.; Ahn, J.H.; Bank, M.S.; Ok, Y.S. Effects of microplastics on the terrestrial environment: A critical review. Environ. Res. 2022, 209, 112734. [Google Scholar] [CrossRef] [PubMed]
- Piergiacomo, F.; Brusetti, L.; Pagani, L. Understanding the Interplay between Antimicrobial Resistance, Microplastics and Xenobiotic Contaminants: A Leap towards One Health? Int. J. Environ. Res. Public Health 2023, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhang, L.; Xiang, K.; Zhang, Y.; Wang, G.; Chen, L. Microplastic-contaminated antibiotics as an emerging threat to mammalian liver: Enhanced oxidative and inflammatory damages. Biomater. Sci. 2023, 11, 4298–4307. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xiao, M.; Hu, S.; Wang, M. Keap1-Nrf2 pathway: A key mechanism in the occurrence and development of cancer. Front. Oncol. 2024, 14, 1381467. [Google Scholar] [CrossRef]
- Culletta, G.; Buttari, B.; Arese, M.; Brogi, S.; Almerico, A.M.; Saso, L.; Tutone, M. Natural products as non-covalent and covalent modulators of the KEAP1/NRF2 pathway exerting antioxidant effects. Eur. J. Med. Chem. 2024, 270, 116355. [Google Scholar] [CrossRef]
- Xia, Y.; Lan, Y.; Xu, Y.; Liu, F.; Chen, X.; Luo, J.; Xu, H.; Liu, Y. Effects of microplastics and tetracycline induced intestinal damage, intestinal microbiota dysbiosis, and antibiotic resistome: Metagenomic analysis in young mice. Environ. Int. 2025, 199, 109512. [Google Scholar] [CrossRef]
- Sun, W.; Yan, S.; Meng, Z.; Tian, S.; Jia, M.; Huang, S.; Wang, Y.; Zhou, Z.; Diao, J.; Zhu, W. Combined ingestion of polystyrene microplastics and epoxiconazole increases health risk to mice: Based on their synergistic bioaccumulation in vivo. Environ. Int. 2022, 166, 107391. [Google Scholar] [CrossRef]
- Sun, H.; Yang, B.; Zhu, X.; Li, Q.; Song, E.; Song, Y. Oral exposure of polystyrene microplastics and doxycycline affects mice neurological function via gut microbiota disruption: The orchestrating role of fecal microbiota transplantation. J. Hazard. Mater. 2024, 467, 133714. [Google Scholar] [CrossRef]
- Zhang, W.; Teng, M.; Yan, J. Combined effect and mechanism of microplastic with different particle sizes and levofloxacin on developing Rana nigromaculata: Insights from thyroid axis regulation and immune system. J. Environ. Manag. 2024, 366, 121833. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, W.; Teng, M.; Xu, J.; Wang, J.; Yang, J.; Liu, Y. The effect and mechanism of variable particle size microplastics and levofloxacin on the neurotoxicity of Rana nigromaculata based on the microorganism-intestine-brain axis. J. Environ. Manag. 2024, 354, 120329. [Google Scholar] [CrossRef]
- Liu, B.; Yu, D.; Ge, C.; Luo, X.; Du, L.; Zhang, X.; Hui, C. Combined effects of microplastics and chlortetracycline on the intestinal barrier, gut microbiota, and antibiotic resistome of Muscovy ducks (Cairina moschata). Sci. Total Environ. 2023, 887, 164050. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Yang, D.; Yu, L.; Song, L.; Yang, L.; Yang, Q. Effect of polyethylene microplastics on tebuconazole bioaccumulation, oxidative stress, and intestinal bacterial community in earthworms. J. Hazard. Mater. 2024, 480, 136056. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, C.; Zhang, E.; Smagghe, G.; Gui, S.; Wu, X.; Chen, X. Pyraclostrobin and polyethylene nanoplastics jointly interfere with the antibiotic resistome in earthworm gut. Biol. Fertil. Soils 2025. [Google Scholar] [CrossRef]
- Bao, X.; Zhou, R.; Cui, Y.; Wang, Z.; Shi, J.; Gao, S.; Wang, X.; Meng, Z.; Chen, X. The carrier effects of aged polyethylene microplastics regulate the toxicological effects of azoxystrobin on earthworms: Interaction relationship of adsorption-desorption behavior and combined toxicity. Environ. Chem. Ecotoxicol. 2025, 7, 1506–1517. [Google Scholar] [CrossRef]
- Sun, W.; Meng, Z.; Li, R.; Zhang, R.; Jia, M.; Yan, S.; Tian, S.; Zhou, Z.; Zhu, W. Joint effects of microplastic and dufulin on bioaccumulation, oxidative stress and metabolic profile of the earthworm (Eisenia fetida). Chemosphere 2021, 263, 128171. [Google Scholar] [CrossRef]
- Ma, J.; Sheng, G.D.; O’Connor, P. Microplastics combined with tetracycline in soils facilitate the formation of antibiotic resistance in the Enchytraeus crypticus microbiome. Environ. Pollut. 2020, 264, 114689. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Brookes, P.C.; Xu, J. The sorption kinetics and isotherms of sulfamethoxazole with polyethylene microplastics. Mar. Pollut. Bull. 2018, 131, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; You, X.-y. Recent progress of microplastic toxicity on human exposure base on in vitro and in vivo studies. Sci. Total Environ. 2023, 903, 166766. [Google Scholar] [CrossRef]
- Bhagat, J.; Zang, L.; Nishimura, N.; Shimada, Y. Zebrafish: An emerging model to study microplastic and nanoplastic toxicity. Sci. Total Environ. 2020, 728, 138707. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Faggio, C.; Betancourt-Lozano, M.; González-Mille, D.J.; Ilizaliturri-Hernández, C.A. Ecotoxicological perspectives of microplastic pollution in amphibians. J. Toxicol. Environ. Health Part B 2022, 25, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Beggel, S.; Kalis, E.J.J.; Geist, J. Towards harmonized ecotoxicological effect assessment of micro- and nanoplastics in aquatic systems. Environ. Pollut. 2025, 366, 125504. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, K.H.D. Combined Toxicity of Microplastics and Antimicrobials on Animals: A Review. Antibiotics 2025, 14, 896. https://doi.org/10.3390/antibiotics14090896
Tang KHD. Combined Toxicity of Microplastics and Antimicrobials on Animals: A Review. Antibiotics. 2025; 14(9):896. https://doi.org/10.3390/antibiotics14090896
Chicago/Turabian StyleTang, Kuok Ho Daniel. 2025. "Combined Toxicity of Microplastics and Antimicrobials on Animals: A Review" Antibiotics 14, no. 9: 896. https://doi.org/10.3390/antibiotics14090896
APA StyleTang, K. H. D. (2025). Combined Toxicity of Microplastics and Antimicrobials on Animals: A Review. Antibiotics, 14(9), 896. https://doi.org/10.3390/antibiotics14090896








