In Vitro and In Vivo Characterization of Novel Cathelicidin-Based Peptides with Antimicrobial Activity Against Pseudomonas aeruginosa
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Susceptibility Profile
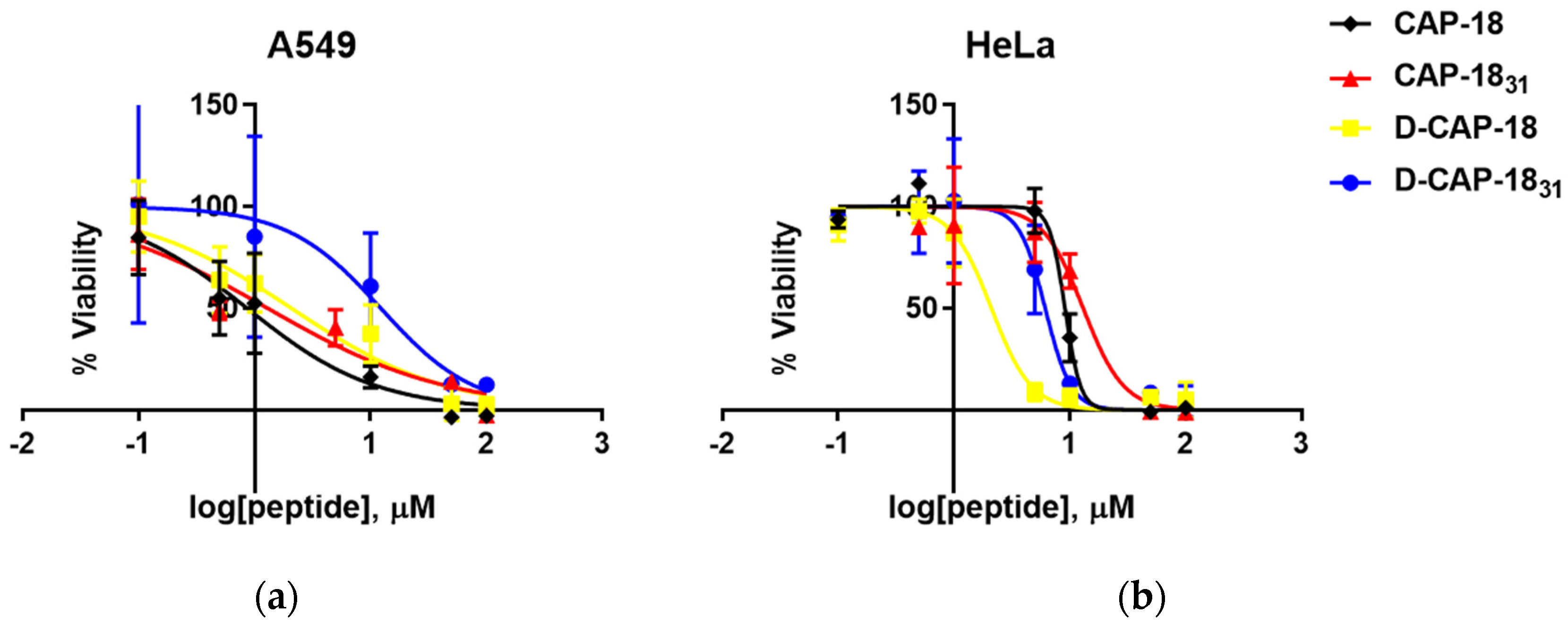
2.2. Hemolytic and Cytotoxic Effect in Human Cells
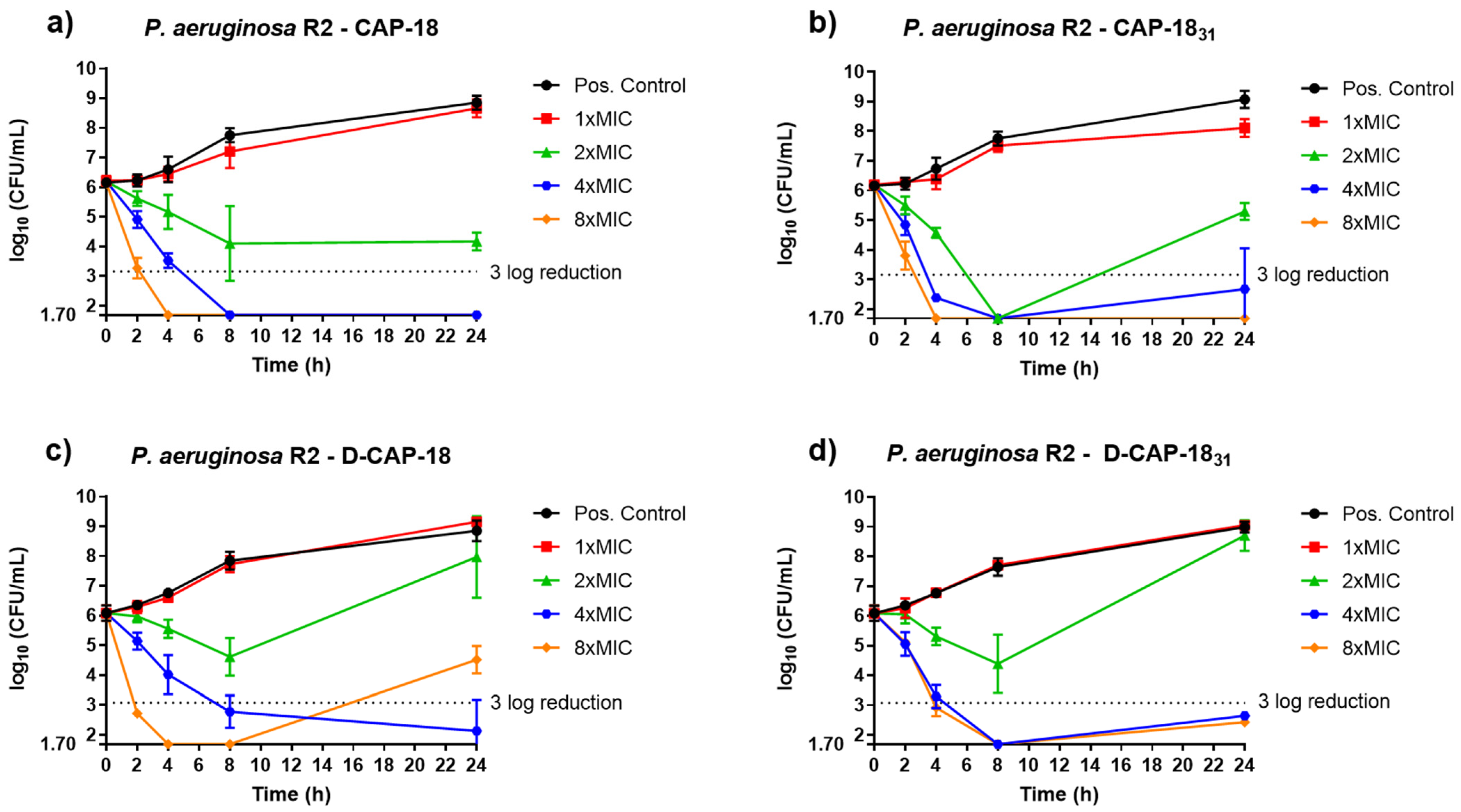
2.3. Time-Kill Kinetics Assays
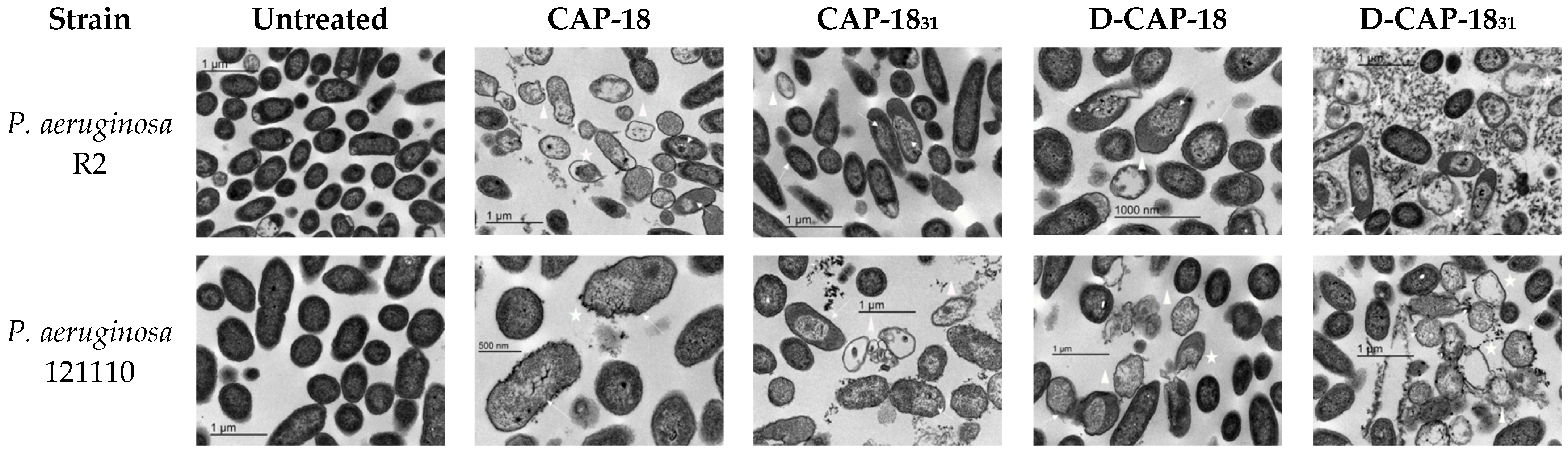
2.4. Bacterial Membrane Damage Through Transmission Electron Microscopy (TEM)
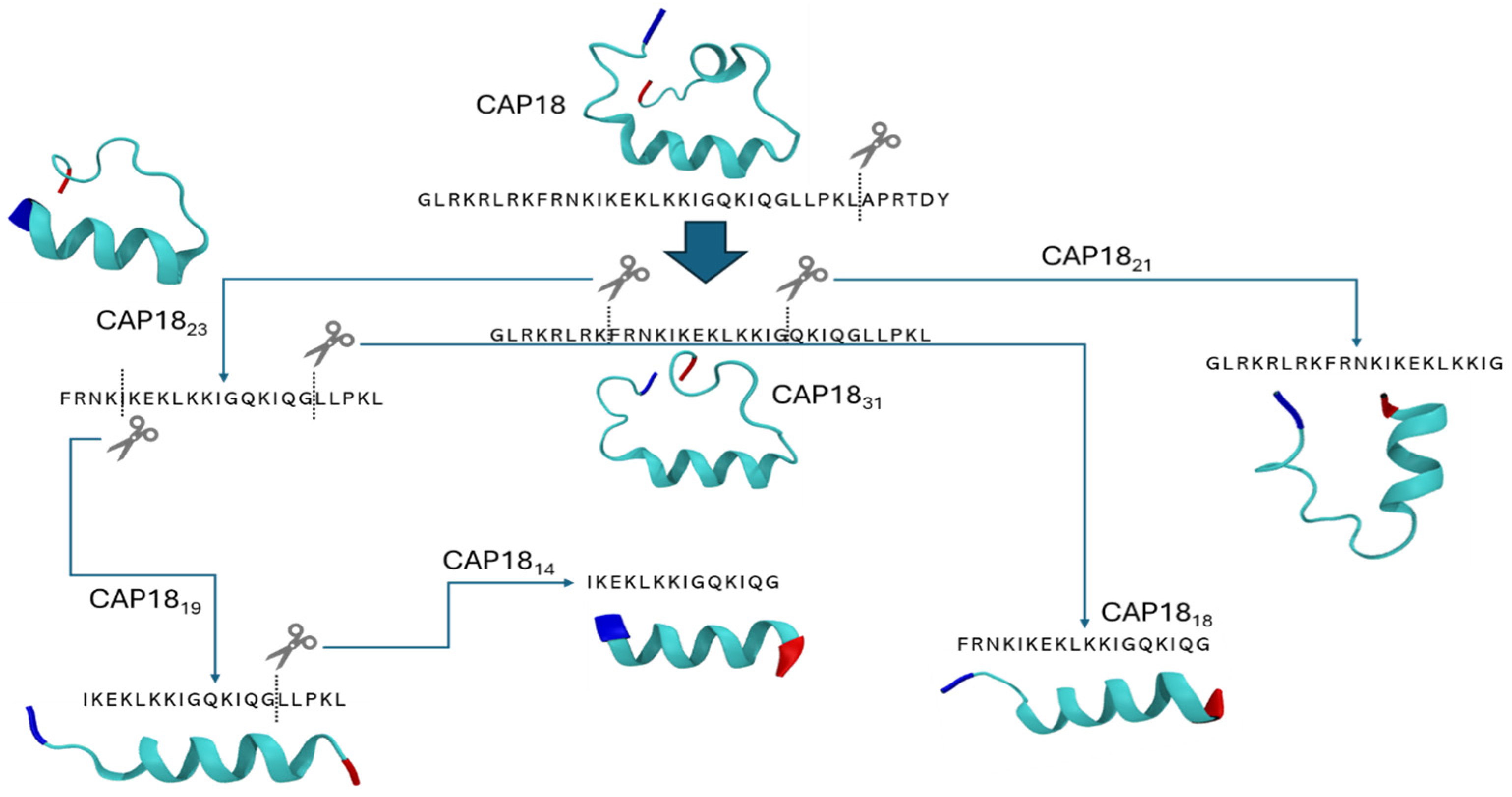
2.5. Modeling the Effect of Sequence Truncation on Helical Content
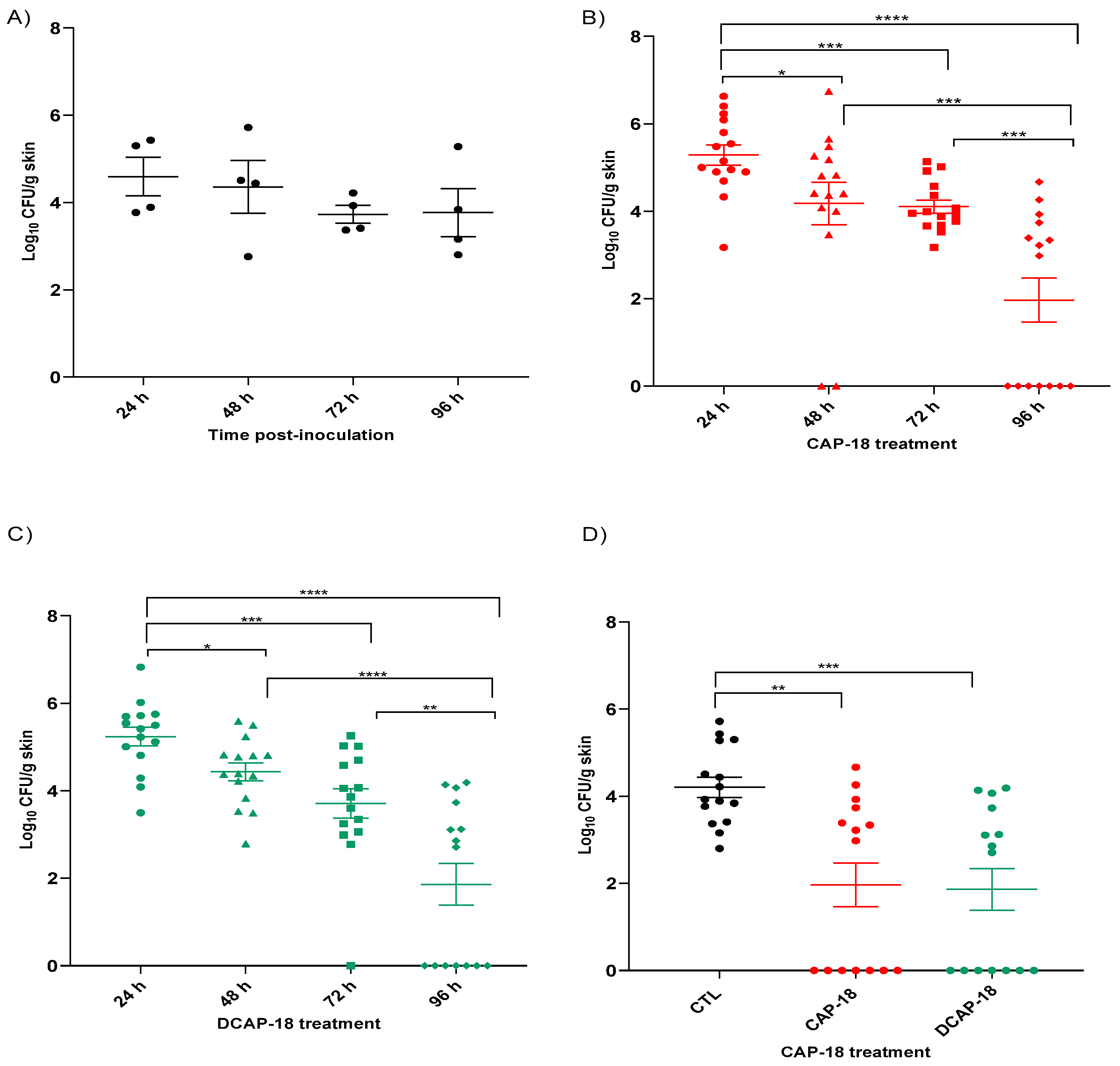
2.6. In Vivo Assays—Toxicity Studies
2.7. Efficacy Studies in Skin Murine Model by P. aeruginosa
3. Discussion
4. Materials and Methods
4.1. Peptides and Peptide Synthesis
4.2. Bacterial Strains
4.3. Antimicrobial Susceptibility Testing
4.4. Hemolysis Assays
4.5. Cytotoxicity Assays
4.6. Time-Kill Kinetic Assays
4.7. Transmission Electron Microscopy Sample Visualization
4.8. Secondary Structure Prediction
4.9. In Vivo Assays
4.10. Toxicity Studies
4.11. Efficacy Studies in Skin Murine Infection Model with P. aeruginosa
4.12. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial Resistance |
| WHO | World Health Organization |
| MDR | Multidrug resistant |
| AMP | Antimicrobial peptide |
| MIC | Minimum Inhibitory Concentration |
| TKC | Time-kill curve |
| ATCC | American Type Culture Collection |
| TEM | Transmission Electron Microscopy |
| CDR | Carboxyl-terminal disordered region |
| VMD | Visual Molecular Dynamics |
Appendix A
| MIC (mg/L) | |||||
|---|---|---|---|---|---|
| P. aeruginosa Strains | Colistin | CAP-18 | CAP-1831 | D-CAP-18 | D-CAP-1831 |
| 12-1110 S1 | ≤0.125 | ≤0.125 | 0.5 | 0.25 | 0.25 |
| 21-0505 S2 | ≤0.125 | ≤0.125 | 0.5 | 0.25 | 0.5 |
| 21-0308 R2 | 64 | 2 | 8 | 2 | 4 |
| 15-0803 S3 | ≤0.125 | 0.5 | 2 | 0.25 | 4 |
| 15-0307 S4 | ≤0.125 | ≤0.125 | 0.5 | ≤0.125 | 0.5 |
| 21-0410 S5 | ≤0.125 | 0.25 | 1 | 0.5 | 0.5 |
| 16359347-2 R3 | 64 | 1 | 8 | 2 | 4 |
| 36a | 0.25 | 0.5 | 1 | 1 | 2 |
| 38a | ≤0.125 | 1 | 4 | 1 | 4 |
| 27853 | ≤0.125 | 1 | 4 | 2 | 4 |
| C1 | 0.25 | 4 | 16 | 2 | 16 |
| C2 | 0.5 | 0.25 | 16 | 1 | 16 |
| C3 | ≤0.125 | 0.25 | 2 | 2 | 8 |
| C4 | 0.25 | 4 | 4 | 1 | 8 |
| C5 | ≤0.125 | 1 | 2 | 1 | 4 |
Appendix B

Appendix C
| Peptide | Sequence | Formula | MW (Da) |
|---|---|---|---|
| CAP-18 | GLRKRLRKFRNKIKEKLKKIGQKIQGLLPKLAPRTDY | C202H356N64O47 | 4433.45 |
| CAP-1831 | GLRKRLRKFRNKIKEKLKKIGQKIQGLLPKL | C171H311N55O37 | 3729.70 |
| CAP-1823 | FRNKIKEKLKKIGQKIQGLLPKL | C127H226N36O29 | 2721.42 |
| CAP-1821 | GLRKRLRKFRNKIKEKLKKIG | C118H217N41O25 | 2610.28 |
| CAP-1819 | IKEKLKKIGQKIQGLLPKL | C102H187N27O24 | 2175.77 |
| CAP-1818 | FRNKIKEKLKKIGQKIQG | C98H174N30O24 | 2156.65 |
| CAP-1814 | IKEKLKKIGQKIQG | C73H135N21O19 | 1611.00 |
| D-CAP-18 | Glrkrlrkfrnkikeklkkigqkiqgllpklaprtdy | C202H356N64O47 | 4433.45 |
| D-CAP-1831 | Glrkrlrkfrnkikeklkkigqkiqgllpkl | C171H311N55O37 | 3729.70 |
| R-CAP-18 | Ydtrpalkpllgqikqgikklkekiknrfkrlrkrlg | C202H356N64O47 | 4433.45 |
| R-CAP-1831 | Lkpllgqikqgikklkekiknrfkrlrkrlg | C171H311N55O37 | 3729.70 |
Appendix C.1
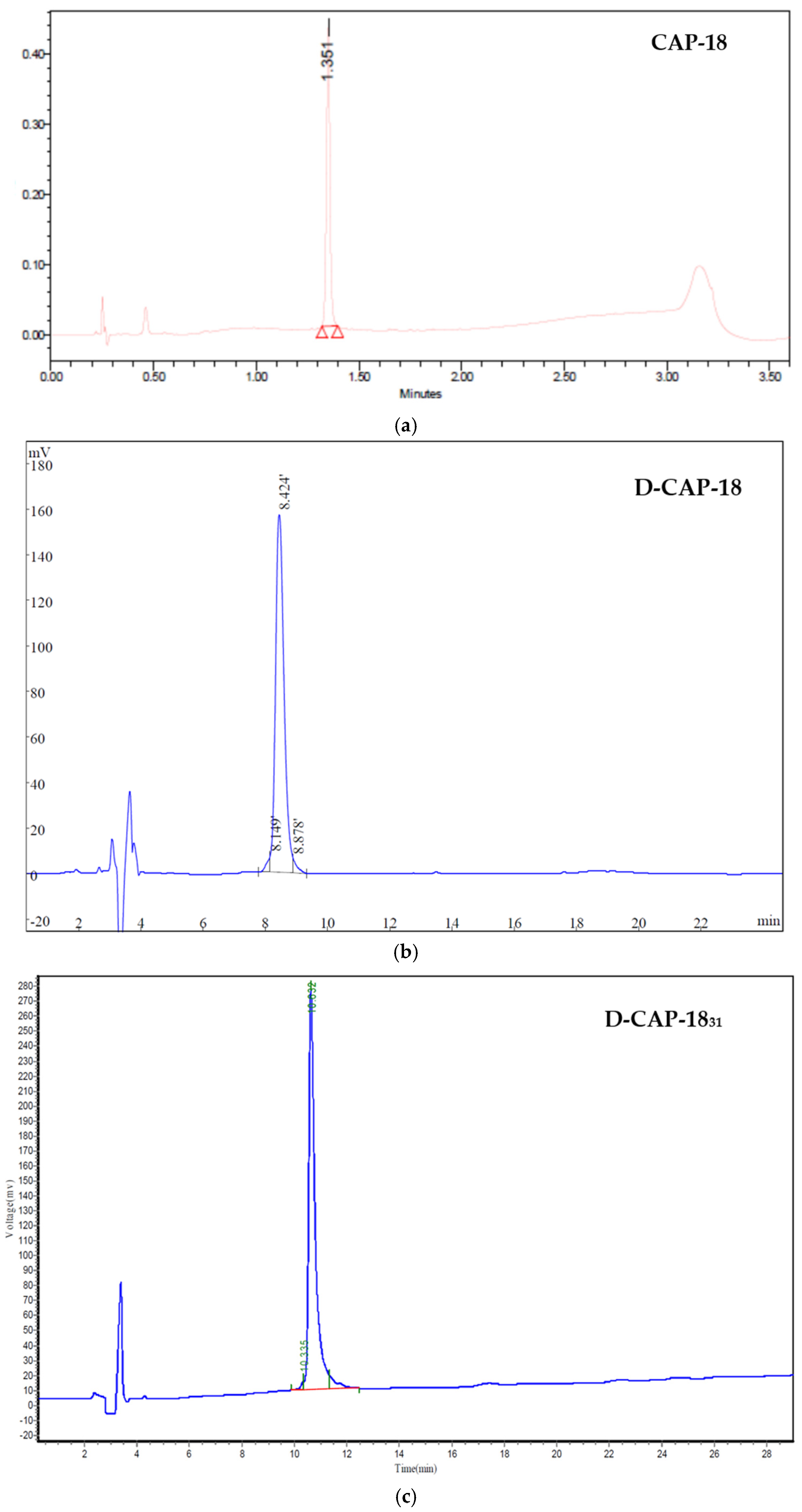

Appendix C.2


References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Mestrovic, T.; Aguilar, G.R.; Swetschinski, L.R.; Ikuta, K.S.; Gray, A.P.; Weaver, N.D.; Han, C.; Wool, E.E.; Hayoon, A.G.; Hay, S.I.; et al. The Burden of Bacterial Antimicrobial Resistance in the WHO European Region in 2019: A Cross-Country Systematic Analysis. Lancet Public Health 2022, 7, e897–e913. [Google Scholar] [CrossRef]
- Cook, M.A.; Wright, G.D. The Past, Present, and Future of Antibiotics. Sci. Transl. Med. 2022, 14, eabo7793. [Google Scholar] [CrossRef]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heuer, O.E.; et al. The Global Threat of Antimicrobial Resistance: Science for Intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef]
- Sharland, M.; Pulcini, C.; Harbarth, S.; Zeng, M.; Gandra, S.; Mathur, S.; Magrini, N. 21st WHO Expert Committee on Selection and Use of Essential Medicines Classifying Antibiotics in the WHO Essential Medicines List for Optimal Use-Be AWaRe. Lancet Infect. Dis. 2018, 18, 18–20. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- World Health Organization (WHO). 2021 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis, 1st ed.; WHO: Geneva, Switzerland, 2022; ISBN 978-92-4-004765-5. [Google Scholar]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated with Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Vincent, J.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.; Marshall, J.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef]
- Williams, F.N.; Herndon, D.N.; Hawkins, H.K.; Lee, J.O.; Cox, R.A.; Kulp, G.A.; Finnerty, C.C.; Chinkes, D.L.; Jeschke, M.G. The Leading Causes of Death after Burn Injury in a Single Pediatric Burn Center. Crit. Care Lond. Engl. 2009, 13, R183. [Google Scholar] [CrossRef]
- Reynolds, D.; Kollef, M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas Aeruginosa Infections: An Update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Platforms for Antibiotic Discovery. Nat. Rev. Drug Discov. 2013, 12, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Moreno-Morales, J.; Ballesté-Delpierre, C. Current Landscape in the Discovery of Novel Antibacterial Agents. Clin. Microbiol. Infect. 2020, 26, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Rodríguez-Rojas, A.; Makarova, O.; Rolff, J. Antimicrobials, Stress and Mutagenesis. PLoS Pathog. 2014, 10, e1004445. [Google Scholar] [CrossRef]
- Nizet, V. Mechanisms and Significance of Bacterial Resistance to Human Cationic Antimicrobial Peptides. In Antimicrobial Peptides and Innate Immunity; Hiemstra, P.S., Zaat, S.A.J., Eds.; Progress in Inflammation Research; Springer: Cham, Switzerland, 2013; pp. 11–26. ISBN 978-3-0348-0540-7. [Google Scholar]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Beisswenger, C.; Bals, R. Functions of Antimicrobial Peptides in Host Defense and Immunity. Curr. Protein Pept. Sci. 2005, 6, 255–264. [Google Scholar] [CrossRef]
- Wimley, W.C.; Hristova, K. Antimicrobial Peptides: Successes, Challenges and Unanswered Questions. J. Membr. Biol. 2011, 239, 27–34. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned from Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef]
- Ebenhan, T.; Gheysens, O.; Kruger, H.G.; Zeevaart, J.R.; Sathekge, M.M. Antimicrobial Peptides: Their Role as Infection-Selective Tracers for Molecular Imaging. BioMed Res. Int. 2014, 2014, 867381. [Google Scholar] [CrossRef]
- Fry, D.E. Antimicrobial Peptides. Surg. Infect. 2018, 19, 804–811. [Google Scholar] [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of Antimicrobial Peptides. A Review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef]
- Zanetti, M.; Gennaro, R.; Romeo, D. Cathelicidins: A Novel Protein Family with a Common Proregion and a Variable C-Terminal Antimicrobial Domain. FEBS Lett. 1995, 374, 1–5. [Google Scholar] [CrossRef]
- Boman, H. Peptide Antibiotics and Their Role in Innate Immunity. Annu. Rev. Immunol. 1995, 13, 61–92. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M. The Role of Cathelicidins in the Innate Host Defenses of Mammals. Curr. Issues Mol. Biol. 2005, 7, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Scocchi, M.; Skerlavaj, B.; Romeo, D.; Gennaro, R. Proteolytic Cleavage by Neutrophil Elastase Converts Inactive Storage Proforms to Antibacterial Bactenecins. Eur. J. Biochem. 1992, 209, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Hultmark, D.; Steiner, H.; Rasmuson, T.; Boman, H.G. Insect Immunity. Purification and Properties of Three Inducible Bactericidal Proteins from Hemolymph of Immunized Pupae of Hyalophora Cecropia. Eur. J. Biochem. 1980, 106, 7–16. [Google Scholar] [CrossRef]
- Zasloff, M. Magainins, a Class of Antimicrobial Peptides from Xenopus Skin: Isolation, Characterization of Two Active Forms, and Partial cDNA Sequence of a Precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef]
- Hirata, M.; Tsunoda, N.; Yoshida, M. Anticoagulant, Cationic Protein (CAP) Isolated from Granulocytes. Blood Vessel 1987, 18, 592–594. [Google Scholar] [CrossRef]
- Hirata, M.; Yoshida, M.; Inada, K.; Kirikae, T. Investigation of Endotoxin Binding Cationic Proteins from Granulocytes; Agglutination of Erythrocytes Sensitized with Re-LPS. In Endotoxin; Friedman, H., Klein, T.W., Nakano, M., Nowotny, A., Eds.; Springer: Boston, MA, USA, 1990; pp. 287–299. ISBN 978-1-4757-5140-6. [Google Scholar]
- Larrick, J.; Morgan, J.; Palings, I.; Hirata, M.; Yen, M. Complementary DNA Sequence of Rabbit CAP18--a Unique Lipopolysaccharide Binding Protein. Biochem. Biophys. Res. Commun. 1991, 179, 170–175. [Google Scholar] [CrossRef]
- Gennaro, R.; Skerlavaj, B.; Romeo, D. Purification, Composition, and Activity of Two Bactenecins, Antibacterial Peptides of Bovine Neutrophils. Infect. Immun. 1989, 57, 3142–3146. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Boman, A.; Sun, C.X.; Andersson, M.; Jörnvall, H.; Mutt, V.; Boman, H.G. Antibacterial Peptides from Pig Intestine: Isolation of a Mammalian Cecropin. Proc. Natl. Acad. Sci. USA 1989, 86, 9159–9162. [Google Scholar] [CrossRef]
- Larrick, J.W.; Hirata, M.; Zheng, H.; Zhong, J.; Bolin, D.; Cavaillon, J.M.; Warren, H.S.; Wright, S.C. A Novel Granulocyte-Derived Peptide with Lipopolysaccharide-Neutralizing Activity. J. Immunol. 1994, 152, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Shimomura, Y.; Yoshida, M.; Morgan, J.G.; Palings, I.; Wilson, D.; Yen, M.H.; Wright, S.C.; Larrick, J.W. Characterization of a Rabbit Cationic Protein (CAP18) with Lipopolysaccharide-Inhibitory Activity. Infect. Immun. 1994, 62, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Zarember, K.A.; Katz, S.S.; Tack, B.F.; Doukhan, L.; Weiss, J.; Elsbach, P. Host Defense Functions of Proteolytically Processed and Parent (Unprocessed) Cathelicidins of Rabbit Granulocytes. Infect. Immun. 2002, 70, 569–576. [Google Scholar] [CrossRef]
- Chen, C.; Brock, R.; Luh, F.; Chou, P.J.; Larrick, J.W.; Huang, R.F.; Huang, T.H. The Solution Structure of the Active Domain of CAP18—A Lipopolysaccharide Binding Protein from Rabbit Leukocytes. FEBS Lett. 1995, 370, 46–52. [Google Scholar] [CrossRef]
- Lamers, C. Overcoming the Shortcomings of Peptide-Based Therapeutics. Future Drug Discov. 2022, 4, FDD75. [Google Scholar] [CrossRef]
- Prades, R.; Oller-Salvia, B.; Schwarzmaier, S.M.; Selva, J.; Moros, M.; Balbi, M.; Grazú, V.; de La Fuente, J.M.; Egea, G.; Plesnila, N.; et al. Applying the Retro-Enantio Approach to Obtain a Peptide Capable of Overcoming the Blood-Brain Barrier. Angew. Chem. Int. Ed. Engl. 2015, 54, 3967–3972. [Google Scholar] [CrossRef]
- Arranz-Gibert, P.; Ciudad, S.; Seco, J.; García, J.; Giralt, E.; Teixidó, M. Immunosilencing Peptides by Stereochemical Inversion and Sequence Reversal: Retro-D-Peptides. Sci. Rep. 2018, 8, 6446. [Google Scholar] [CrossRef]
- Bacalum, M.; Radu, M. Cationic Antimicrobial Peptides Cytotoxicity on Mammalian Cells: An Analysis Using Therapeutic Index Integrative Concept. Int. J. Pept. Res. Ther. 2015, 21, 47–55. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, B. Positive Selection in Cathelicidin Host Defense Peptides: Adaptation to Exogenous Pathogens or Endogenous Receptors? Heredity 2017, 118, 453–465. [Google Scholar] [CrossRef]
- Rey, J.; Murail, S.; de Vries, S.; Derreumaux, P.; Tuffery, P. PEP-FOLD4: A pH-Dependent Force Field for Peptide Structure Prediction in Aqueous Solution. Nucleic Acids Res. 2023, 51, W432–W437. [Google Scholar] [CrossRef]
- Larrick, J.W.; Hirata, M.; Shimomoura, Y.; Yoshida, M.; Zheng, H.; Zhong, J.; Wright, S.C. Antimicrobial Activity of Rabbit CAP18-Derived Peptides. Antimicrob. Agents Chemother. 1993, 37, 2534–2539. [Google Scholar] [CrossRef]
- Tossi, A.; Scocchi, M.; Skerlavaj, B.; Gennaro, R. Identification and Characterization of a Primary Antibacterial Domain in CAP18, a Lipopolysaccharide Binding Protein from Rabbit Leukocytes. FEBS Lett. 1994, 339, 108–112. [Google Scholar] [CrossRef]
- Nagaoka, I.; Tamura, H.; Reich, J. Therapeutic Potential of Cathelicidin Peptide LL-37, an Antimicrobial Agent, in a Murine Sepsis Model. Int. J. Mol. Sci. 2020, 21, 5973. [Google Scholar] [CrossRef]
- Zharkova, M.S.; Orlov, D.S.; Golubeva, O.Y.; Chakchir, O.B.; Eliseev, I.E.; Grinchuk, T.M.; Shamova, O.V. Application of Antimicrobial Peptides of the Innate Immune System in Combination with Conventional Antibiotics-A Novel Way to Combat Antibiotic Resistance? Front. Cell. Infect. Microbiol. 2019, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Wnorowska, U.; Niemirowicz, K.; Myint, M.; Diamond, S.; Wróblewska, M.; Savage, P.; Janmey, P.; Bucki, R. Bactericidal Activities of Cathelicidin LL-37 and Select Cationic Lipids against the Hypervirulent Pseudomonas Aeruginosa Strain LESB58. Antimicrob. Agents Chemother. 2015, 59, 3808–3815. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-Q.; Kyung, S.M.; Kim, S.; Kim, G.; Lee, S.Y.; Yoo, H.S. Effects of Synthetic Peptide RP557 and Its Origin, LL-37, on Carbapenem-Resistant Pseudomonas Aeruginosa. Microbiol. Spectr. 2023, 11, e0043023. [Google Scholar] [CrossRef] [PubMed]
- Luther, A.; Urfer, M.; Zahn, M.; Müller, M.; Wang, S.-Y.; Mondal, M.; Vitale, A.; Hartmann, J.-B.; Sharpe, T.; Monte, F.L.; et al. Chimeric Peptidomimetic Antibiotics against Gram-Negative Bacteria. Nature 2019, 576, 452–458. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, F.; Chen, Y.-C.; Peng, H.; Wang, K.-J. The Long-Term Effect of a Nine Amino-Acid Antimicrobial Peptide AS-Hepc3(48–56) Against Pseudomonas Aeruginosa with No Detectable Resistance. Front. Cell. Infect. Microbiol. 2021, 11, 752637. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, F.; Li, S.; Peng, H.; Wang, K.-J. A Novel Antimicrobial Peptide Sparanegtin Identified in Scylla Paramamosain Showing Antimicrobial Activity and Immunoprotective Role In Vitro and Vivo. Int. J. Mol. Sci. 2021, 23, 15. [Google Scholar] [CrossRef]
- Mandel, S.; Michaeli, J.; Nur, N.; Erbetti, I.; Zazoun, J.; Ferrari, L.; Felici, A.; Cohen-Kutner, M.; Bachnoff, N. OMN6 a Novel Bioengineered Peptide for the Treatment of Multidrug Resistant Gram Negative Bacteria. Sci. Rep. 2021, 11, 6603. [Google Scholar] [CrossRef]
- Eylar, E.H.; Madoff, M.A.; Brody, O.V.; Oncley, J.L. The Contribution of Sialic Acid to the Surface Charge of the Erythrocyte. J. Biol. Chem. 1962, 237, 1992–2000. [Google Scholar] [CrossRef]
- Van Moll, L.; De Smet, J.; Paas, A.; Tegtmeier, D.; Vilcinskas, A.; Cos, P.; Van Campenhout, L. In Vitro Evaluation of Antimicrobial Peptides from the Black Soldier Fly (Hermetia Illucens) against a Selection of Human Pathogens. Microbiol. Spectr. 2022, 10, e0166421. [Google Scholar] [CrossRef]
- Vila-Farrés, X.; López-Rojas, R.; Pachón-Ibáñez, M.E.; Teixidó, M.; Pachón, J.; Vila, J.; Giralt, E. Sequence-Activity Relationship, and Mechanism of Action of Mastoparan Analogues against Extended-Drug Resistant Acinetobacter baumannii. Eur. J. Med. Chem. 2015, 101, 34–40. [Google Scholar] [CrossRef]
- Zhang, W.; An, Z.; Bai, Y.; Zhou, Y.; Chen, F.; Wang, K.-J. A Novel Antimicrobial Peptide Scyreptin1–30 from Scylla Paramamosain Exhibiting Potential Therapy of Pseudomonas Aeruginosa Early Infection in a Mouse Burn Wound Model. Biochem. Pharmacol. 2023, 218, 115917. [Google Scholar] [CrossRef]
- Heselpoth, R.D.; Euler, C.W.; Fischetti, V.A. PaP1, a Broad-Spectrum Lysin-Derived Cationic Peptide to Treat Polymicrobial Skin Infections. Front. Microbiol. 2022, 13, 817228. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST) Recommendations for MIC Determination of Colistin (Polymyxin E): As Recommended by the Joint CLSI–EUCAST Polymyxin Breakpoints Working Group. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf (accessed on 28 May 2025).
- Lamiable, A.; Thévenet, P.; Rey, J.; Vavrusa, M.; Derreumaux, P.; Tufféry, P. PEP-FOLD3: Faster de Novo Structure Prediction for Linear Peptides in Solution and in Complex. Nucleic Acids Res. 2016, 44, W449–W454. [Google Scholar] [CrossRef] [PubMed]
- Binette, V.; Mousseau, N.; Tuffery, P. A Generalized Attraction–Repulsion Potential and Revisited Fragment Library Improves PEP-FOLD Peptide Structure Prediction. J. Chem. Theory Comput. 2022, 18, 2720–2736. [Google Scholar] [CrossRef]
- Tufféry, P.; Derreumaux, P. A Refined pH-Dependent Coarse-Grained Model for Peptide Structure Prediction in Aqueous Solution. Front. Bioinform. 2023, 3, 1113928. [Google Scholar] [CrossRef]
- Camproux, A.C.; Gautier, R.; Tufféry, P. A Hidden Markov Model Derived Structural Alphabet for Proteins. J. Mol. Biol. 2004, 339, 591–605. [Google Scholar] [CrossRef] [PubMed]
- National Research Council; Division on Earth and Life Studies; Institute for Laboratory Animal Research; Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Kugelberg, E.; Norström, T.; Petersen, T.K.; Duvold, T.; Andersson, D.I.; Hughes, D. Establishment of a Superficial Skin Infection Model in Mice by Using Staphylococcus Aureus and Streptococcus Pyogenes. Antimicrob. Agents Chemother. 2005, 49, 3435–3441. [Google Scholar] [CrossRef]


| MIC (mg/L) [μM] | ||
|---|---|---|
| Peptide | Colistin Susceptible P. aeruginosa | Colistin-Resistant P. aeruginosa |
| r-CRAMP | >256 [>65] | 64 [16] |
| α-defensin 3 | >50 [>14] | >50 [>14] |
| α-defensin 1 | >50 [>15] | >50 [>15] |
| PR39 | 64 [14] | 32 [7] |
| β-defensin 4 | >50 [>11] | >50 [>11] |
| CRAMP | 256 [>66] | 32 [8] |
| β-defensin 2 | >50 [>12] | >50 [>12] |
| α-defensin 6 | >50 [>13] | >50 [>13] |
| β-defensin 3 | >50 [>10] | >50 [>10] |
| Dermicidin | >256 [>53] | >256 [>53] |
| Mundticin | >256 [>60] | >256 [>60] |
| CRAMP 1-39 | 64 [>14] | 32 [7] |
| α-defensin 2 | >50 [>15] | >50 [>15] |
| CAP-18 | 8 [1.8] | 4 [0.9] |
| HP 2-20 | >256 [>110] | >256 [>110] |
| Hepcidin | >256 [>92] | >256 [>92] |
| α-defensin 5 | 50 [14] | >50 [14] |
| Colistin | 0.75 [0.7] | 32 [28] |
| MIC (mg/L) [μM] | |||
|---|---|---|---|
| Peptide | Sequence | Strain 121007 | Strain ATCC 121110 |
| CAP-18 | GLRKRLRKFRNKIKEKLKKIGQKIQGLLPKLAPRTDY | 1 [0.2] | <0.125 [<0.03] |
| CAP-1831 * | GLRKRLRKFRNKIKEKLKKIGQKIQGLLPKL | 8 [2.1] | 0.5 [0.1] |
| CAP-1823 * | FRNKIKEKLKKIGQKIQGLLPKL | >64 [>24] | >32 [>12] |
| CAP-1821 * | GLRKRLRKFRNKIKEKLKKIG | 32 [12] | 32 [12] |
| CAP-1819 * | IKEKLKKIGQKIQGLLPKL | >64 [>29] | >64 [>29] |
| CAP-1818 * | FRNKIKEKLKKIGQKIQG | >64 [>30] | >64 [>30] |
| CAP-1814 * | IKEKLKKIGQKIQG | >64 [40] | >64 [>40] |
| D-CAP-18 | Glrkrlrkfrnkikeklkkigqkiqgllpklaprtdy | 2 [0.4] | 0.25 [0.06] |
| D-CAP-1831 | Glrkrlrkfrnkikeklkkigqkiqgllpkl | 2 [0.5] | 0.25 [0.07] |
| R-CAP-18 | Ydtrpalkpllgqikqgikklkekiknrfkrlrkrlg | 2 [0.4] | 32 [7.2] |
| R-CAP-1831 | Lkpllgqikqgikklkekiknrfkrlrkrlg | 0.5 [0.1] | 4 [1.1] |
| P. aeruginosa Strain Panel | ||
|---|---|---|
| Peptide | MIC50 (mg/L) [μM] | MIC90 (mg/L) [μM] |
| CAP-18 | 0.5 [0.1] | 4 [0.9] |
| CAP-1831 | 2 [0.5] | 16 [4.3] |
| D-CAP-18 | 1 [0.2] | 2 [0.45] |
| D-CAP-1831 | 4 [1.1] | 16 [4.3] |
| Colistin | ≤0.125 [≤0.11] | 64 [55] |
| Peptide | Hemolysis IC50 (μM) [mg/L] | A549 IC50 (µM) [mg/L] | Therapeutic Index (IC50/MIC90) P. aeruginosa |
|---|---|---|---|
| CAP-18 | 35.73 [158.41] | 0.87 [3.86] | 39.6 |
| CAP-1831 | 185.26 [690.97] | 1.31 [4.89] | 43.2 |
| D-CAP-18 | 61.97 [274.76] | 2.09 [9.26] | 137.4 |
| D-CAP-1831 | 108.44 [404.48] | 12.32 [45.94] | 25.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Morales, J.; Martín-Vilardell, N.; Guardiola, S.; Vila-Farrés, X.; Cebrero, T.; Babić, M.; Ballesté-Delpierre, C.; Kalafatović, D.; Giralt, E.; Pachón-Ibañez, M.E.; et al. In Vitro and In Vivo Characterization of Novel Cathelicidin-Based Peptides with Antimicrobial Activity Against Pseudomonas aeruginosa. Antibiotics 2025, 14, 838. https://doi.org/10.3390/antibiotics14080838
Moreno-Morales J, Martín-Vilardell N, Guardiola S, Vila-Farrés X, Cebrero T, Babić M, Ballesté-Delpierre C, Kalafatović D, Giralt E, Pachón-Ibañez ME, et al. In Vitro and In Vivo Characterization of Novel Cathelicidin-Based Peptides with Antimicrobial Activity Against Pseudomonas aeruginosa. Antibiotics. 2025; 14(8):838. https://doi.org/10.3390/antibiotics14080838
Chicago/Turabian StyleMoreno-Morales, Javier, Núria Martín-Vilardell, Salvador Guardiola, Xavier Vila-Farrés, Tania Cebrero, Marko Babić, Clara Ballesté-Delpierre, Daniela Kalafatović, Ernest Giralt, María Eugenia Pachón-Ibañez, and et al. 2025. "In Vitro and In Vivo Characterization of Novel Cathelicidin-Based Peptides with Antimicrobial Activity Against Pseudomonas aeruginosa" Antibiotics 14, no. 8: 838. https://doi.org/10.3390/antibiotics14080838
APA StyleMoreno-Morales, J., Martín-Vilardell, N., Guardiola, S., Vila-Farrés, X., Cebrero, T., Babić, M., Ballesté-Delpierre, C., Kalafatović, D., Giralt, E., Pachón-Ibañez, M. E., & Vila, J. (2025). In Vitro and In Vivo Characterization of Novel Cathelicidin-Based Peptides with Antimicrobial Activity Against Pseudomonas aeruginosa. Antibiotics, 14(8), 838. https://doi.org/10.3390/antibiotics14080838









