From Pandemic to Resistance: Addressing Multidrug-Resistant Urinary Tract Infections in the Balkans
Abstract
1. Introduction
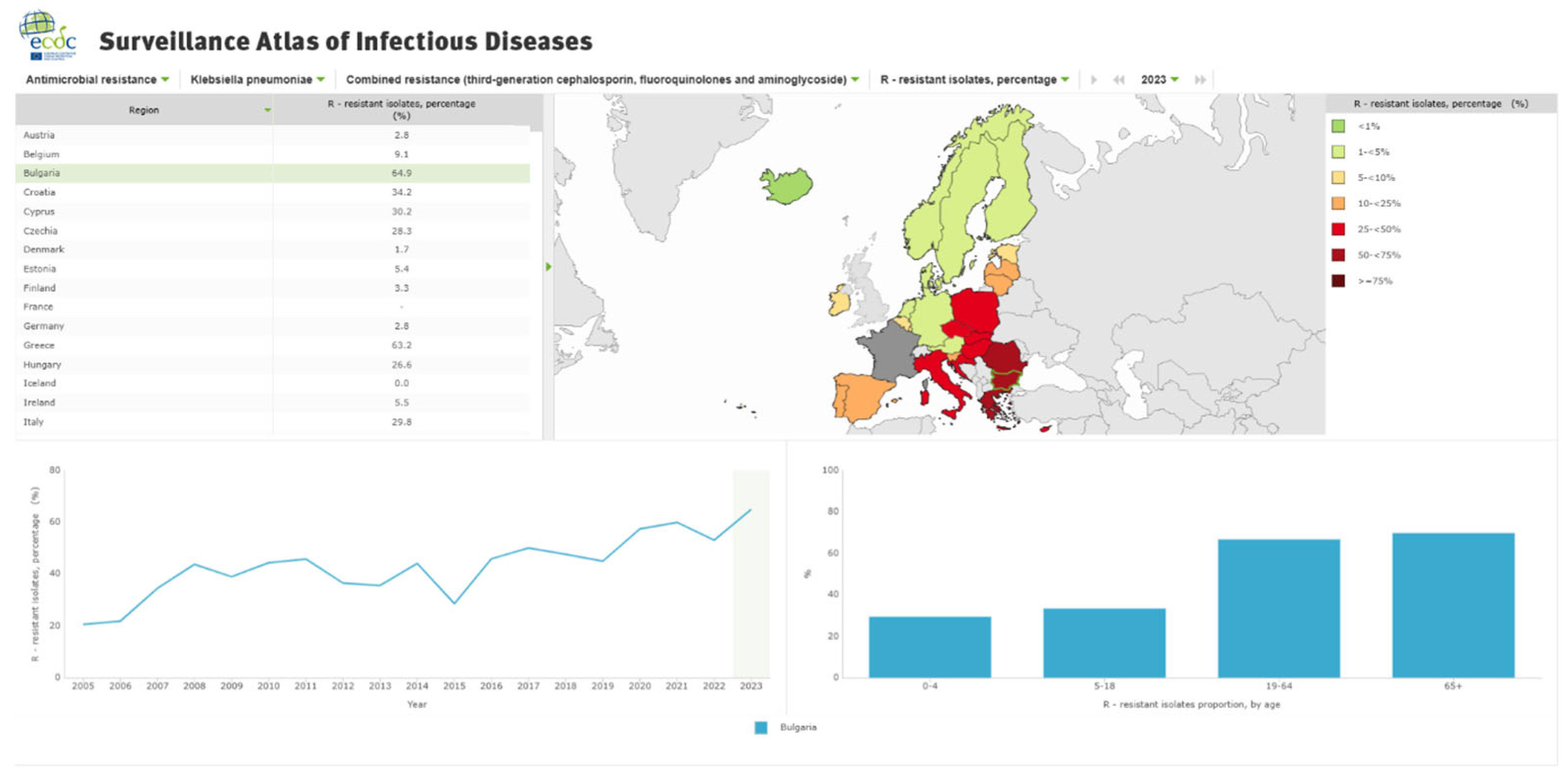
Epidemiology of MDR UTIs
2. Results
2.1. Risk Factors for MDR UTIs
2.2. Surveillance and Control Strategies
2.3. Clinical and Public Health Implications of Multidrug-Resistant Urinary Tract Infections in the Balkans
2.4. The Benefits of Rapid Diagnostic Testing and Therapy—New Approaches
2.5. Policy Recommendations and Implementation of Roadmap for the Balkans
- 1.
- Establish a Regional AMR Taskforce:
- 2.
- Mandatory surveillance and real-time data integration
- 3.
- Investment in rapid diagnostic technologies
- 4.
- Strengthen AMS programs in hospitals and outpatient clinics
- 5.
- Public and professional education campaigns
- 6.
- Facilitate access to non-antibiotic alternatives
2.6. The Ethical, Economic and Global Health Implications of Multidrug-Resistant Urinary Tract Infections (UTIs) in the Balkans
- 1.
- Ethical Imperatives: Equity and informed decision-making
- 2.
- Economic pressure on health systems and households
- 3.
- Global Health Interdependence and Responsibility
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| AMS | Antimicrobial stewardship |
| EARS-Net | European Antimicrobial Resistance Surveillance Network |
| ECDC | European Centre for Disease Prevention and Control |
| ESBL | Extended-spectrum beta-lactamase producing bacteria |
| MDR | Multidrug-resistant |
| MDROs | Multidrug-resistant organisms |
| UTIs | Urinary tract infections |
| WHO | World health organization |
References
- Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef]
- Medina, M.; Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Guclu, E.; Halis, F.; Kose, E.; Ogutlu, A.; Karabay, O. Risk factors of multidrug-resistant bacteria in community-acquired urinary tract infections. Afr. Health Sci. 2021, 21, 214–219. [Google Scholar] [CrossRef]
- Available online: https://www.cidrap.umn.edu/many-factors-tied-multidrug-resistant-urinary-tract-infections?utm_source=chatgpt.com (accessed on 21 April 2025).
- Yang, X.; Li, X.; Qiu, S.; Liu, C.; Chen, S.; Xia, H.; Zeng, Y.; Shi, L.; Chen, J.; Zheng, J.; et al. Global antimicrobial resistance and antibiotic use in COVID-19 patients within health facilities: A systematic review and meta-analysis of aggregated participant data. J. Infect. 2024, 89, 106183. [Google Scholar] [CrossRef]
- Petrakis, V.; Panopoulou, M.; Rafailidis, P.; Lemonakis, N.; Lazaridis, G.; Terzi, I.; Papazoglou, D.; Panagopoulos, P. The Impact of the COVID-19 Pandemic on Antimicrobial Resistance and Management of Bloodstream Infections. Pathogens 2023, 12, 780. [Google Scholar] [CrossRef]
- Gajic, I.; Jovicevic, M.; Popadic, V.; Trudic, A.; Kabic, J.; Kekic, D.; Ilic, A.; Klasnja, S.; Hadnadjev, M.; Popadic, D.J.; et al. The emergence of multi-drug-resistant bacteria causing healthcare-associated infections in COVID-19 patients: A retrospective multi-centre study. J. Hosp. Infect. 2023, 137, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://atlas.ecdc.europa.eu/ (accessed on 29 May 2025).
- Mareș, C.; Petca, R.-C.; Popescu, R.-I.; Petca, A.; Geavlete, B.F.; Jinga, V. Uropathogens’ Antibiotic Resistance Evolution in a Female Population: A Sequential Multi-Year Comparative Analysis. Antibiotics 2023, 12, 948. [Google Scholar] [CrossRef]
- Petca, R.-C.; Negoita, S.; Mares, C.; Petca, A.; Popescu, R.-I.; Chibelean, C.B. Heterogeneity of antibiotics multidrug-resistance profile of uropathogens in Romanian population. Antibiotics 2021, 10, 523. [Google Scholar] [CrossRef]
- Mareș, C.; Petca, R.-C.; Petca, A.; Popescu, R.-I.; Jinga, V. Does the COVID Pandemic Modify the Antibiotic Resistance of Uropathogens in Female Patients? A New Storm? Antibiotics 2022, 11, 376. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Stoichitoiu, L.E.; Pinte, L.; Ceasovschih, A.; Cernat, R.C.; Vlad, N.D.; Padureanu, V.; Sorodoc, L.; Hristea, A.; Purcarea, A.; Badea, C.; et al. In-Hospital antibiotic use for COVID-19: Facts and rationales assessed through a mixed-methods study. J. Clin. Med. 2022, 11, 3194. [Google Scholar] [CrossRef]
- Fukushige, M.; Ngo, N.H.; Lukmanto, D.; Fukuda, S.; Ohneda, O. Effect of the COVID-19 pandemic on antibiotic consumption: A systematic review comparing 2019 and 2020 data. Front. Public Health 2022, 10, 946077. [Google Scholar] [CrossRef]
- Gajdács, M.; Urbán, E.; Stájer, A.; Baráth, Z. Antimicrobial resistance in the context of the sustainable development goals: A brief review. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 71–82. [Google Scholar] [CrossRef]
- Martin, E.; Philbin, M.; Hughes, G.; Bergin, C.; Fe Talento, A. Antimicrobial stewardship challenges and innovative initiatives in the acute hospital setting during the COVID-19 pandemic. J. Antimicrob. Chemother. 2021, 76, 272–275. [Google Scholar] [CrossRef]
- Abdel Gawad, A.M.; Ashry, W.M.O.; El-Ghannam, S.; Hussein, M.; Yousef, A. Antibiotic resistance profile of common uropathogens during COVID-19 pandemic: Hospital based epidemiologic study. BMC Microbiol. 2023, 23, 28. [Google Scholar] [CrossRef]
- El Omari, L.; Sakhi, A.; Miloudi, M.; Elkamouni, Y.; Zouhair, S.; Arsalane, L. The impact of the COVID pandemic on the uropathogenic bacterial resistance profile: Experience of the bacteriology lab of the military hospital Avicenne in Marrakech. GSC Adv. Res. Rev. 2023, 14, 59–65. [Google Scholar] [CrossRef]
- Khoshbakht, R.; Kabiri, M.; Neshani, A.; Khaksari, M.N.; Sadrzadeh, S.M.; Mousavi, S.M.; Ghazvini, K.; Ghavidel, M. Assessment of antibiotic resistance changes during the Covid-19 pandemic in northeast of Iran during 2020–2022: An epidemiological study. Antimicrob. Resist. Infect. Control 2022, 11, 121. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Surveillance in Europe 2023–2021 Data. European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2023-2021-data (accessed on 16 July 2025).
- WHO/Europe CAESAR Initiative. Available online: https://www.who.int/europe/groups/central-asian-and-european-surveillance-of-antimicrobial-resistance-(caesar) (accessed on 11 June 2025).
- Brkic, S.; Cirkovic, I. Carbapenem-Resistant Enterobacterales in the Western Balkans: Addressing Gaps in European AMR Surveillance Map. Antibiotics 2024, 13, 895. [Google Scholar] [CrossRef]
- Surveillance Atlas of Infectious Disease. ECDC Atlas. Available online: https://www.ecdc.europa.eu/en/surveillance-atlas-infectious-diseases (accessed on 16 July 2025).
- Langford, B.J.; Soucy, J.P.R.; Leung, V.; So, M.; Kwan, A.T.; Portnoff, J.S.; Bertagnolio, S.; Raybardhan, S.; MacFadden, D.R.; Daneman, N. Antibiotic resistance associated with the COVID-19 pandemic: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2023, 29, 302–309. [Google Scholar] [CrossRef]
- Centers for Disease Control Prevention. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022. U.S. Department of Health and Human Services. 2022. Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/COVID-19.html (accessed on 16 July 2025).
- Milovanovic, T.; Dumic, I.; Veličkovic, J.; Lalosevic, M.S.; Nikolic, V.; Palibrk, I. Epidemiology and risk factors for multi-drug resistant hospital-acquired urinary tract infection in patients with liver cirrhosis: Single center experience in Serbia. BMC Infect. Dis. 2019, 19, 141. [Google Scholar] [CrossRef]
- Flores-Mireles, A.; Hreha, T.N.; Hunstad, D.A. Pathophysiology, Treatment, and Prevention of Catheter-Associated Urinary Tract Infection. Top. Spinal Cord Inj. Rehabil. Summer 2019, 25, 228–240. [Google Scholar] [CrossRef]
- Petca, R.C.; Mareș, C.; Petca, A.; Negoiță, S.; Popescu, R.I.; Boț, M.; Barabás, E.; Chibelean, C.B. Spectrum and Antibiotic Resistance of Uropathogens in Romanian Females. Antibiotics 2020, 9, 472. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens 2023, 1, 623. [Google Scholar] [CrossRef] [PubMed]
- Gajic, I.; Tomic, N.; Lukovic, B.; Jovicevic, M.; Kekic, D.; Petrovic, M.; Jankovic, M.; Trudic, A.; Mitic Culafic, D.; Milenkovic, M.; et al. A Comprehensive Overview of Antibacterial Agents for Combating Multidrug-Resistant Bacteria: The Current Landscape, Development, Future Opportunities, and Challenges. Antibiotics 2025, 14, 221. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Filev, R.; Lyubomirova, M.; Bogov, B.; Kolevski, A.; Pencheva, V.; Kalinov, K.; Rostaing, L. Urinary Tract Infections Caused by Klebsiella pneumoniae and Prolonged Treatment with Trimethoprim/Sulfamethoxazole. Microorganisms 2025, 13, 422. [Google Scholar] [CrossRef]
- Helmi, R.T.; Al-Maqbali, J.S.; Gamal, S.; Ba Wazir, H.; Al Sulemani, Y.; Al Za’abi, M. Short-term effects of antimicrobial stewardship programs on antibiotics usage, clinical outcomes, and multidrug resistant organisms in the post COVID-19 era. J. Infect. Public Health 2024, 17, 819–824. [Google Scholar] [CrossRef]
- Mandelli, G.; Dore, F.; Langer, M.; Garbero, E.; Alagna, L.; Bianchin, A.; Ciceri, R.; Di Paolo, A.; Giani, T.; Giugni, A.; et al. Effectiveness of a Multifaced Antibiotic Stewardship Program: A Pre-Post Study in Seven Italian ICUs. J. Clin. Med. 2022, 11, 4409. [Google Scholar] [CrossRef]
- Dik, J.W.H.; Vemer, P.; Friedrich, A.W.; Hendrix, R.; Lo-Ten-Foe, J.R.; Sinha, B.; Postma, M.J. Financial evaluations of antibiotic stewardship programs-a systematic review. Front. Microbiol. 2015, 6, 317. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.; Liu, C.; Liu, S.; Liu, X.; Li, X. The impact of pharmacist-led antimicrobial stewardship program on antibiotic use in a county-level tertiary general hospital in China: A retrospective study using difference-in-differences design. Front. Public Health 2022, 10, 1012690. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Training Courses for Antibiotic Stewardship. Available online: https://www.ecdc.europa.eu/en/publications-data/directory-guidance-prevention-and-control/training-antimicrobial-stewardship? (accessed on 23 July 2025).
- Antibiotic resistance. Rapid Tests Take Aim at Antibiotic-Resistant ‘Superbugs’. Financial Times. Available online: https://www.ft.com/medical-science?page=8 (accessed on 23 July 2025).
- Alonso-Tarrés, C.; Benjumea Moreno, C.; Navarro, F.; Habison, A.C.; Gonzàlez-Bertran, E.; Blanco, F.; Borràs, J.; Garrigó, M.; Saker, J. Bacteriuria and phenotypic antimicrobial susceptibility testing in 45 min by point-of-care Sysmex PA-100 System: First clinical evaluation. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 1533–1543. [Google Scholar] [CrossRef]
- Elsisi, G.H.; Zaky, H.S.; Polo, J.M. Budget impact analysis on the use of Sysmex PA-100 AST system as a point of care for uncomplicated urinary tract infections detection and treatment in Spanish females. J. Med. Econ. 2024, 27, 1434–1443. [Google Scholar] [CrossRef]
- Portsmouth, S.; Van Veenhuyzen, D.; Echols, R.; Machida, M.; Ferreira, J.C.A.; Ariyasu, M.; Nagata, T.D. Clinical Response of Cefiderocol Compared with Imipenem/Cilastatin in the Treatment of Adults with Complicated Urinary Tract Infections with or without Pyelonephritis or Acute Uncomplicated Pyelonephritis: Results from a Multicenter, Double-blind, Randomized Study (APEKS-cUTI). Open Forum Infect. Dis. 2017, 4, S537–S538. [Google Scholar] [CrossRef]
- Viale, P.; Sandrock, C.E.; Ramirez, P.; Rossolini, G.M.; Lodise, T.P. Treatment of critically ill patients with cefiderocol for infections caused by multidrug-resistant pathogens: Review of the evidence. Ann. Intensive Care 2023, 13, 52. [Google Scholar] [CrossRef]
- Perry, C.; Hossain, M.; Powell, M.; Raychaudhuri, A.; Scangarella-Oman, N.; Tiffany, C.; Xu, S.; Dumont, E.; Janmohamed, S. Design of Two Phase III, Randomized, Multicenter Studies Comparing Gepotidacin with Nitrofurantoin for the Treatment of Uncomplicated Urinary Tract Infection in Female Participants. Infect. Dis. Ther. 2022, 11, 2297–2310. [Google Scholar] [CrossRef]
- Lodise, T.P.; Das, A.F.; Frimodt-Møller, N.; Gupta, K.; Rodvold, K.A.; Santerre Henriksen, A.; Sommer, M.O.; Wagenlehner, F.; Kaye, K.S. Pivmecillinam for Treatment of Uncomplicated Urinary Tract Infection: New Efficacy Analysis. Clin. Infect. Dis. 2025, ciaf280. [Google Scholar] [CrossRef]
- Al-Anany, A.M.; Hooey, P.B.; Cook, J.D.; Burrows, L.L.; Martyniuk, J.; Hynes, A.P.; German, G.J. Phage Therapy in the Management of Urinary Tract Infections: A Comprehensive Systematic Review. Phage 2023, 4, 112–127. [Google Scholar] [CrossRef]
- Zalewska-Piątek, B.; Nagórka, M. Phages as potential life-saving therapeutic option in the treatment of multidrug-resistant urinary tract infections. Acta Biochim. Pol. 2025, 72, 14264. [Google Scholar] [CrossRef]
- Larcher, R.; Dinh, A.; Monnin, B.; Laffont-Lozes, P.; Loubet, P.; Lavigne, J.P.; Bruyere, F.; Sotto, A. Phage therapy in patients with urinary tract infections: A systematic review. Expert Rev. Anti Infect. Ther. 2025, 1–12, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.W.; Alloussi, S.; Egger, G.; Blümlein, H.M.; Cozma, G.; Schulman, C.C.; Multicenter UTI Study Group. A long-term, multicenter, double-blind study of an Escherichia coli extract (OM-89) in female patients with recurrent urinary tract infections. Eur. Urol. 2005, 47, 542–548. [Google Scholar] [CrossRef]
- Prattley, S.; Geraghty, R.; Moore, M.; Somani, B.K. Role of Vaccines for Recurrent Urinary Tract Infections: A Systematic Review. Eur. Urol. Focus 2020, 6, 593–604. [Google Scholar] [CrossRef]
- Nestler, S.; Peschel, C.; Horstmann, A.H.; Vahlensieck, W.; Fabry, W.; Neisius, A. Prospective multicentre randomized double-blind placebo-controlled parallel group study on the efficacy and tolerability of StroVac® in patients with recurrent symptomatic uncomplicated bacterial urinary tract infections. Int. Urol. Nephrol. 2023, 55, 9–16, Erratum in Int. Urol. Nephrol. 2023, 55, 1159. https://doi.org/10.1007/s11255-023-03469-5. [Google Scholar] [CrossRef]
- Nestler, S.; Grüne, B.; Schilchegger, L.; Suna, A.; Perez, A.; Neisius, A. Efficacy of vaccination with StroVac for recurrent urinary tract infections in women: A comparative single-centre study. Int. Urol. Nephrol. 2021, 53, 2267–2272. [Google Scholar] [CrossRef]
- Eggers, A.; Ballüer, M.; Mohamed, B.A.; Nau, R.; Seele, J. A suspension of inactivated bacteria used for vaccination against recurrent urinary tract infections increases the phagocytic activity of murine macrophages. Front. Immunol. 2023, 14, 1180785. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filev, R.; Bogov, B.; Lyubomirova, M.; Rostaing, L. From Pandemic to Resistance: Addressing Multidrug-Resistant Urinary Tract Infections in the Balkans. Antibiotics 2025, 14, 849. https://doi.org/10.3390/antibiotics14090849
Filev R, Bogov B, Lyubomirova M, Rostaing L. From Pandemic to Resistance: Addressing Multidrug-Resistant Urinary Tract Infections in the Balkans. Antibiotics. 2025; 14(9):849. https://doi.org/10.3390/antibiotics14090849
Chicago/Turabian StyleFilev, Rumen, Boris Bogov, Mila Lyubomirova, and Lionel Rostaing. 2025. "From Pandemic to Resistance: Addressing Multidrug-Resistant Urinary Tract Infections in the Balkans" Antibiotics 14, no. 9: 849. https://doi.org/10.3390/antibiotics14090849
APA StyleFilev, R., Bogov, B., Lyubomirova, M., & Rostaing, L. (2025). From Pandemic to Resistance: Addressing Multidrug-Resistant Urinary Tract Infections in the Balkans. Antibiotics, 14(9), 849. https://doi.org/10.3390/antibiotics14090849







