Abstract
Background/Objectives: Vancomycin-resistant enterococci (VRE) are significant nosocomial pathogens worldwide, potentially transmitted by food-producing animals and related products. This study investigates the epidemiological role of bovine raw milk in the transmission of VRE to humans. Methods: Bulk milk samples were screened for van gene presence using a multiplex PCR. Mastitogenic enterococci isolated from individual milk samples were tested for antimicrobial susceptibility using the broth microdilution method. Strains not susceptible to vancomycin were whole genome sequenced. Results: Overall, vanC genes were detected in 299/1026 (29.14%) bulk milk samples. Specifically, vanC1 was found in 204 samples (19.88%) and vanC2/3 in 57 samples (5.56%), with both detected simultaneously in 38 samples (3.70%). Clinically significant vanA and vanB genes were not identified. A total of 163 mastitogenic Enterococcus strains were isolated from individual milk samples. Eight different Enterococcus species were detected, with E. faecium (104/163, 63.80%) and E. faecalis (34/163, 20.86%) being the most common. Multidrug resistance was observed in 106/163 (65.03%) isolates. The most common resistance frequencies were to ciprofloxacin and erythromycin (102/163, 62.58% both), followed by quinupristin/dalfopristin (93/163, 57.06%), linezolid (65/163, 39.88%), tetracycline (58/163, 35.58%), daptomycin (46/163, 28.22%), chloramphenicol (33/163, 20.25%), ampicillin, tigecycline, and high-dosage gentamycin (8/163, 4.91% all). Resistance to teicoplanin was not observed. Two vancomycin non-susceptible strains were identified: one vanC2/3 E. casseliflavus and one vanC1 E. gallinarum. Whole genome sequencing confirmed the presence of the complete vanC gene cluster and several virulence genes in both strains. Conclusions: Our findings suggest that while raw milk is unlikely to be a source of vancomycin resistance genes of highest clinical importance (vanA or vanB), it may contribute to the spread of vanC enterococci, which are increasingly associated with human infections.
1. Introduction
Enterococci are natural components of the human and animal microbiota, primarily located in the gastrointestinal tract, as well as in the oral and genital mucosae [1,2]. They are also commonly detected in the environment, including water, soil, and plants [3,4,5]. The genus Enterococcus (E.) comprises over 50 species, with E. faecium and E. faecalis being the most representative [6]. Although typically considered commensals, enterococci can act as opportunistic pathogens under favourable conditions. Globally, enterococci is one of the leading causes of nosocomial infections, being associated with considerable mortality, morbidity, and growing costs for the healthcare system [7]. These bacteria are responsible for a broad spectrum of infections, including genitourinary infections, bloodstream infections, endocarditis, peritonitis, meningitis, and neonatal sepsis [6], with high mortality rates (up to 20%) [8].
The clinical relevance of enterococci is underscored by their intrinsic resistance to a wide range of antimicrobial agents, including aminoglycosides, cephalosporins, macrolides and sulphonamides [9]. Concerningly, over the past decade, enterococci have increasingly acquired multidrug-resistant (MDR) profiles, including Critically Important Antimicrobials (CIAs).
In particular, vancomycin is one of the last-line therapeutic options for severe infections caused by enterococci and other MDR Gram-positive bacteria [7]. In recent years, the prevalence of vancomycin-resistant enterococci (VRE) has increased concerningly in the European Union (EU), including in the community setting.
Vancomycin resistance has become particularly widespread in E. faecium, the primary pathogenic species of the genus Enterococcus responsible for clinical infections in humans [10,11]. In 2021, vancomycin-resistant E. faecium was reported in 39% of the countries in Europe, with prevalence rates ranging from 25% to 66% [10,12]. In Italy, the incidence of clinical vancomycin-resistant E. faecium has shown a significant increase, rising from 11.1% in 2015 to 32.5% in 2023 [10]. Therefore, E. faecium has been included in the “WHO global priority pathogens list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics” [13]. Additionally, the spread of vancomycin resistance among enterococci also raises concerns about potential horizontal transfer of antimicrobial resistance gene (ARG) to other pathogens, such as Staphylococcus aureus [14,15]. Notably, clinical isolates of the Enterococcus genus also showed high resistance rates (>50%) to other critical antimicrobials, such as aminopenicillins and high-dose gentamicin, despite these trends having remained relatively stable in recent years [10,11].
Food-production animals and related products are strongly suspected of playing a role in the transmission of VRE to humans. Historically, the emergence of vancomycin resistance has been linked to the widespread use of similar molecules (such as avoparcin) as growth promoters in livestock until the late 1990s. Given the potential antimicrobial resistance (AMR) transmission through the food chain, the EU banned the use of avoparcin in animal husbandry in 1997 [16,17].
Currently, the potential transmission of VRE from livestock and related products to humans is monitored through the European surveillance programme (Commission Implementing Decision 2020/1729/EU), targeting vancomycin-resistant E. faecalis and E. faecium in selected meat-producing animals (broiler chickens, laying hens, fattening turkeys, and cattle under one year of age). The presence of enterococci in meat may be associated with faecal carcass contamination during critical slaughtering procedures (i.e., skinning or evisceration) or with faecal contamination of the environments where meat is processed [18].
However, other livestock sectors, such as dairy production, may also contribute to the transmission of critical resistances to humans.
Milk is considered a primary necessity food, with European production reaching 145 million tonnes in 2023, 8.9% of which is attributed to the Italian dairy industry [19]. The microbiological safety of this product is essential to protect consumers, as milk has already been associated with bacterial infections transmitted through raw dairy product consumption, including E. coli O157 [20,21,22].
Additionally, in the last decade, dairy cattle and related products have been identified as potential food sources of VRE [23,24,25,26,27,28]. Enterococci are known causative agents of both clinical and subclinical mastitis in cattle and small ruminants, typically originating from contaminated environments [29]. Once the mammary gland is colonised, enterococci can proliferate in the cistern and mammary ducts and be excreted in high concentrations in milk [27]. Additionally, faecal contamination during milking, especially under poor hygiene conditions, may further compromise milk quality [30].
Enterococcal infections in dairy cows may pose an occupational hazard for breeders and veterinarians and also represent a potential threat to individuals in close contact with livestock, especially if immunocompromised. Moreover, the growing consumer demand for unpasteurised milk and artisanal cheeses, often driven by preferences for “natural” or minimally processed foods, represents a risk of foodborne transmission [31].
The lack of data on foodborne or occupational infections in humans associated with milk-origin VRE—likely due to underreporting and underestimation—highlights the importance of further investigating this issue.
This study aims to investigate the epidemiological role of raw bovine milk in the potential transmission of VRE and/or enterococci associated with other critical resistance profiles to humans. The phenotypic and genotypic characterisation of vancomycin resistance and virulence determinants in milk-derived VRE strains will provide valuable insights into the mechanisms of pathogenicity, therapeutic challenges, and the epidemiology of VRE infections.
2. Results
2.1. Bulk Milk
van Gene Distribution
van genes were detected in 299/1026 (29.14%) bulk milk samples. Specifically, vanC1 was identified in 204 samples (19.88%), vanC2/3 in 57 samples (5.56%), and both vanC1 and vanC2/3 in 38 samples (3.70%). vanA and vanB genes were not detected.
2.2. Individual Milk
2.2.1. Enterococcus Species Distribution
A total of 163 Enterococcus spp. strains were isolated, belonging to eight different species. The most common were E. faecium (104/163, 63.80%) and E. faecalis (34/163, 20.86%), followed by E. hirae (9/163, 5.52%), E. cecorum (6/163, 3.68%), E. saccharolitycus (4/163, 2.45%), E. canintestini (3/163, 1.84%), E. casseliflavus (2/163, 1.23%), and E. gallinarum (1/163, 0.61%) (Table 1).

Table 1.
Number of isolates, number of isolates susceptible to all the antimicrobial panel tested (S), number of multidrug-resistant (MDR) strains, number of strains not susceptible to ≥5 different antimicrobial classes (≥5 R), according to Enterococcus species.
2.2.2. AMR Profiles of Enterococcus spp. Strains
A total of 158 out of 163 (96.93%) strains were non-susceptible to at least one antimicrobial, meanwhile 106/163 (65.03%) isolates were MDR from three up to nine different antimicrobial classes. Most strains showing non-susceptibility to at least five different antimicrobial classes belonged to E. faecium (26/33, 78.79%) (Table 1).
The highest percentages of non-susceptibility strains resulted in ciprofloxacin and erythromycin (102/163, 62.58% both), quinupristin/dalfopristin (93/163, 57.06%), linezolid (65/163, 39.88%), tetracycline (58/163, 35.58%), daptomycin (46/163, 28.22%), chloramphenicol (33/163, 20.25%), ampicillin, tigecycline, and high-dosage gentamycin (8/163, 4.91% both). All strains were susceptible to teicoplanin (Figure 1).
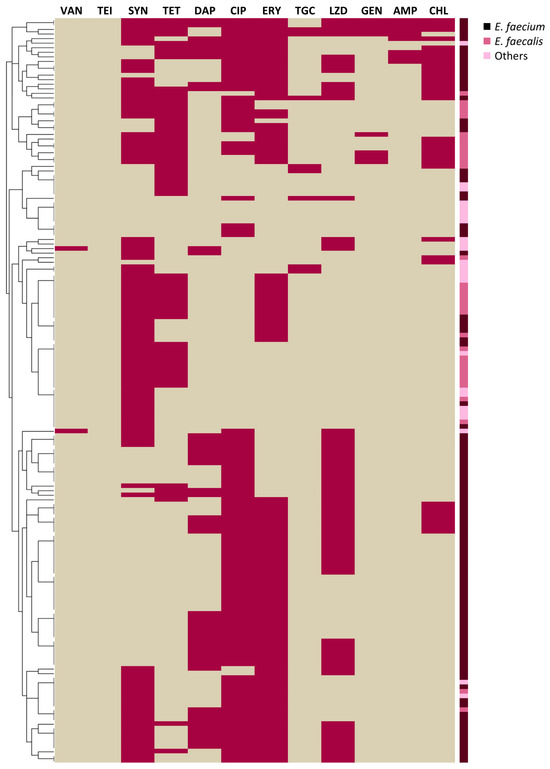
Figure 1.
Heatmap depicting non-susceptibility (violet) or susceptibility (beige) to different antimicrobials of Enterococcus isolates, clustered according to the similarities of their antimicrobial resistance profiles. Enterococcus species are colour-coded. VAN: vancomycin, TEI: teicoplanin, SYN: quinupristin/dalfopristin, TET: tetracycline, DAP: daptomycin, CIP: ciprofloxacin, ERY: erythromycin, TGC: tigecycline, LZD: linezolid, GEN: high-dosage gentamycin, AMP: ampicillin, CHL: chloramphenicol.
Interestingly, two strains were non-susceptible to vancomycin: one E. casseliflavus (MIC = 8 μg/mL) and one E. gallinarum (MIC = 16 μg/mL), carrying vanC2/3 and vanC1, respectively. Both strains showed MDR profiles, being also resistant to quinupristin/dalfopristin and linezolid (for both E. casseliflavus and E. gallinarum), and ciprofloxacin (for E. casseliflavus) and daptomycin (for E. gallinarum).
2.2.3. WGS of VRE Strains
Both vanC2/3 E. casseliflavus and vanC1 E. gallinarum harboured the entire vanC gene cluster, characterised by five genes, namely vanC, vanXY, vanT, vanR, and vanY. Different virulence genes (VAGs) involved in pathogenesis were also detected and reported in Table 2.

Table 2.
Virulence genes (VAGs) and related functions carried by vancomycin non-susceptible E. gallinarum and E. casseliflavus.
2.3. Statistical Analysis Results
Both E. faecium and E. faecalis demonstrated high multidrug resistance (MDR) rates, with 73.08% (76/104) and 67.65% (23/34), respectively. Nevertheless, the MDR profile did not show a significant association with either species. Pearson’s Chi-square test revealed significant differences in antimicrobial resistance (AMR) patterns between the two species. Specifically, resistance to quinupristin/dalfopristin and tetracycline was significantly more frequent in E. faecalis compared to E. faecium (p < 0.001), whereas E. faecium showed significantly higher resistance to daptomycin, ciprofloxacin, and linezolid (p < 0.001). No statistically significant differences in resistance to other antimicrobials were observed between the two species, based on Pearson’s Chi-square or Fisher’s exact tests.
3. Discussion
Enterococci, although commensals of the human gut, can cause serious opportunistic infections such as urinary tract infections, bacteremia, and endocarditis [6]. Their intrinsic resistance to different antimicrobials, along with a high capacity to acquire new ARGs, makes them clinically significant [32]. Particularly concerning is the increasing detection of VRE, considered major nosocomial pathogens worldwide [11]. Given the rising incidence of VRE infections and the limited availability of effective treatments, significant research has been focused on identifying VRE reservoirs and transmission routes, including those beyond healthcare settings.
The food animal sector has long been suspected of playing a role in the dissemination of VRE to humans. While poultry, swine, and bovine meat have been extensively studied and monitored for VRE contamination [33,34,35,36,37,38,39,40,41,42,43,44,45,46], dairy products, particularly raw milk, and raw milk cheeses, have received comparatively less attention. However, these products may represent potential vectors for VRE transmission.
Indeed, raw milk can easily become contaminated with faecal enterococci during milking procedures due to inadequate hygienic practices, such as improper udder cleaning, use of contaminated equipment (e.g., teat cups, milking machines) or poor sanitation of milking parlour surfaces [47]. Moreover, the high tolerance of enterococci to adverse conditions [48,49], combined with their biofilm-forming ability that promotes strong surface adhesion [50], supports their prolonged environmental persistence and increases the risk of milk contamination during milking.
Vancomycin resistance screening revealed a high prevalence (29.14%) of vanC genes in bulk milk samples, while vanA and vanB genes were not detected. vanC genes are typically chromosomally encoded and primarily found in E. gallinarum, E. casseliflavus, and E. flavescens. They confer low level vancomycin resistance (MIC 4–32 mg/L) and susceptibility to teicoplanin [51]. Therefore, they are considered “less virulent” compared to the plasmid-encoded vanA and vanB genes, associated with high level of vancomycin resistance, MDR profile and most human outbreaks [52]. However, the past decade has witnessed an increasing number of vanC isolates associated with human disease [53,54,55,56,57,58,59,60,61,62,63,64,65]. This trend highlights the growing clinical relevance of vanC enterococci, even though vanA and vanB VRE remain the primary concern.
Despite the high prevalence of vanC genes detected in bulk milk samples, their occurrence in mastitic milk was limited to just two Enterococcus strains, identified as vanC2/3 E. casseliflavus, and vanC1 E. gallinarum. Once again, vanA and vanB genes were not identified in individual milk samples.
Notably, vanC strains were MDR to several critical antimicrobials, including vancomycin, quinupristin/dalfopristin, linezolid, daptomycin, and ciprofloxacin, challenging the long-standing assumption of VanC phenotypes as “low-virulent” pathogens. From a genomic perspective, both vanC strains harboured the whole vanC operon consisting of five genes (vanC, vanXY, vanT, vanR, and vanS) encoding for proteins involved in vancomycin resistance (VanC ligase, VanXY D, D-peptidase, and VanT serine racemase), as well as in the regulation of resistance gene expression (VanR/VanS two-component regulatory system). These findings are consistent with previous reports on vancomycin resistance gene clusters in these Enterococcus species [66]. Additionally, vanC enterococci were found to harbour a wide range of VAGs associated with key pathogenic mechanisms such as adhesion, immune evasion, biofilm formation, invasion, and surface protein anchoring. These genetic determinants, detailed in Table 2, likely contribute to the ability of vanC2/3 E. casseliflavus and vanC1 E. gallinarum isolated in this study to establish infection and cause mastitis.
Our data suggest that raw bovine milk and related products may serve as a source of emerging pathogenic vanC enterococci, while they are unlikely to represent a significant reservoir of the most clinically relevant vanA and vanB genes, as also reported by other authors [25,28,67,68,69].
Notably, mastitogenic enterococci from individual milk were associated with other critical resistances. E. faecium and E. faecalis, representing the most common Enterococcus species responsible for human infections [70], showed high MDR rate (E. faecium, 76/104, 73.08%; E. faecalis, 23/34, 67.65%), including to antimicrobials commonly used for enterococcosis treatments.
Ampicillin, high-dosage gentamycin/ampicillin association and vancomycin are considered recommended therapeutic options in case of enterococcal infections [71]. Resistance to these antimicrobials is routinely monitored by the European Antimicrobial Resistance Surveillance Network (EARS-Net). According to the last EARS Net-Annual Epidemiological Report, in 2023, more than nine-tenths (92.8%) of the invasive E. faecium isolates reported in the European countries were resistant to at least one of the antimicrobial groups under surveillance, meanwhile the percentage of high-level gentamicin resistance in E. faecalis was 24.3% [11]. Interestingly, in our study, resistance to high-dosage gentamycin and ampicillin was observed in nine and eight strains, respectively, mostly associated with E. faecium isolates. Notably, four E. faecium strains were resistant to both antimicrobials.
Treatment options for VRE infections are limited, with daptomycin and linezolid being the preferred first-line antibiotics [71]. Other antimicrobials such as quinupristin/dalfopristin and tigecycline might also be useful as salvage therapy [71], as well as fluoroquinolones and tetracycline in combination with other molecules in case of sepsis [72]. Concerningly, in this study, Enterococcus strains isolated from mastitic milk showed non-susceptibility to all aforementioned antimicrobials, with resistance rates ranging from 5% to 63%. The significant associations between E. faecalis and quinupristin/dalfopristin, tetracycline resistance and E. faecium, and ciprofloxacin and linezolid resistance were in accordance with previous results [73,74,75]. Linezolid resistance is typically reported as comparable between the two species [75,76,77,78] and our findings were consistent with this observation.
High resistance rates detected in mastitogenic enterococci are concerning as these bacteria could represent a source of AMR for humans. Mastitic milk is not intended for human consumption, as it may contain pathogens, potentially leading to foodborne illness and diffusion of AMR to humans, especially through the consumption of raw milk and related products [79,80]. In cases of severe clinical mastitis, abnormalities in the milk are easily detectable and the milk is typically discarded [81]. However, milk from cows with subclinical mastitis, where no visible changes are present, may inadvertently be mixed with bulk tank milk, entering the food chain and posing a potential hazard to consumers [82].
Furthermore, the excretion of mastitogenic AMR enterococci by infected cows can result in environmental contamination (especially of organic bedding materials, manure, wet or damp areas within barns or pastures and milking equipment) [83,84], which may subsequently lead to the contamination of milk intended for human consumption.
Therefore, mastitogenic milk may pose a risk to human health, either by directly contaminating milk intended for human consumption or by indirectly affecting the hygiene of the milking environment.
Preventing and controlling mastitis is therefore essential to ensure safe milk production and to reduce the spread of antimicrobial-resistant pathogens to humans [85].
Finally, our findings highlight the importance of molecular screening of van genes in bulk milk as an effective method for monitoring VRE in the dairy supply chain. Studies investigating the presence of van genes in bulk milk are limited and often focus solely on a narrow range of Enterococcus species, primarily E. faecalis and E. faecium [86,87,88,89]. Moreover, many of them rely exclusively on microbiological approaches, without considering molecular screening for van gene detection [86,87,88,89,90,91,92]. These limitations may hinder a comprehensive understanding of the dynamics of VRE transmission through raw milk, as evidenced by the absence or low detection rates of vanC enterococci reported in previous investigations. Conversely, molecular techniques offer high sensitivity and specificity, enable the rapid analysis of large numbers of samples, and provide valuable epidemiological insights into the role of raw milk in the transmission dynamics of VRE to humans.
4. Materials and Methods
4.1. Sample Collection
The study included bovine milk samples submitted to the IZSLER Laboratories in the Emilia Romagna region, Italy, between December 2022 and June 2024. Two types of samples were included in the study: (i) bulk milk samples, assessed for chemical parameters in accordance with the Italian payment programme based on milk quality, (ii) individual milk samples, analysed for routine mastitis prevention and control programmes. Samples were collected from dairy farms located in different Italian regions, predominantly in Emilia-Romagna, for a total of 1026 bulk milk samples and more than 80,000 individual milk samples. They were transported and stored at 4 °C until bacteriological and chemical evaluation. Information on the sampling procedures, farm treatments, and biosecurity measures in the farms included in the study was not provided by suppliers.
4.2. Van Gene Screening of Bulk Milk Samples
After homogenisation, 1 mL of each bulk milk sample was pre-enriched in 9 mL of Enterococcosel Broth (Oxoid), supplemented with 20 µg/mL vancomycin (Thermo Fisher Scientific, Waltham, MA, USA) and incubated at 37 ± 1 °C for 48 h. Genomic DNA was then extracted using the Biosprint 96 (Qiagen, Singapore) automated system with the MagMax CORE Nucleic Acid Purification Kit (Applied Biosystems, Thermo Fisher Scientific), following the manufacturer’s instructions. The presence of vancomycin resistance genes (vanA, vanB, vanC1, and vanC2/3) was investigated by multiplex PCR, following the protocol described by Dutka-Malen et al. [93].
4.3. Isolation and Identification of Enterococcus Isolates from Individual Milk Samples
Individual milk samples, tested for routine mastitis prevention and control programmes, were plated onto Aesculin Blood Agar (Oxoid) and incubated at 37 ± 1 °C for 24–48 h, following the National Mastitis Council protocols [29]. Aesculin-fermenting colonies were tested for Gram stain, catalase activity, and growth on Kanamycin Aesculin Azide Agar (Oxoid), incubated at 37 ± 1 °C for 24 h. Presumptive Enterococcus isolates were identified by matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometry.
4.3.1. Antimicrobial Resistance Phenotyping and Van Gene Detection in Enterococcus Isolates
AMR profiles were evaluated by broth microdilution method on Sensititre® EU Surveillance EUVENC plates (Thermo Fisher Scientific), according to the Clinical and Laboratory Standard Institute (CLSI) guideline [94]. Minimal inhibitory concentration (MIC) was assessed for 12 antibiotics, namely vancomycin, teicoplanin, quinupristin/dalfopristin, tetracycline, daptomycin, ciprofloxacin, erythromycin, tigecycline, linezolid, gentamicin, ampicillin, and chloramphenicol. The antimicrobial panel was proposed by Commission Implementing Decision 2020/1729/EU on the monitoring and reporting of AMR in zoonotic and commensal bacteria. MIC results were interpreted according to CLSI clinical breakpoints [94,95], or when not available, to the EUCAST clinical ones [96]. Strains were categorised as “susceptible” or “non-susceptible”, where the term “non-susceptible” encompasses both resistant and intermediate isolates, according to the definition of Sweeney et al. [97]. Isolates that were non-susceptible to at least three different antimicrobial classes were considered multidrug-resistant (MDR). Only acquired AMRs were considered to address a strain as MDR [98].
The AMR profiles of the whole collection are shown as a heatmap (Figure 1), depicting non-susceptibility (violet) or susceptibility (beige) to different antimicrobials. The dendogram on the left clusters the strains according to the similarities in their AMR profiles. This graphical representation was generated using R software version 3.5.1 (R Foundation for Statistical Computing) and RStudio version 1.1.463 (RStudio Inc., Boston, MA, USA). Finally, strains not susceptible to vancomycin (MIC ≥ 8 µg/mL) were tested for van gene presence by multiplex PCR [93].
4.3.2. Whole Genome Sequencing (WGS) and Assembly
The whole genomes of VRE strains were sequenced to deeply analyse their genomic features. Bacterial isolates were suspended in 1 mL of physiological solution and genomic DNA was extracted using the Nucleospin Tissue kit (Machery-Naghel), according to the manufacturer’s instructions. DNA concentrations were quantified with the QuantiFluor® ONE dsDNA System using the Quantus Fluorometer (Promega, Madison, WI, USA).
Genomic libraries were prepared using the Illumina DNA Prep (M) Tagmentation Kit (Illumina, San Diego, CA) and WGS was performed on the MiniSeq System (Illumina), generating 2 × 150 bp paired-end reads.
Raw reads were uploaded to the KBase platform [99]. Read quality was assessed using FastQC (version 0.12.1) (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/, accessed on 12 February 2025), which evaluated average read length, duplication levels, and GC content distribution. Trimmomatic v0.36 [100] was employed to trim and filter raw reads using the following parameters: sliding window size = 4, sliding window minimum quality = 20, minimum read length = 50. Quality-filtered reads were assembled using SPAdes version 3.15.3 [101], specifying the kmer list (21, 33, 55, 77, 99) and a minimum contig length of 1000 bp. The quality of the assemblies was assessed using QUAST version 4.4 [102].
4.3.3. Detection of Antimicrobial Resistance Genes, Virulence Factors, and Multi-Locus Sequence Typing (MLST)
Antimicrobial resistance genes were identified using the Resistance Gene Identifier tool (RGI, version 6.0.3, “main mode”) with the Comprehensive Antibiotic Resistance Database (CARD, version 4.0.0) [103]. The dataset included “strict” and “perfect” hits, while “loose” and “nudged” hits were excluded. Hits defined “nudged”, characterised by an identity > 95%, were reintroduced in the dataset only if the “percentage length of reference sequence” was >80%. Virulence genes were detected using the VFanalyzer pipeline (https://www.mgc.ac.cn/cgi-bin/VFs/v5/main.cgi, accessed on 12 February 2025) and the Virulence Factors Database (VFDB) (http://www.mgc.ac.cn/VFs/, accessed on 12 February 2025). Only hits that delivered an output with a gene name (rather than a numeric ID) were considered.
MLST analysis was performed using the PubMLST web interface (https://pubmlst.org/organisms, accessed on 12 February 2025) and the BIGSdb software [104] to characterise the isolates and determine their sequence types (STs). All the sequencing data of the Enterococcus isolates from this study are deposited in the National Centre for Biotechnology Information (NCBI) database as BioProject PRJNA1272009.
4.4. Statistical Analysis
Descriptive statistics (absolute frequencies and percentages) were performed considering (i) van gene distribution in bulk milk samples, (ii) enterococcal species, occurrence, and AMR characteristics (non-susceptibility to antimicrobials, MDR profiles) of Enterococcus spp. isolates from individual milk samples. Pearson’s Chi-square test or Fisher’s exact test was applied to compare the phenotypic AMR and MDR profiles of E. faecalis and E. faecium strains in order to identify statistically significant differences between the two species. Significance was set at a p-value < 0.001. Statistical analyses were performed with SPSS version 27 (IBM).
5. Conclusions
This study provides important insights into the potential role of raw milk in the dissemination of vancomycin-resistant enterococci, particularly those harbouring vanC genes.
Notably, the dairy supply chain does not seem to be involved in the transmission of vancomycin resistance genes of the highest clinical importance (i.e., vanA and vanB genes). However, a high prevalence of vanC genes was detected in bulk milk samples, as well as in two Enterococcus strains isolated from mastitic milk. These findings suggest that raw milk may represent a potential route of transmission for vanC enterococci, which have increasingly been implicated in human clinical infections and nosocomial outbreaks in recent years.
Furthermore, the high prevalence of MDR E. faecium and E. faecalis strains, showing resistance to critical antimicrobials such as linezolid, daptomycin, and high-dosage gentamicin, raises significant public health concerns. MDR strains from mastitic milk, particularly in subclinical infections, may accidentally contaminate milk intended for human consumption and consequently be transmitted to humans through dairy products.
Finally, the present study suggests how molecular-based methods are useful for VRE monitoring in the dairy supply chain. Such approaches show enhanced sensitivity and specificity compared to conventional microbiological methods and provide important epidemiological insight into the role of raw milk in the transmission of VRE to humans.
Author Contributions
Conceptualization, E.M.; methodology, E.M.; software, M.R., A.D.C., T.S. and G.B.; formal analysis, E.M., M.R., A.D.C., T.S. and G.B.; investigation, E.M., S.R., A.F., C.A.G., P.B., E.T., C.T., G.P., G.R., V.C., C.B. and A.L.; resources, E.M.; data curation, E.M., A.D.C., T.S. and G.B.; writing—original draft preparation, E.M.; writing—review and editing, E.M., S.R., A.F., C.A.G., P.B., E.T., C.T., G.P., G.R., V.C., C.B., A.D.C., T.S., G.B. and A.L.; visualization, E.M.; supervision, A.L.; project administration, E.M.; funding acquisition, E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The Genus Enterococcus: Between Probiotic Potential and Safety Concerns-An Update. Front. Microbiol. 2018, 9, 1791. [Google Scholar] [CrossRef]
- Sirichoat, A.; Florez, A.B.; Vazquez, L.; Buppasiri, P.; Panya, M.; Lulitanond, V.; Mayo, B. Antibiotic Resistance-Susceptibility Profiles of Enterococcus faecalis and Streptococcus spp. From the Human Vagina, and Genome Analysis of the Genetic Basis of Intrinsic and Acquired Resistances. Front. Microbiol. 2020, 11, 1438. [Google Scholar] [CrossRef]
- Sadowy, E.; Luczkiewicz, A. Drug-resistant and hospital-associated Enterococcus faecium from wastewater, riverine estuary and anthropogenically impacted marine catchment basin. BMC Microbiol. 2014, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Micallef, S.A.; Goldstein, R.E.; George, A.; Ewing, L.; Tall, B.D.; Boyer, M.S.; Joseph, S.W.; Sapkota, A.R. Diversity, distribution and antibiotic resistance of Enterococcus spp. recovered from tomatoes, leaves, water and soil on U.S. Mid-Atlantic farms. Food Microbiol. 2013, 36, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Byappanahalli, M.N.; Nevers, M.B.; Korajkic, A.; Staley, Z.R.; Harwood, V.J. Enterococci in the environment. Microbiol. Mol. Biol. Rev. 2012, 76, 685–706. [Google Scholar] [CrossRef]
- Said, M.S.; Tirthani, E.; Lesho, E. Enterococcus Infections; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Brinkwirth, S.; Ayobami, O.; Eckmanns, T.; Markwart, R. Hospital-acquired infections caused by enterococci: A systematic review and meta-analysis, WHO European Region, 1 January 2010 to 4 February 2020. Eurosurveillanca 2021, 26, 2001628. [Google Scholar] [CrossRef] [PubMed]
- Suppli, M.; Aabenhus, R.; Harboe, Z.B.; Andersen, L.P.; Tvede, M.; Jensen, J.U. Mortality in enterococcal bloodstream infections increases with inappropriate antimicrobial therapy. Clin. Microbiol. Infect. 2011, 17, 1078–1083. [Google Scholar] [CrossRef]
- EUCAST. Expected Resistant Phenotypes, Version 1.2 January 2023. Available online: https://www.eucast.org/expert_rules_and_expected_phenotypes/expected_phenotypes (accessed on 10 June 2025).
- ECDC. Surveillance Atlas of Infectious Diseases. Available online: https://www.ecdc.europa.eu/en/surveillance-atlas-infectious-diseases (accessed on 10 June 2025).
- ECDC. Antimicrobial Resistance in the EU/EEA (EARS-Net)-Annual Epidemiological Report 2023. Available online: https://www.eucast.org/ (accessed on 10 May 2025).
- ECDC. Antimicrobial Resistance Surveillance in Europe 2023, 2021 Data. Available online: https://www.eucast.org/ (accessed on 10 June 2025).
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: https://www.who.int/ (accessed on 10 June 2025).
- Li, G.; Walker, M.J.; De Oliveira, D.M.P. Vancomycin Resistance in Enterococcus and Staphylococcus aureus. Microorganisms 2023, 11, 24. [Google Scholar] [CrossRef]
- Noble, W.C.; Virani, Z.; Cree, R.G. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol. Lett. 1992, 72, 195–198. [Google Scholar] [CrossRef]
- Acar, J.; Casewell, M.; Freeman, J.; Friis, C.; Goossens, H. Avoparcin and virginiamycin as animal growth promoters: A plea for science in decision-making. Clin. Microbiol. Infect. 2000, 6, 477–482. [Google Scholar] [CrossRef]
- Collignon, P.J. Vancomycin-resistant enterococci and use of avoparcin in animal feed: Is there a link? Med. J. Aust. 1999, 171, 144–146. [Google Scholar] [CrossRef]
- Cebeci, T. Species prevalence, virulence genes, and antibiotic resistance of enterococci from food-producing animals at a slaughterhouse in Turkey. Sci. Rep. 2024, 14, 13191. [Google Scholar] [CrossRef]
- Milk and Milk Product Statistics. Available online: https://ec.europa.eu/eurostat (accessed on 22 July 2025).
- Giacometti, F.; Bonilauri, P.; Piva, S.; Scavia, G.; Amatiste, S.; Bianchi, D.; Losio, M.; Bilei, S.; Cascone, G.; Comin, D. Paediatric HUS cases related to the consumption of raw milk sold by vending machines in Italy: Quantitative risk assessment based on Escherichia coli O157 official controls over 7 years. Zoonoses Public Health 2017, 64, 505–516. [Google Scholar] [CrossRef]
- Guh, A.; Phan, Q.; Randall, N.; Purviance, K.; Milardo, E.; Kinney, S.; Mshar, P.; Kasacek, W.; Cartter, M. Outbreak of Escherichia coli O157 associated with raw milk, Connecticut, 2008. Clin. Infect. Dis. 2010, 51, 1411–1417. [Google Scholar] [CrossRef]
- Jaakkonen, A.; Salmenlinna, S.; Rimhanen-Finne, R.; Lundström, H.; Heinikainen, S.; Hakkinen, M.; Hallanvuo, S. Severe outbreak of sorbitol-fermenting Escherichia coli O157 via unpasteurized milk and farm visits, Finland 2012. Zoonoses Public Health 2017, 64, 468–475. [Google Scholar] [CrossRef]
- Jung, W.K.; Lim, J.Y.; Kwon, N.H.; Kim, J.M.; Hong, S.K.; Koo, H.C.; Kim, S.H.; Park, Y.H. Vancomycin-resistant enterococci from animal sources in Korea. Int. J. Food Microbiol. 2007, 113, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.J.; Cho, S.S.; Kim, B.S. Isolation, Identification and Characterization of Ynncomycin-Resistant Enterococci from Raw Milk. J. Microbiol. 2002, 40, 170–172. [Google Scholar]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; García-Solache, M. Ready-to-eat dairy products as a source of multidrug-resistant Enterococcus strains: Phenotypic and genotypic characteristics. J. Dairy Sci. 2020, 103, 4068–4077. [Google Scholar] [CrossRef]
- Moraes, P.M.; Perin, L.M.; Todorov, S.D.; Silva Jr, A.; Franco, B.D.G.d.M.; Nero, L.A. Bacteriocinogenic and virulence potential of Enterococcus isolates obtained from raw milk and cheese. J. Appl. Microbiol. 2012, 113, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Paschoalini, B.R.; Nunez, K.V.M.; Maffei, J.T.; Langoni, H.; Guimaraes, F.F.; Gebara, C.; Freitas, N.E.; Dos Santos, M.V.; Fidelis, C.E.; Kappes, R.; et al. The Emergence of Antimicrobial Resistance and Virulence Characteristics in Enterococcus Species Isolated from Bovine Milk. Antibiotics 2023, 12, 1243. [Google Scholar] [CrossRef]
- Gołaś-Prądzyńska, M.; Łuszczyńska, M.; Rola, J.G. Dairy Products: A potential source of Multidrug-Resistant Enterococcus faecalis and Enterococcus faecium Strains. Foods 2022, 11, 4116. [Google Scholar] [CrossRef]
- Adkins, P.R.F.; Middleton, J.R.; Fox, L.K.; Pighetti, G.; Petersson-Wolfe, C.; Council, N.M. Laboratory Handbook on Bovine Mastitis; National Mastitis Council: New Prague, MN, USA, 2017. [Google Scholar]
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Guimarães, J.T.; Cruz, A.G.; Sant’Ana, A.S. Hazards of a ‘healthy’trend? An appraisal of the risks of raw milk consumption and the potential of novel treatment technologies to serve as alternatives to pasteurization. Trends Food Sci. Technol. 2018, 82, 148–166. [Google Scholar] [CrossRef]
- García-Solache, M.; Rice, L.B. The Enterococcus: A model of adaptability to its environment. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef]
- Robredo, B.; Singh, K.V.; Baquero, F.; Murray, B.E.; Torres, C. Vancomycin-resistant enterococci isolated from animals and food. Int. J. Food Microbiol. 2000, 54, 197–204. [Google Scholar] [CrossRef]
- Messi, P.; Guerrieri, E.; de Niederhausern, S.; Sabia, C.; Bondi, M. Vancomycin-resistant enterococci (VRE) in meat and environmental samples. Int. J. Food Microbiol. 2006, 107, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Kanki, M.; Kawai, T.; Taguchi, M.; Asao, T.; Kumeda, Y. Isolation of VanA-type vancomycin-resistant Enterococcus strains from domestic poultry products with enrichment by incubation in buffered peptone water at 42 °C. Appl. Environ. Microbiol. 2010, 76, 5317–5320. [Google Scholar] [CrossRef][Green Version]
- Aslam, M.; Diarra, M.S.; Checkley, S.; Bohaychuk, V.; Masson, L. Characterization of antimicrobial resistance and virulence genes in Enterococcus spp. isolated from retail meats in Alberta, Canada. Int. J. Food Microbiol. 2012, 156, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Klibi, N.; Said, L.B.; Jouini, A.; Slama, K.B.; Lopez, M.; Sallem, R.B.; Boudabous, A.; Torres, C. Species distribution, antibiotic resistance and virulence traits in enterococci from meat in Tunisia. Meat Sci. 2013, 93, 675–680. [Google Scholar] [CrossRef]
- Pesavento, G.; Calonico, C.; Ducci, B.; Magnanini, A.; Lo Nostro, A. Prevalence and antibiotic resistance of Enterococcus spp. isolated from retail cheese, ready-to-eat salads, ham, and raw meat. Food Microbiol. 2014, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zarfel, G.; Galler, H.; Luxner, J.; Petternel, C.; Reinthaler, F.F.; Haas, D.; Kittinger, C.; Grisold, A.J.; Pless, P.; Feierl, G. Multiresistant bacteria isolated from chicken meat in Austria. Int. J. Environ. Res. Public Health 2014, 11, 12582–12593. [Google Scholar] [CrossRef]
- Bortolaia, V.; Espinosa-Gongora, C.; Guardabassi, L. Human health risks associated with antimicrobial-resistant enterococci and Staphylococcus aureus on poultry meat. Clin. Microbiol. Infect. 2016, 22, 130–140. [Google Scholar] [CrossRef]
- Rizzotti, L.; Rossi, F.; Torriani, S. Biocide and antibiotic resistance of Enterococcus faecalis and Enterococcus faecium isolated from the swine meat chain. Food Microbiol. 2016, 60, 160–164. [Google Scholar] [CrossRef]
- Madanipour, E.; Mehrabi, M.R.; Mirzaee, M. The antibiotic susceptibility pattern and prevalence of vanA, vanB, and vanC genes among Enterococcus faecalis strains isolated from consumed meat. Infect. Epidemiol. Microbiol. 2017, 3, 117–121. [Google Scholar]
- Tyson, G.H.; Nyirabahizi, E.; Crarey, E.; Kabera, C.; Lam, C.; Rice-Trujillo, C.; McDermott, P.F.; Tate, H. Prevalence and antimicrobial resistance of enterococci isolated from retail meats in the United States, 2002 to 2014. Appl. Environ. Microbiol. 2018, 84, e01902–e01917. [Google Scholar] [CrossRef]
- SWAB. NethMap 2019: Consumption of Antimicrobial Agents and Antimicrobial Resistance Among Medically Important Bacteria in the Netherlands/MARAN 2019: Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2018. Available online: https://edepot.wur.nl/ (accessed on 10 June 2025).
- Sabença, C.; de Sousa, T.; Oliveira, S.; Viala, D.; Theron, L.; Chambon, C.; Hébraud, M.; Beyrouthy, R.; Bonnet, R.; Caniça, M. Next-generation sequencing and MALDI mass spectrometry in the study of multiresistant processed meat vancomycin-resistant enterococci (VRE). Biology 2020, 9, 89. [Google Scholar] [CrossRef]
- Caddey, B.; Shaukat, W.; Tang, K.L.; Barkema, H.W. Vancomycin-resistant Enterococcus prevalence and its association along the food chain: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2025, 80, 908–918. [Google Scholar] [CrossRef]
- de Lurdes Enes Dapkevicius, M.; Sgardioli, B.; Câmara, S.P.; Poeta, P.; Malcata, F.X. Current trends of enterococci in dairy products: A comprehensive review of their multiple roles. Foods 2021, 10, 821. [Google Scholar] [CrossRef]
- Heller, L.C.; Edelblute, C.M. Long-term metabolic persistence of gram-positive bacteria on health care-relevant plastic. Am. J. Infect. Control 2018, 46, 50–53. [Google Scholar] [CrossRef]
- Grund, A.; Rautenschlein, S.; Jung, A. Tenacity of Enterococcus cecorum at different environmental conditions. J. Appl. Microbiol. 2021, 130, 1494–1507. [Google Scholar] [CrossRef]
- Geraldes, C.; Tavares, L.; Gil, S.; Oliveira, M. Enterococcus virulence and resistant traits associated with its permanence in the hospital environment. Antibiotics 2022, 11, 857. [Google Scholar] [CrossRef]
- Cetinkaya, Y.; Falk, P.; Mayhall, C.G. Vancomycin-resistant enterococci. Clin. Microbiol. Rev. 2000, 13, 686–707. [Google Scholar] [CrossRef] [PubMed]
- Mazuski, J.E. Vancomycin-resistant Enterococcus: Risk factors, surveillance, infections, and treatment. Surg. Infect. 2008, 9, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Pappas, G.; Liberopoulos, E.; Tsianos, E.; Elisaf, M. Enterococcus casseliflavus bacteremia. Case report and literature review. J. Infect. 2004, 48, 206–208. [Google Scholar] [CrossRef]
- Gikas, A.; Christidou, A.; Scoulica, E.; Nikolaidis, P.; Skoutelis, A.; Levidiotou, S.; Kartali, S.; Maltezos, E.; Metalidis, S.; Kioumis, J.; et al. Epidemiology and molecular analysis of intestinal colonization by vancomycin-resistant enterococci in Greek hospitals. J. Clin. Microbiol. 2005, 43, 5796–5799. [Google Scholar] [CrossRef][Green Version]
- Contreras, G.A.; DiazGranados, C.A.; Cortes, L.; Reyes, J.; Vanegas, S.; Panesso, D.; Rincon, S.; Diaz, L.; Prada, G.; Murray, B.E.; et al. Nosocomial outbreak of Enteroccocus gallinarum: Untaming of rare species of enterococci. J. Hosp. Infect. 2008, 70, 346–352. [Google Scholar] [CrossRef]
- Koganemaru, H.; Hitomi, S. Bacteremia caused by VanC-type enterococci in a university hospital in Japan: A 6-year survey. J. Infect. Chemother. 2008, 14, 413–417. [Google Scholar] [CrossRef]
- Sutter, S.T.; Frei, R.; Dangel, M.; Gratwohl, A.; Bonten, M.; Widmer, A. Not all patients with vancomycin-resistant enterococci need to be isolated. Clin. Infect. Dis. 2010, 51, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.K.; Lai, C.C.; Wang, J.Y.; Lin, S.H.; Liao, C.H.; Huang, Y.T.; Wang, C.Y.; Lin, H.I.; Hsueh, P.R. Bacteremia caused by non-faecalis and non-faecium Enterococcus species at a Medical center in Taiwan, 2000 to 2008. J. Infect. 2010, 61, 34–43. [Google Scholar] [CrossRef]
- Hillier, R.J.; Arjmand, P.; Rebick, G.; Ostrowski, M.; Muni, R.H. Post-traumatic vancomycin-resistant enterococcal endophthalmitis. J. Ophthalmic Inflamm. Infect. 2013, 3, 42. [Google Scholar] [CrossRef]
- Berenger, B.M.; Kulkarni, S.; Hinz, B.J.; MD, S.E.F. Exogenous endophthalmitis caused by Enterococcus casseliflavus: A case report and discussion regarding treatment of intraocular infection with vancomycin-resistant enterococci. Can. J. Infect. Dis. Med. Microbiol. 2015, 26, 330–332. [Google Scholar] [CrossRef]
- Nguyen, J.; Hartnett, M.E. Successful management of post-traumatic vancomycin-resistant enterococcus endophthalmitis. Am. J. Ophthalmol. Case Rep. 2017, 5, 117–118. [Google Scholar] [CrossRef]
- Zhao, B.; Ye, M.S.; Zheng, R. Enterococcus gallinarum meningitis: A case report and literature review. BMC Infect. Dis. 2018, 18, 231. [Google Scholar] [CrossRef]
- Okumura, N.; Watanabe, T.; Teranishi, S.; Suzuki, D.; Hashimoto, T.; Takahashi, K.; Hara, T. Successful treatment of aortic valve endocarditis caused by Enterococcus casseliflavus: A case report. BMC Infect. Dis. 2021, 21, 447. [Google Scholar] [CrossRef]
- Şahin, M.H.; Temtek, U. Enterococcus gallinarum group meningitis after transanal migration of the ventriculoperitoneal shunt: A pediatric case report. Childs Nerv. Syst. 2023, 39, 1093–1096. [Google Scholar] [CrossRef]
- Xu, S.; Huang, B.; Cao, Y.; Zhong, Z.; Yin, J. Polycystic intrahepatic infection caused by Enterococcus casseliflavus: A case report and literature review. BMC Nephrol. 2024, 25, 88. [Google Scholar] [CrossRef]
- Courvalin, P. Vancomycin resistance in gram-positive cocci. Clin. Infect. Dis. 2006, 42 (Suppl. S1), S25–S34. [Google Scholar] [CrossRef] [PubMed]
- Morandi, S.; Brasca, M.; Andrighetto, C.; Lombardi, A.; Lodi, R. Technological and molecular characterisation of enterococci isolated from north–west Italian dairy products. Int. Dairy J. 2006, 16, 867–875. [Google Scholar] [CrossRef]
- Hammad, A.M.; Hassan, H.A.; Shimamoto, T. Prevalence, antibiotic resistance and virulence of Enterococcus spp. in Egyptian fresh raw milk cheese. Food Control 2015, 50, 815–820. [Google Scholar] [CrossRef]
- Morandi, S.; Silvetti, T.; Lopreiato, V.; Piccioli-Cappelli, F.; Trevisi, E.; Brasca, M. Biodiversity and antibiotic resistance profile provide new evidence for a different origin of enterococci in bovine raw milk and feces. Food Microbiol. 2024, 120, 104492. [Google Scholar] [CrossRef] [PubMed]
- Klaas, I.C.; Zadoks, R.N. An update on environmental mastitis: Challenging perceptions. Transbound. Emerg. Dis. 2018, 65 (Suppl. S1), 166–185. [Google Scholar] [CrossRef] [PubMed]
- Turco, E.; Bartoletti, M.; Dahl, A.; Cervera, C.; Pericàs, J. How do I manage a patient with enterococcal bacteraemia. Clin. Microbiol. Infect. 2021, 27, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.; Contreras, G.A.; Murray, B.E. Management of multidrug-resistant enterococcal infections. Clin. Microbiol. Infect. 2010, 16, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Mengeloğlu, F.Z.; Çakır, D.; Terzi, H.A. Comparison of resistance in isolates of Enterococcus faecalis and Enterococcus faecium. J. Microbiol. Infect. Dis. 2011, 1, 10–13. [Google Scholar] [CrossRef]
- Jabbari Shiadeh, S.M.; Pormohammad, A.; Hashemi, A.; Lak, P. Global prevalence of antibiotic resistance in blood-isolated Enterococcus faecalis and Enterococcus faecium: A systematic review and meta-analysis. Infect. Drug Resist 2019, 12, 2713–2725. [Google Scholar] [CrossRef]
- Dadashi, M.; Sharifian, P.; Bostanshirin, N.; Hajikhani, B.; Bostanghadiri, N.; Khosravi-Dehaghi, N.; van Belkum, A.; Darban-Sarokhalil, D. The Global prevalence of daptomycin, tigecycline, and linezolid-resistant Enterococcus faecalis and Enterococcus faecium strains from human clinical samples: A systematic review and meta-analysis. Front. Med. 2021, 8, 720647. [Google Scholar] [CrossRef]
- Rathnayake, I.U.; Hargreaves, M.; Huygens, F. Antibiotic resistance and virulence traits in clinical and environmental Enterococcus faecalis and Enterococcus faecium isolates. Syst. Appl. Microbiol. 2012, 35, 326–333. [Google Scholar] [CrossRef]
- Jia, W.; Li, G.; Wang, W. Prevalence and antimicrobial resistance of Enterococcus species: A hospital-based study in China. Int. J. Environ. Res. Public Health 2014, 11, 3424–3442. [Google Scholar] [CrossRef]
- Georges, M.; Odoyo, E.; Matano, D.; Tiria, F.; Kyany’a, C.; Mbwika, D.; Mutai, W.C.; Musila, L. Determination of Enterococcus faecalis and Enterococcus faecium antimicrobial resistance and virulence factors and their association with clinical and demographic factors in Kenya. J. Pathog. 2022, 2022, 3129439. [Google Scholar] [CrossRef]
- Leedom, J.M. Milk of nonhuman origin and infectious diseases in humans. Clin. Infect. Dis. 2006, 43, 610–615. [Google Scholar] [CrossRef]
- Ashraf, A.; Imran, M. Causes, types, etiological agents, prevalence, diagnosis, treatment, prevention, effects on human health and future aspects of bovine mastitis. Anim. Health Res. Rev. 2020, 21, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, L.; Loir, L. Mastitis impact on technological properties of milk and quality of milk products—A review. Dairy Sci. Technol. 2011, 91, 247–282. [Google Scholar] [CrossRef]
- Guzmán-Luna, P.; Nag, R.; Martínez, I.; Mauricio-Iglesias, M.; Hospido, A.; Cummins, E. Quantifying current and future raw milk losses due to bovine mastitis on European dairy farms under climate change scenarios. Sci. Total Environ. 2022, 833, 155149. [Google Scholar] [CrossRef]
- Asfaw, M.; Negash, A. Review on impact of bovine mastitis in dairy production. Adv. Biol. Res. 2017, 11, 126–131. [Google Scholar]
- Pal, M.; Regasa, A.; Gizaw, F. Etiology, pathogenesis, risk factors, diagnosis and management of bovine mastitis: A comprehensive review. Int. J. Anim. Vet. Sci. 2019, 6, 40–55. [Google Scholar]
- EFSA. Scientific Opinion on the Public Health Risks Related to the Consumption of Raw Drinking Milk. Available online: https://www.efsa.europa.eu/ (accessed on 10 June 2025).
- Kang, H.J.; Yoon, S.; Kim, K.; Lee, Y.J. Characteristics of high-level aminoglycoside-resistant Enterococcus faecalis isolated from bulk tank milk in Korea. Animals 2021, 11, 1724. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Lee, Y.J. Molecular characteristics of Enterococcus faecalis and Enterococcus faecium from bulk tank milk in Korea. Animals 2021, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- Hammad; Aly, S.S.; Hassan, H.A.; Abbas, N.H.; Eltahan, A.; Khalifa, E.; Shimamoto, T. Occurrence, phenotypic and molecular characteristics of vancomycin-resistant enterococci isolated from retail raw milk in Egypt. Foodborne Pathog. Dis. 2022, 19, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, K.; Lee, Y.J. Dissemination and characteristics of high-level erythromycin-resistant Enterococcus faecalis from bulk tank milk of dairy companies in Korea. Can. J. Vet. Res. 2023, 87, 51–58. [Google Scholar]
- Schlegelova, J.; Babak, V.; Klimova, E.; Lukasova, J.; Navratilova, P.; Sustackova, A.; Sediva, I.; Rysanek, D. Prevalence of and resistance to anti-microbial drugs in selected microbial species isolated from bulk milk samples. J. Vet. Med. B Infect. Dis. Vet. Public Health 2002, 49, 216–225. [Google Scholar] [CrossRef]
- Kim, H.J.; Youn, H.Y.; Kang, H.J.; Moon, J.S.; Jang, Y.S.; Song, K.Y.; Seo, K.H. Prevalence and virulence characteristics of Enterococcus faecalis and Enterococcus faecium in bovine mastitis milk compared to bovine normal raw milk in South Korea. Animals 2022, 12, 1407. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, G.N.; Guimarães, F.; Fornazari, F.; Joaquim, S.F.; Guerra, S.T.; de França, D.A.; Possebon, F.S.; Pantoja, J.C.d.F.; Lucheis, S.B.; Rall, V.L.M. Antimicrobial susceptibility profile of Enterococcus species isolated from cows with clinical mastitis and from bulk milk tanks in Brazil. Afr. J. Microbiol. Res. 2023, 17, 29–34. [Google Scholar] [CrossRef]
- Dutka-Malen, S.; Evers, S.; Courvalin, P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 1995, 33, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Clinical & Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; CLSI Report VET01 ED5:2023; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Clinical & Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; CLSI Report M100 ED30:2020; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- EUCAST. Clinical Breakpoints (v 15.0). Available online: https://www.eucast.org/ (accessed on 10 June 2025).
- Sweeney, M.T.; Lubbers, B.V.; Schwarz, S.; Watts, J.L. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J. Antimicrob. Chemother. 2018, 73, 1460–1463. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Arkin, A.P.; Cottingham, R.W.; Henry, C.S.; Harris, N.L.; Stevens, R.L.; Maslov, S.; Dehal, P.; Ware, D.; Perez, F.; Canon, S.; et al. KBase: The United States Department of Energy Systems Biology Knowledgebase. Nat. Biotechnol. 2018, 36, 566–569. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A.; Edalatmand, A.; Petkau, A.; Syed, S.A.; Tsang, K.K. CARD 2023: Expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C. Open-access bacterial population genomics: BIGSdb software, the PubMLST. org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).